Abstract
Perseverative behavior has been highly implicated in addiction. Activation of serotonin 2C receptors (5-HT2CRs) attenuates cocaine and high caloric food intake, but whether a 5-HT2CR agonist can reduce high caloric diet (HCD) or methamphetamine (METH) intake and response perseveration remains unknown. Clarifying the role of 5-HT2CRs in these behaviors will improve knowledge of neurochemical processes that regulate flexible decision-making and whether improvements in decision-making are accompanied by decreases in HCD or METH intake. This study evaluated the effects of long-term HCD and METH intake on reversal learning in female rhesus monkeys. The effects of the 5-HT2CR agonist WAY163909 on reversal learning before and after extended HCD or METH intake, and on food intake, was also tested. Moreover, we examined whether the 5-HT2CR is necessary for the effects of WAY163909. WAY163909 was given prior to reversal learning at baseline and after extended HCD or METH intake, and prior to measures of food intake. Extended intake of METH or the HCD increased perseverative errors during reversal. WAY163909 increased correct responses and decreased perseverative errors, both before and after extended HCD or METH intake. Similarly, WAY163909 decreased consumption of a HCD, but not a low caloric diet. The effects of WAY163909 on all these measures were blocked by co-administration with a 5-HT2CR antagonist. These data indicate that long-term HCD or METH intake disrupts flexible decision-making. Further, the results suggest that reductions in food intake produced by WAY163909 are associated with parallel improvements in decision-making strategies, underscoring the role of the 5-HT2CR for these behavioral effects.
Subject terms: Cognitive control, Feeding behaviour
Introduction
Addiction costs the US over $600 billion per year [1] and FDA-approved treatments are limited. Current treatments ignore the co-morbidity between different addictions [2, 3] and their shared neurobiology [4], and do not address the underlying causes of the disorder [5, 6]. Identifying the core neurochemical processes underlying these behaviors could advance the search for novel therapeutics to treat various addictions.
Dopamine (DA) neurotransmission is critical to the reinforcing effects of psychostimulant drugs [7], palatable, high caloric foods [8, 9], and non-drug reinforcers like sex [10, 11] and gambling [12]. Attempts to leverage DA drugs for treatment of addiction have yielded no medications that lack abuse liability [13, 14] and exhibit efficacy to reduce craving and prevent relapse.
The serotonin 2C receptor (5-HT2CR) is a promising therapeutic candidate for treatment of addiction [15, 16]. 5-HT2CRs are most highly expressed on GABAergic interneurons in the prefrontal cortex (PFC) and striatal areas, including the ventral tegmental area (VTA), nucleus accumbens (NAcc), caudate and putamen [17, 18]. Activation of 5-HT2CRs should inhibit mesolimbic DA signaling, attenuating the abuse-related effects of reinforcers. Electrophysiological and microdialysis studies [16, 19–21], and receptor knockout studies [22], provide support for this hypothesis. Moreover, 5-HT2CR agonists decrease psychostimulant self-administration and reinstatement [21] in non-human primates (NHPs), food intake in humans [23, 24], and nicotine [25] and alcohol [26] self-administration in rats.
Perseverative behavior, which has been implicated in addiction, can be defined as a general inability to alter responding with changing reinforcement contingencies. It is measured using reversal learning tasks and often interpreted as a measure of compulsivity [27, 28]. A progression from recreational use of reinforcers to compulsive use is thought to underlie the development and maintenance of addiction [29–31]. Reinforcer-induced increases in DA signaling within the striatum could play a crucial role in decision-making and intake of reinforcers. For example, prenatal exposure to methamphetamine (METH) and methylphenidate in mice [32] and METH pretreatment in rats [33] increase perseverative behavior. The 5-HT system modulates perseverative behavior and the abuse-related effects of reinforcers, likely through its effects on DA neurotransmission, given that selective depletion of 5-HT in the orbital PFC (oPFC) increases perseverative responding in animal models [34, 35]. Studies addressing the effects of 5-HT2CRs on perseverative behavior in rodents have yielded mixed results. The 5-HT2CR agonists Ro 60-0175 [36] and CP 809.101 [37] decrease perseverative responding, whereas another study found that the 5-HT2C antagonist SB242084 has the same effect [38]. Thus far, there have been no studies on the effects of 5-HT2CR agonists or antagonists on perseverative behavior in NHPs, or on whether the 5-HT2CR is necessary for these effects.
