Abstract
Although falling in love is one of the most important and psychologically potent events in human life, the somatic implications of new romantic love remain poorly understood. Psychological, immunological, and reproductive perspectives offer competing predictions of the specific transcriptional regulatory shifts that might accompany the experience of falling in love. To characterize the impact of romantic love on human genome function, we conducted genome-wide transcriptome profiling of 115 circulating immune cell samples collected from 47 young women over the course of a 2-year longitudinal study. Analyses revealed a selective alteration in immune cell gene regulation characterized by up-regulation of Type I interferon response genes associated with CD1C+/BDCA-1+ dendritic cells (DCs) and CLEC4C+/BDCA-2+ DCs, and a reciprocal down-regulation of α-defensin-related transcripts associated with neutrophil granulocytes. These effects emerged above and beyond the effects of changes in illness, perceived social isolation, and sexual contact. These findings are consistent with a selective up-regulation of innate immune responses to viral infections (e.g., Type I interferons and DC) and with DC facilitation of sexual reproduction, and provide insight into the immunoregulatory correlates of one of the keystone experiences in human life.
Keywords: Romantic love, social genomics, immune system regulation, health
1. Introduction
Falling in love is one of the most psychologically potent experiences in human life (Aron et al., 2005; Hatfield, 1987; Jankowiak & Fischer, 1992; Leckman & Mayes, 1999). New romantic love is accompanied not only by psychological changes, but physiological changes as well (e.g., Marazziti & Canale, 2004; Schneiderman et al., 2011; Ulmer-Yaniv et al., 2016). However, the somatic impact of falling in love remains poorly understood. Here, we investigate the impact of new romantic love on immune-related gene regulation.
Several studies have begun to map the multiple neurobiological implications of new romantic love. Research investigating the endocrinological correlates of love suggest that that circulating oxytocin is higher in people in new romantic relationships compared to other groups (such as new parents and single individuals; Schneiderman et al., 2012; Ulmer-Yaniv et al., 2016). Work investigating circulating levels of neurotrophins has found elevated plasma levels of Nerve Growth Factor in participants who had recently fallen in love relative to single participants or participants in long term relationships (no differences between groups in other neurotrophin levels were detected; Emanuele et al., 2006). Follow-up samples revealed that those new-love participants who remained in the same relationship had NGF levels at (12–24 month follow-up) that were indistinguishable from other groups. Marazziti and Canale (2004) found elevated levels of cortisol in both men and women who had recently fallen in love, and reduced testosterone and follicle-stimulating hormone in men, but increased testosterone in women (these differences were eliminated at 12–24 month follow-up). Other work has found that new romantic love is associated with lower densities of specific serotonin transporter binding sites—densities that were equivalent to those found in individuals suffering from obsessive-compulsive disorder (Marazziti et al., 1999).
Another growing body of work has begun to illuminate the distinct neural and central nervous system (CNS) alterations associated with new romantic love. Some work suggests that new romantic love is associated with lowered autonomic reactivity to emotions (Schneiderman et al., 2011). The early stage of new romantic relationships has been associated with greater neural activity in both the left posterior cingulate cortex and caudate regions relative to later stages of these relationships (e.g., Aron et al., 2005; Bartels and Zeki, 2000, 2004; Kim et al., 2009). This pattern of neural activity parallels in some respects the pattern associated with new maternal love (Bartels & Zeki, 2004), and these brain areas are also associated with high concentrations of reward-based neuromodulators (e.g., oxytocin, vasopressin, and dopamine; see Zeki, 2007). Animal models also attest to the role of oxytocin, alterations in CNS processes, and accompanying changes in HPA axis activity in pair-ponding (for reviews see de Boer et al., 2012; Carter, 1998).
While each of the above studies provides evidence for specific neurobiological changes associated with new romantic love, the broader somatic impact of falling in love remains poorly understood. The development of mating bonds has complex consequences for human physiology, and at least three different perspectives offer competing theories of what the somatic implications of falling in love might be. Theories focusing on the psychological experience of deep interpersonal connection, for example, might predict a reduction in stress-related physiological processes associated with social isolation (Cacioppo & Hawkley, 2009), such as activation of the hypothalamic-pituitary-adrenal (HPA)-axis and sympathetic nervous system (SNS; Cacioppo et al., 2015), or expression of the leukocyte conserved transcriptional response to adversity (CTRA) by CD16- “classical” monocytes (Cole et al., 2015). In contrast, an immunologic life history perspective derived from evolutionary biology (McDade, 2003) might predict an alternative regulatory shift toward generalized immune activation as the body adapts to a new microbial symbiosis, which would be characterized by up-regulating both inflammatory and antiviral gene modules in granulocytes and dendritic cells (DCs; Amit et al., 2009). Finally, a third perspective from reproductive life history theory (Abrams & Miller, 2011; Lorenz et al., 2015) implies a distinct set of gene regulatory responses that prepare the body for sexual reproduction (e.g., by downregulating systemic inflammation and up-regulating natural killer cells and DCs to facilitate pregnancy; Lorenz et al., 2015; Mor et al., 2011; Plaks et al., 2008). Although psychological, immunologic, and reproductive perspectives imply distinct patterns of biological adaptation to new love, little is known about which pattern prevails; to date, no studies have comprehensively examined the gene regulatory impact of new romantic love.
