Abstract
While memory encoding and consolidation processes have been linked with dopaminergic signaling for a long time, the role of dopamine in episodic memory retrieval remained mostly unexplored. Based on previous observations of striatal activity during memory retrieval, we used pharmacological functional magnetic resonance imaging to investigate the effects of dopamine on retrieval performance and metacognitive memory confidence in healthy humans. Dopaminergic modulation by the D2 antagonist haloperidol administered acutely during the retrieval phase improved recognition accuracy of previously learned pictures significantly and was associated with increased activity in the substantia nigra/ventral tegmental area, locus coeruleus, hippocampus, and amygdala during retrieval. In contrast, confidence for new decisions was impaired by unsystematically increased activity of the striatum across confidence levels and restricted range of responsiveness in frontostriatal networks under haloperidol. These findings offer new insights into the mechanisms underlying memory retrieval and metacognition and provide a broader perspective on the presence of memory problems in dopamine-related diseases and the treatment of memory disorders.
Subject terms: Long-term memory, Cognitive neuroscience
Introduction
The neurotransmitter dopamine has been linked with learning and memory for a long time [1]. In particular, encoding and consolidation of memories require the stimulation of dopamine receptors as part of a hippocampal–striatal–prefrontal loop that orchestrates the formation of new memories [2, 3]. Dopaminergic effects on working memory (WM) are well established in animals and humans [4], but dopamine has also been reported to affect other domains of memory (e.g., [5, 6]). For episodic memory, while several pharmacological studies reported dopaminergic effects on encoding or consolidation [7–10], effects on retrieval performance were often absent or inconclusive [11–15]. Importantly, however, in all these human pharmacological studies dopaminergic involvement in the retrieval of stored episodic memories could not be examined in isolation from effects on encoding and consolidation because dopamine is usually manipulated already during the encoding phase (but see [16–18] for a notable exception in false memory research). Also, disorders linked with aberrant dopamine function such as schizophrenia or Parkinson’s disease often feature impairments in memory [19–21], but these memory deficits cannot be constrained to retrieval-specific dopaminergic effects. To bridge this gap, we designed a placebo-controlled pharmacological functional magnetic resonance imaging (fMRI) study on episodic memory retrieval in which dopamine is modulated only during the retrieval phase to test specifically effects on recognition of pre-drug-encoded pictures.
Previous evidence for a retrieval-specific role of dopamine comes from two branches of research. On the one hand, rat studies suggested that post-encoding administration of D2-receptor antagonist haloperidol facilitated memory retrieval [22, 23]. On the other hand, human fMRI studies from our own lab [24, 25] as well as from other labs [26–28] consistently reported very robust striatal activity during the retrieval of nonvalent episodic information. This striatal activity might stem from dopamine signaling arriving from the midbrain in the dopamine-innervated striatum [29, 30] and has been suggested to reflect motivational aspects of retrieval such as higher subjective value of successfully retrieving old than rejecting new items [26] or higher memory confidence during retrieval [25]. Intriguingly, however, dopamine might also affect the actual retrieval of episodic information. In a previous fMRI study, we demonstrated independent retrieval-related and memory confidence signals in overlapping striatal regions [24] in an established picture recognition paradigm [25]. We here investigated whether these striatally mediated memory processes are indeed dopaminergic using acute administration of 2 mg of the D2-receptor antagonist haloperidol in combination with fMRI measurements during retrieval.
Importantly, acute low doses of D2 antagonists are thought to preferably block the presynaptic D2 autoreceptors [31, 32], which regulate dopamine release (in addition to synthesis and reuptake, see [33]) and therefore potentiate phasic dopamine release [34–38]. With regard to our memory task, this means that a presumably phasic dopamine release evoked by the picture recognition might be amplified by the D2 antagonist blocking the autoreceptors. In terms of fMRI activity, the measured signal will reflect the net effect of presynaptic and postsynaptic dopaminergic receptor activation [39, 40]. Striatal cerebral blood volume (CBV) and blood oxygenation level-dependent (BOLD) MRI signal increases have been shown to parallel dopamine release into the striatum evoked by amphetamine [41, 42]. Moreover, this amphetamine-induced CBV increase is potentiated by D2 antagonists and attenuated by D2 agonists, respectively [38, 43], while application of low-dose D2 antagonists alone lead only to slightly enhanced striatal CBV/BOLD [38, 44, 45]. Therefore, we assume that a presumably phasic dopamine release evoked by the picture recognition paradigm should be potentiated by haloperidol and this should be reflected by increased BOLD activity in dopaminergic regions involved in memory retrieval or memory confidence.
Materials and methods
Participants and group allocation
Fifty-four healthy volunteers without previous or current physical or mental diseases, medication, or drug use (14 males, mean age 24 ± 2.9 years) participated in the study (note that the higher proportion of females in the participant sample came about by chance). The study was approved by the local ethics committee of the Hamburg Medical Association and written consent was given by each participant before the start of the study. All participants were informed about the purpose and the course of the study and about the potential risks and side effects of haloperidol and they were instructed to restrain from caffeine, nicotine, and alcohol on the day of the fMRI testing. Random group assignment was conducted by TS, who did not interact with the participants at any time. All participants were monitored for side effects and mood effects after drug administration and had to indicate the substance (haloperidol or placebo) they thought they had received at the end of the study (see Supplementary Materials and Methods).
Experimental design
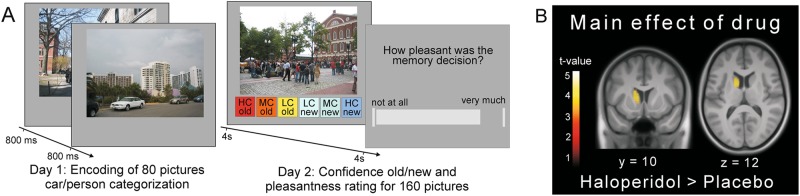
Encoding for the fMRI experiment took place outside the scanner on the first day (Fig. 1 and Supplementary Materials and Methods) within a 1-h baseline testing session (no medication given). The next day, the participants started the picture recognition inside the scanner approximately 2.5 h after tablet ingestion (2 mg haloperidol or placebo). During the recognition task (Fig. 1), all 80 previously encoded pictures were presented randomly intermixed with 80 new pictures in five functional runs (see Supplementary Materials and Methods for all details of the MR acquisition and fMRI analysis). For each picture (presented for 4 s, interstimulus intervals 2–4 s), participants indicated the old/new status and their subjective memory confidence on a combined 6-point confidence scale (1—“high confidence old,” 6—“high confidence new”) by pressing one of six buttons. The boxes corresponding to the various levels of confidence old/new were horizontally arranged in each trial in random order to minimize transfer effects to the next rating. Feedback about the correctness of the response was not given. Two to four seconds after each confidence old/new rating participants indicated how pleasant the preceding memory retrieval was on a visual analog scale ranging from 1 (“not at all”) to 100 (“very much”). The completion of this task took approximately 45 min.
