Abstract
Background aim.
Translation of therapeutic cell therapies to clinical scale products is critical to realizing widespread success. Currently, however, there are limited tools that are accessible at the research level and readily scalable to clinical scale needs.
Methods.
We herein developed and assessed a closed loop bioreactor system in which 1) a highly gas permeable silicone material was used to fabricate cell culture bags and 2) dynamic flow was introduced to allow for dissociation of activated T-Cell aggregates.
Results.
Using this system, we find superior T-Cell proliferation compared to conventional bag materials and flasks, especially at later time points. Furthermore, intermittent dynamic flow could easily break apart T-Cell clusters.
Conclusions.
Our novel closed loop bioreactor system is amenable to enhanced T-Cell proliferation and has broader implications for being easily scaled for use in larger need settings.
Keywords: bioreactors, biomanufacturing, T-Cells, immunotherapy
Introduction
Current advances in clinical T-cell therapies holds great promise towards the near term eradication of specific cancers [1–3]. While a continuous stream of new cell therapeutic candidates emerges, there has been less focus on the underlying biomanufacturing methods and tools to expand human T cells at commercial scale. Many benchtop scale experiments typically still use T-flasks, cell culture bags, or small bioreactors whereas larger scale processes with increased regulatory constraints have shifted to clinical scale, closed-loop, and automated systems[4–6]. While large scale clinical and industrial scale process are often robust, they are also encumbered by rigid protocols, whereas smaller scale tools are highly adaptable to rapidly evolving needs.
A scalable system for the expansion of T cells, like other cell types, must match the nutrient needs of the cells as they grow, especially the transport of gases such as oxygen and carbon dioxide. The transport of gases in cell culture is inherently related to the cell culture container; the most widely used bench scale culture vessels are T-flasks and cell culture bags. While T-flasks are common place tools in nearly every biological laboratory environment, scaling can become cumbersome when large cell quantities are required [7,8]. While large vessels exist, such as multi layered flasks and cell factories, these systems are all openloop and require manual intervention for media exchanges and cell harvesting. Cell culture bag systems open up the opportunity for a different approach to scaling, ranging from as small as 5mL up to several liters from commercially available source, to theoretically even larger volumes for custom designs. Scaling becomes more straightforward as the bag sizes can be easily increased, although physical handling of such systems may become an issue at significantly larger volumes. Furthermore, culture bags are amenable to closed loops systems as they can be easily fitted with ports for sterile access. Presently, culture bags are limited in terms of their material composition; typically: polyolefin/EVA or FEP. While all these materials will allow for gas permeation and cell growth, they are less than ideal due to their reduced gas permeation as compared to filter capped flasks[9]. We herein take advantage of a highly gas permeable silicone rubber material that has demonstrated great success in the culture and maintenance of cells to fabricate our own custom cell culture bag[9–11]. Another significant part of bench level cell expansion is the normalization of cell concentration and media replenishment[12,13]. This process requires the disaggregation of T-cell clusters in order to properly enumerate the culture. There is currently no system, let alone a closed loop one, that is able to perform this, except from a manual pipetting process. Numerous commercial devices include culture agitation which aims to promote nutrient diffusion into the media but does not shear aggregates apart. We herein assess a new custom and highly gas permeable cell culture bag with the ability to be integrated into a closed loop system to facilitate the disaggregation of T-cell clusters.
Methods
Cell Culture
Media for all cell culture followed the same recipe: RPMI 1640 (Gibco, Thermo Fisher Scientific), 1% penicillin-streptomycin (Gibco, Thermo Fisher Scientific), 1% HEPES (Gibco, Thermo Fisher Scientific), 1% sodium pyruvate (Gibco, Thermo Fisher Scientific), and 10% heat-inactivated FBS (Peak FBS).
Jurkat cells (ATCC) were initially seeded at a density of ~250k/mL, counted every other day, and renormalized to a concentration of ~250k/mL after each count.
PBMCs were obtained from fresh health donors (Massachusetts General Hospital). Approval for the consented collection of blood from healthy volunteers and the testing of biospecimens was obtained from the Institutional Review Board of Massachusetts General Hospital (reg. No. 2011B000346). All methods involving these samples were performed in accordance with institutional and biosafety regulations. PBMCs were isolated per standard Ficoll density separation methods.