To characterize the impact of romantic love on human genome function, we conducted a two-year longitudinal study of young women in new romantic relationships. We performed genome-wide transcriptome profiling of 115 circulating immune cell samples collected from 47 young women at three different relationship stages (which varied within individuals over time): not in love (but in a new romantic relationship), newly in love, and out-of-love. Analyses involved both unbiased characterization of the empirical transcriptome alterations associated with falling in love and targeted tests of specific competing hypotheses derived from psychological, immunological, and reproductive life history theories. Analyses focused in particular on gene-regulatory dynamics occurring in myeloid lineage immune cells (monocytes, granulocytes, and DCs), which have previously been found to show distinct profiles of transcriptional regulation in response to microbial exposures (i.e., immunologic activation, pro-inflammatory gene expression, and activation of Type I interferon-related antiviral genes; Amit et al., 2009), deep social connection (i.e., reductions in the CTRA profile of up-regulated inflammation and down-regulated Type I interferon activity; Cole et al., 2015), and pregnancy (i.e., down-regulated inflammation and granulocyte function and up-regulated monocyte and DC function; Mor et al., 2011).
2. Material and Methods
2.1. Participants and Procedures
Participants were 47 young women (mean age = 20.5 yrs) who were attending a U.S. university (sample characteristics in Table 1). At study enrollment, all participants had recently begun a new (< 1 month) exclusive romantic relationship and reported having not yet fallen in love with their partners (by answering “no” to the question, “Would you consider yourself to have fallen in love with your partner?” during prescreening via either telephone or email). Additional eligibility criteria assessed during prescreening included 1) being heterosexual, 2) not being pregnant or breastfeeding, 3) being a non-smoker, 4) having no immune, cardiovascular, or psychiatric medication, and 5) planning to remain in the city for at least 6 months. Analyses utilized a repeated measures design to relate intra-individual changes in immune cell gene expression profiles to intra-individual changes in in-love status (thereby controlling for baseline individual differences). Sixty-one eligible women enrolled and provided an online baseline questionnaire and blood sample; among those, 47 (77%) provided at least one follow-up blood sample (and accompanying online questionnaire) and could thus be included in within-subject repeated measures analyses of transcriptome change over time. All procedures were approved by the UCLA Biomedical Institutional Review Board (#13–001122).
Table 1.
Participant demographics and baseline characteristics.
| Age | 20.5 years (SD = 2.6) |
|---|---|
| Ethnicity | 36.2% White or Caucasian, 25.5% Asian 17.0% multi-racial 12.8% Hispanic or Latino 6.4% Black or African American 2.1% American Indian |
| BMI | 22.1 kg/m2 (SD = 3.6) |
| Cigarettes smoked (previous 2 wk) | 0.0 (SD = 0) |
| Alcoholic beverages consumed (previous 2 wk) | 7.0 (SD = 9.3; maximum = 35) |
| Using hormonal contraceptive | 40.4% |
Data represent mean (standard deviation) of continuous variables or % of total sample for categorical variables.
Participants provided written informed consent and a baseline blood sample and then completed within 24 hours a comprehensive online survey of demographic, psychological, sexual, and relationship parameters. After providing the baseline blood sample, participants completed up to 48 twice-monthly surveys. The central question of interest in these twice-monthly surveys assessed whether participants felt that they had fallen in love with their partners since they completed the previous survey (Question: “When did you first feel you fell in love with your partner?” Response options: “This has not happened,” “Within the last two days,” “Within the last week,” “Within the last two weeks”). These surveys also assessed perceived changes in physical and mental health (and accompanied medications), relationship status, sexual activity, and other related psychological and behavioral parameters. Participants were scheduled to provide a follow-up blood sample and comprehensive survey when any of the following occurred: 1) they reported having fallen in love with their partner, 2) they reported the relationship had broken up, 3) they were selected as a yoked control participant for another participant who had fallen in love, or 4) 12 months had elapsed. For those who had broken up, participation concluded after the 2nd blood draw. After the second blood draw, those still in relationships continued twice-monthly brief surveys until they either reported a break-up, they reported falling in love (if they were not in love at their second blood draw), or 12 months had elapsed after the second blood draw. Participants were scheduled for a 3rd and final blood draw and comprehensive survey after any of these events, and were compensated based on the number of blood samples and on-time surveys provided (maximum $196).