Fig. 1.
Task design and general drug effects. a Participants encoded pictures of outdoor scenes outside the scanner. Picture recognition took place under haloperidol/placebo in the fMRI scanner the next day. b Haloperidol effect across all trials on brain activity compared to placebo. Note that increased activity in the right dorsal striatum was also present, but its extent (k = 76 voxels) and height (z = 3.52) did not pass the FWE-corrected threshold of p < 0.05. The inverse contrast (placebo > haloperidol) revealed no significant activity. Activation maps are thresholded at p < 0.05 (FWE-corrected at the cluster level using a cluster forming threshold at voxel level of p < 0.001). HC high confidence, MC medium confidence, LC low confidence
Recognition memory analyses
Recognition trials were sorted post hoc into the response categories: hits (correct old responses), correct rejections (CRs, correct new responses), false alarms (FA, incorrect old responses), and misses (incorrect new responses). Discrimination sensitivity (d′) and response bias (c) were computed according to the signal detection approach [46]. Discrimination sensitivity was calculated as d’ = z(H) − z(FA), where z represents the inverse of the cumulative normal distribution and H = p(response = old|stimulus = old) and FA = p(response = old|stimulus = new). Response bias was calculated as c = −0.5[z(H) + z(FA)]. We moreover analyzed the effects of haloperidol on recollection vs. familiarity processes [47] using the dual-process signal detection model as implemented in the ROC Toolbox [48]. The individual familiarity parameters were transformed from z-standardized scores to probabilities to make them comparable to the recollection parameters prior to the statistical analysis.
Metamemory for old and new picture trials was quantified by calculating response-specific meta-d′ values per trial type (old/new) [49] using the MATLAB code available at http://www.columbia.edu/~bsm2105/type2sdt. Meta-d′ aims to quantify metacognitive sensitivity, that is, how well the observer’s confidence ratings discriminate between correct and incorrect responses. Meta-d′ can be evaluated with respect to d′ in order to take into account the observer’s discrimination sensitivity by calculating meta-d′ − d′ (meta-d′ difference) as meta-d′ is expressed in the same units as d′. Suboptimal metacognition is reflected by meta-d′ difference values below zero and enhanced metacognition is reflected by meta-d′ difference values above zero [50]. For one placebo participant, the modeled meta-d′ for old picture trials was more than 4 standard deviations above the mean (z-score > 4.4) and thus this participant was excluded from the group analysis.
Results
General drug effects
The groups did not differ with regard to age, sex, weight, or in terms of baseline WM (Table 1). Moreover, participants were not able to guess the substance received above chance level and there were no group differences in reported side effects or subjective feelings relative to baseline (Table S1). With regard to overall group differences, our fMRI results showed significantly higher activity under haloperidol compared to placebo across all recognition trials (see Supplementary Materials and Methods) specifically and selectively in the dorsal striatum (p < 0.05 whole-brain family-wise error (FWE)-corrected; Fig. 1b). No significant activity differences were found for the inverse contrast (placebo > haloperidol) in any brain region.
Table 1.
Demographics and baseline performance
| Placebo, n = 27 | Haloperidol, n = 27 | Statistics | |
|---|---|---|---|
| Age | 24.37 ± 3.3 years | 23.56 ± 2.5 years | T(52) = 1.03, p = 0.31 |
| Sex | 8 males, 19 females | 6 males, 21 females | χ2(1) = 0.39, p = 0.54 |
| Weight | 65.70 ± 6.8 kg | 65.74 ± 10.6 kg | T(52) = -0.15, p = 0.99 |
| Baseline WM: Complex span/symmetry accuracy/RT symmetry rating | 25.78 ± 7.3 | 27.85 ± 7.0 | T(52) = -1.07, p = 0.29 |
| 96.1 ± 4.2% | 96.7 ± 3.7% | T(52) = -0.51, p = 0.61 | |
| 2.17 ± 1.44 s | 1.67 ± 0.63 s | T(35.49) = 1.63, p = 0.11 | |
| Baseline WM: digit span forward/backward | 8.26 ± 1.3 | 8.30 ± 1.7 | T(52) = -0.09, p = 0.93 |
| 9.15 ± 2.2 | 8.67 ± 1.7 | T(52) = 0.37, p = 0.93 | |
| Baseline WM: block span forward/backward | 9.73 ± 1.8 | 10.44 ± 2.0 | T(51) = -1.35, p = 0.18 |
| 9.15 ± 1.9 | 9.81 ± 1.6 | T(51) = -1.37, p = 0.18 | |
| Baseline WM: z-summary score | −0.06 ± 0.63 | 0.04 ± 0.58 | T(52) = -0.63, p = 0.53 |
| Baseline encoding: categorization accuracy picture/RT/categorization accuracy arrows/RT | 76.78 ± 22.0% | 80.23 ± 15.9% | T(51) = -0.66, p = 0.51 |
| 0.58 ± 0.04 s | 0.57 ± 0.06 s | T(51) = 0.71, p = 0.48 | |
| 86 ± 9.0% | 86 ± 10.5% | T(51) = 0.12, p = 0.91 | |
| 0.38 ± 0.01 s | 0.38 ± 0.02 s | T(51) = 0.05, p = 0.96 |
Plus or minus denotes the standard variation
Differential effects of haloperidol on recognition performance, metamemory, and WM
