Abstract
Though bile acids have been well known as digestive juice, recent studies have demonstrated that bile acids bind to their endogenous receptors, including Farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (GPBAR1; TGR5) and serve as hormone to control various biological processes, including cholesterol/bile acid metabolism, glucose/lipid metabolism, immune responses, and energy metabolism. Deficiency of those bile acid receptors has been reported to induce diverse metabolic syndromes such as obesity, hyperlipidemia, hyperglycemia, and insulin resistance. As consistent, numerous studies have reported alteration of bile acid signaling pathways in type II diabetes patients. Interestingly, bile acids have shown to activate TGR5 in intestinal L cells and enhance secretion of glucagon-like peptide 1 (GLP-1) to potentiate insulin secretion in response to glucose. Moreover, FXR has been shown to crosstalk with TGR5 to control GLP-1 secretion. Altogether, bile acid receptors, FXR and TGR5 are potent therapeutic targets for the treatment of metabolic diseases, including type II diabetes.
Keywords: Bile acids, Farnesoid X receptor, G protein-coupled bile acid receptor, Glucagon-like peptide 1, obesity, diabetes
The origin of bile acids is cholesterol in the liver. By numerous cytochrome P450 enzymes, including CYP7A1, CYP8B1 and CYP27A1, bile acids are converted from cholesterol in the liver [1,2,3,4]. Since bile acids are hydrophobic and hydrophilic, they detergent to serve as digestive juice to emulsify lipid for lipid absorption in the intestinal tract [1]. In 1999, bile acids have been identified as endogenous ligands for nuclear receptor, Farnesoid X receptor (FXR) [5]. In 2006, bile acids have been reported as endogenous ligands for G-protein bile acid receptor (TGR5) [6]. Numerous studies have demonstrated that bile acids promote secretion of glucagon-like peptide 1 (GLP-1) to potentiate insulin secretion in pancreatic β cells, leading to decreased blood glucose level in enteroendocrinal cell lines and animal models [7,8]. Furthermore, bile acid nuclear receptor FXR has been recently reported to crosstalk with TGR5 to secrete GLP-1 [9,10]. The discovery of bile acid-mediated endocrine functions of FXR and TGR5 has proposed new perspectives to understand physiological roles of bile receptors to control GLP-1 secretion for the maintenance of glycemic homeostasis.
The biology of bile acids
Abovementioned, bile acids are converted from the cholesterol in the liver by numerous cytochrome P450s. Once bile acids are synthesized, they are immediately conjugated to taurine or glycine for secretion into bile canaliculi [1,3]. Bile acids are stored in the gall bladder until feeding signal stimulates bile acid secretion for emulsifying lipid and fat absorption in the small intestine. When bile acids reach the small intestine, 95% of secreted bile acids are reabsorbed and transported back to the liver by portal vein. The remaining 5% of secreted bile acids is excreted with feces. Thus, total bile acids pool size loses 5% of bile acids in each enterohepatic circulation. The conversion of cholesterol to bile acids compensates equivalent amount of loss of bile acids excreted with feces. Thus, conversion of bile acid from cholesterol is to maintain constant bile acid pool size [1,3].