The present study evaluated whether long-term high caloric diet (HCD) or methamphetamine (METH) intake increases perseverative behavior, and whether a 5-HT2CR agonist decreases response perseveration in female rhesus monkeys. The present study also determined whether 5-HT2CR activation decreases intake of a low caloric diet (LCD) and/or a choice diet, where both a LCD and a palatable HCD are available. To demonstrate that the 5-HT2CR is necessary for these effects, pretreatment with a selective 5-HT2C receptor antagonist was used.
Materials and methods
Subjects
Adult female rhesus macaques (Macaca mulatta) weighing 7–14 kg (N = 10) served as subjects. Menstrual cycle was not monitored. At the onset, subjects were experimentally naive, having never had any access to drugs of abuse or a palatable HCD. All procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Eighth Edition, revised in 2010), and were approved by the Institutional Animal Care and Use Committee of Emory University.
Subjects in the METH group (N = 5) were pair-housed in indoor stainless-steel home cages at the Yerkes National Primate Research Center (YNPRC) Main Station. They were fed Purina monkey chow (Ralston Purina, St. Louis, MO, USA) supplemented with fruits and vegetables, and had water continually available in the colony, maintained at an ambient temperature of 22 ± 2 °C at 45–50% humidity and a 12-h light/dark cycle (lights on at hour 0700; lights off at hour 1900). Environmental enrichment was provided on a regular rotating basis. Subjects were accessed for testing using a pole and collar (primate products) method and positioned into a primate chair (primate products) placed inside testing chambers.
Subjects in the HCD group (N = 5) were housed in indoor–outdoor 144 ft2 (12 × 12 ft.) pens at the YNPRC Field Station in groups of 5–6 females. Environmental enrichment was provided on a regular rotating basis. Socially housed female rhesus monkeys form a dominance hierarchy, with subordinates showing a distinct behavioral and physiological phenotype [39], thus only middle ranking animals were utilized. Subjects were accessed for testing using a transport cage (primate products) method and carried to testing chambers.
Drugs
The 5-HT2CR agonist WAY163909 hydrochloride [(7b– R,10a –R)-1,2,3,4,8,9,10,10a—octahydro-7bHcyclopenta[b][1,4] diazepino [6,7,1hi] indole] was a generous gift from Pfizer Inc. (New York, NY, USA). WAY163909 was dissolved in 10 mg/mL beta-cyclodextrin and administered intramuscularly 45 min prior to testing. WAY163909 is selective for the 5-HT2CR, having a much higher affinity for the 5-HT2CR (Ki = 10.5) than the 5-HT2AR (Ki = 212) or the 5-HT2BR (Ki = 2101) [40]. The selective 5-HT2C antagonist SB242084 [6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride hydrate] was purchased from Tocris Bioscience (Ellisville, MO, USA), dissolved in at a concentration of 1.0 mg/mL in a 20:20:60 mixture of 95% ethanol, Tween 80 (Sigma-Aldrich®, St. Louis, MO), and 0.9% saline, and further diluted to appropriate concentrations using 0.9% saline. SB242084 is also selective for the 5-HT2CR, having a much higher affinity for the 5-HT2CR (Ki = 7.1) than the 5-HT2AR (Ki = 160) or the 5-HT2BR (Ki = 100) [40]. SB242084 was administered intramuscularly 15 min prior to WAY163909. (±)METH hydrochloride was provided by the National Institute on Drug Abuse (Research Technology Branch, Research Triangle Park, NC, USA), dissolved in 0.9% saline, and administered intravenously.