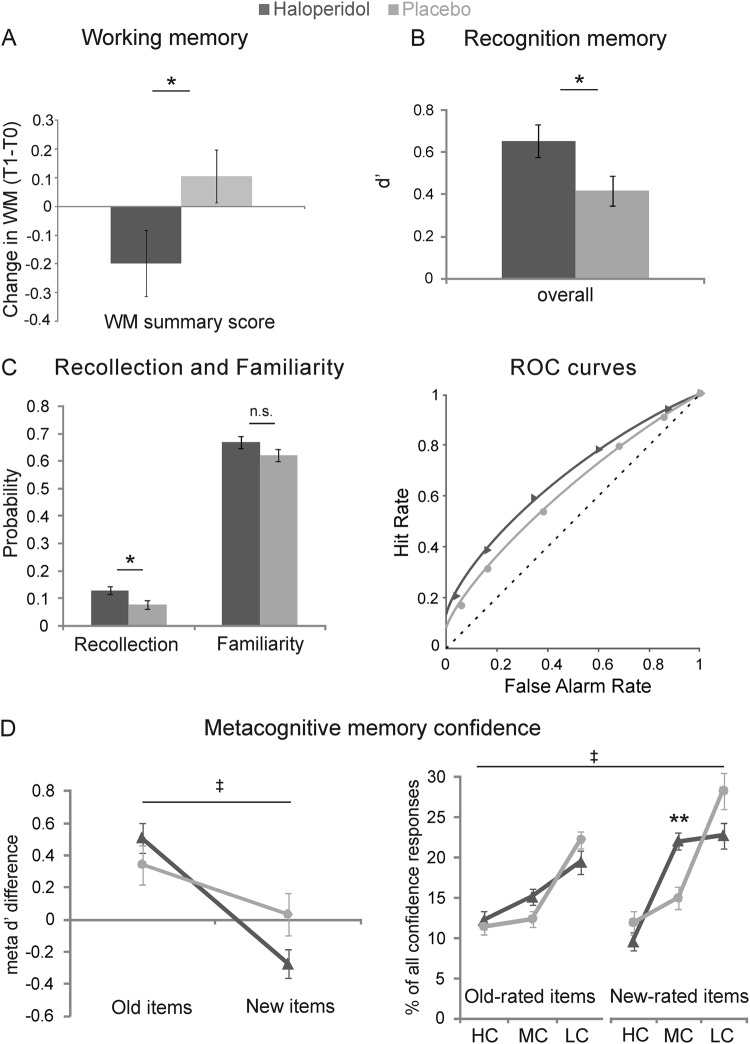
Recognition memory performance (d′) was significantly enhanced in the haloperidol group (t(52) = −2.27, p = 0.028, Cohen’s d = −0.63/Pearson’s r = 0.30 (medium effect size); Fig. 2b and Fig. S1B), but showed no group difference in response bias (Fig. S1C). The superior memory discrimination in the haloperidol group was similarly due to more correct responses for old and new items (significant group × accuracy interaction (F(1,52) = 6.19, p = 0.016), but there were no significant group differences for the hit or FA rate (all p < 0.10); Table 2). Restricting this analysis to only high confidence responses also revealed a similar pattern without significant group differences for the hit or FA rate (all p < 0.10) (Fig. S2).
Fig. 2.
Behavioral effects under haloperidol (dark gray) and placebo (light gray). a Working memory: Difference in mean z-transformed WM performance after drug administration relative to baseline. b Memory accuracy: mean d′. c Mean recollection and familiarity parameters (left) and dual-process model-predicted ROC curves (right) with mean observed ROC values (triangles/circles). d Metacognition: mean meta-d′ difference (meta-d′ − d′) for old and new items (left) and frequency of confidence ratings for old and new responses (right). */**Significant group difference at p < 0.05/at p < 0.01 (for post-hoc tests after Bonferroni correction); ǂSignificant group × condition interaction at p < 0.05. NS non-significant. Error bars denote the SEM. HC high, MC medium, LC low confidence
Table 2.
Descriptive statistics of behavioral responses in the recognition memory task
| Response category | Hits | CR | FA | Miss |
|---|---|---|---|---|
| Placebo: % of responsesa | 53.59 (3.1) | 61.71 (2.7) | 38.54 (2.9) | 46.84 (3.3) |
| Haloperidol: % of responsesa | 59.15 (2.0) | 65.58 (2.5) | 35.12 (2.6) | 40.34 (2.0) |
| Placebo: mean confidenceb | 1.89 (0.06) | 1.73 (0.07) | 1.58 (0.06) | 1.59 (0.07) |
| Haloperidol: mean confidenceb | 1.99 (0.05) | 1.80 (0.04) | 1.56 (0.05) | 1.65 (0.05) |
| Placebo: mean pleasantnessc | 63.34 (2.3) | 57.66 (3.1) | 55.33 (2.2) | 54.15 (2.9) |
| Haloperidol: mean pleasantnessc | 63.74 (2.3) | 55.79 (2.6) | 52.76 (2.2) | 51.51 (2.5) |
| Placebo: mean RTd (s) | 2.12 (0.09) | 2.17 (0.06) | 2.15 (0.08) | 2.16 (0.07) |
| Haloperidol: mean RTd (s) | 1.98 (0.08) | 2.06 (0.08) | 2.05 (0.08) | 2.07 (0.08) |
Numbers within parentheses denote the standard error. Confidence: 1 = low, 3 = high; pleasantness: 1 = low, 100 = high.
a Hit/CR/FA/miss rates: significant group × accuracy interaction: F(1,52) = 6.19, p = 0.02; but no significant group differences for individual rates (all p > 0.10)
b Confidence: main effect of group: F(1,52) = 0.64, p = 0.43; group × old/new rating × accuracy interaction: F(1, 52) = 2.98 (p = 0.09)
c Pleasantness: main effect of group: t(52) = 0.48, p = 0.64; all group × condition interactions (p > 0.20)
d RT: main effect of group: t(52) = 1.16, p = 0.25; all group × condition interactions (p > 0.15)
We additionally analyzed the recognition performance within the framework of the dual-process signal detection model [48]. Although there was no group × memory-type (recollection/familiarity) interaction (F(1, 52) < 0.01, p=0.957, we analyzed the recollection and familiarity effects separately. The haloperidol group had significantly higher estimates of recollection only (t(52) = −2.29, p = 0.026, Cohen’s d = −0.64/Pearson’s r = 0.30 (medium effect size); Fig. 2c); there was no group difference in the estimates of familiarity (t (52) = −1.55, p = 0.128). However, due to the absence of the interaction effect we do not draw any conclusions from this observation.
In contrast, metacognitive memory confidence showed some impairment under haloperidol. Meta-d′ difference (meta-d′ − d′) showed a significant group × old/new interaction (F(1, 51) = 4.46, p = 0.04, partial η2 = 0.08 (medium effect size)), demonstrating comparably enhanced metamemory for old items in the haloperidol group as in the placebo group, but suboptimal metamemory for new items (Fig. 2d). In a similar vein, the frequency of confidence responses showed a significant shift towards medium confidence (MC) responses particularly for new-rated items, indicating reduced confidence differentiation under haloperidol (group × confidence × old/new rating interaction: F(2, 51) = 4.50, p = 0.016, partial η2 = 0.15 (large effect size); post-hoc independent-samples t test MC new trials: t(52) = −3.44, p = 0.006, Cohen’s d = −0.95/Pearson’s r = 0.43 (large effect size) (Bonferroni-corrected); Fig. 2d). As this shift was due to a relative reduction of both HC and LC new responses, this effect was not reflected by a significant group difference in mean confidence (Table 2). Together, these analyses point to an impairing effect of acute haloperidol administration on metacognitive confidence for new decisions.