Primary T-cells were isolated from PBMCs using a negative selection, pan T-cell isolation kit per vendor recommended protocol (STEMCELL Technologies). T-cells were seeded at an initial density of 1M/mL in basal media supplemented with 50IU/mL IL-2 (aldesleukin, Prometheus Laboratories) and activated using a CD3/CD28 T-cell activator (ImmunoCult, STEMCELL Technologies) or soluble CD3/CD28 (R&D Systems). T-cells were initially left undisturbed for the first three days, then counted and renormalized to 500k/mL every other day thereafter. We did not further dissect out populations to CD4, CD8, or CD56 (natural killer) cells.
Cell Culture Vessels
Polystyrene culture flasks with vented caps were used as control standard vessels (Corning). Three bags were herein used: polyolefin/EVA copolymer (Evolve, Origen), custom FEP (American Durafilm), and custom silicone rubber bags were fabricated in house from sheets (Rogers Corporation). Custom silicone bags were fitted with silicone tubing (Cole Parmer), and sealed with adhesive sealant designed for silicone (DAP). Culture flasks and Origen bags came pre-sterilized. FEP and silicone bags/tubing were sterilized through exposure to 0.5M NaOH for a minimum of one hour. NaOH sterilized vessels were subsequently flushed out three times with sterile PBS to remove all trace NaOH. Specific details on vessels can be found in Table 1.
Table 1.
Culture Vessel Properties
| Wall Thickness (mil) |
Maximum Volume (mL) |
O2 Permeability[8,9] (cm3·mm/mm2·day·atm) |
CO2 Permeability[8,9] (cm3·mm/mm2·day·atm) |
|
|---|---|---|---|---|
| Culture Flask | N/A | 280 | N/A | N/A |
| Silicone | 10 | 220 | 4 × 104 | 2 × 105 |
| Polyolefin/EVA | 5 | 250 | 2 × 102 | 1 × 103 |
| FEP | 5 | 175 | 3 × 102 | 8 × 102 |
Properties of cell culture vessels used for experimentation including: wall thickness, maximum allowable system volume, O2, and CO2 permeability.
Perfusion with silicone cell culture bags was accomplished through peristaltic pumping (Cole Parmer). Flow rate was set at 100mL/min and run for 1–2 minutes total.
Media Gas/Analyte Measurements
Dissolved O2 and CO2, pH, and HCO3 were measured using a handheld blood/gas analyzer (iStat, Abbott). A CG4+ cartridge (Abbott) was used for all measurements.
Results
Intermittent Fluidic Culture Improves T-Cell Yields and Minimizes Cell Aggregation
The effects of a continuous flow culture on the soluble CD3/CD28 activated proliferative capacity of T-cells was first examined. A clear negative effect was observed with continuous flow on T-cell proliferation resulting in roughly a quarter of the initial cells present by day 9, Fig 1A. We hypothesized that nutrient deprivation was an issue as stimulated T cells formed large clonal aggregates and therefore considered an intermittent flow strategy to disaggregate T-cell clusters and promote improved nutrient distribution. This was accomplished through integration of a magnetically actuated centrifugal pump and a cell culture bag (polyolefin/EVA) to facilitate gas transfer. The magnetic pump we used is preferable to other forms of pumps (i.e. other centrifugal or peristaltic pumps) due to its compact size and reductions in mechanical forces which can readily lead to significant cell shearing and death [14]. Applying intermittent flow (100mL/min) for 5 minutes every other day, we see a significant difference. A modest 1.3-fold increase in cell number was observed in the intermittent flow group, Fig 1A. Intermittent flow adequately dissociated T-cell clusters as expected. After 5 minutes of continuous flow, we find a near homogenous single cell suspension that was once full of large clusters Fig 1B. Overall, cell proliferation is rather low compared to historical static culture methods in flasks, so we further examined more significant ways to impact improved nutrient transport by other means.
Figure 1. Comparison between continuous and intermittent fluidic cell culture conditions.
