Abstract
The rotator cuff is composed of several distinct muscles and tendons that function in concert to coordinate shoulder motion. Injuries to these tendons frequently result in permanent dysfunction and persistent pain. Despite considerable advances in operation techniques, surgical repair alone still does not fully restore rotator cuff function. This review focuses on recent research in the use of biologics and stem cell-based therapies to augment repair, highlighting promising avenues for future work and remaining challenges. While a number of animal models are used for rotator cuff studies, the anatomy of the rotator cuff varies dramatically between species. Since the rodent rotator cuff shares the most anatomical features with the human, this review will focus primarily on rodent models to enable consistent interpretation of outcome measures.
Keywords: rotator cuff, tendon, injury, stem cells
Graphical abstract
Injuries to the tendons of the rotator cuff frequently result in permanent dysfunction and persistent pain. This review focuses on recent research in the use of biologics and stem cell-based therapies to augment surgical repair of the rotator cuff, highlighting promising avenues for future work and remaining challenges. Since the rodent rotator cuff shares the most anatomical features with the human, this review will focus primarily on rodent models to enable consistent interpretation of outcome measures.
Introduction
The rotator cuff is a collection of four muscles (the supraspinatus, infraspinatus, teres minor, and subscapularis) and their tendons. Each of these muscles plays a unique role in shoulder movement, but together, they provide strength and stability to the joint during motion. Common injuries to the rotator cuff typically involve the tendon; tendons can tear as a result of mechanical overuse (i.e., wear and tear) or become inflamed.1; 2 Injury to the rotator cuff tendons represents a significant socioeconomic burden, with over 4.5 million physician visits annually.3 With injury, shoulder function is impaired, resulting in weakness, decreased range of motion, and pain. In the absence of treatment, primary tears to tendons result in secondary degeneration of the muscle and atrophy. Of the four tendons, the supraspinatus tendon is the most frequently injured due to its unique anatomical location beneath the acromion bone, which impinges on the tendon during overhead motion (Fig. 1).4 For such tendon tears, surgical repair is often required to reattach the tendon back to its bony insertion. Unfortunately, surgical outcomes remain variable and failure rates can be extremely high in certain at-risk populations (up to 90% in the elderly).5 The key challenge in rotator cuff tendon healing is the failure to restore a mechanically functional attachment between the tendon and bone after injury. This critical attachment (termed the enthesis) is normally composed of a specialized transitional gradient of tendon, fibrocartilage, mineralized cartilage, and bone (Fig. 1).6–8 This gradient integrates two tissues of highly dissimilar material properties and allows for dissipation of high stresses that arise at the interface. Although surgical repair physically reattaches the tendon to bone (thereby restoring some function), the enthesis gradient is permanently disrupted. Instead the enthesis is replaced by disorganized scar tissue with impaired mechanical properties that may lead to re-rupture over time.9; 10
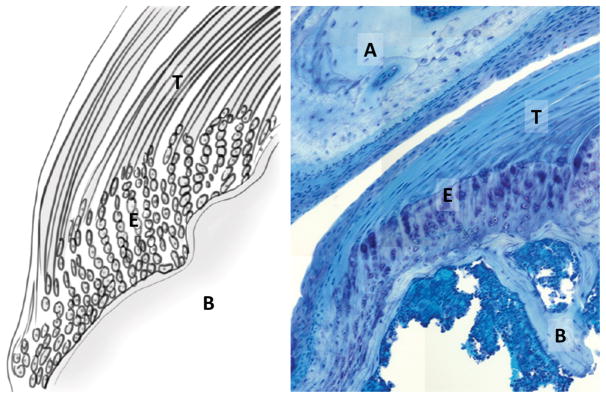
Figure 1.
Anatomy and cell morphology of the supraspinatus tendon enthesis. (Left) Schematic of the tendon-to-bone (T/B) insertion via fibrocartilage enthesis gradient. (Right) Toludine blue staining of the supraspinatus tendon inserting into the humeral head, beneath the acromion (plastic section, 20X). T: supraspinatus tendon, B: humeral bone; E: enthesis; A: acromion.
In recent years, the clinical community has focused mainly on advancing operation and suturing technologies to better restore the native biomechanical footprint of tendon attachment to bone. However, efficacy of these methods has been low or mixed, highlighting the limitations in suture- and cell-based approaches.11–15 Further improvements to rotator cuff repair will likely reside in the development of new cell- or biologically-based technologies to augment surgery. Advancing this area of research thus has vast potential to revolutionize orthopedic practice. In this review, we synthesize the literature on biomolecules and stem cell-based therapies in the most anatomically relevant preclinical model of rotator cuff repair, the rat, and identify current challenges and future directions for this exciting research.
Rotator cuff injury models
Animal models
To date, the most widely used animal models for rotator cuff injury and repair include the rat, sheep, and rabbit, with 276, 111, and 106 search results, respectively in Pubmed (as of April 2018). By comparison, search results for alternative models such as dog, cat, and goat totaled 64 collectively, highlighting the relative dominance of the top three models. While large animals such as sheep or goat are advantageous and necessary for testing medical devices (due to the similarity in scale to human), the anatomical positioning of the bony, tendinous/ligamentous, and muscular shoulder components is not comparable to humans, which limits their use in other contexts. A pioneering study evaluating 33 animal models revealed that only the rat possessed a shoulder anatomy similar to human, with a prominent supraspinatus tendon passing beneath an enclosed bony arch and inserting into the greater tuberosity of the proximal humerus.16 In humans and rats, this specific anatomy leads to impingement of the supraspinatus tendon with overhead forelimb activity, which results in biomechanically-mediated degeneration of the tendon over time. Thus, the absence of these important anatomic features limits the use of non-rat models for modeling clinically relevant rotator cuff conditions. In addition to these important differences in gross anatomy, the rotator cuff tendons in non-rat animal models are also extra-articular and the collagen structure is highly aligned, rather than interdigitated.17 Suture-based repairs are therefore challenging to model since the repairs fail within a very short period of time due to the highly aligned nature of the tendon structure.
Although the majority of rodent rotator cuff studies have been carried out in rat, the mouse shoulder anatomy was recently shown to be comparable to rat and human. The very small size of the mouse rotator cuff precluded its use in earlier studies; however, novel microsurgical techniques have now been applied to successfully create partial and full detachment injuries with surgical repair, thereby opening new avenues for mechanistic research.18–24 Despite the numerous advantages of rat models, limitations include scar-mediated healing (human tears do not undergo spontaneous healing), quadrupedal gait, and small scale relative to human.17 While joint replacement or other medical devices cannot be tested in rodent models, preclinical studies testing the efficacy of cell and biomolecule-based therapies are acceptable in rodents for meeting FDA standards. Since this review will focus on the potential of biologic therapies, the advantages of rodent models for these studies outweigh their limitations; this review will therefore focus on rat studies to allow consistent comparisons across studies.