There were no group differences for response times or for pleasantness (Table 2 and Fig. S4B). Including the baseline WM span summary score as a covariate to control for individual differences in baseline dopamine level revealed no effect on any of the observed group differences, indicating either that baseline dopamine level did not influence the acute haloperidol effects or that the WM span was not a good proxy for baseline dopamine level. Of interest, however, there was an detrimental effect of acute haloperidol on WM measured directly after scanning relative to the baseline WM measured before drug administration: the WM summary score demonstrated decreased WM span under haloperidol (time × group interaction: F(1,50) = 4.28, p = 0.044, partial η2 = 0.079 (medium effect size); Fig. 2a and Fig. S1A).
Increased activity in midbrain and mnemonic regions is linked with better recognition performance
We examined fMRI effects of memory by grouping trial onsets into hit, CR, FA, and miss trials (see Supplementary Materials and Methods). Across both groups, retrieval success (hits > CR) [28] revealed activity in various brain regions including the striatum, prefrontal cortex (PFC), hippocampus, substantia nigra/ventral tegmental area (SN/VTA) and anterior cingulate cortex (ACC) (p < 0.05 whole-brain FWE-corrected; Fig. 3a). The haloperidol group had significantly higher activity for retrieval success ((haloperidol hits > CR) > (placebo hits > CR) in a cluster localized specifically in the SN/VTA, the hippocampus, amygdala and locus coeruleus (LC) (p < 0.05 whole-brain FWE-corrected; Fig. 3b and Table 3), suggesting that superior memory performance under haloperidol was linked with higher activity in these midbrain and mnemonic regions. Of note, we observed no group differences for this retrieval success activity in the striatum even at an uncorrected threshold despite overall increased striatal activity under haloperidol (see also β plot in Fig. 3a). Supporting the role for dopaminergic and hippocampal activity in retrieval performance, the individual activity in the SN/VTA for retrieval success correlated significantly with memory discrimination (d′) and with right anterior (CA1) hippocampal activity for retrieval success in the haloperidol group (SN/VTA × d′: haloperidol group: r = 0.47, p = 0.014, placebo group: r = 0.16, p = 0.445; SN/VTA × hippocampus: haloperidol group: r = 0.50, p = 0.008, placebo group: r = 0.26, p = 0.192; Fig. 3b). As these correlations were not significantly different between groups, these correlation findings merely suggest that participants with higher SN/VTA activity showed better recognition memory and had higher hippocampal activity during retrieval in both groups, although this relationship was more pronounced on a descriptive level in the haloperidol group.
Fig. 3.
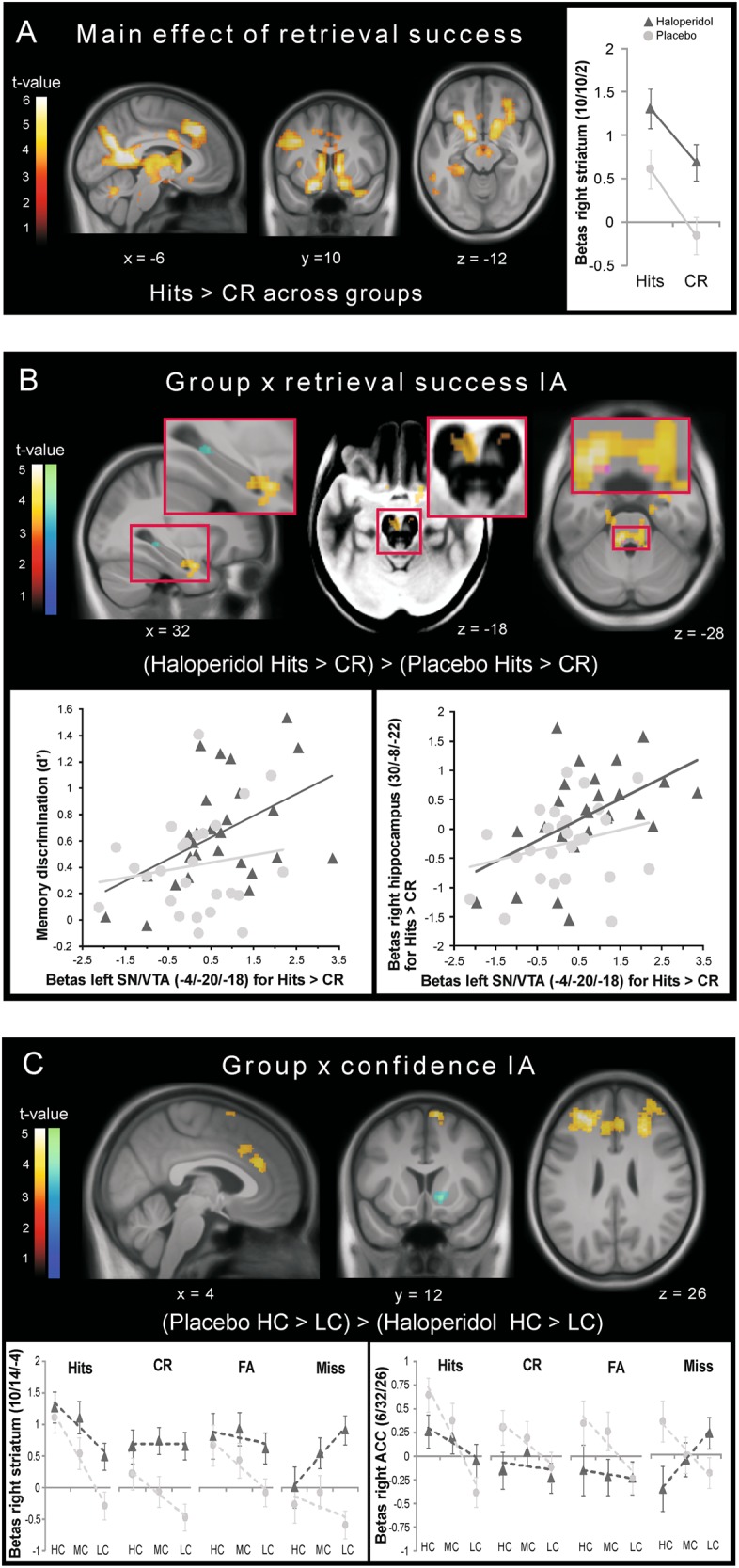
fMRI results. a Activity pattern of retrieval success across groups and β estimates from the peak voxel in the right striatum. b Significantly increased activity for retrieval success under haloperidol in the hippocampus and amygdala (left), SN/VTA (middle; activation displayed on the mean MT image showing the SN/VTA as a bright region) and LC (right; activation overlaid with the LC mask [73] in magenta). Scatter plots of the individual SN/VTA response for retrieval success and memory accuracy (left) and the individual anterior hippocampus response for retrieval success (right) in the haloperidol (dark gray triangles) and the placebo group (light gray circles). c Regions showing higher confidence activity under placebo and β plots illustrating the confidence pattern in the right striatum and the ACC. Dashed lines represent the linear trend across confidence levels. All activation maps are thresholded at p < 0.05 (warm colors: FWE-corrected at the cluster level using a cluster forming threshold at voxel level of p < 0.001; cold colors: small-volume FWE-corrected using anatomical masks). The inverse contrasts revealed no significant activations. HC high confidence, MC medium confidence, LC low confidence, IA interaction
Table 3.