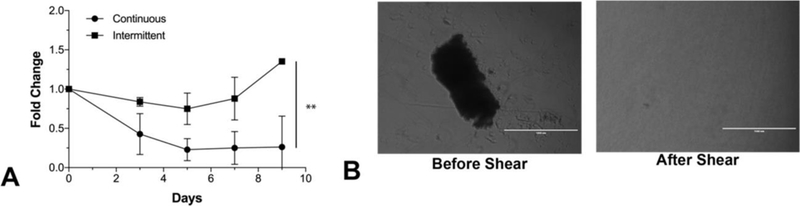
The proliferative capacity of primary T-cells was compared in a continuous perfusion setup and intermittent perfusion setup. Continuous perfusion was performed at 100mL/min using a magnetically actuated pump while intermittent perfusion was performed every other day (starting at Day 3) at 100mL/min for 5 minutes. A) We observe a 0.25× change in the continuous group, 1.3× in intermittent, and 13× in flask culture. It is clear that continuous perfusion is detrimental to T-cell proliferation. Interestingly, intermittent perfusion is only modestly better and significantly worse than standard T-flask culture – this difference may arise due to the material properties of the different culture vessels. ** = P<0.001 B) We observe the ability of shearing action to dissociate T-cell blast clusters. We were able to visually and microscopically identify clusters, whereby after 5 minutes of continuous flow, we find a near homogenous single cell suspension.
Effect of cell culture bag material on cell proliferation
Our attention next focused on cell culture bag materials as a way to improve gas transport. We determined the growth characteristics of a T-cell line, Jurkat, in four distinct cell culture vessels. The material characteristics are shown in Table 1, and were chosen to have very different gas transfer properties. Silicon, for example, even at twice the material thickness over polyolefin and FEP bags, has O2 and CO2 permeability that is improved by over an order of magnitude (Table 1). Over the course of 6 days, we observed a large expansion as expected of this cell line. Interestingly, we observed that the flask and silicone bags are comparable at roughly 40-fold growth while polyolefin/EVA and FEP are comparable at around 20-fold. These results yielded doubling times of: 1.12 +/− 0.07 days (flask), 0.94 +/−0.28 days (silicone), 1.38 +/− 0.27 days (polyolefin/EVA), and 1.37 +/− 0.18 days (FEP) (Figure 2A). All conditions were readily fit by an exponential dose curve with R2 all above 0.96.
Figure 2. Comparison of cell culture vessels using a Jurkat T cell line.
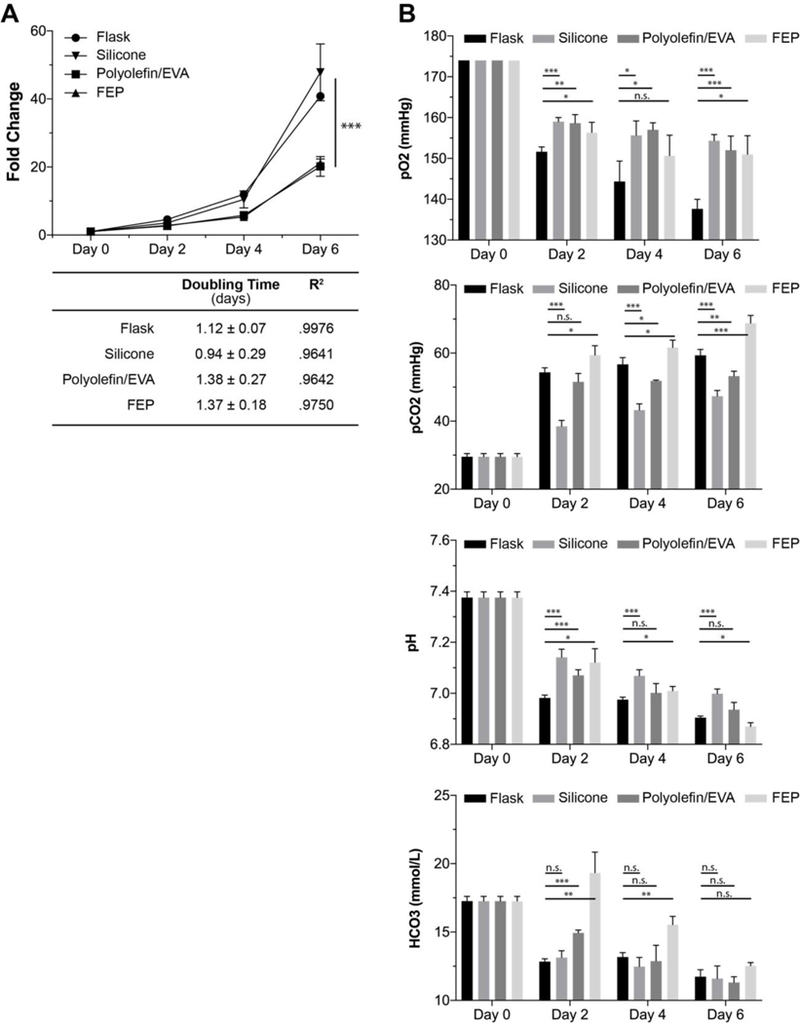
We herein assessed the growth characteristics and media properties in a standard tissue culture flask and silicone, polyolefin/EVA, and FEP cell culture bags. A) Comparability between flask/silicone and polyolefin/FEP is observed wherein the flask/silicone has significantly improved cell proliferative capacity. Both flasks and silicone bags were significantly different compared to polyolefin/EVA and FEP (*** = P<0.001). B) Comparison of several media characteristics including: dissolved O2, CO2, pH, and HCO3. Comparability is seen in O2, with the flask being overall lower across all days. CO2 demonstrates an advantage to silicone with overall lower values across the experiment. pH values show comparability with an overall downward trend likely due to increased cell burden. HCO3 demonstrates drastic difference at early time points but normalizes by the end of the culture. * = P<0.05, ** = P<0.01, *** = P<0.005.