Chronic/overuse models of tendon degeneration
In humans, rotator cuff tendon tears are typically preceded by degeneration of the tendon. Tendon degeneration in turn, is thought to be initiated by repeated mechanical impingement of tendon against bone, leading to pathological changes in the tissue structure and tendon cells.16; 25; 26 To model these degenerative changes, a number of approaches have been established that use either mechanical or chemical methods to induce tendon degeneration. To mechanically induce degeneration, initial studies in the rat rotator cuff focused on artificially creating subacromial impingement by attaching a tendon or bony piece under the acromion to constrict the supraspinatus tendon passing through the bony arch.26–28 This constriction led to degenerative changes in the tendon, evidenced by increased cellularity, rounder cell shape, and decreased collagen organization.16; 28 Subsequently, another model of overuse tendinopathy was developed using downhill treadmill running, which caused similar degenerative changes; this method can be applied either on its own29–31 or in combination with an artificial subacromial impingement.26; 28 Treadmill running is non-invasive, reproducible, and mimics the local mechanical conditions of supraspinatus impingement in humans, with decreases in tendon properties.29 Although this model does not result in spontaneous tears, it remains the gold standard for inducing chronic rotator cuff tendon pathology. To chemically induce degeneration, collagenase can be injected directly into the supraspinatus tendon to degrade the collagen structure.16; 32 Although collagenase treatment is experimentally less laborious and results in much more rapid changes to the tendon structure compared to the mechanical methods described above, this method is not commonly used since it does not recapitulate the clinical condition, which is largely mechanical in etiology. The degenerative effects of chronic/overuse models are also typically resolved within a relatively short time frame, which is a significant limitation.32
Models of acute injury and repair
Although overuse models of tendon degeneration best capture the early sequelae of damage, spontaneous tears in the tendon do not typically develop after chronic damage in rats. Therefore, the majority of studies for rotator cuff tendon tears employ an acute laceration injury of otherwise healthy tendon. These models include detachment of the supraspinatus tendon, either alone or in tandem with the infraspinatus or other tendons, to model massive rotator cuff tears.18–20; 33; 34 Reattachment surgeries are typically carried out immediately after detachment, and overall healing evaluated at later timepoints.21; 22; 35–41 Because the laceration injuries and repairs are quite invasive, the environment after injury differs in significant ways from degeneration-induced tears observed in the clinic. Acute injuries result in considerable inflammation and recruitment of immune cells, which secrete numerous factors that impact the healing response and drive scar formation. Since the tendon cells are typically healthy, the response to injury may not recapitulate the response of degenerated tendon in which cells are also diseased. One way to overcome these limitations may be to combine overuse and acute injury models; in one study, tendinopathy was induced by forced treadmill running, followed by full detachment/repair surgery to model tendon tear.31 Finally, immediate repair after laceration also does not mimic the clinical scenario in which tendon tears are usually left untreated for weeks or months prior to repair. To model delayed repair, a few groups recently carried out supraspinatus tendon detachment followed by surgical repair 3 to 16 weeks later.34; 42–47 Prolonged unloading resulted in tendon retraction, muscle atrophy, fatty infiltration, and fibrotic scarring. Despite eventual repair, these studies generally suggested that the pathological events associated with unloading cannot be reversed by simple reattachment.18; 20; 33; 34
Biomolecule-based therapies for rotator cuff repair
The delivery of biologics to augment surgical repair of rotator cuff tendons has been the focus of intense interest and research. Advantages of biomolecule delivery include ease of use and the off-the-shelf nature of such products. For biomolecule-based therapies, there is no need to isolate and culture cells, thus minimizing the time required for treatment, cost, and the number of invasive procedures. The goal of this approach is to create a more regenerative healing environment after rotator cuff repair surgery through the use of exogenous factors that promote cell proliferation and appropriate differentiation, while repressing inflammation and scar formation. In this section, we briefly discuss clinical outcomes related to platelet-rich plasma, which is one of the main biologics used to improve healing in human patients. We will then focus on current findings in rodents related to the delivery of growth factors, such as transforming growth factor-β (TGF-β), bone morphogenetic protein (BMP), and fibroblast growth factor (FGF), as well as immunomodulatory factors. Together, these comprise the most commonly tested, defined biomolecules for rat rotator cuff repairs.
Platelet-Rich Plasma
Platelet-rich plasma (PRP) is an undefined mixture of growth factors, including but not limited to TGF-β, FGF, and platelet-derived growth factor (PDGF), as well as varying leukocyte, monocyte, and macrophage populations.14; 15; 48 PRP is the most commonly tested, undefined biological adjuvant for rotator cuff repairs, and several randomized clinical trials have studied the functional effects and patient outcomes of this treatment, with the goal of increasing healing of the enthesis and reducing pain and inflammation of patients after repair.14; 15; 48 There are several reviews that summarize patient outcomes and compare data across multiple studies, with few finding significant improvements in patient outcomes and pain levels in patients with PRP-treated rotator cuff repairs versus control repairs.15; 49 The majority of studies fail to find significant results, as most clinical studies have shown that PRP has no significant effects on healing, re-tear rate, pain, or functional outcomes at long-term follow-up tests.50; 51 However, interesting differences have been observed when comparing treatment subgroups. These include the finding that PRP applied directly to the enthesis site produces better outcomes than PRP injected over the tendon, and that PRP treatment better reduces re-tear rates in small and medium tears compared to large tears.50 The undefined composition of PRP presents more inconsistencies between studies and is a likely limitation of PRP treatment. For example, leukocyte-reduced PRP may lower re-tear rates more effectively than regular PRP.52; 53 Overall, the findings of clinical studies and reviews suggest that PRP treatments have no consistently significant benefit on functional outcomes or re-tear rates in patients. This review will omit further discussion of PRP, due to the conflicting reports of efficacy in the clinic and the undefined composition of PRP formulations.14; 48; 54
Delivery vehicles
While we focus this section on the efficacy of biomolecules, it is also clear that the vehicle of delivery also plays a crucial role in outcomes. The ideal vehicle would allow continuous and controlled delivery to maintain an effective concentration of therapeutic agent at the healing site. However, most studies do not test bioactivity over time and dosing studies are not frequently presented. For inhibition of systemic processes (such as inflammation), repeated intraperitoneal injections of drug is an effective way to maintain adequate levels of drug. However, for most growth factors, local application is highly desired to prevent unwanted off-target effects. Growth factors are therefore delivered via (1) implantation of pumps that release a continuous supply over time, (2) local bolus injections, (3) adenoviral recombination, (4) implantation of a biomaterial carrier, or (5) delivery of transduced cells (which will be covered in greater detail in the next section).55–59 Although pumps are advantageous for controlled delivery, their use in rats can be challenging due to the small size of the animal and the potential for dislocation from the desired site.60 Local bolus injections may have a short bioactive half-life; further, it can be challenging to restrict activity to the healing site. Adenoviral recombination can also be challenging clinically since permanent expression of growth factors is likely undesirable past a certain stage in healing. In contrast, biomaterial carriers with biomimetic properties are particularly attractive for their ability to exert additional therapeutic responses, as well as the ability to design important features such as controlled release of factors or degradation of biomaterial. To date, none of the commonly used delivery vehicles have the capacity to target delivery to specific cell populations. Our review of the literature finds that the choice of vehicle can have considerable impact on healing and the efficacy of delivered growth factors. Where findings diverged, we identify the mode of delivery used to provide potential explanations for these discrepancies.