Peak activations group differences
| Region | x | y | z | z-score | Cluster size |
|---|---|---|---|---|---|
| All trials: H > P | |||||
| L caudate nucleus | −12 | 2 | 18 | 4.74 | 348 |
| Memory effects: Group x retrieval success (H (Hits > CR) > P (Hits > CR)) | |||||
| Brain stem | −8 | -36 | −28 | 4.81 | 420 |
| L LC | −6 | −38 | −30 | 3.75 | |
| R LC | 8 | −38 | −28 | 3.64 | |
| L SN/VTA | −4 | −20 | −18 | 3.41 | |
| R Amygdala (LB [74]) | 30 | 0 | −24 | 4.02 | 189 |
| R anterior hippocampus (CA1 [74]) | 30 | −8 | −22 | 3.68 | |
| R posterior hippocampus (CA2 [74]) | 34 | −34 | −6 | 3.78a | 11 |
| Confidence effects: Group x confidence (P (HC > LC) > H (HC > LC)) | |||||
| L MFG | −26 | 32 | 24 | 5.27 | 2173 |
| L SFG | −22 | 36 | 26 | 5.22 | |
| R SFG | 16 | 32 | 40 | 4.54 | |
| R ACC | 6 | 32 | 26 | 4.46 | |
| L ACC | −8 | 30 | 26 | 4.33 | |
| R SMA | 10 | 14 | 68 | 4.56 | 230 |
| R NAcc | 10 | 14 | −4 | 4.18b | 27 |
| R putamen | 14 | 12 | −6 | 3.93b | 23 |
| R caudate nucleus | 12 | 14 | −2 | 3.91b | 36 |
x, y, z coordinates refer to the peak voxel in MNI space thresholded at P < 0.05 (FWE-corrected at the cluster level/small volume FWE-corrected). No significant activations were observed for the inverse contrasts.
R right, L left, H haloperidol, P placebo, LC locus coeruleus, SN/VTA substantia nigra/ventral tegmental area, MFG middle frontal gyrus, SFG superior frontal gyrus, ACC anterior cingulate gyrus, SMA supplementary motor area, NAcc nucleus accumbens
aSmall volume FWE-corrected using the bilateral anatomical hippocampus mask
bSmall volume FWE-corrected using the bilateral anatomical striatum mask.
We additionally examined the data for drug effects on false alarms by computing the group by false memory interactions: (haloperidol FA > CR) > (placebo FA > CR) and (placebo FA > CR) > (haloperidol FA > CR). There were no significant group differences in false memory activation at an FWE-corrected level (whole-brain or small-volume), but there was some indication of reduced striatal activity in the haloperidol group at an uncorrected level of p < 0.01 (Fig. S3).
Aberrant activity in frontostriatal circuit is linked with impaired metamemory for new decisions
For the analysis of confidence, we split the hit, CR, FA, and miss trials further into high confidence (HC), medium confidence (MC), and low confidence (LC) trials (see Supplementary Materials and Methods). The main effect of confidence (HC > LC) across groups revealed similar frontostriatal–parietal regions as previous studies [24, 25] (Fig. S4A). The haloperidol group had significantly lower confidence activity ((placebo HC > LC) > (haloperidol HC > LC)) in the PFC and ACC (p < 0.05 whole-brain FWE-corrected) and in the right ventral striatum (p < 0.05 small-volume FWE-corrected; Fig. 3c and Table 3). Importantly, the β parameters in Fig. 3c indicate that this reduced striatal confidence response under haloperidol was due to reduced differentiation between confidence levels rather than due to reduced striatal signal per se: replicating our previous non-pharmacological study [25], the placebo group showed linear decreasing activity in the striatum with decreasing confidence across response category conditions, but this linear confidence effect was clearly reduced under haloperidol due to an unsystematic activity increase across confidence levels. Note that this effect was particularly evident for correct new decisions (CR trials) and that miss trials show a somewhat peculiar pattern under haloperidol. A similar reduced confidence differentiation, but without overall increased activity was observed in the ACC.
Discussion
We showed that retrieval of episodic information can be improved post learning by acute D2 antagonist administration. The retrieval-specific recognition enhancement of haloperidol is in agreement with previous reports of haloperidol-induced post-learning memory retrieval facilitation in the rat [22, 23] and with preliminary evidence of recognition impairments under decreased dopaminergic transmission in humans using a low-dose dopaminergic agonist [13]. However, the current retrieval-specific results go beyond this latter study where dopamine levels were not exclusively manipulated during retrieval and therefore the observed recognition deficit might also be due to dopaminergic effects on encoding. Inconclusive or absent retrieval performance effects of dopamine in other human pharmacological studies [11, 12, 14, 15] might likewise have resulted from a failure to constrain dopaminergic modulation to the retrieval phase specifically.
Of interest, the few human pharmacological studies that examined the retrieval effects specifically using dopamine agonists such as amphetamine, THC, or MDMA [16–18] observed increased false recognition under drug in the absence of retrieval accuracy effects. In contrast, our present results give no clear indication of a haloperidol effect on false alarm rates. These contradictory findings might be explained by a different impact on prefrontal retrieval monitoring mechanisms by these drugs. Specifically, while these dopamine agonists might target in particular frontal circuits linked with monitoring and false memory recognition [17], haloperidol has only marginal direct effects on frontal circuits but acts primarily on the striatum [37, 51]. Additionally, it should be noted that low-dose dopamine agonists can have likewise paradoxical effects due to the primary activation of the high-affinity presynaptic autoreceptors (e.g., [13, 40]) and therefore it cannot be excluded that these previously reported false recognition effects [16–18] result from decreased (rather than increased) dopaminergic neurotransmission.