Farnesoid X receptor: a member of nuclear receptor superfamily
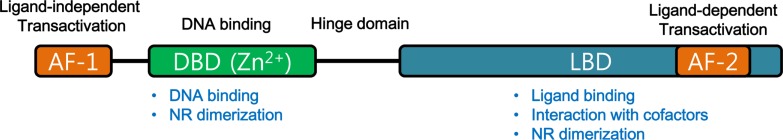
Nuclear receptors are well-known transcriptional regulators and regulate diverse biological functions, such as physiological homeostasis, reproduction, development, inflammation and metabolism [11,12,13,14]. In general, nuclear receptors function as ligand-activated transcriptional regulator. Upon binding with their endogenous ligands, nuclear receptors recruit various coactivators to induce their target gene expressions. In the absence of their ligands, nuclear receptors interact with corepressor complex to suppress expression of their target genes. Nuclear receptors share common structure. Though the N-terminal domains are highly variable (A/B domain), nuclear receptors share constitutively active transactivation function domain (AF-1). In C-terminal domain, nuclear receptors share ligand-dependent activation function domain (AF2) (Figure 1). The most conserved domain of nuclear receptors is DNA-binding domain (DBD), which has two cysteine-Zn2+ finger motifs to recognize specific DNA elements and allow nuclear receptors binding to the DNA for gene regulation. Besides, DBD domain allows nuclear receptors dimerization to control target gene expression (Figure 1). Ligand-binding domain is the most critical region to control transcriptional activities of nuclear receptors. Upon binding of their ligands, conformational changes of LBD lead to modulation of transcriptional activities of their nuclear receptors (Figure 1). Binding of agonist, the structure changes of LBD recruit coactivators whereas antagonists-bound LBD domain recruits corepressors for their gene expression regulation. Between DBD and LBD, hinge region is located to allow 3D structural changes of nuclear receptors to modulate target gene expressions in response to diverse signals (Figure 1).
Figure 1. Structure of Nuclear receptor superfamily.
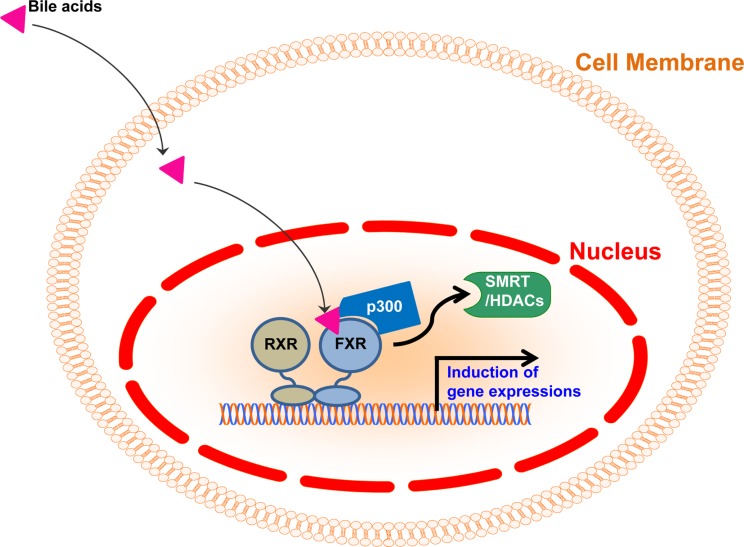
FXR has been identified as a member of nuclear receptor superfamily in 1995 [15]. In the absence of ligands, FXR generally heterodimerizes with retinoid X receptor (RXR) and binds to the FXR response element (FXRE) in the promoter regions with corepressors, including Silencing Mediators of Retinoic acid and Thyroid hormone receptor (SMRT) and histone deacetylases (HDACs) to suppress target gene expression [2,15,16,17] (Figure 2). Upon binding with bile acids, FXR interacts with various coactivators including histone acetyltransferase p300, and induces target gene expressions in response to bile acids [18] (Figure 2). FXR is ubiquitously expressed in diverse tissues, such as liver, intestine, kidney, adipose tissues and even immune cells, FXR-mediated bile acid signaling plays pivotal roles to control physiological homeostasis [17,19,20,21,22,23,24]. Previous studies have reported that FXR-null mice exhibited dysregulated lipid homeostasis [19]. Given that FXR controls a set of genes involved in lipoprotein metabolism including SREBP-1c, PLTP, SCD-1, VLDLR, ApoCII and ApoE, FXR-null mice exhibited elevated cholesterol and triglyceride in the blood [16,17,19,25]. Moreover, it has been shown that FXR-null mice exhibited impaired insulin signaling and dysregulated glucose homeostasis [19,21,22,24]. Therefore, FXR is a key transcriptional factor to maintain lipid and glucose homeostasis.