TGF-β Superfamily
The TGF-β superfamily has been implicated in the development, differentiation, and healing of all musculoskeletal tissues, such as bone, cartilage, tendon, and muscle.61–66 Members of the superfamily are generally organized into two branches based on their downstream Smad signaling.67 While members of the TGF-β branch (including TGF-βs, activin, myostatin, and nodal) activate the receptor Smads 2/3, members of the BMP branch activate the receptor Smads 1/5/8. After phosphorylation, receptor Smads then bind to the co-Smad Smad4, and the entire complex translocates into the nucleus to initiate transcription.67 Although Smad-mediated signaling remains the best known pathway associated with the superfamily, TGF-β family signaling can also activate a number of non-Smad pathways.68 Recent data also indicates that the receptor Smads can activate transcription independently of Smad4 binding.69
For rat rotator cuff healing, TGF-βs and BMPs are attractive targets due to their well-established roles in tendon, cartilage, and bone differentiation. Since the rotator cuff enthesis is a transition of all three tissues, in vivo delivery of ligands to enhance TGF-β or BMP signaling seemed promising as a therapeutic strategy to enhance tissue regeneration. For TGF-β, initial landmark studies testing this hypothesis showed that bolus delivery of TGF-βs by osmotic pump (without scaffold) is more closely associated with fibrosis (indicated by increased type III collagen deposition, cellularity, and vascularity) and a heightened inflammatory response.57 These studies suggested that the cells responsive to TGF-β signaling may be immune or scar-forming cells rather than stem/progenitor cells with tenogenic or chondrogenic potential. In the context of function however, application of exogeneous TGF-β ligands generally improve mechanical properties, which may be due to increased scar tissue.56; 58; 70 By contrast, inhibition of TGF-β signaling via delivery of neutralizing antibodies impaired mechanical properties, indicating that early TGF-β signaling is likely required to initiate the healing cascade.57 Interestingly, a few studies combining TGF-β ligands with bone biomimetic carriers showed enhanced enthesis fibrocartilage and bone deposition.55; 70 Therefore, the use of such bone-promoting scaffolds in combination with TGF-βs may alter the type of cells recruited during healing and initiate a more regenerative response.
Members of the BMP branch (including BMPs 2, 4, and 7) are typically associated with cartilage and bone formation, as well as tendon inhibition.66; 71; 72 Of this group, only BMP2 and BMP7 have been tested in the context of rat rotator cuff healing. Although BMP2 delivery via transduced stem cells dramatically impaired healing (as evidenced by reduced mechanical properties and loss of bone), sustained delivery of BMP7 enhanced fibrocartilage deposition at the tendon-to-bone site.59; 73 However, biomechanics were not significantly improved relative to controls.73 These limited outcomes suggest that additional research will be required to determine whether these chondrogenic/osteogenic BMPs have therapeutic value.
In addition to these classic BMPs, another subset of BMPs, formerly known as GDFs 5, 6, and 7 (now BMPs 14, 13, and 12, respectively), were shown to have potential roles in tendon development.61; 74–76 Of this group, only BMP13 was tested for rat rotator cuff healing. Similar to the other BMPs, results from these studies were inconsistent; while BMP13 delivered by sutures or adenovirus improved biomechanical healing, BMP13-transduced stem cells delivered to the injury failed to show any improvement.58; 77; 78 Collectively, the inconclusive outcomes from BMP treatments highlight several challenges and open questions, including the extent to which different modes of delivery, timing, and dosing may significantly impact BMP’s biologic functions in vivo. Interestingly, the use of stem cells as a delivery vehicle for BMPs proved least successful.
FGFs
The FGF signaling pathway consists of 22 ligands, the majority of which are secreted factors that bind and signal through receptor tyrosine kinases. Binding of ligand to one of four receptors results in phosphorylation at the intracellular tyrosine kinase domain and recruitment of key molecules that interact with specific domains. These signaling complexes then trigger a cascade of phosphorylation events and induces downstream activation of several pathways, including the Ras/MAP kinase, PI3 kinase/Akt, PLCγ, and STAT pathways. For more details on FGF signaling, please see the excellent and comprehensive review by Ornitz and Itoh.79 Like TGF-βs and BMPs, FGFs have been implicated in multiple aspects of musculoskeletal development, as mutations in specific FGF ligands or receptors result in severe defects in limb outgrowth, skeletal differentiation, and muscle development/regeneration.79 For tendon, FGF was the first signaling molecule identified to regulate somitic tendon induction in chick.80 Due to potential redundancy between multiple FGF ligands/receptors and their requirement in skeletal development, the role of FGFs in mammalian tendon or enthesis development has not been easily elucidated. It is therefore unclear whether FGF signaling molecules have any role in mammalian tendon induction, differentiation, or growth/maturation, either alone or in tandem with other signaling molecules such as TGF-β.81
To date, there are only three studies that have tested the potential of FGF2 to enhance rat rotator cuff healing. Although the number of studies is limited, supplementation of supraspinatus repairs with FGF2 universally improved healing outcomes, regardless of mode of delivery (hydrogels or osmotic pump). Improved healing was demonstrated by improved biomechanics, enhanced cell proliferation, upregulation of tendon-specific genes, and improved structural organization.60; 82; 83 These results suggest that FGF2 may be a promising therapeutic molecule for follow up investigation, although additional studies are certainly required to identify mechanisms of action.