Importantly, the fMRI data acquired in the present recognition study give an indication of the direction of the dopaminergic effects associated with the memory effects observed on the behavioral level. In particular, the increased striatal and SN/VTA activations indicate that haloperidol indeed potentiated dopamine release from the SN/VTA by blocking the presynaptic autoreceptors [37, 38, 43]. The resulting increased stimulation of (postsynaptic) dopamine receptors in the hippocampus and amygdala might have improved memory discrimination, possibly by augmenting the signal-to-noise ratio [52–54] of the mnemonic representation in these structures. This explanation is in accordance with the higher fMRI activity for retrieval success in these regions and in the dopaminergic midbrain under haloperidol. Still, it should be noted that the haloperidol-induced dopamine increase (e.g., [34, 35, 37]) as well as the tight correlation between dopaminergic stimulation and BOLD activity [38, 41–43] is thoroughly established in the striatum, but comparably less clear for the hippocampus or amygdala (though see [55, 56]). In contrast, the fMRI activity of memory confidence suggested that enhanced dopaminergic stimulation of frontostriatal networks under haloperidol resulted in behavioral impairments in metamemory for new decisions and probably also contributed to the decreased WM performance [31] in the haloperidol group. In particular, the unsystematically increased striatal activity across all confidence levels (“reduced confidence differentiation”) under haloperidol might have limited the dynamic response range of the striatum [52] to signal the previously reported linear increasing striatal confidence activity from low to high confidence [25] and altered striatal interactions with the PFC and ACC underlying metacognition. This frontostriatal metacognitive process seems to be more important for new decisions than for old decisions, as only the former were impaired under haloperidol. Of interest, an improvement of prefrontal metacognition for memory retrieval through the dopamine agonist l-dopa was reported by [57] while another recent study [58] did not observe an effect of dopamine on metacognition in a perceptual dot motion task. This could point to different mechanisms underlying the introspective metacognition in memory retrieval compared to perceptual metacognition (see also [59]).
Together, these data demonstrate that the dopaminergic modulation here has facilitating effects on midbrain–hippocampal memory retrieval, but detrimental effects on frontostriatally mediated metamemory. These opposing findings are in agreement with recently demonstrated dissociable brain mechanisms underlying recognition performance and memory confidence in non-human primates [60], as well as with long-established behavioral dissociations between memory accuracy and confidence in human cognitive studies (e.g., [61–64]). Beyond this dissociation, our findings might help to explain the presence of episodic memory [65] and metamemory [66] impairments in dopamine-related diseases, as well as offer new perspectives for the treatment of memory disorders.
Limitations
Our interpretation rests on the assumption that acute administration of 2 mg haloperidol will actually increase dopaminergic signaling. However, this effect has been directly demonstrated only in animals. Previous human fMRI studies using similar doses of haloperidol usually assumed that the drug decreases dopaminergic signaling (e.g., [67, 68]). It is possible that the effect of haloperidol is relatively dependent on the task and the kind of stimuli. Moreover, D2 antagonist effects on autoreceptor actions are certainly complex and not understood in all details. Depending on the state of the dopamine neurons and the exact stimulation pattern, regulation effects of the autoreceptors may vary and also feature additional components shaping the neuron’s activity [69]. Also, the observed memory effects might stem from interactions with other neurotransmitter systems (especially noradrenaline [23]) and be tightly linked to specific doses determining the amount of presynaptic vs. postsynaptic blockade [70]. Eventually, additional studies using multi-modal methodological approaches [71] will be necessary to resolve these contradictory drug findings as well to examine the specificity of dopaminergic effects on memory retrieval in more detail.
Finally, drug studies are prone to vascular baseline effects confounding the MR signal [72]. The critical fMRI group differences we report here for memory retrieval and confidence are well controlled for such potential baseline differences as we compared hit with CR trials and high with low confidence trials, respectively. General drug effects affecting the vascular reactivity should affect both conditions similarly and are therefore removed before the groups are compared. Still, future studies might consider to control these potential confounds more rigorously by, for example, measuring the baseline brain perfusion using arterial spin labeling and by recording and correcting for physiological parameters such as heart or breathing rate [72].
Funding and disclosure
This study was supported by a grant from the Deutsche Forschungsgemeinschaft to TS and NB (DFG SO 952/3-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to the University of Minnesota Center for Magnetic Resonance Research for providing the image reconstruction algorithm for the simultaneous multi-slice acquisitions. The authors declare no competing interests.
Electronic supplementary material
Acknowledgments
Author contributions
Conceptualization, MC and TS; methodology, MC and TS; formal Analysis, MC; investigation, MC; writing—original draft, MC; writing—review and editing, MC, NB, and TS; resources—NB and TS; supervison—TS; funding acquisition, NB and TS.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0246-y).