We then went on to assess media properties using a clinical blood-gas analyzer. We observe comparability of dissolved oxygen (pO2) in all conditions at all times except for flasks which are noticeably lower (Figure 2B). We then move onto to dissolved carbon dioxide (pCO2) and notice an interesting hierarchal trend. From highest to lowest across all time points, we find in descending order: FEP, polyolefin/EVA, flask, and silicone (Figure 2B) which may possibly tie into why particular vessels outperform. Bicarbonate, which contributes to total CO2 and carbonic acid content, may also be at play here and potentially explain some of the deficiencies in polyolefin/EVA and FEP bags at earlier time points (Figure 2B).
Taking note of the pH, we see a varied response wherein flask conditions are typically lower across the board (Figure 2B). There is an overall decreasing trend over time, which is likely resultant from increasing cell mass. The reduced proliferation in polyolefin/EVA and FEP is unlikely due to a biocompatible issue to their wide availably as a commercial product for the culture of cells (Origen, Miltenyi, Saint Gobain, etc.).
Closed loop system for the expansion of primary T-Cells
From the materials testing phase with Jurkat cells, we were able to observe that flasks and silicone bags were superior to polyolefin/EVA and FEP vessels with respect to overall cell proliferation. We then transitioned to more relevant primary immunocult CD3/CD28 activated T-cells and extended the duration of the experiment due to a potential slight advantage to silicone observed in our initial Jurkat study. Figure 3A depicts our silicone bag setup with perfusion loop. The tan colored tubing fits into the pump head of the peristaltic system whereas the blue port is a needleless syringe port used for taking samples and introducing new media.
Figure 3. Growth comparison of primary human T-cells.
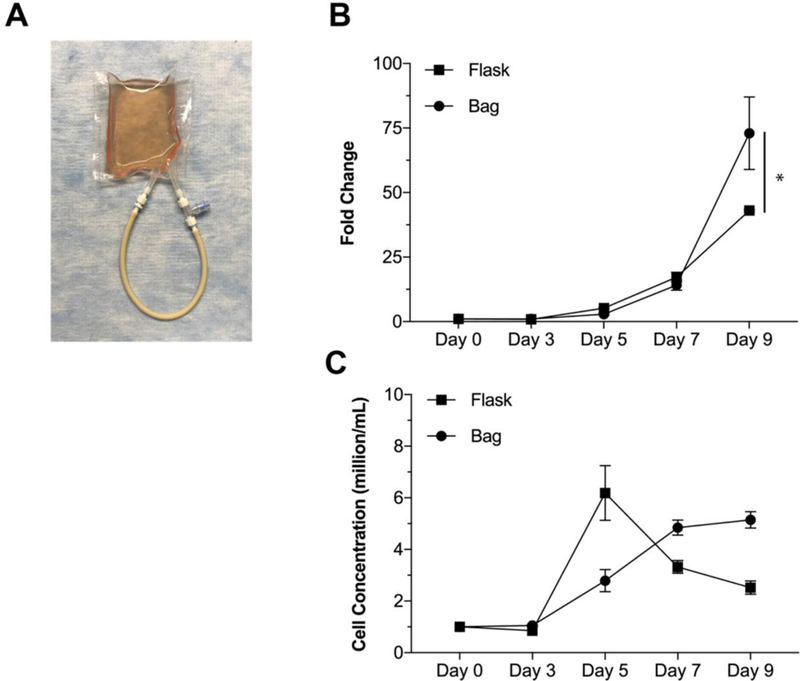
Proliferative comparison of flask versus silicone bag for the growth of healthy, primary T-cells. A) Picture depicting the custom designed and manufactured silicone bag. Tan loop represents the portion interfacing with the peristaltic pump for flowing action. Medium introduction and sampling were accomplished through a needleless syringe port. B) Growth comparison over a 9-day culture. Medium and fresh IL-2 was added on the days 3, 5, 7, and 9. Flask growth holds an advantage at early time points, however, silicone systems show a drastic increase in cell yield by the latest time point. * = P<0.05. C) Growth comparison over a 9day culture as measured by cell concentration. We observe that the bag has an increasing cell concentration at each time point whereas flask conditions spike and decrease. Note that cell concentration was normalized to 1M/mL on days 3, 5, 7, and 9.