Immunomodulatory Factors
In addition to growth factors, immunomodulatory factors have also been extensively evaluated, based on their capacity to alter the inflammatory environment post-injury and improve healing outcomes. Much attention has been focused on the use of nonsteroidal anti-inflammatory drugs (NSAIDs), since drugs in this class are routinely prescribed as part of post-operative care. NSAIDs function by inhibition of cyclooxygenase (Cox) enzymes, which are present in two forms, Cox1 and Cox2.84 Traditional nonselective NSAIDs inhibit both Cox1 and Cox2, while selective NSAIDs only target Cox2 (Table 1). For rotator cuff repair, a number of NSAIDs, including diclofenac, meloxicam, ibuprofen, indomethacin, and celecoxib showed largely detrimental effects on mechanical properties and tendon healing after acute injury.85–88 These effects may be somewhat time-dependent; while most studies showed impaired healing with immediate delivery post-injury, delivery at later stages of healing did not seem to affect functional properties.87 Indeed, delayed repair coupled with licofelone injections (a dual Cox and lipoxygenase inhibitor) improved mechanical properties and fibrocartilage deposition.89 However, despite reductions in fatty infiltration and muscle fibrosis, some degree of muscle atrophy remained, which is consistent with other studies showing that chronic unloading of tendon results in permanent changes to the rotator cuff unit. Collectively, this body of work indicates that early inflammation is critical for tendon healing and functional repair. Consistent with this concept, activation of Cox2 by treatment with statins showed improved mechanical properties and cell proliferation.90 While most studies have focused on NSAID delivery to modulate local inflammation, one study repressed the activity of TNF-α via delivery of the receptor TNFR1 and showed a transient improvement in mechanical properties at early stages that was not sustained at later timepoints.40 This suggests that distinct inflammatory pathways may be activated during healing.
Table 1.
Non-selective and selective NSAIDs
| Nonselective NSAIDs | Selective NSAIDs |
|---|---|
|
| |
| Ibuprofen | Meloxicam |
| Diclofenac | Celecoxib |
| Indomethacin | |
| Ketoprofen | |
| Aspirin | |
| Piroxicam | |
| Naproxen | |
Stem cell-based therapies for rotator cuff repair
The promise of stem cells for regenerative medicine has inspired considerable attention over the past 20+ years, due to their ability to self-renew and undergo differentiation along multiple lineages. While stem cell types such as embryonic stem cells or induced pluripotent stem cells retain the capacity to create any tissue lineage, postnatal tissue-specific stem cells are typically more restricted.91–93 Tissue-specific stem cells can either undergo multilineage differentiation (such as mesenchymal stem/stromal cells, MSCs), or are largely restricted to a single lineage (such as muscle satellite cells).94; 95 For musculoskeletal regeneration, the most commonly used stem cells are multilineage stem cells derived from bone marrow (bMSCs) or fat (adipose-derived stem/stromal cells, ASCs).94; 96 Collectively, these two stem cell types have been differentiated into almost every connective tissue lineage, including bone, cartilage, tendon, and the intervertebral disc.97–101 More recently, cells with self-renewal capacity, clonogenicity, and multi-lineage potential have been isolated from tendon tissue.102–106 In the original characterization of these tendon stem/progenitor cells (TSPCs), it was suggested that these cells may reside within the tendon proper and comprise a sub-population of tenocytes.102 Other studies, however, suggest that TSPCs may be located within the epitenon, which is the thin epithelial layer surrounding all tendons.107–109 For bMSCs, ASCs, and TSPCs, definitive markers are not well established and these cells are typically derived and characterized based on plastic adherence, expansion potential, and multilineage potential. Markers that have been used to identify putative bMSCs include various cell surface markers (Sca-1, Stro-1, CD146, CD90, and CD44), PDGFRα, and region-specific Hox expression (Table 2).102; 110; 111 TSPCs were found to share several markers with bMSCs, with the additional expression of markers Scx, Comp, and Sox9.102 Recently, single-cell RNA-seq screening implicated nestin as a potential marker for distinguishing TSPCs; however the in vivo location of these cells was not determined.112 The use of stem cells clinically remains controversial, with few high quality studies. Two clinical studies provide insight into patient outcomes after surgical augmentation with MSCs. In one, bMSCs applied during arthroscopic single-row rotator cuff repair drastically reduced re-tear rates at a ten year follow-up.113 A more recent study also found a significant decrease in re-tear rate after surgical augmentation with ASCs.114 The paucity of clinical data from studies performed in the US is largely due to the high risks associated with stem cell-based therapies, including growth of tumors, administration site reactions, and other adverse effects.115 Rigorous pre-clinical studies testing stem cell delivery and functional repair in animal models will be required to inform potential therapies and clinical translation. In this section, we therefore focus on in vivo applications of commonly used MSC populations (bMSCs, ASCs, TSPCs) for rat rotator cuff repair and current findings in this area. Tissue engineering efforts that focus solely on in vitro differentiation strategies will not be discussed.
Table 2.
Commonly used markers for MSC validation
| Negative selection markers | Positive selection markers | |
|---|---|---|
|
| ||
| CD34 (hematopoietic cells) | Sca-1 | CD146 |
| CD45 (leukocytes) | Stro-1 | CD44 |
| CD11b (monocytes/macrophages) | PDGFRα | CD90 |
| Hox | CD105 | |
Directed differentiation of stem cells in vivo
One strategy for stem cell-based rotator cuff repair harnesses their multipotent capacity to direct differentiation toward a tendon or fibrocartilage phenotype. Differentiation can be carried out in vitro to create an engineered tendon/enthesis construct prior to implantation, or naive MSCs can be delivered within a biomimetic matrix to promote the proper cell fate in situ.116–119 Commonly used scaffolds for rotator cuff include electrospun fibers, xenograft tendon scaffolds, or hydrogels (typically fibrin or type I collagen gels).43; 59; 120–123 The use of a carrier vehicle is advantageous since it ensures proper localization of delivered cells; the environment provided by the scaffolds has the additional potential to improve host cell proliferation or differentiation. While MSCs delivered within such carriers generally showed some positive indicators of improved healing (such as improved histology, fibrocartilage deposition, or expression of enthesis markers),46; 120; 122 functional outcomes have been mixed. Although some studies reported improved mechanical properties,120; 123 others observed no differences relative to carrier or suture controls by the final timepoint.39; 46; 121 An alternative strategy to direct differentiation is transduction of transcription factors that may induce a tenogenic cell fate, such as the transcription factors Scx or Mkx.71; 124; 125 For rotator cuff, bMSCs transduced with Scx improved fibrocartilage and mechanical outcomes after acute injury (compared to naive bMSCs), suggesting that delivery of tenogenic cells may have greater therapeutic value relative to naive stem cells.126 Similarly, delivery of TSPCs in fibrin improved rotator cuff mechanical properties after injury,127 however direct comparisons against bMSCs or ASCs were not determined. Though promising, additional research is required given the paucity of studies using TSPCs for rotator cuff repair.