References
- 1.Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–90. doi: 10.1016/S0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 2.Axmacher N, Cohen MX, Fell J, Haupt S, Dümpelmann M, Elger CE, et al. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010;65:541–9. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Mehta MA, Riedel WJ. Dopaminergic enhancement of cognitive function. Curr Pharm Des. 2006;12:2487–500. doi: 10.2174/138161206777698891. [DOI] [PubMed] [Google Scholar]
- 5.Clos M, Sommer T, Schneider SL, Rose M. Enhanced transformation of incidentally learned knowledge into explicit memory by dopaminergic modulation. PLoS ONE. 2018;13:e0199013. doi: 10.1371/journal.pone.0199013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNamara CG, Tejero-Cantero Aacute, Trouche S, Campo-Urriza N, Dupret D. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat Neurosci. 2014;17:1658–60. doi: 10.1038/nn.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Düzel E. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32:14193–204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knecht S, Breitenstein C, Bushuven S, Wailke S, Kamping S, Flöel A, et al. Levodopa: faster and better word learning in normal humans. Ann Neurol. 2004;56:20–26. doi: 10.1002/ana.20125. [DOI] [PubMed] [Google Scholar]
- 9.Linssen AMW, Vuurman EFPM, Sambeth A, Riedel WJ. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology (Berl) 2012;221:611–9. doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White NM, Packard MG, Seamans J. Memory enhancement by post-training peripheral administration of low doses of dopamine agonists: possible autoreceptor effect. Behav Neural Biol. 1993;59:230–41. doi: 10.1016/0163-1047(93)90998-W. [DOI] [PubMed] [Google Scholar]
- 11.Andreou C, Moritz S, Veith K, Veckenstedt R, Naber D. Dopaminergic modulation of probabilistic reasoning and overconfidence in errors: a double-blind study. Schizophr Bull. 2014;40:558–65. doi: 10.1093/schbul/sbt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linssen AMW, Riedel WJ, Sambeth A. Effects of tyrosine/phenylalanine depletion on electrophysiological correlates of memory in healthy volunteers. J Psychopharmacol (Oxf) 2011;25:230–8. doi: 10.1177/0269881109348160. [DOI] [PubMed] [Google Scholar]
- 13.Montoya A, Lal S, Menear M, Duplessis E, Thavundayil J, Schmitz N, et al. Apomorphine effects on episodic memory in young healthy volunteers. Neuropsychologia. 2008;46:292–300. doi: 10.1016/j.neuropsychologia.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Morcom AM, Bullmore ET, Huppert FA, Lennox B, Praseedom A, Linnington H, et al. Memory encoding and dopamine in the aging brain: a psychopharmacological neuroimaging study. Cereb Cortex. 2010;20:743–57. doi: 10.1093/cercor/bhp139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rammsayer TH, Rodewald S, Groh D. Dopamine—antagonistic, anticholinergic, and GABAergic effects on declarative and procedural memory functions. Brain Res Cogn Brain Res. 2000;9:61–71. doi: 10.1016/S0926-6410(99)00045-2. [DOI] [PubMed] [Google Scholar]
- 16.Doss MK, Weafer J, Gallo DA, de Wit H. MDMA Impairs both the encoding and retrieval of emotional recollections. Neuropsychopharmacology. 2018;43:791–800. doi: 10.1038/npp.2017.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doss MK, Weafer J, Gallo DA, de Wit H. Δ9-Tetrahydrocannabinol at retrieval drives false recollection of neutral and emotional memories. Biol Psychiatry. 2018. 10.1016/j.biopsych.2018.04.020. [DOI] [PubMed]
- 18.Ballard ME, Gallo DA, de Wit H. Amphetamine increases errors during episodic memory retrieval. J Clin Psychopharmacol. 2014;34:85–92. doi: 10.1097/JCP.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fioravanti M, Bianchi V, Cinti ME. Cognitive deficits in schizophrenia: an updated metanalysis of the scientific evidence. BMC Psychiatry. 2012;12:64. doi: 10.1186/1471-244X-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald AA, Seergobin KN, Owen AM, Tamjeedi R, Monchi O, Ganjavi H, et al. Differential effects of Parkinson’s disease and dopamine replacement on memory encoding and retrieval. PLoS ONE. 2013;8:e74044. doi: 10.1371/journal.pone.0074044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittington CJ, Podd J, Kan MM. Recognition memory impairment in Parkinson’s disease: power and meta-analyses. Neuropsychology. 2000;14:233–46. doi: 10.1037/0894-4105.14.2.233. [DOI] [PubMed] [Google Scholar]
- 22.Chugh Y, Saha N, Sankaranarayanan A, Sharma PL. Possible mechanism of haloperidol-induced enhancement of memory retrieval. Methods Find Exp Clin Pharmacol. 1991;13:161–4. [PubMed] [Google Scholar]
- 23.Sara SJ. Haloperidol facilitates memory retrieval in the rat. Psychopharmacology (Berl) 1986;89:307–10. doi: 10.1007/BF00174365. [DOI] [PubMed] [Google Scholar]
- 24.Clos M, Schwarze U, Gluth S, Bunzeck N, Sommer T. Goal- and retrieval-dependent activity in the striatum during memory recognition. Neuropsychologia. 2015;72:1–11. doi: 10.1016/j.neuropsychologia.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Schwarze U, Bingel U, Badre D, Sommer T. Ventral striatal activity correlates with memory confidence for old- and new-responses in a difficult recognition test. PLoS ONE. 2013;8:e54324. doi: 10.1371/journal.pone.0054324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Huettel SA, Raposo A, Adcock RA, Dobbins IG. Functional significance of striatal responses during episodic decisions: recovery or goal attainment? J Neurosci. 2010;30:4767–75. doi: 10.1523/JNEUROSCI.3077-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H. Differential neural activity in the recognition of old versus new events: an activation likelihood estimation meta-analysis. Hum Brain Mapp. 2013;34:814–36. doi: 10.1002/hbm.21474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698. doi: 10.1126/science.aac9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lohani S, Poplawsky AJ, Kim S-G, Moghaddam B. Unexpected global impact of VTA dopamine neuron activation as measured by opto-fMRI. Mol Psychiatry. 2016. 10.1038/mp.2016.102. [DOI] [PMC free article] [PubMed]
- 31.Frank MJ, O’Reilly RC. A mechanistic account of striatal dopamine function in human cognition: psychopharmacological studies with cabergoline and haloperidol. Behav Neurosci. 2006;120:497–517. doi: 10.1037/0735-7044.120.3.497. [DOI] [PubMed] [Google Scholar]
- 32.Knutson B, Gibbs SEB. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–22. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- 33.Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22. doi: 10.1016/j.neuroscience.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dugast C, Brun P, Sotty F, Renaud B, Suaud-Chagny MF. On the involvement of a tonic dopamine D2-autoinhibition in the regulation of pulse-to-pulse-evoked dopamine release in the rat striatum in vivo. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:716–9. doi: 10.1007/PL00005004. [DOI] [PubMed] [Google Scholar]
- 35.Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, et al. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–29. doi: 10.1016/S0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 36.Jaworski JN, Gonzales RA, Randall PK. Effect of dopamine D2/D3 receptor antagonist sulpiride on amphetamine-induced changes in striatal extracellular dopamine. Eur J Pharmacol. 2001;418:201–6. doi: 10.1016/S0014-2999(01)00936-0. [DOI] [PubMed] [Google Scholar]
- 37.Pehek EA. Comparison of effects of haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J Pharmacol Exp Ther. 1999;289:14–23. [PubMed] [Google Scholar]
- 38.Chen YCI, Choi JK, Andersen SL, Rosen BR, Jenkins BG. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 2005;180:705–15. doi: 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- 39.Mandeville JB, Sander CYM, Jenkins BG, Hooker JM, Catana C, Vanduffel W, et al. A receptor-based model for dopamine-induced fMRI signal. Neuroimage. 2013;75:46–57. doi: 10.1016/j.neuroimage.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren J, Xu H, Choi JK, Jenkins BG, Chen YI. Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse. 2009;63:764–72. doi: 10.1002/syn.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–12. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 42.Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–98. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J, et al. Selective dopamine D(3) receptor antagonist SB-277011-A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse. 2004;54:1–10. doi: 10.1002/syn.20055. [DOI] [PubMed] [Google Scholar]
- 44.Choi JK, Mandeville JB, Chen YI, Grundt P, Sarkar SK, Newman AH, et al. Imaging brain regional and cortical laminar effects of selective D3 agonists and antagonists. Psychopharmacology (Berl) 2010;212:59–72. doi: 10.1007/s00213-010-1924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Handley R, Zelaya FO, Reinders AATS, Marques TR, Mehta MA, O’Gorman R, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272–82. doi: 10.1002/hbm.21436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Macmillan NA, Creelman CD. Detection Theory: a user’s guide. New York: Psychology Press; 2004.