We observe an even greater improvement in the silicone bag compared to the T-flask by day 9 (73.0 +/−14.0 vs 43.0 +/− 0.44 fold change, Figure 3B). No differences in cell viability were observed between these groups (data not shown). We find once again that this occurs at the later time points, wherein the earlier time points demonstrate comparability between the two systems. We also find that our bags result in an increased cell concentration over time while the flasks spike and decrease (Figure 3C); note that cell concentration was normalized to 1M/mL at days 3,5,7, and 9. This assessment was based on an initially purified pan CD3 population, we did not further measure CD4, CD8, or CD56 at the end of the experiment.
Discussion
Our work describes the ability of a closed loop bioreactor system to facilitate T-cell aggregate dissociation and improved gas transfer properties leading to higher cell proliferation by using a silicone based cell culture bag material. We herein take away two major conclusions from flow conditions: 1) the notion that proper Tcell activation and proliferation require physical interaction between cells and blast formation[15–19], which continuous flow does not provide and 2) material properties of the culture vessels may underlie the major differences seen between intermittent flow and flask culture. Note that we based our results on a initial pan CD3 population but did not further dissect into CD4, CD8, or CD56 populations. Based on the stimulation reagent (immunocult, CD3/CD28, we anticipate expansion to only be limited to CD3 and potentially a small NKT cell population.
Silicone rubbers are highly gas permeable [9,20]. As such, this class of material which we used to fabricate our bags has been implemented into next generation culture systems to enable high transfer of oxygen to cells, such as the G-Rex from Wilson Wolf. We interestingly find an added benefit of improved release of CO2 to the atmosphere. Accumulation of dissolved carbon dioxide is a known negative regulator of cell proliferation [21,22]. Thus, by allowing the permeation of excess CO2 to the environment, longer term growth can thus be sustained. This issue becomes exacerbated by increases in cell mass and may thus explain why the silicone bag may appear to outperform the T-flask at later time points. This suggests the importance of CO2 in cell culture and demonstrates silicone rubber as a means to temper accelerated accumulation. However, CO2 does indeed still rise over time in the improved silicone bag indicating elevated CO2 production and retention rate above the permeation rate through the membrane. Next generation methods of a cell culture system may include CO2 monitoring after which a threshold is reached, media replacement may be performed to renormalize cell waste. Additional technologies may also be added such as a rocking device to enhance convection of the media within the bag to potentially improve gaseous transfer with the ambient incubator environment. While pO2 is a useful metric to monitor cell cultures, it may provide confounding information as an actual comparator between bag conditions. We notice a depression in early on in flasks which may be partially due to the increased cell proliferation at early time points – current technologies are using pO2 as a method to determine cell concentration[23]. There is a slight gradual downward trend in all conditions, although not significant, which is likely explained by the increasing cell burden within the culture.
Although many industrial closed systems do not disturb the cell aggregates that form during T-Cell activation, we chose to mimic a protocol typically performed at the benchtop scale and found in numerous manufacturer protocols [12,13]. These protocols involve de-aggregating cells at set intervals to count and renormalize cell concentration with new media. While we believe that normalizing to fresh media is highly critical, it is this aspect of minimizing cell aggregate size to ensure improved nutrient diffusion into aggregates to be of importance as well [24–26]. This may lead to improved industrial designs in which aggregate shearing technologies are implemented to facilitate this improved nutrient access to cells. Further studies must be performed to assess the effect of aggregate sizing and frequency of shear stress applied to cells on cell phenotype and function.