Stem cell delivery to modulate local environment
In addition to direct differentiation, MSC delivery may have other therapeutic benefits. For example, naive MSCs are thought to be immunoprivileged and may exert immunomodulatory effects on the local environment via secreted factors.128 One study demonstrated a therapeutic effect of MSC-conditioned media applied to injured rotator cuff.47 While enthesis healing was not determined, application of the MSC secretome inhibited muscle degeneration and atrophy. While still not definitively established, the immunomodulatory benefits of MSCs may be due to their effect on macrophage recruitment or polarization. Co-culture of ASCs with macrophages suggest that ASCs can shift macrophages toward an anti-inflammatory M2 phenotype and exert a protective effect on tendon fibroblasts.56 Delivery of ASCs to augment rotator cuff repair in vivo generally reduces inflammation and the presence of inflammatory cells; however, functional properties do not improve.32; 129 Similarly, delivery of bMSCs showed increased numbers of anti-inflammatory M2 macrophages at later timepoints, in parallel with upregulated enthesis markers.122 Collectively, these studies indicate that MSCs derived from bone marrow or fat may have immunoprotective properties, but the impact on functional restoration may be minimal.
Finally, naive stem cells can also be used as a vehicle for local biomolecule production via gene transduction, which can have cell-autonomous or non-autonomous effects on healing. For rotator cuff healing, biomolecules of interest include growth factors or molecules that regulate cell proliferation, differentiation, or matrix degradation/remodeling.58; 59; 130; 131 While stem cells transduced with BMPs had no or detrimental effects on healing,58; 59 there have been some success with other molecules such as an shRNA for TOB1 (a protein that inhibits proliferation) and MT1-MMP (otherwise known as MMP14).130; 131 One of the challenges for this delivery strategy is temporal control; molecules will be produced by stem cells as long as they are present in the injury site. This is in contrast to delivery strategies such as bolus injections, which have a relatively brief half-life, or biomaterials that can be engineered to degrade and disappear over time. In some cases, prolonged or excessive production of molecules can provoke undesirable responses by host cells. In the case of BMPs, which regulates important biological functions for many different cell types, uncontrolled response may explain some of the poor healing outcomes.
Conclusions
Although there is considerable interest in biological augmentation of rotator cuff repair, outcome measures have thus far been mixed. Our review of the literature suggests that the method of delivery (for biologics as well as cells) may have a considerable impact on success. To date, much of the outcome measures have focused on structural and functional recovery. While these remain critical measures, advancing biologic and cell-based therapies will also require mechanistic studies that follow cell fate. The recent adaptation of rotator cuff injury and repair in mouse will enable such studies; genetic manipulations and cell lineage tracing can be easily accomplished in mouse to directly test promising signaling pathways, identify responsive cells, distinguish host from donor cells, and identify local cell populations that are relevant for healing. Despite current limitations, biological augmentation remains a highly exciting research area and represents the next frontier in functional rotator cuff restoration. Successful strategies will likely require a combination of biomolecules, intelligent biomaterials, and stem cells (Fig. 2).
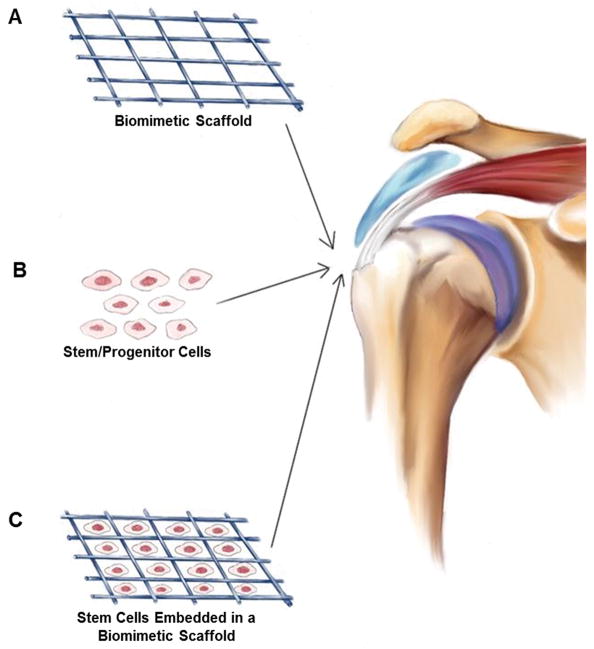
Figure 2.
Overview schematic highlighting biologic and stem cell strategies for rotator cuff repair. (A) Cell-free biomimetic scaffolds can be implanted to control release of biomolecules or exert their own beneficial proliferative or differentiating effects on local tissues. (B) Stem/progenitor cells delivered to the injury site can modulate the local environment or undergo direct cell differentiation. (C) The combination of biomaterial scaffold and stem/progenitor cells has been explored in recent studies to increase healing after rotator cuff injury and repair.
Acknowledgments
Supported by funding from the National Institutes of Health (R01AR069537) to AHH and a fellowship by Neuenschwander Stiftung to HLM. SB, HLM, LMG, and AHH contributed to conception and manuscript preparation. Literature review was performed by SB and HLM. We would also like to acknowledge Deborah Tompkins Bianco for illustrations depicted in Figure 2.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Neer CS., 2nd Anterior acromioplasty for the chronic impingement syndrome in the shoulder: a preliminary report. J Bone Joint Surg Am. 1972;54:41–50. [PubMed] [Google Scholar]
- 2.Oliva F, Piccirilli E, Bossa M, et al. I.S.Mu.L.T - Rotator Cuff Tears Guidelines. Muscles Ligaments Tendons J. 2015;5:227–263. doi: 10.11138/mltj/2015.5.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh LS, Wolf BR, Hall MP, et al. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;455:52–63. doi: 10.1097/BLO.0b013e31802fc175. [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Wang VM, April EW, et al. Anterior and posterior musculotendinous anatomy of the supraspinatus. J Shoulder Elbow Surg. 2000;9:436–440. doi: 10.1067/mse.2000.108387. [DOI] [PubMed] [Google Scholar]
- 5.Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86-A:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- 7.Benjamin M, Kumai T, Milz S, et al. The skeletal attachment of tendons--tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin M, Toumi H, Ralphs JR, et al. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber C, Schneeberger AG, Perren SM, et al. Experimental rotator cuff repair. A preliminary study. J Bone Joint Surg Am. 1999;81:1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter JE, Thomopoulos S, Flanagan CL, et al. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 11.Burks RT, Crim J, Brown N, et al. A prospective randomized clinical trial comparing arthroscopic single- and double-row rotator cuff repair: magnetic resonance imaging and early clinical evaluation. Am J Sports Med. 2009;37:674–682. doi: 10.1177/0363546508328115. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi F, Ruzzini L, Longo UG, et al. Equivalent clinical results of arthroscopic single-row and double-row suture anchor repair for rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2007;35:1254–1260. doi: 10.1177/0363546507302218. [DOI] [PubMed] [Google Scholar]
- 13.Grasso A, Milano G, Salvatore M, et al. Single-row versus double-row arthroscopic rotator cuff repair: a prospective randomized clinical study. Arthroscopy. 2009;25:4–12. doi: 10.1016/j.arthro.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2016 doi: 10.1007/s00167-016-4261-4. [DOI] [PubMed] [Google Scholar]