Figure 2. Transcriptional regulation by FXR in response to bile acids.
Cell membrane bile acid receptor TGR5
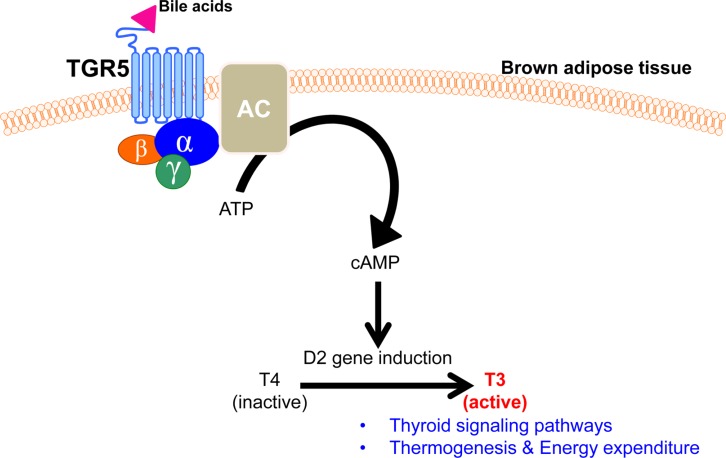
Besides nuclear bile acid receptor FXR, intrinsic bile acid receptor is located in the cell membrane. G protein-coupled bile acid receptor 1 (GPBAR1; TGR5) is a member of the rhodopsin-like superfamily member of GPCR protein and is ubiquitously expressed in diverse tissues, including endocrine organs, muscle, adipose tissue, immune cells, and intestinal tract [8,26,27,28,29,30]. Upon binding with lithocholic acids (LCA) and taurolithocholic acid (TLCA), TGR5 is activated to transduce signal transduction into the nucleus and control diverse gene expression [30]. It has been shown that bile acids bind to TGR5 and stimulate cAMP signaling to activate mitogen-activated protein kinase (MAPK) pathway. Elevated intracellular cAMP levels then activate protein kinase A (PKA) to phosphorylate cAMP response element binding protein (CREB) and control their target gene expressions [31]. Previously, TGR5 has been demonstrated to activate thermogenesis and thyroid signaling pathways to enhance energy expenditure in brown adipose tissue [6] (Figure 3). In brown adipose tissue, TGR5 activation induce Dio2 gene expression which converts thyroxine (T4) to tri-iodothyronine (T3), resulting in an increased energy expenditure [6]. In addition to Dio2, numerous genes involved in thermogenesis were largely increased by TGR5 activation: peroxisome proliferator-activated receptor γ coactivator-1α (PCG1α) and 1β (PGC1β), uncoupling protein-1 (UCP1) and -3 (UCP3) and straight-chain acyl-CoA oxidase 1 [6]. Quite interestingly, these gene expressions were not changed in Dio2 knockout mice, suggesting that TGR5-mediated Dio2 functions are critical to induce numerous thermogenic gene expressions [6]. Thus, these findings propose that bile acids control TGR5-cAMP-Dio2-thyroid hormone signaling axis to control energy homeostasis. Consistently, TGR5-deficient mice exhibited severe metabolic syndromes including obesity, severe insulin resistance and impaired glucose and lipid homeostasis [32]. Therefore, these reports clearly have proposed that TGR5 is a potent therapeutic target for the treatment of metabolic syndromes including obesity and type II diabetes.
Figure 3. Bile acids activate TGR5 to control thermogenesis and energy expenditure in brown adipose tissue.