We herein demonstrate the feasibility of an enhanced cell culture bag in combination with a dynamic circulating flow system that allows for high yield cell expansion and cell cluster disaggregation in a closed loop. There is surprisingly no commercially available, silicone rubber cell culture bag despite its vastly superior gas transport characteristics relative to readily available products. We believe one potential reason for this is due to manufacturing and costs. Polyolefin/EVA and FEP materials can easily be cut to shape and heat sealed to form a fully leakproof container. Silicone rubbers, on the other hand, must be cured to the final desired configuration which typically requires molds and can be prohibitively expensive. Thus, without significant market impetus to recoup tooling costs, it becomes more apparent why there may be a lack of such a vessel on the market. While we were able to construct our bags with simple silicone specific glue, this process is highly manual and inconsistent across batches – which is not amenable to large scale production. Increased market demand for this type of system may result in the tools taking hold.
Our resulting closed flow system may, at face value, only hold use for aggregating cells which may need dissociation. One can, however, imagine an alternate system in which the combination of continuous flow with a highly gas permeable bag may now contend with stir tanked bioreactors for the large-scale expansion of cells seeded on microcarriers. The continuous flow will keep microcarriers in suspension allowing for proper nutrient transport while the silicone bag may provide an alternative to gas sparging in tanks which can be detrimental to cells due to bubble formation. These continuous flow systems also do away with reactor impellers which add a high level of complexity to bioreactor optimization design in maximizing cell dispersion and nutrient availability while minimizing damage to cells. Current systems, such as the Prodigy or Xuri, apply peristaltic flow for media/buffer exchange during cell culture; none currently apply direct shear stress in a dynamic manner as described herein this system. Greater investigation into such systems may lead to efficient yet significantly simpler bioreactor systems. We believe that such systems should be scalable up due to the simple nature of the bag fabrication. Furthermore, these magnetic fluidic systems are also available in sizes aimed to process higher volumes, albeit at elevated flow rates.
We thus find that silicone rubber makes a highly suitable material for use as a cell culture bag with comparable and improved performance to T-flasks especially for long term culture. We further demonstrate that such a system is readily amenable for use a closed loop system to be paired with a perfusion system to facilitate shear disaggregation of cell clumps to promote improved nutrient transport and allow for cell density enumeration.
Acknowledgements
This work was supported in part by the Shriners Hospitals for Children (BP) and the National Institutes of Health Grants R01EB012521 and T32-EB016652 (ML)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional Information
BP is a founder and equity holder of Sentien Biotechnologies, Inc whom have licensed patents pertaining to MSC therapeutics. The authors declare no other competing financial interests. Supplementary information accompanies this paper online. Reprints and permissions information is available online. Correspondence and requests for materials should be addressed to B.P.
Data Statement
Data and materials are available upon request for independent reproduction of results.
References
- 1.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric Antigen Receptor Therapy for Cancer. Annu Rev Med 2014;65: 333–347. doi: 10.1146/annurev-med-060512-150254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newick K, O’Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med 2017;68: 139–152. doi: 10.1146/annurev-med-062315-120245 [DOI] [PubMed] [Google Scholar]
- 3.Sadelain M Chimeric Antigen Receptors: A Paradigm Shift in Immunotherapy. Annu Rev Cancer Biol 2017;1: 447–466. doi: 10.1146/annurev-cancerbio-050216-034351 [DOI] [Google Scholar]
- 4.Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Mol Ther -Methods Clin Dev 2017;4: 92–101. doi: 10.1016/j.omtm.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 2018;53: 164–181. doi: 10.1016/j.copbio.2018.01.025 [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther -Oncolytics 2016;3: 16015. doi: 10.1038/mto.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ratcliffe E, Thomas RJ, Williams DJ. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull 2011;100: 137–155. doi: 10.1093/bmb/ldr037 [DOI] [PubMed] [Google Scholar]