- 15.Charles MD, Christian DR, Cole BJ. The Role of Biologic Therapy in Rotator Cuff Tears and Repairs. Curr Rev Musculoskelet Med. 2018;11:150–161. doi: 10.1007/s12178-018-9469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 17.Derwin KA, Baker AR, Iannotti JP, et al. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B Rev. 2010;16:21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Manzano G, Kim HT, et al. A rat model of massive rotator cuff tears. J Orthop Res. 2011;29:588–595. doi: 10.1002/jor.21266. [DOI] [PubMed] [Google Scholar]
- 19.Zingman A, Li H, Sundem L, et al. Shoulder arthritis secondary to rotator cuff tear: A reproducible murine model and histopathologic scoring system. J Orthop Res. 2017;35:506–514. doi: 10.1002/jor.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuenzler MB, Nuss K, Karol A, et al. Neer Award 2016: reduced muscle degeneration and decreased fatty infiltration after rotator cuff tear in a poly(ADP-ribose) polymerase 1 (PARP-1) knock-out mouse model. J Shoulder Elbow Surg. 2017;26:733–744. doi: 10.1016/j.jse.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Bell R, Taub P, Cagle P, et al. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res. 2015;33:25–32. doi: 10.1002/jor.22727. [DOI] [PubMed] [Google Scholar]
- 22.Lebaschi AH, Deng XH, Camp CL, et al. Biomechanical, Histologic, and Molecular Evaluation of Tendon Healing in a New Murine Model of Rotator Cuff Repair. Arthroscopy. 2018;34:1173–1183. doi: 10.1016/j.arthro.2017.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida R, Alaee F, Dyrna F, et al. Murine supraspinatus tendon injury model to identify the cellular origins of rotator cuff healing. Connect Tissue Res. 2016;57:507–515. doi: 10.1080/03008207.2016.1189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AG, Galatz LM, Thomopoulos S. Enthesis regeneration: a role for Gli1+ progenitor cells. Development. 2017;144:1159–1164. doi: 10.1242/dev.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neer CS, 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–1244. [PubMed] [Google Scholar]
- 26.Carpenter JE, Flanagan CL, Thomopoulos S, et al. The effects of overuse combined with intrinsic or extrinsic alterations in an animal model of rotator cuff tendinosis. Am J Sports Med. 1998;26:801–807. doi: 10.1177/03635465980260061101. [DOI] [PubMed] [Google Scholar]
- 27.Schneeberger AG, Nyffeler RW, Gerber C. Structural changes of the rotator cuff caused by experimental subacromial impingement in the rat. J Shoulder Elbow Surg. 1998;7:375–380. doi: 10.1016/s1058-2746(98)90026-x. [DOI] [PubMed] [Google Scholar]
- 28.Soslowsky LJ, Thomopoulos S, Esmail A, et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng. 2002;30:1057–1063. doi: 10.1114/1.1509765. [DOI] [PubMed] [Google Scholar]
- 29.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 30.Parks AN, McFaline-Figueroa J, Coogan A, et al. Supraspinatus tendon overuse results in degenerative changes to tendon insertion region and adjacent humeral cartilage in a rat model. J Orthop Res. 2017;35:1910–1918. doi: 10.1002/jor.23496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tucker JJ, Riggin CN, Connizzo BK, et al. Effect of overuse-induced tendinopathy on tendon healing in a rat supraspinatus repair model. J Orthop Res. 2016;34:161–166. doi: 10.1002/jor.22993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen HS, Su YT, Chan TM, et al. Human adipose-derived stem cells accelerate the restoration of tensile strength of tendon and alleviate the progression of rotator cuff injury in a rat model. Cell Transplant. 2015;24:509–520. doi: 10.3727/096368915X686968. [DOI] [PubMed] [Google Scholar]
- 33.Sevivas N, Serra SC, Portugal R, et al. Animal model for chronic massive rotator cuff tear: behavioural and histologic analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23:608–618. doi: 10.1007/s00167-014-3441-3. [DOI] [PubMed] [Google Scholar]
- 34.Thangarajah T, Henshaw F, Sanghani-Kerai A, et al. Supraspinatus detachment causes musculotendinous degeneration and a reduction in bone mineral density at the enthesis in a rat model of chronic rotator cuff degeneration. Shoulder Elbow. 2017;9:178–187. doi: 10.1177/1758573217696450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomopoulos S, Hattersley G, Rosen V, et al. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res. 2002;20:454–463. doi: 10.1016/S0736-0266(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 36.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 37.Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 38.Bedi A, Kovacevic D, Hettrich C, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Gulotta LV, Kovacevic D, Ehteshami JR, et al. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37:2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 40.Gulotta LV, Kovacevic D, Cordasco F, et al. Evaluation of tumor necrosis factor alpha blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy. 2011;27:1351–1357. doi: 10.1016/j.arthro.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 41.Beck J, Evans D, Tonino PM, et al. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med. 2012;40:2037–2044. doi: 10.1177/0363546512453300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Killian ML, Cavinatto L, Shah SA, et al. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res. 2014;32:439–447. doi: 10.1002/jor.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tornero-Esteban P, Hoyas JA, Villafuertes E, et al. Efficacy of supraspinatus tendon repair using mesenchymal stem cells along with a collagen I scaffold. J Orthop Surg Res. 2015;10:124. doi: 10.1186/s13018-015-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Killian ML, Cavinatto LM, Ward SR, et al. Chronic Degeneration Leads to Poor Healing of Repaired Massive Rotator Cuff Tears in Rats. Am J Sports Med. 2015;43:2401–2410. doi: 10.1177/0363546515596408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gumucio JP, Flood MD, Roche SM, et al. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int Orthop. 2016;40:759–764. doi: 10.1007/s00264-015-2937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smietana MJ, Moncada-Larrotiz P, Arruda EM, et al. Tissue-Engineered Tendon for Enthesis Regeneration in a Rat Rotator Cuff Model. Biores Open Access. 2017;6:47–57. doi: 10.1089/biores.2016.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sevivas N, Teixeira FG, Portugal R, et al. Mesenchymal Stem Cell Secretome Improves Tendon Cell Viability In Vitro and Tendon-Bone Healing In Vivo When a Tissue Engineering Strategy Is Used in a Rat Model of Chronic Massive Rotator Cuff Tear. Am J Sports Med. 2018;46:449–459. doi: 10.1177/0363546517735850. [DOI] [PubMed] [Google Scholar]
- 48.Boswell SG, Cole BJ, Sundman EA, et al. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28:429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Hurley ET, Lim Fat D, Moran CJ, et al. The Efficacy of Platelet-Rich Plasma and Platelet-Rich Fibrin in Arthroscopic Rotator Cuff Repair: A Meta-analysis of Randomized Controlled Trials. Am J Sports Med. 2018 doi: 10.1177/0363546517751397. 363546517751397. [DOI] [PubMed] [Google Scholar]