Among the 47 women who were included in the repeated measures analyses, 17 (36%) subsequently reported falling in love with their partners (median time from enrollment = 94 d; SD = 112 d) and 25 (53%) never indicated being in love during their longitudinal follow-up period (median follow-up = 97 d; SD = 67 d). Five women (11%) reported falling in love between enrollment and their first follow-up survey, of which 2 (4%) subsequently reported falling out of love and 3 (6%) reported still being in love at their longest-term follow-up. Blood samples were collected from participants at baseline and again within 14 d of the first report of being in love (or within 14 d of being selected as a yoked control participant). Among 115 total samples assayed, 2 yielded technically invalid results, and 2 had no paired longitudinal sample, leaving 111 samples for analyses of intra-individual change over time. (Detailed eligibility criteria and procedural details are provided in the Supplementary Materials).
2.2. RNA Profiling and Bioinformatics
Transcriptome profiling analyses were conducted on 115 peripheral blood mononuclear cell (PBMC) samples collected from the sample. Total RNA was isolated from PBMC obtained from 60 mL blood samples (Qiagen RNeasy), tested for suitable mass and integrity (Nanodrop ND1000 and Agilent TapeStation), and subject to genome-wide transcriptional profiling using a standard Illumina microarray system (Ambion TotalPrep cRNA hybridized to Illumina HT-12 v4 BeadArrays) in the UCLA Neuroscience Genomics Core Laboratory, following the manufacturers’ standard protocol. Quantile-normalized gene expression data were analyzed using standard repeated measures linear modeling to relate change in log2 gene expression values (follow-up – baseline) to change in in-love status, with statistical control where indicated for parallel changes in loneliness, illness symptoms, sexual intercourse (either frequency or presence/absence). Genes showing > 15% change in average expression as a function of falling in love served as inputs into higher-order bioinformatics analyses identifying co-regulation of gene sets as a function of transcription factor activity (TELiS, as previously described; Cacioppo & Hawkley, 2009; Cole et al., 2005), cell type of origin (TOA; Cole et al., 2011), or differential cell type abundance (TRA; Powell et al., 2013). TOA and TRA analyses utilized cell type-specific reference transcriptome profiles derived from previous studies (GSE1133; Su et al., 2004) and flow-cytometric sorting of PBMC into major myeloid cell populations including 3 major DC subsets and 2 major monocyte subsets (GSE101489). Analyses also assessed changes in expression of a 53-gene contrast score representing the CTRA profile of up-regulated pro-inflammatory genes (19 transcripts) and down-regulated Type I interferon response genes (31 transcripts) and antibody-related genes (3 transcripts) as previously described (Cole et al., 2007). All samples yielded valid data and data are publicly available as Gene Expression Omnibus GSE102689. Additional details are provided in the Supplementary Materials.
3. Results
Preliminary analyses of intraindividual psychological change revealed that falling in love was associated with a nonsignificant decrease in self-reported stress (mean = 2.4 ± 0.16 SE in love vs. 2.6 ± 0.14 SE at baseline, p = .21). Falling in love was similarly associated with a nonsignificant decrease in self-reported depressive symptoms (0.20 ± 0.036 SE in love vs. 0.25 ± 0.058 SE at baseline, p = .29).
3.1. Empirical Transcriptome Alterations
Analysis of leukocyte transcriptome profiles identified 61 gene transcripts that showed > 15% difference in magnitude of change from baseline to follow-up as participants transitioned from being out of love to in love (20 up-regulated and 41 down-regulated; see Supplementary Table S1). Genes up-regulated in association with falling in love included transcripts involved in Type I interferon signaling (IFI30, ISG15, TNFAIP2) and DC markers (CD1C/BDCA-1, CLEC4C/BDCA-2, IGSF6, TNFRSF21; representative examples in Fig 1A). Down-regulated genes included α-defensins (DEFA1, DEFA1B, DEFA3) and other granulocyte-related transcripts (CPA3, TCN1, HDC), cell membrane-associated receptors and ion channels (CXCR6, EBP42, SLC4A1, SLC25A39, SLC45A3, TSPAN5, XK), the α-synuclein component of amyloid (SNCA), and the FOS component of the AP-1 transcription factor. Although biologically coherent, these transcriptomic correlates of falling in love were quantitatively modest in magnitude (generally < 50% change in average expression).