- 47.Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. doi: 10.1006/jmla.2002.2864. [DOI] [Google Scholar]
- 48.Koen JD, Barrett FS, Harlow IM, Yonelinas AP. The ROC Toolbox: atoolbox for analyzing receiver-operating characteristics derived from confidence ratings. Behav Res Methods. 2016. 10.3758/s13428-016-0796-z. [DOI] [PMC free article] [PubMed]
- 49.Maniscalco B, Lau H (2014). Signal detection theory analysis of type 1 and type 2 data: meta-d′, response-specific meta-d′, and the unequal variance SDT Model. Cogn Neurosci Metacogn. 2014; 25–66. 10.1007/978-3-642-45190-4_3.
- 50.Maniscalco B, Lau H. A signal detection theoretic approach for estimating metacognitive sensitivity from confidence ratings. Conscious Cogn. 2012;21:422–30. doi: 10.1016/j.concog.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 51.Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–60. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 52.Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17:51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- 53.Warren CM, Eldar E, Brink RL, van den, Tona KD, Wee NJ, van der, Giltay EJ, et al. Catecholamine-mediated increases in gain enhance the precision of cortical representations. J Neurosci. 2016;36:5699–708. doi: 10.1523/JNEUROSCI.3475-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yousif N, Fu RZ, Abou-El-Ela Bourquin B, Bhrugubanda V, Schultz SR, Seemungal BM. Dopamine activation preserves visual motion perception despite noise interference of human V5/MT. J Neurosci. 2016;36:9303–12. doi: 10.1523/JNEUROSCI.4452-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fotros A, Casey KF, Larcher K, Verhaeghe JAJ, Cox SML, Gravel P, et al. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [18F]fallypride study in cocaine dependent participants. Neuropsychopharmacology. 2013;38:1780–8. doi: 10.1038/npp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, et al. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–9. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joensson M, Thomsen KR, Andersen LM, Gross J, Mouridsen K, Sandberg K, et al. Making sense: dopamine activates conscious self-monitoring through medial prefrontal cortex. Hum Brain Mapp. 2015;36:1866–77. doi: 10.1002/hbm.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hauser TU, Allen M, Purg N, Moutoussis M, Rees G, Dolan RJ (2017). Noradrenaline blockade specifically enhances metacognitive performance. Elife. 2017;6:e24901. [DOI] [PMC free article] [PubMed]
- 59.Morales J, Lau H, Fleming SM. Domain-general and domain-specific patterns of activity supporting metacognition in human prefrontal cortex. J Neurosci. 2018;38:3534–46. doi: 10.1523/JNEUROSCI.2360-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miyamoto K, Osada T, Setsuie R, Takeda M, Tamura K, Adachi Y, et al. Causal neural network of metamemory for retrospection in primates. Science. 2017;355:188–93. doi: 10.1126/science.aal0162. [DOI] [PubMed] [Google Scholar]
- 61.Dobbins IG, Kroll NE, Liu Q. Confidence-accuracy inversions in scene recognition: a remember-know analysis. J Exp Psychol Learn Mem Cogn. 1998;24:1306–15. doi: 10.1037/0278-7393.24.5.1306. [DOI] [PubMed] [Google Scholar]
- 62.Busey TA, Tunnicliff J, Loftus GR, Loftus EF. Accounts of the confidence-accuracy relation in recognition memory. Psychon Bull Rev. 2000;7:26–48. doi: 10.3758/BF03210724. [DOI] [PubMed] [Google Scholar]
- 63.Roediger HL, DeSoto KA. Confidence and memory: assessing positive and negative correlations. Memory. 2014;22:76–91. doi: 10.1080/09658211.2013.795974. [DOI] [PubMed] [Google Scholar]
- 64.Chandler CC. Studying related pictures can reduce accuracy, but increase confidence, in a modified recognition test. Mem Cogn. 1994;22:273–80. doi: 10.3758/BF03200854. [DOI] [PubMed] [Google Scholar]
- 65.Scimeca JM, Badre D. Striatal contributions to declarative memory retrieval. Neuron. 2012;75:380–92. doi: 10.1016/j.neuron.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eisenacher S, Zink M. The importance of metamemory functioning to the pathogenesis of psychosis. Front Psychol. 2017;8:304. doi: 10.3389/fpsyg.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oei NYL, Rombouts SA, Soeter RP, Gerven JM, van, Both S. Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology. 2012;37:1729–37. doi: 10.1038/npp.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pleger B, Ruff CC, Blankenburg F, Klöppel S, Driver J, Dolan RJ. Influence of dopaminergically mediated reward on somatosensory decision-making. PLoS Biol. 2009;7:e1000164. doi: 10.1371/journal.pbio.1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kita JM, Parker LE, Phillips PEM, Garris PA, Wightman RM. Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem. 2007;102:1115–24. doi: 10.1111/j.1471-4159.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- 70.Shi WX, Smith PL, Pun CL, Millet B, Bunney BS. D1–D2 interaction in feedback control of midbrain dopamine neurons. J Neurosci. 1997;17:7988–94. doi: 10.1523/JNEUROSCI.17-20-07988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bruinsma TJ, Sarma VV, Oh Y, Jang DP, Chang SY, Worrell GA, et al. The relationship between dopamine neurotransmitter dynamics and the blood-oxygen-level-dependent (BOLD) signal: a review of pharmacological functional magnetic resonance imaging. Front Neurosci. 2018;12:238. doi: 10.3389/fnins.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iannetti GD, Wise RG. BOLD functional MRI in disease and pharmacological studies: room for improvement? Magn Reson Imag. 2007;25:978–88. doi: 10.1016/j.mri.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 73.Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. Neuroimage. 2009;47:1261–7. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–52. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.