Bile acids-activated TGR5 to control GLP-1 secretion from the intestinal L cells
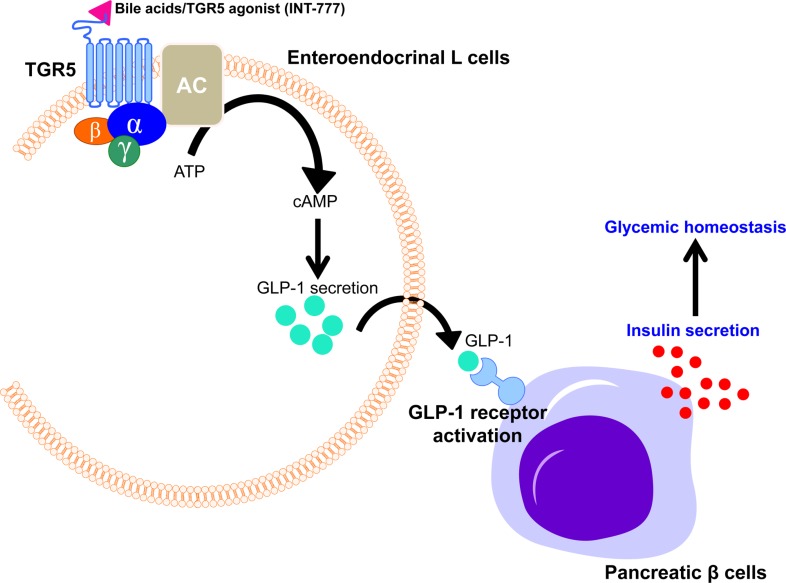
Besides energy expenditure, bile acids have been reported to control incretin secretion, such as glucagon-like peptide 1 (GLP-1). Bile acid-mediated TGR5 activation has been demonstrated to stimulate the secretion of GLP-1, a member of the incretin family [7,8]. Food intake signals stimulate bile acid secretion from the gall bladder to small intestine, which activates TGR5 in the intestinal entero-endocrine cells to secrete insulinotropic hormones into the bloodstream. In rodent model, bile acids have been shown to activate TGR5 which triggers cAMP signaling pathway to stimulate GLP-1 secretion in intestinal L cells [7]. As consistent, synthetic TGR5 agonist, INT-777 has been shown to potentiate GLP-1 secretion in the intestinal L cells, resulting in GLP-1 receptor activation followed by an increase of insulin secretion in pancreatic β cells [7]. These studies clearly propose that bile acid-mediated TGR5 activation is critical to maintain glucose homeostasis in response to food intake via control of incretin secretion (Figure 4).
Figure 4. Bile acids activate TGR5 to enhance GLP-1 secretion in entoroendocrinal L cells.
Crosstalk between FXR and TGR5 to potentiate GLP-1 secretion from the intestinal L cells
Though bile acid-mediated TGR5 activation on GLP-1 secretion is clear, physiological roles of bile acidmediated FXR activation to control GLP-1 secretion is still controversial. Previous studies have shown that FXR activation repressed transcription of GLP-1 in intestinal L cells [31,33]. As gene expression of GLP-1 has been reduced, FXR agonist GW4064 treatment largely reduced GLP-1 secretion in intestinal L cells [33]. In contrast, gut-specific FXR-null mice exhibited elevated GLP-1 secretion, implying that FXR activation is opposite to the TGR5 activation on the control of GLP-1 secretion [33].