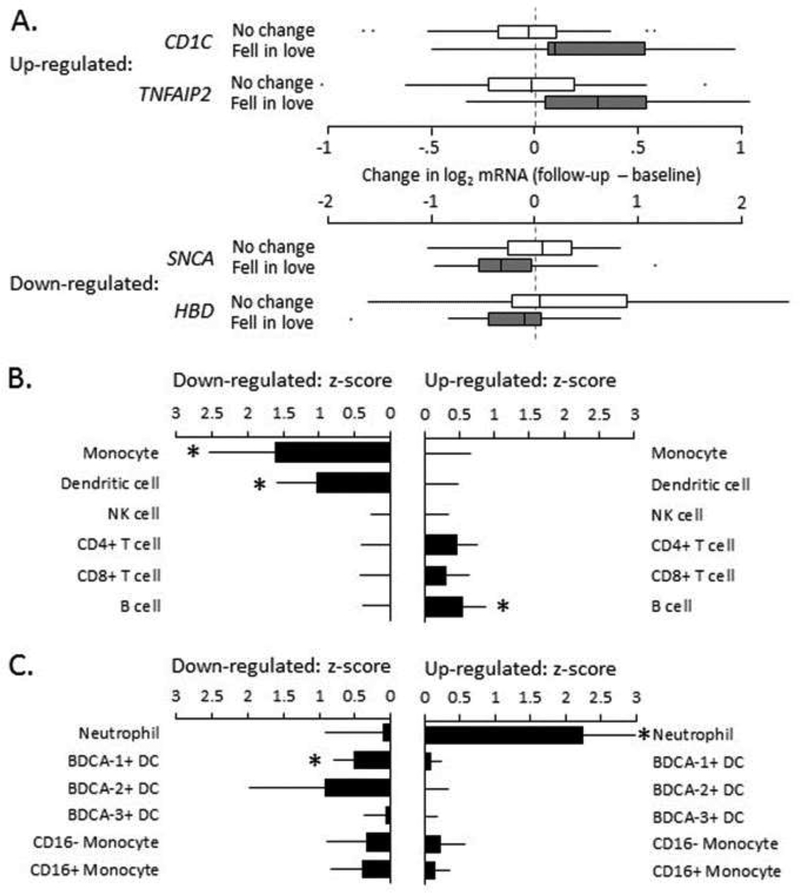
Fig 1 – Gene transcriptional correlates of falling in love.
(A.) Representative examples of 61 gene transcripts showing > 15% differential change from baseline to follow-up in 94 longitudinal PBMC samples from 47 women who fell in love (n=17) vs. did not (n=30). Boxes span 25th-75th percentiles, internal bar = median, and dashes indicate the no-change reference point. Transcript Origin Analysis (TOA) mapped the cellular origins of the 20 up-regulated and 41 down-regulated genes (listed in Table S1) over major leukocyte subsets (B.) and a more refined fractionation of myeloid lineage immune cells (C.). Data represent mean ± SE TOA cell diagnostic z-score; * indicates p < .05.
To clarify the cellular architecture of love-related changes in the total PBMC transcriptome, we conducted Transcript Origin Analysis (TOA; Cole et al., 2011) using reference RNA profiles derived from isolated subsets of PBMC. Results (Fig 1B) identified DCs and monocytes as the primary cellular origin of gene transcripts up-regulated in association with falling in love, and B lymphocytes as primary cellular origins of down-regulated transcripts. Follow-up TOAs utilizing more differentiated reference data on specific myeloid lineage cell subsets (Fig 1C) localized up-regulated transcripts to CD1C+ (BDCA-1+) DCs and down-regulated transcripts to neutrophil granulocytes. CLEC4C+ (BDCA-2+) DCs also appeared to contribute, although their analytic signal showed greater sampling variability and did not reach statistical significance. These effects appear to stem from selective regulation of specific gene modules within cell types as parallel Transcriptome Representation Analyses (TRA; Powell et al., 2013) indicated no change in overall prevalence of any cell type within the circulating leukocyte pool (all differences < 2% in magnitude and p > .30).
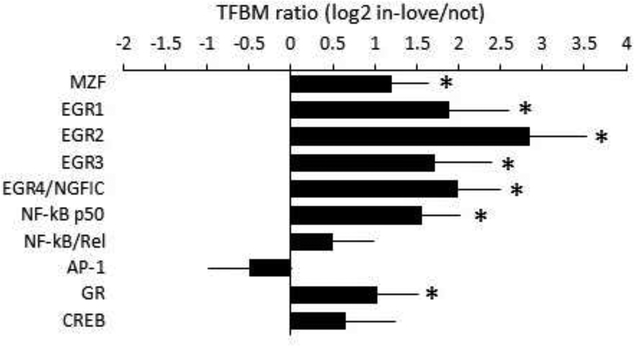
To identify transcription control pathways involved in love-related changes in gene expression, we conducted TELiS bioinformatics analysis (Cole et al., 2005) of transcription factor-binding motif patterns within core promoters of up- and down-regulated genes (Fig 2). Analyses focused specifically on transcription factors known to play a role in myeloid cell gene regulation and indicated activation of the myeloid differentiation factor, MZF-1; the myeloid differentiation- and activation-related factors, EGR1, EGR2, EGR3, and EGR4/NGFIC; and the anti-inflammatory p50 component of the NF-κB family (Cao et al., 2006). Consistent with previously reported increases in glucocorticoid output during new romantic love (Marazziti & Canale, 2004), results also indicated increased glucocorticoid receptor activity. No significant differences were observed in activity of the SNS-regulated CREB family of transcription factors or the pro-inflammatory NF-κB/Rel or AP-1 family factors (Fig 2).
Fig 2 – Transcription control pathways in love-related gene regulation.
TELiS bioinformatics analysis analyzed the prevalence of transcription factor-binding motifs (TFBMs) for representative transcription factors involved in myeloid immune cell development and effector function and neuroendocrine regulation. Data represent the average (± SE) log2 ratio of TFBM frequency in promoters of 20 genes up-regulated in association with falling in love vs. 41 genes down-regulated (genes listed in Table S1). * indicates p < .05 difference from null hypothesis value of 0 log-ratio.