- 8.Fekete N, Béland AV, Campbell K, Clark SL, Hoesli CA. Bags versus flasks: a comparison of cell culture systems for the production of dendritic cell-based immunotherapies: CANCER IMMUNOTHERAPY CULTURE VESSELS. Transfusion (Paris) 2018; doi: 10.1111/trf.14621 [DOI] [PubMed] [Google Scholar]
- 9.Avgoustiniatos ES, Hering BJ, Rozak PR, Wilson JR, Tempelman LA, Balamurugan AN, et al. Commercially Available Gas-Permeable Cell Culture Bags May Not Prevent Anoxia in Cultured or Shipped Islets. Transplant Proc 2008;40: 395–400. doi: 10.1016/j.transproceed.2008.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson Wolf G-Rex [Internet] Available: http://assets.ngin.com/attachments/document/0003/8900/GRex_Instructions_for_use_12.16.2013.pdf
- 11.Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J, et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012;14: 1131–1143. doi: 10.3109/14653249.2012.700767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thermo, Dynabeads Protocol [Internet] Available: https://www.thermofisher.com/us/en/home/references/protocols/proteins-expression-isolation-andanalysis/t-cell-activation-and-expansion/dynabeads-human-t-activator-cd3-cd28.html#prot2
- 13.STEMCELL Tech, Immunocult Protocol [Internet] Available: https://cdn.stemcell.com/media/files/pis/DX20349-PIS_1_0_2.pdf?_ga=2.60969452.1317456412.1526655624-238722423.1512144574
- 14.Blaschczok K, Kaiser SC, Löffelholz C, Imseng N, Burkart J, Bösch P, et al. Investigations on Mechanical Stress Caused to CHO Suspension Cells by Standard and Single-Use Pumps. Chem Ing Tech 2013;85: 144–152. doi: 10.1002/cite.201200135 [DOI] [Google Scholar]
- 15.Zumwalde NA, Domae E, Mescher MF, Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J Immunol Baltim Md 1950 2013;191: 3681–3693. doi: 10.4049/jimmunol.1201954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purtic B, Pitcher LA, van Oers NSC, Wulfing C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proc Natl Acad Sci 2005;102: 2904–2909. doi: 10.1073/pnas.0406867102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grönberg A, Halapi E, Ferm M, Petersson M, Patarroyo M. Regulation of lymphocyte aggregation and proliferation through adhesion molecule CD54 (ICAM-1). Cell Immunol 1993;147: 12–24. [DOI] [PubMed] [Google Scholar]
- 18.Böhmer RM, Bandala-Sanchez E, Harrison LC. Forward light scatter is a simple measure of T-cell activation and proliferation but is not universally suited for doublet discrimination. Cytom Part J Int Soc Anal Cytol 2011;79: 646–652. doi: 10.1002/cyto.a.21096 [DOI] [PubMed] [Google Scholar]
- 19.Teague TK, Munn L, Zygourakis K, McIntyre BW. Analysis of lymphocyte activation and proliferation by video microscopy and digital imaging. Cytometry 1993;14: 772–782. doi: 10.1002/cyto.990140710 [DOI] [PubMed] [Google Scholar]
- 20.Zhang H The Permeability Characteristics of Silicone RUbber [Internet]. Society for the Advancement of Material Process Engineering; 2006. Available: https://imageserv5.teamlogic.com/mediaLibrary/99/D116_20Haibing_20Zhang_20et_20al.pdf [Google Scholar]
- 21.Matanguihan R, Sajan E, Zachariou M, Olson C, Michaels J, Thrift J, et al. Solution to the high Dissolved CO2 Problem in High-Density Perfusion Culture of Mammalian Cells. In: Lindner-Olsson E, Chatzissavidou N, Lüllau E, editors. Animal Cell Technology: From Target to Market. Dordrecht: Springer Netherlands; 2001. pp. 399–402. doi: 10.1007/978-94-010-0369-8_95 [DOI] [Google Scholar]
- 22.Gray DR, Chen S, Howarth W, Inlow D, Maiorella BL. CO2 in large-scale and high-density CHO cell perfusion culture. Cytotechnology 1996;22: 65–78. doi: 10.1007/BF00353925 [DOI] [PubMed] [Google Scholar]
- 23.GE, Xuri Interview [Internet] Available: http://www.sciencemag.org/sites/default/files/Transcript_Science%20Immunotherapy%20webinar%20on%201%20Oct%202014.pdf
- 24.Lin R-Z, Chang H-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008;3: 1172–1184. doi: 10.1002/biot.200700228 [DOI] [PubMed] [Google Scholar]
- 25.Carlsson J, Acker H. Relations between pH, oxygen partial pressure and growth in cultured cell spheroids. Int J Cancer 1988;42: 715–720. [DOI] [PubMed] [Google Scholar]
- 26.Mueller-Klieser W Three-dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol-Cell Physiol 1997;273: C1109–C1123. doi: 10.1152/ajpcell.1997.273.4.C1109 [DOI] [PubMed] [Google Scholar]