Reversal learning
Subjects were trained on a discrimination reversal learning (DRL) task [41]. The Wisconsin General Test Apparatus (WGTA) was fitted with a stimulus tray containing three wells. DRL sessions were conducted once/day (Monday–Friday at the Yerkes Field Station and Monday–Sunday at the Yerkes Main Station). Each session consisted of 30 trials separated by 15 s intervals. Subjects had a 2-min limited hold to emit their response before the trial ended. The positions of objects during each trial were randomized.
During acquisition of the task, one of three distinct objects was paired with a hidden reward (M&Ms, Skittles, etc.). If the subject’s first response during a trial was the rewarded object, the trial would end, followed by a 15 s time-out, and the next trial. If the subject’s first response was one of the unrewarded objects, there would be a 15 s time-out, followed by a repetition of that trial with a forced correction in which the chosen incorrect object was moved to reveal the empty well underneath. Subjects could repeat each trial, making an unlimited number of erroneous responses until they chose the rewarded object, moving on to the next trial. Acquisition continued until the subject reached a performance criterion of 90% correct responses (the rewarded object was chosen first). After meeting these criteria, they underwent a reversal the following day.
During reversal, the reward was hidden under one of the previously unrewarded objects. Reversal lasted for one 30-trial session with no required performance criterion. Reversal trials were conducted identically to acquisition trials. Possible errors included choosing the previously rewarded object (perseverative error) or the third unrewarded (random error). The inclusion of forced correction trial repetitions resulted in a finite number of possible correct responses (30), but an unlimited number of possible perseverative errors and random errors.
Reversal learning was measured before (pre-exposure) and after intake of METH or the HCD for 6 months (post-HCD exposure or post-METH exposure). All pretreatments with vehicle, WAY163909, or SB242084 were given prior to reversal sessions. A new set of three objects was used for each subsequence round of acquisition and reversal.
5-HT2CR interventions
Following baseline reversal DRL task measures, during which no pretreatments were given, all subjects were evaluated after administration of WAY163909 or its vehicle. WAY163909 pretreatments (vehicle, 0.1, 0.3, and 1.0 mg/kg) were randomized across subjects in each group. Doses were chosen based on psychostimulant self-administration and reinstatement studies, and microdialysis studies on psychostimulant-induced DA overflow in the NAcc [21]. For one subject (Iv8) in the HCD group, the dose range was reduced because the highest dose of WAY163909 produced behavioral suppression during the task. She was evaluated under the following doses: 0.03, 0.1, and 0.3 mg/kg WAY163909. A second, final baseline measure was collected for all subjects following all other treatments. For all pre-exposure group DRL graphs presented, N = 10 for all experimental conditions, except the 1.0 mg/kg WAY163909 treatment condition, for which N = 9. For the HCD group, baseline food intake of a LCD only, during which no pretreatments were given, was measured. Then, LCD intake was evaluated after administration of WAY163909 or its vehicle.
After 6 months of METH SA or HCD consumption, subjects were re-tested on the DRL task after administration of WAY163909 (0.3 mg/kg for Iv8, 1.0 mg/kg for all others), its vehicle, or a combination of WAY163909 and SB242084 (0.1 mg/kg). For the HCD group, food intake was measured under no pretreatment conditions. Then, food intake was evaluated following administration of WAY163909 (0.3 mg/kg for Iv8, 1.0 mg/kg for all others), its vehicle, or a combination of WAY163909 and SB242084 (0.1 mg/kg). The dose of SB242084 used was chosen based on previous drug self-administration experiments in squirrel monkeys [19, 20]. All pretreatments were counterbalanced across subjects.
Intravenous drug self-administration
Following baseline reversal learning testing, subjects in the METH group self-administered METH (0.01 mg/kg/infusion i.v.) for a period of 6 months. The apparatus and self-administration (SA) procedure are described in detail by Howell and Wilcox [42]. Briefly, subjects responded under a fixed-ratio (FR) 20 schedule of drug delivery during 60-min daily sessions in the morning (starting between 0700 and 1000 h) Monday–Friday. The effects of WAY163909 on METH SA were not tested here because they have been previously described [21].