3.2. Social Connection
To determine whether falling in love opposed the biological effects of loneliness, we tested for down-regulation of the CTRA gene expression profile previously linked to perceived social isolation (Cole et al., 2015). Results indicated no significant change in CTRA gene expression as a function of falling in love (change in the 53-gene CTRA indicator score: −1.2% ± SE 2.3% log2 mRNA abundance, p = .543; change in the 19-gene CTRA inflammatory subcomponent: +1.0% ± 4.3%, p = .836; change in the 34-gene CTRA interferon/antibody-related subcomponent: +2.5% ± 4.2%, p = .510; TOA assessment of classical monocyte activation: 0.34 ± 0.55 z-score units, p = .275). These negligible changes in loneliness-related gene expression are consistent with the fact that falling in love was not associated with any significant reduction in experienced loneliness (r = −.06, p = .712).
3.3. Immunologic Adaptation
To determine how changing microbial exposures might contribute to love-related transcriptome alterations, we analyzed variations in sexual contact and somatic illness rates. Falling in love was associated with greater rates of self-reported illness (20.0% among those in love at follow-up vs. 7.4% among those not in love; although this difference did not reach statistical significance, p = .202), and illness was associated with increased expression of Type I interferon response genes (+9.7% ± 4.7% change in CTRA interferon/antibody-related mRNAs, p = .035). Illness was also associated with increased expression of transcripts derived from DCs (0.74 ± 0.33 TOA z-score units, p = .014) and monocytes (1.62 ± 0.81, p = .025). However, in analyses that controlled for changes in illness rates, results continued to indicate up-regulation of the specific interferon- and DC-related genes associated with love in unadjusted analyses (i.e., 19 of the 21 genes up-regulated by > 15% in unadjusted analyses also showed > 15% up-regulation in adjusted analyses, with an average change of +20.0% ± 5.8% after control for illness, p = .002; and TOA continued to indicate activation of CD1C+/BDCA-1+ DCs: 0.69 ± 0.30 z-score units, p = .016).
Falling in love was associated with a 7.5-fold increase in sexual intercourse frequency (5.0 ± 0.9 acts per 2-wk interval for those in love at follow-up vs. 0.7 ± 0.4 for those not in love; difference, p < .001). However, analyses controlling for intercourse frequency continued to indicate up-regulated expression of the interferon- and DC-related gene transcripts associated with love in unadjusted analyses (19 of the 21 genes up-regulated by > 15% in unadjusted analyses also showed > 15% up-regulation in adjusted analyses, with an average change of +21.8% ± 6.4% after control for intercourse frequency, p = .001; and TOA continued to indicate activation of CD1C+/BDCA-1+ DCs: 0.76 ± 0.28 z-score units, p = .005). Similar results emerged from analyses that additionally controlled for frequencies of oral sex and semen exposure. Parallel analyses of sexual intercourse as a discrete variable (present/absent) showed a trend toward reduced inflammatory gene expression among those having intercourse (−7.8% ± 3.3% mRNA for the CTRA pro-inflammatory gene set, p = .054) but showed no change in interferon/antibody-related gene expression (+0.3% ± 3.9%, p = .880). Analyses that controlled for differential intercourse rates also continued to link falling in love to up-regulated expression of the interferon- and DC-related transcripts identified in unadjusted analyses (average change: +19.5% ± 6.1% after control for intercourse, p = .002; TOA indication for CD1C+/BDCA-1+ DCs: 0.65 ± 0.28 z-score units, p = .012).
Among those who were not in love at the first follow-up but fell in love by the second follow-up, transcriptome change again resembled those reported above in showing up-regulation of genes predominately expressed by DCs (0.67 ± 0.30 TOA z-score units, p = .012) and monocytes (0.88 ± 0.43, p = .021), and more specifically by CD1C+/BDCA-1+ (0.23 ± 0.15, p = .064) and CLEC4C+/BDCA-2+ (0.86 ± 0.43, p = .022) DC subsets. Down-regulated transcripts derived predominantly from B lymphocytes (0.50 ± 0.20, p = .007). Results also showed a trend toward up-regulated expression of the same set of 21 Type I interferon- and DC-related genes identified in analyses of change from baseline to first follow-up (+22.9% ± 14.2%, p = .072).
3.4. Falling out of love
Using statistical models that estimated separate change parameters for transitions from being in-love to out-of-love and from being out-of-love to in-love, analyses of 34 paired follow-up samples from 17 participants linked falling out of love to reduced expression of Type I interferon response genes (−15.6% ± 5.1% change in CTRA interferon gene set, p = .012) and reduced activity of interferon response factor (IRF; −1.63 ± 0.36 log2 ratio of promoter binding sites in up- vs. down-regulated genes, p < .001) and signal transducer and activator of transcription 1 (STAT1; −0.50 ± 0.20, p = .014) transcription factors. However, falling out of love was not associated with down-regulation of mRNA for DC markers CD1C or CLEC4C or with any indication that down-regulated transcripts derived predominately from DCs (−0.22 ± .14 TOA z-score units, p = .941).