- 50.Saltzman BM, Jain A, Campbell KA, et al. Does the Use of Platelet-Rich Plasma at the Time of Surgery Improve Clinical Outcomes in Arthroscopic Rotator Cuff Repair When Compared With Control Cohorts? A Systematic Review of Meta-analyses. Arthroscopy. 2016;32:906–918. doi: 10.1016/j.arthro.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Warth RJ, Dornan GJ, James EW, et al. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31:306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Jo CH, Shin JS, Shin WH, et al. Corrigendum. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2016;44:NP3. doi: 10.1177/0363546515621880. [DOI] [PubMed] [Google Scholar]
- 53.Malavolta EA, Gracitelli ME, Ferreira Neto AA, et al. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. Am J Sports Med. 2014;42:2446–2454. doi: 10.1177/0363546514541777. [DOI] [PubMed] [Google Scholar]
- 54.Kesikburun S, Tan AK, Yilmaz B, et al. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. Am J Sports Med. 2013;41:2609–2616. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 55.Kovacevic D, Fox AJ, Bedi A, et al. Calcium-phosphate matrix with or without TGF-beta3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39:811–819. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 56.Manning CN, Kim HM, Sakiyama-Elbert S, et al. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. J Orthop Res. 2011;29:1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 57.Kim HM, Galatz LM, Das R, et al. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res. 2011;52:87–98. doi: 10.3109/03008207.2010.483026. [DOI] [PubMed] [Google Scholar]
- 58.Gulotta LV, Kovacevic D, Packer JD, et al. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:180–187. doi: 10.1177/0363546510379339. [DOI] [PubMed] [Google Scholar]
- 59.Lipner J, Shen H, Cavinatto L, et al. In Vivo Evaluation of Adipose-Derived Stromal Cells Delivered with a Nanofiber Scaffold for Tendon-to-Bone Repair. Tissue Eng Part A. 2015;21:2766–2774. doi: 10.1089/ten.tea.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buchmann S, Sandmann GH, Walz L, et al. Refixation of the supraspinatus tendon in a rat model--influence of continuous growth factor application on tendon structure. J Orthop Res. 2013;31:300–305. doi: 10.1002/jor.22211. [DOI] [PubMed] [Google Scholar]
- 61.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blitz E, Viukov S, Sharir A, et al. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev Cell. 2009;17:861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo HS, Serra R. Deletion of Tgfbr2 in Prx1-cre expressing mesenchyme results in defects in development of the long bones and joints. Dev Biol. 2007;310:304–316. doi: 10.1016/j.ydbio.2007.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 66.Rosen V, Thies RS, Lyons K. Signaling pathways in skeletal formation: a role for BMP receptors. Ann N Y Acad Sci. 1996;785:59–69. doi: 10.1111/j.1749-6632.1996.tb56244.x. [DOI] [PubMed] [Google Scholar]
- 67.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Retting KN, Song B, Yoon BS, et al. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136:1093–1104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.You X, Shen Y, Yu W, et al. Enhancement of tendonbone healing following rotator cuff repair using hydroxyapatite with TGFbeta1. Mol Med Rep. 2018;17:4981–4988. doi: 10.3892/mmr.2018.8499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto-Shiraishi Y, Kuroiwa A. Wnt and BMP signaling cooperate with Hox in the control of Six2 expression in limb tendon precursor. Dev Biol. 2013;377:363–374. doi: 10.1016/j.ydbio.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 73.Kabuto Y, Morihara T, Sukenari T, et al. Stimulation of Rotator Cuff Repair by Sustained Release of Bone Morphogenetic Protein-7 Using a Gelatin Hydrogel Sheet. Tissue Eng Part A. 2015;21:2025–2033. doi: 10.1089/ten.tea.2014.0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–841. doi: 10.1002/jor.20092. [DOI] [PubMed] [Google Scholar]
- 75.Mikic B, Rossmeier K, Bierwert L. Sexual dimorphism in the effect of GDF-6 deficiency on murine tendon. J Orthop Res. 2009;27:1603–1611. doi: 10.1002/jor.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chhabra A, Tsou D, Clark RT, et al. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–835. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 77.Lamplot JD, Angeline M, Angeles J, et al. Distinct effects of platelet-rich plasma and BMP13 on rotator cuff tendon injury healing in a rat model. Am J Sports Med. 2014;42:2877–2887. doi: 10.1177/0363546514547171. [DOI] [PubMed] [Google Scholar]
- 78.Murray DH, Kubiak EN, Jazrawi LM, et al. The effect of cartilage-derived morphogenetic protein 2 on initial healing of a rotator cuff defect in a rat model. J Shoulder Elbow Surg. 2007;16:251–254. doi: 10.1016/j.jse.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 81.Havis E, Bonnin MA, Olivera-Martinez I, et al. Transcriptomic analysis of mouse limb tendon cells during development. Development. 2014;141:3683–3696. doi: 10.1242/dev.108654. [DOI] [PubMed] [Google Scholar]
- 82.Tokunaga T, Shukunami C, Okamoto N, et al. FGF-2 Stimulates the Growth of Tenogenic Progenitor Cells to Facilitate the Generation of Tenomodulin-Positive Tenocytes in a Rat Rotator Cuff Healing Model. Am J Sports Med. 2015;43:2411–2422. doi: 10.1177/0363546515597488. [DOI] [PubMed] [Google Scholar]
- 83.Ide J, Kikukawa K, Hirose J, et al. The effects of fibroblast growth factor-2 on rotator cuff reconstruction with acellular dermal matrix grafts. Arthroscopy. 2009;25:608–616. doi: 10.1016/j.arthro.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Peterson K, McDonagh M, Thakurta S, et al. Drug Class Review: Nonsteroidal Antiinflammatory Drugs (NSAIDs): Final Update 4 Report. Portland (OR): 2010. [PubMed] [Google Scholar]
- 85.Cabuk H, Avci A, Durmaz H, et al. The effect of diclofenac on matrix metalloproteinase levels in the rotator cuff. Arch Orthop Trauma Surg. 2014;134:1739–1744. doi: 10.1007/s00402-014-2099-0. [DOI] [PubMed] [Google Scholar]
- 86.Chechik O, Dolkart O, Mozes G, et al. Timing matters: NSAIDs interfere with the late proliferation stage of a repaired rotator cuff tendon healing in rats. Arch Orthop Trauma Surg. 2014;134:515–520. doi: 10.1007/s00402-014-1928-5. [DOI] [PubMed] [Google Scholar]
- 87.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic Ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014;472:2433–2439. doi: 10.1007/s11999-013-3258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen DB, Kawamura S, Ehteshami JR, et al. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med. 2006;34:362–369. doi: 10.1177/0363546505280428. [DOI] [PubMed] [Google Scholar]