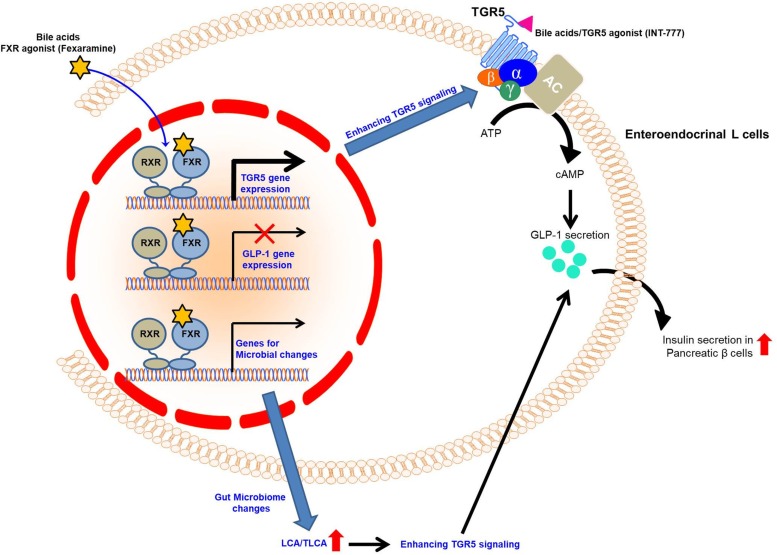
Quite interestingly, another recent study has demonstrated that FXR activation mediated by a gut-specific FXR agonist, Fexaramine stimulates TGR5 expression in the intestinal L cells to potentiate TGR5-mediated cAMP signaling and increases GLP-1 secretion in intestinal L cells [9,10,34]. Consistently, Fexaramine treatment enhanced GLP-1 secretion in genetic obese mice [10]. Moreover, this study has demonstrated that Fexaramine treatment induces gut microbiome remodeling to change bile acid composition and increases the levels of LCA and TLCA which are potent agonists for TGR5. Thus, Fexaramine-mediated microbiome changes lead to change of bile acids composition, resulting in enhanced TGR5 signaling in vivo [10]. Thus, crosstalk between bile acid receptors FXR and TGR5 potentiates GLP-1 secretion in enteroendocrinal L cells to control glucose homeostasis (Figure 5).
Figure 5. Crosstalk between FXR and TGR5 to potentiate GLP-1 secretion for the maintenance of glycemic homeostasis.
Conclusion
It has been largely accepted that bile acid signaling is critical for the beneficial improvements of sleeve gastrectomy surgery in rodent models [35]. Thus, physiological roles of bile acid receptors FXR and TGR5 have been considered as novel therapeutic targets for diverse metabolic syndromes. Consistent with rodent models, there are sufficient evidence suggesting that bile acid signaling has been improved in both human patients and animal models with bariatric surgery [36,37]. Patients with beneficial improvements including body weight loss and reduced blood glucose levels exhibited high levels of circulating bile acids and enhanced GLP-1 secretion [36,37,38]. As consistent, patients with severe obesity generally exhibit a decreased postprandial bile acids levels and GLP-1 secretion [38]. Given that crosstalk between FXR and TGR5 is critical to control GLP-1 secretion in response to bile acid signaling pathway, both FXR and TGR5 will provide novel approach to develop therapeutic strategies for treatment of metabolic syndromes such as obesity and type II diabetes.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF-2018R1A2B6003447) and faculty research grant of Yonsei University College of Medicine (6-2017-0099).
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Chiang JY. Bile acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23(4):443–463. doi: 10.1210/er.2000-0035. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug Metab Rev. 2013;45(1):145–155. doi: 10.3109/03602532.2012.740048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 4.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6(3):507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 5.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 7.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329(1):386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 9.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292(26):11055–11069. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018;68(4):1574–1588. doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SH, Ahmadian M, Yu RT, Atkins AR, Downes M, Evans RM. Nuclear receptors and metabolism: from feast to famine. Diabetologia. 2014;57(5):860–867. doi: 10.1007/s00125-014-3209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Lamia KA, Evans RM. Nuclear receptors, metabolism, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:387–394. doi: 10.1101/sqb.2007.72.058. [DOI] [PubMed] [Google Scholar]
- 13.Ordentlich P, Downes M, Evans RM. Corepressors and nuclear hormone receptor function. Curr Top Microbiol Immunol. 2001;254:101–116. doi: 10.1007/978-3-662-10595-5_5. [DOI] [PubMed] [Google Scholar]
- 14.McKenna NJ, Evans RM, O'Malley BW. Nuclear Receptor Signaling: a home for nuclear receptor and coregulator signaling research. Nucl Recept Signal. 2014;12:e006. doi: 10.1621/nrs.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell. 1995;81(5):687–693. doi: 10.1016/0092-8674(95)90530-8. [DOI] [PubMed] [Google Scholar]
- 16.Edwards PA, Kast HR, Anisfeld AM. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43(1):2–12. [PubMed] [Google Scholar]