4. Discussion
These analyses mapped the human transcriptomic response to falling in love and identified a small but biologically coherent alteration in immune cell gene regulation characterized by up-regulation of Type I interferon response genes associated with CD1C+/BDCA-1+ DCs and CLEC4C+/BDCA-2+ DCs, and a reciprocal down-regulation of α-defensin-related transcripts associated with neutrophil granulocytes. These shifts in myeloid cell gene regulation occurred above and beyond the effects of changes in illness and sexual contact, perceived social isolation, and leukocyte subset prevalence (which did not change with falling in love). Transcription control pathway analyses implicated EGR- and MZF-family transcription factors and the anti-inflammatory glucocorticoid receptor and NF-κB p50 subunit as molecular mediators.
The transcriptomic alterations observed here are consistent with a selective up-regulation of innate immune responses to viral infections (e.g., Type I interferons and DCs; Amit et al., 2009). This pattern is most consistent with reproductive life-history perspectives which implicate the physiological modulation of DC function to facilitate sexual reproduction (Mor et al., 2011). Future research will be required to define more clearly the extent to which changes in DC activation observed here represent a response to viral exposures or an anticipatory physiological preparation for reproduction. This pattern of transcriptional alteration is not consistent with psychological perspectives implying that romantic love is antithetical to loneliness or reduces the transcriptional correlates of social isolation (e.g., the CTRA profile emerging from CD16- classical monocytes), nor is the pattern consistent with immunologic life-history perspectives implying that increases in commensal microbe exposure would activate myeloid lineage inflammatory signaling (e.g., in neutrophils or monocytes) or lymphoid lineage adaptive immune activation (e.g., in T or B cells).
The observation that falling in love was not associated with any reduction in loneliness-related transcriptional alterations may seem surprising, but it is consistent with other observations in this study and with previous research. In this sample, women who fell in love reported no significant reduction in general loneliness (note that the UCLA Loneliness Scale measures generalized perceptions of social isolation, not relationship-specific social bonds) and their leukocyte transcriptome alterations indicated an increase in glucocorticoid-mediated gene expression (possibly reflecting increased HPA-axis output, which would be the opposite of what would be expected if love simply “makes everything OK” psychosocially). Previous studies have also documented elevated glucocorticoid levels (Carter, 1998; Marazziti & Canale, 2004) and an amalgam of positive and negative emotions (including relationship anxiety and intrusive/obsessive preoccupation) in the early stages of romantic love (e.g., Aron et al., 2005; Hatfield & Sprecher, 1986; Leckman & Mayes, 1999). In fact, some have suggested that a certain level of stress is necessary at the initial stages of a romantic relationship in order for strong bonds to form (e.g., see Esch & Stefano, 2005; Simpson & Rholes, 1994). Among those already in a relationship, higher levels of passionate love are also associated with greater physiological stress reactions when asked to think of one’s romantic partner (Loving et al., 2009). Some research suggests that physiological changes associated with new romantic love may be attenuated as the relationship matures (Emaneule et al., 2006; Ulmer-Yaniv et al., 2016). Although the present data are consistent with previous research suggesting that—physiologically and psychologically speaking—falling in love is not simply the psychobiological antithesis of loneliness, it remains to be determined whether the biological correlates of love observed here might abate with the maturation of a longer-term more stable and secure mate bond, as has been found in previous work (Emaneule et al., 2006; Ulmer-Yaniv et al., 2016).
Falling out of love was associated with a reduction in Type I interferon-related gene expression and related IRF and STAT transcription factor activity, but no decrease in DC prevalence or activation. These results suggest that intracellular transcription control pathways may be more responsive than cell population dynamics to changing social conditions (as in other studies examining changing social status; Snyder-Mackler et al., 2016; Tung et al., 2012). However, changes in cell prevalence or activation status may develop over longer durations than observed in the present study’s blood sampling protocol. This study may also have lacked power to resolve the cellular effects of falling out of love due to the fact that such analyses were only feasible in the subset of participants (47%) who fell in love within this study’s first 2 visits.
Several limitations deserve note. The first is the study’s correlational design, which precludes any definitive conclusion that falling in love is the specific cause of the transcriptional changes observed here. However, it seems far more theoretically plausible that subjective appraisals of being in love would induce leukocyte transcriptome changes, rather than leukocyte transcriptome changes inducing feelings of love. A second limitation is the self-report assessment of love, which was necessitated by the absence of any objective biological or behavioral indication of this inherently experiential state. However, previous work suggests that subjective assessments of psychological states are sometimes more strongly related to genome regulation than are objective assessments (e.g., perceived social isolation vs. objective social network density; Cole et al., 2007). A related limitation of this self-reported assessment of love was the dichotomous nature of the question measuring the presence or absence of love (this dichotomy was necessitated by the current study’s within/between subject design). Although colloquially it is common for people to speak about the existence of romantic love in a dichotomous way, how accurately such a dichotomous treatment reflects the neurobiology of love remains to be seen. Future research using more continuous measures of intense romantic love (such as the Passionate Love Scale, Hatfield & Sprecher, 1986) may reveal that the reported “magnitude” of romantic or passionate love predicts the magnitude of transcriptional changes reported here.