Food intake
Prior to and during pre-exposure reversal learning testing, subjects in the HCD group had ad libitum access to standard monkey chow (LCD; 3.45 kcal/g, Purina 5038) via previously validated automated feeders that allowed for constitutive quantification of caloric intake [43]. Briefly, activation of a radio-frequency (RF) antenna via RF identification chip within each subject’s wrist signaled a computer to dispense a single pellet of food. The caloric composition of the LCD was 12% fat, 18% protein, 4.14% sugar carbohydrate, and 65.9% fiber carbohydrate. Following pre-exposure reversal learning testing, subjects in the HCD group were given access to both the LCD and a HCD (4.47 kcal/g, D07091204S Research Diets, New Brunswick NJ), with a caloric composition of 36% fat, 18% protein, 16.4% sugar carbohydrate, and 29.9% fiber-starch carbohydrate, for 6 months. In monkeys, availability of diet choice sustains excess calorie intake and promotes obesity compared to availability of only a HCD [44, 45]. Measures of food intake were taken for 24 h following vehicle, WAY163909, or SB242084 + WAY163909 pretreatments.
Statistical analysis
All statistical analyses were conducted using GraphPad Prism (GraphPad Software, version 7). Paired t-tests and repeated measures analysis of variance (RM ANOVA) with Dunnett’s post hoc test were used when appropriate. Significance for all tests was set a priori at the 95% confidence level (p < 0.05).
Results
Reversal learning measures before METH or HCD exposure
Pre-exposure DRL task measures are reported as group mean ± SEM (Fig. 1). During reversal sessions, subjects (N = 10) made 13.5 ± 1.71 correct responses at baseline, when no pretreatments were given, and 11.2 ± 1.99 after vehicle pretreatment; 23.8 ± 3.09 perseverative errors at baseline and 26.6 ± 3.8 after vehicle pretreatment; 7.05 ± 1.27 random errors at baseline and 6.2 ± 1.24 after vehicle pretreatment. Subjects took 3.35 ± 0.35 days to acquire discriminations prior to baseline reversal, and 4.4 ± 1.01 days to acquire discriminations prior to vehicle pretreatment. RM ANOVA with Dunnett’s post hoc test revealed no significant difference between any baseline or vehicle DRL task measures (p < 0.05).
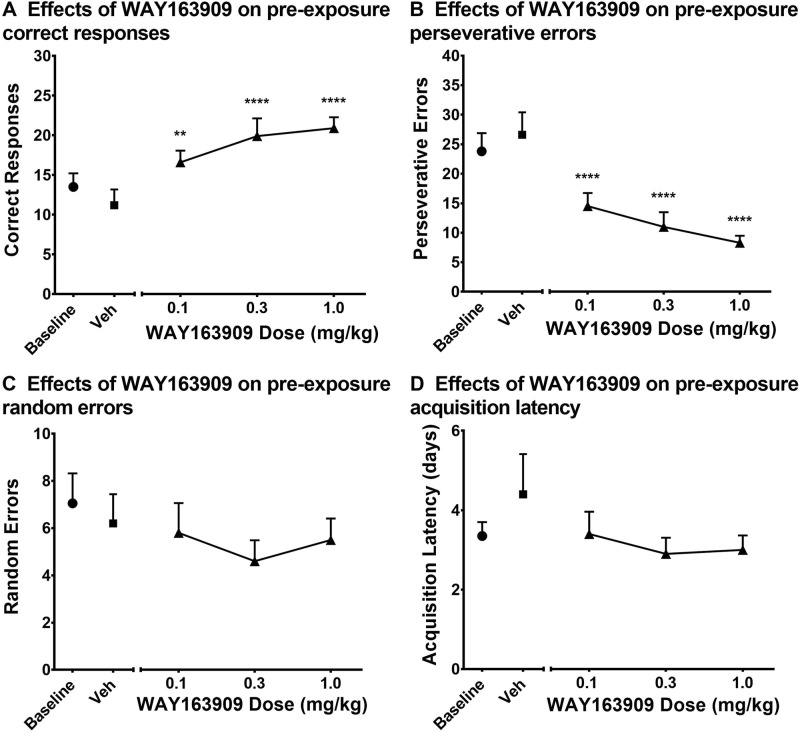
Fig. 1.