- 17.Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31(10):572–580. doi: 10.1016/j.tibs.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Fang S, Tsang S, Jones R, Ponugoti B, Yoon H, Wu SY, Chiang CM, Willson TM, Kemper JK. The p300 acetylase is critical for ligand-activated farnesoid X receptor (FXR) induction of SHP. J Biol Chem. 2008;283(50):35086–35095. doi: 10.1074/jbc.M803531200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103(4):1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renga B, Mencarelli A, Vavassori P, Brancaleone V, Fiorucci S. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802(3):363–372. doi: 10.1016/j.bbadis.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116(4):1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duran-Sandoval D, Cariou B, Percevault F, Hennuyer N, Grefhorst A, van Dijk TH, Gonzalez FJ, Fruchart JC, Kuipers F, Staels B. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem. 2005;280(33):29971–29979. doi: 10.1074/jbc.M501931200. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, Li J, Iwahashi M, Sutherland E, Arend L, Levi M. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56(10):2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 24.Lambert G, Amar MJ, Guo G, Brewer HB, Jr, Gonzalez FJ, Sinal CJ. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278(4):2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 25.Claudel T, Staels B, Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25(10):2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 26.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6(10):e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keitel V, Donner M, Winandy S, Kubitz R, Häussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372(1):78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 28.Lou G, Ma X, Fu X, Meng Z, Zhang W, Wang YD, Van Ness C, Yu D, Xu R, Huang W. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One. 2014;9(4):e93567. doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keitel V, Reinehr R, Gatsios P, Rupprecht C, Görg B, Selbach O, Häussinger D, Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45(3):695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 30.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46(4):302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Zhu L, Yang X, Li W, Sun X, Yi B, Zhu S. Farnesoid X Receptor (FXR) Interacts with Camp Response Element Binding Protein (CREB) to Modulate Glucagon-Like Peptide-1 (7-36) Amide (GLP-1) Secretion by Intestinal L Cell. Cell Physiol Biochem. 2018;47(4):1442–1452. doi: 10.1159/000490836. [DOI] [PubMed] [Google Scholar]
- 32.Velazquez-Villegas LA, Perino A, Lemos V, Zietak M, Nomura M, Pols TWH, Schoonjans K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat Commun. 2018;9(1):245. doi: 10.1038/s41467-017-02068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trabelsi MS, Daoudi M, Prawitt J, Ducastel S, Touche V, Sayin SI, Perino A, Brighton CA4, Sebti Y, Kluza J, Briand O, Dehondt H, Vallez E, Dorchies E, Baud G, Spinelli V, Hennuyer N, Caron S, Bantubungi K, Caiazzo R, Reimann F, Marchetti P, Lefebvre P, Bäckhed F, Gribble FM, Schoonjans K, Pattou F, Tailleux A, Staels B, Lestavel S. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun. 2015;6:7629. doi: 10.1038/ncomms8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Pérez HE, Sandoval DA, Kohli R, Bäckhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerhard GS, Styer AM, Wood GC, Roesch SL, Petrick AT, Gabrielsen J, Strodel WE, Still CD, Argyropoulos G. A role for fibroblast growth factor 19 and bile acids in diabetes remission after Roux-en-Y gastric bypass. Diabetes Care. 2013;36(7):1859–1864. doi: 10.2337/dc12-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98(4):E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabiee A, Magruder JT, Salas-Carrillo R, Carlson O, Egan JM, Askin FB, Elahi D, Andersen DK. Hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass: unraveling the role of gut hormonal and pancreatic endocrine dysfunction. J Surg Res. 2011;167(2):199–205. doi: 10.1016/j.jss.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]