Finally, the research sample was comprised of a restricted socio-demographic range of young, healthy, and well-educated American women. Future research in more demographically diverse samples will be required to assess the scope of these results. Some previous investigations into the neurobiological correlates of love have found opposite patterns in men and women (Maraziti and Canale, 2004, for example, found that circulating testosterone was decreased in new-love men but elevated in new-love women). A crucial next step in establishing the parameters of the current set of results will thus be to investigate whether a similar pattern of transcriptional shifts occurs in newly-in-love men. If the selective upregulation of antiviral defenses reported here reflects a functional, prophylactic defense against potentially novel viral infections then it is logically plausible that this same pattern may be observed in men. However, if this selective antiviral upregulation instead reflects an anticipatory modulation of DC function designed to facilitate reproduction (e.g., Mor et al., 2011), then the pattern of results reported here may be sex-specific.
The magnitude of the overall leukocyte transcriptome shifts observed here was not large, but this may stem in part from the fact that DCs represent a small fraction of circulating white blood cells (< 1%). However, the limited effect size precluded any statistical testing at the level of individual gene transcripts and only bioinformatic inferences regarding gene sets related to cell type, biological function, or transcription factor activity should be regarded as statistically reliable. The biological significance of the observed transcriptome shifts (e.g., for host resistance to infection, successful pregnancy, etc.) also remain to be defined in future research, as do their longitudinal dynamics (e.g., do they change with relationship maturation?). Other gene regulatory responses to falling in love are likely to occur in other tissues (e.g., the nervous and reproductive systems) and the present results can only provide insight into immunoregulatory responses to love. Despite these limitations, the present data provide a preliminary but comprehensive molecular perspective on one of the most significant functional transitions that occurs in the course of individual human development and in the evolutionary biology of pair-bonding species.
Supplementary Material
Highlights.
Falling in love is associated with up-regulation of Type I interferon response genes.
Falling in love is associated with a reciprocal down-regulation of α-defensin-related transcripts
These changes are independent of changes in physical illness or sexual contact
Changes are consistent with selective up-regulation of innate immune responses to viral infections
Changes also consistent with dendritic cell facilitation of sexual reproduction
Acknowledgments
Funding
This research was supported by grants from the UCLA Norman Cousins Center for Psychoneuroimmunology (awarded to MH and SC) and NIH R01-AG043404, R01-AG033590, P30AG017265, P30-CA016042, and P30-AI028697 (awarded to SC).
Footnotes
Competing Interests
Declarations of interest: None
Competing Interests/Conflict of Interest
We have no competing interests.
Ethics Statement
This research was approved by the UCLA Biomedical Institutional Review Board for research on human subjects (Approval #13-001122). Informed consent was obtained for all participants during each clinic visit.
Data
Data can be accessed at Gene Expression Omnibus as series GSE102689.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams ET, Miller EM, 2011. The roles of the immune system in women’s reproduction: Evolutionary constraints and life history trade-offs. Am. J. Phys. Anthropol 146, Suppl 53, 134–154. [DOI] [PubMed] [Google Scholar]
- Amit I, et al. , 2009. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326, 257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL, 2005. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 94, 327–337. [DOI] [PubMed] [Google Scholar]
- Aron A, Paris M, Aron EN, 1995. Falling in love: Prospective studies of self-concept change. J. Pers. Soc. Psychol 69, 1102–1112. [Google Scholar]
- Bartels A, Zeki S 2000. The neural basis of romantic love. Neuroreport 11, 3829–3834. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S 2004. The neural correlates of maternal and romantic love. Neuroimage 21, 1155–1166. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK 1996. Beck depression inventory-II. : Psychological Corporation, San Antonio, TX. [Google Scholar]
- Cacioppo JT, Cacioppo S, Capitanio JP, Cole SW, 2015. The neuroendocrinology of social isolation. Annu. Rev. Psychol 66, 733–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, 2009. Perceived social isolation and cognition. Trends Cogn Sci 13, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Zhang X, Edwards JP, Mosser DM, 2006. NF-kappaB1 homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem 281, 26041–26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, 1998. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrino, 23, 779–818. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav 24, 385–396. [PubMed] [Google Scholar]
- Cole SW, Capitanio JP, Chun K, Arevalo JM, Ma J, Cacioppo JT, 2015. Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proc. Natl. Acad. Sci. USA 112, 15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT, 2011. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc. Natl. Acad. Sci. USA 108, 3080–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LH, Arevalo JM, Sung CY, Rose RM, Cacioppo JT, 2007. Social regulation of gene expression in human leukocytes. Genome Biol. 8, R189. doi: 10.1186/gb-2007-8-9-r189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA, 2005. Expression-based monitoring of transcription factor activity: The TELiS database. Bioinformatics 21, 803–810. [DOI] [PubMed] [Google Scholar]