Effects of WAY163909 on reversal learning measures before METH or HCD exposure. Subjects participated in a DRL task in which several measures were taken during reversal sessions. Dose-effect curves for WAY163909 (0.1, 0.3, and 1.0 mg/kg) were established for the following measures: a correct responses, b perseverative errors, c random errors, and d acquisition latency (days). The data are presented as the group mean ± SEM, where N = 10 for each experimental condition, except for the 1.0 mg/kg WAY163909 dose condition, for which N = 9. **p < 0.01 for correct responses compared to vehicle; ****p < 0.0001 for correct responses and perseverative errors compared to vehicle
5-HT2CR effects on reversal learning measures before METH or HCD exposure
WAY163909 pretreatments dose-dependently increased pre-exposure correct responses [F(4,36) = 14.33, p < 0.0001] compared to vehicle (Fig. 1A). After pretreatment with the 0.1, 0.3, and 1.0 mg/kg WAY163909 doses, subjects made an average of 16.6 ± 1.48, 19.9 ± 2.23, and 20.9 ± 1.37 correct responses, respectively. WAY163909 pretreatments also significantly decreased pre-exposure perseverative errors [F(4,36) = 28.02, p < 0.0001] compared to vehicle (Fig. 1B). After pretreatment with the 0.1, 0.3, and 1.0 mg/kg WAY163909 doses, subjects made an average of 14.5 ± 2.23, 11.0 ± 2.49, and 8.3 ± 1.2 perseverative errors, respectively. Pre-exposure random errors (Fig. 1C) [F(4,36) = 1.295, p = 0.2903] and acquisition latencies (Fig. 1D) [F(4,36) = 1.161, p = 0.3443] were not affected by WAY163909 pretreatments.
5-HT2CR effects on food consumption before HCD exposure
At baseline, subjects in the HCD group only had access to a LCD. RM ANOVA revealed no difference in 24-h total food intake across any of the WAY163909 doses tested [F(4,16) = 0.3243, p = 0.8078] (data not shown).
Extended intake of the HCD or METH
Mean cumulative METH intake (Fig. 2A) over the 6-month period was 48.01 ± 9.335 mg/kg. Under pre-exposure conditions, subjects in the HCD group only had access to the LCD and they consumed an average of 1833.73 ± 223.90 kcal/week (Fig. 2B). During the 6-month choice diet condition (LCD + HCD), mean total food intake increased to 3060.65 ± 287.74 kcal/week [t(4) = 12.08, p = 0.0003] compared to the pre-exposure condition (Fig. 2B).