- 89.Oak NR, Gumucio JP, Flood MD, et al. Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing and reduces muscle fibrosis and lipid accumulation after rotator cuff repair. Am J Sports Med. 2014;42:2860–2868. doi: 10.1177/0363546514549943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dolkart O, Liron T, Chechik O, et al. Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway: an in vivo and in vitro study. Am J Sports Med. 2014;42:2869–2876. doi: 10.1177/0363546514545856. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 92.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 93.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 95.Murphy MM, Lawson JA, Mathew SJ, et al. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bunnell BA, Flaat M, Gagliardi C, et al. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown JP, Galassi TV, Stoppato M, et al. Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res Ther. 2015:6. doi: 10.1186/s13287-015-0043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nerurkar NL, Sen S, Huang AH, et al. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine (Phila Pa 1976) 2010;35:867–873. doi: 10.1097/BRS.0b013e3181d74414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Estes BT, Diekman BO, Gimble JM, et al. Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protoc. 2010;5:1294–1311. doi: 10.1038/nprot.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang AH, Stein A, Mauck RL. Evaluation of the complex transcriptional topography of mesenchymal stem cell chondrogenesis for cartilage tissue engineering. Tissue Eng Part A. 2010;16:2699–2708. doi: 10.1089/ten.tea.2010.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luu YK, Capilla E, Rosen CJ, et al. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res. 2009;24:50–61. doi: 10.1359/JBMR.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007;13:1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 103.Wang JH, Nirmala X. Application of Tendon Stem/Progenitor Cells and Platelet-Rich Plasma to Treat Tendon Injuries. Oper Tech Orthop. 2016;26:68–72. doi: 10.1053/j.oto.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J, Wang JH. Moderate Exercise Mitigates the Detrimental Effects of Aging on Tendon Stem Cells. PLoS One. 2015;10:e0130454. doi: 10.1371/journal.pone.0130454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee JY, Zhou Z, Taub PJ, et al. BMP-12 treatment of adult mesenchymal stem cells in vitro augments tendon-like tissue formation and defect repair in vivo. PLoS One. 2011;6:e17531. doi: 10.1371/journal.pone.0017531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell. 2010;9:911–915. doi: 10.1111/j.1474-9726.2010.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mienaltowski MJ, Adams SM, Birk DE. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A. 2013;19:199–210. doi: 10.1089/ten.tea.2012.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mendias CL, Gumucio JP, Bakhurin KI, et al. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012;30:606–612. doi: 10.1002/jor.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014;32:944–951. doi: 10.1002/jor.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rux DR, Song JY, Swinehart IT, et al. Regionally Restricted Hox Function in Adult Bone Marrow Multipotent Mesenchymal Stem/Stromal Cells. Dev Cell. 2016;39:653–666. doi: 10.1016/j.devcel.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinho S, Lacombe J, Hanoun M, et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin Z, Hu JJ, Yang L, et al. Single-cell analysis reveals a nestin(+) tendon stem/progenitor cell population with strong tenogenic potentiality. Sci Adv. 2016;2:e1600874. doi: 10.1126/sciadv.1600874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim YS, Sung CH, Chung SH, et al. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sports Med. 2017;45:2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
- 114.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 115.FDA Warns About Stem Cell Therapies. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm.
- 116.Spalazzi JP, Vyner MC, Jacobs MT, et al. Mechanoactive scaffold induces tendon remodeling and expression of fibrocartilage markers. Clin Orthop Relat Res. 2008;466:1938–1948. doi: 10.1007/s11999-008-0310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X, Bogdanowicz D, Erisken C, et al. Biomimetic scaffold design for functional and integrative tendon repair. J Shoulder Elbow Surg. 2012;21:266–277. doi: 10.1016/j.jse.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Larkin LM, Calve S, Kostrominova TY, et al. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149–3158. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang G, Rothrauff BB, Lin H, et al. Tendon-Derived Extracellular Matrix Enhances Transforming Growth Factor-beta3-Induced Tenogenic Differentiation of Human Adipose-Derived Stem Cells. Tissue Eng Part A. 2017;23:166–176. doi: 10.1089/ten.tea.2015.0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peach MS, Ramos DM, James R, et al. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PLoS One. 2017;12:e0174789. doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Degen RM, Carbone A, Carballo C, et al. The Effect of Purified Human Bone Marrow-Derived Mesenchymal Stem Cells on Rotator Cuff Tendon Healing in an Athymic Rat. Arthroscopy. 2016;32:2435–2443. doi: 10.1016/j.arthro.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 122.Zong JC, Mosca MJ, Degen RM, et al. Involvement of Indian hedgehog signaling in mesenchymal stem cell-augmented rotator cuff tendon repair in an athymic rat model. J Shoulder Elbow Surg. 2017;26:580–588. doi: 10.1016/j.jse.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 123.Omi R, Gingery A, Steinmann SP, et al. Rotator cuff repair augmentation in a rat model that combines a multilayer xenograft tendon scaffold with bone marrow stromal cells. J Shoulder Elbow Surg. 2016;25:469–477. doi: 10.1016/j.jse.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu W, Watson SS, Lan Y, et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol Cell Biol. 2010;30:4797–4807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ito Y, Toriuchi N, Yoshitaka T, et al. The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc Natl Acad Sci U S A. 2010;107:10538–10542. doi: 10.1073/pnas.1000525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gulotta LV, Kovacevic D, Packer JD, et al. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med. 2011;39:1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 127.Cheng B, Ge H, Zhou J, et al. TSG-6 mediates the effect of tendon derived stem cells for rotator cuff healing. Eur Rev Med Pharmacol Sci. 2014;18:247–251. [PubMed] [Google Scholar]
- 128.Gao F, Chiu SM, Motan DA, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Valencia Mora M, Antuna Antuna S, Garcia Arranz M, et al. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45(Suppl 4):S22–27. doi: 10.1016/S0020-1383(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 130.Gao Y, Zhang Y, Lu Y, et al. TOB1 Deficiency Enhances the Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Tendon-Bone Healing in a Rat Rotator Cuff Repair Model. Cell Physiol Biochem. 2016;38:319–329. doi: 10.1159/000438632. [DOI] [PubMed] [Google Scholar]
- 131.Gulotta LV, Kovacevic D, Montgomery S, et al. Stem cells genetically modified with the developmental gene MT1-MMP improve regeneration of the supraspinatus tendon-to-bone insertion site. Am J Sports Med. 2010;38:1429–1437. doi: 10.1177/0363546510361235. [DOI] [PubMed] [Google Scholar]