- de Boer A, van Buel EM, Ter Horst GJ, 2012. Love is more than just a kiss: A neurobiological perspective on love and affection. Neuroscience 201, 114–124. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ, 1993. An Introduction to the Bootstrap. Chapman & Hall, New York. [Google Scholar]
- Emanuele E, Politi P, Bianchi M, Minoretti P, Bertona M, Geroldi G, 2006. Raised plasma nerve growth factor levels associated with early-stage romantic love. Psychoneuroendocrino. 31, 288–294. [DOI] [PubMed] [Google Scholar]
- Esch T, Stefano GB, 2005. The neurobiology of love. Neuroendocrinol. Letters 26, 175–192. [PubMed] [Google Scholar]
- Galperin A, Haselton M, 2010. Predictors of how often and when people fall in love. Evol. Psychol. 8, 5–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield E, 1987. Passionate and companionate love, in: Sternberg RJ, Barnes ML (Eds.), The Psychology of Love. Yale University Press, New Haven CT, pp. 191–217. [Google Scholar]
- Hatfield E, Sprecher S, 1986. Measuring passionate love in intimate relationships. J. Adolescence 9, 383–410. [DOI] [PubMed] [Google Scholar]
- Jackson JJ, Kirkpatrick LA, 2007The structure and measurement of human mating strategies: Toward a multidimensional model of sociosexuality. Evol. Hum. Behav 28, 382–391. [Google Scholar]
- Jankowiak WR, Fischer EF, 1992. A cross-cultural perspective on romantic love. Ethnology 31, 149–155. [Google Scholar]
- Kim W, Kim S, Jeong J, Lee KU, Ahn KJ, Chung YA, ... Chae JH 2009. Temporal changes in functional magnetic resonance imaging activation of heterosexual couples for visual stimuli of loved partners. Psychiatry Invest. 6, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, 1999. Preoccupations and behaviors associated with romantic and parental love. Perspectives on the origin of obsessive-compulsive disorder. Child & Adol. Psychiatry Clin. North Am 1, 635–665. [PubMed] [Google Scholar]
- Lorenz TK, Worthman CM, Vitzthum VJ, 2015. Links among inflammation, sexual activity and ovulation: Evolutionary trade-offs and clinical implications. Evol. Med. Public Health 16, 304–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loving T, Crockett EE, Paxson AA, 2009. Passionate love and relationship thinkers: Experimental evidence for acute cortisol elevations in women. Psychoneuroendocrino. 34, 939–946. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Akiskal HS, Rossi A, Cassano GB, 1999. Alteration of the platelet serotonin transporter in romantic love. Psychol. Med 29, 741–745. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Canale D, 2004. Hormonal changes when falling in love. Psychoneuroendocrino. 29, 931–936. [DOI] [PubMed] [Google Scholar]
- McDade TW, 2003. Life history theory and the immune system: Steps toward a human ecological immunology. Am. J. Phys. Anthropol. Suppl 37, 100–125. PMID: . [DOI] [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, Guller S, 2011. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci 1221, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S, 2008. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J. Clin. Invest 118, 3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, et al. , 2013. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE, 1980. The revised UCLA Loneliness Scale: Concurrent and discriminant validity evidence. J. Pers. Soc. Psychol 39, 472–480. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R, 2012. Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrino. 37, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zilberstein-Kra Y, Leckman JF, Feldman R (2011). Love alters autonomic reactivity to emotions. Emotion, 11, 1314–1321. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Rholes WS, 1994. Stress and secure base relationships in adulthood In Bartholomew K, & Perlman D (Eds.), Advances in personal relationships Vol 5: Attachment processes in adulthood (pp. 181–204). London: Kingsley. [Google Scholar]
- Snyder-Mackler N, Sanz J, Kohn JN, Brinkworth JF, Morrow S, Shaver AO, Grenier JC, Pique-Regi R, Johnson ZP, Wilson ME, Barreiro LB, Tung J, 2016. Social status alters immune regulation and response to infection in macaques. Science 354, 1041–1045. PubMed PMID: 27885030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su AI, et al. 2004. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc. Natl. Acad. Sci. USA 101, 6062–6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y, 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc. Natl. Acad. Sci. USA 109, 6490–6495. doi: 10.1073/pnas.1202734109. Epub 2012 Apr 9. PubMed PMID: 22493251; PubMed Central PMCID: PMC3340061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer-Yaniv A, Avitsur R, Kanat-Maymon Y, Schneiderman I, Zagoory-Sharon O, Feldman R, 2016. Affiliation, reward, and immune biomarkers coalesce to support social synchrony during periods of bond formation in humans. Brain Behav. Immun 56, 130–139. [DOI] [PubMed] [Google Scholar]
- Zeki S, 2007. The neurobiology of love. FEBS Letters 581, 2575–2579. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB, 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–80. doi: 10.1182/blood-2010-02-258558. Epub 2010 Jul 13. Pub Med PMID: 20628149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.