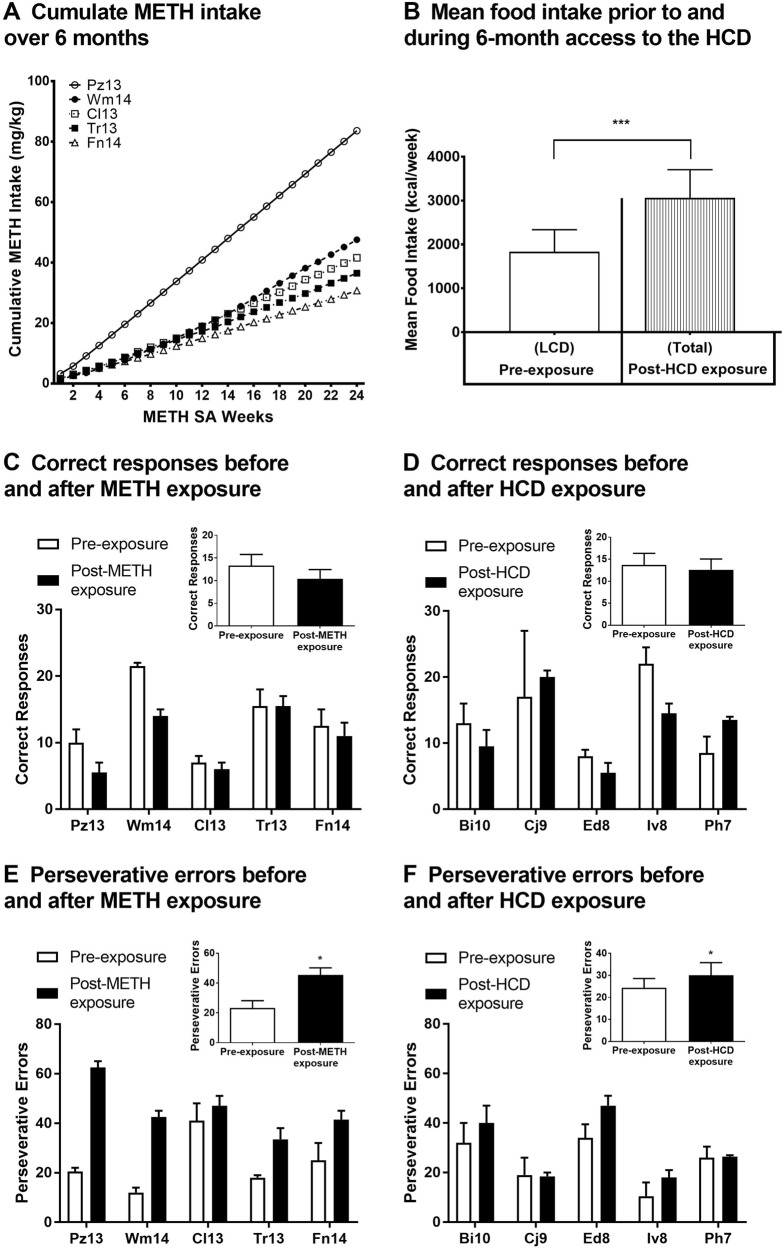
Fig. 2.
Reversal learning measures after 6-month METH or HCD exposure. Subjects in the METH group (N = 5) self-administered METH for 6 months. Data for total cumulative METH intake over this 6-month period a are presented as the 7-day mean ± SEM. Subjects in the HCD group (N = 5) consumed a HCD (under a dietary choice condition) for 6 months. The data for mean food intake b at baseline (LCD only) and during the 6-month choice diet condition (LCD + HCD), are presented as the mean ± SEM. DRL task measures were taken for the METH group. Data for correct responses c and perseverative errors e, under pre-exposure and post-METH exposure conditions, for the group as well as by individual, are presented as the mean ± SEM. DRL task measures were taken for the HCD group. Data for correct responses d and perseverative errors f, under pre-exposure and post-HCD exposure conditions, for the group as well as by individual, are presented as the mean ± SEM. *p < 0.05 for post-HCD and post-METH exposure perseverative errors compared with pre-exposure perseverative errors; ***p < 0.001 for post-HCD exposure mean food intake compared with pre-exposure mean food intake
Reversal learning measures after METH or HCD exposure
Subjects were re-tested on the DRL task after 6-months of either METH SA or HCD consumption. Compared to pre-exposure conditions, perseverative errors increased after either extended METH SA [t(4) = 3.493, p = 0.0125] (Fig. 2E) or HCD consumption [t(4) = 2.259, p = 0.0434] (Fig. 2F). Perseveration in the METH group doubled, from 23.3 ± 4.90 pre-exposure perseverative errors to 45.4 ± 4.80 perseverative errors following 6-months of METH SA. In the HCD group, perseveration increased from 24.3 ± 4.33 pre-exposure perseverative errors to 30 ± 5.82 perseverative errors after 6 months of HCD intake. Post-METH exposure (Fig. 2C), but not post-HCD exposure (Fig. 2D), correct responses trended toward a decrease compared to the pre-exposure condition, however this difference was not significant (p = 0.0611). Random errors and acquisition latency were not affected by extended intake of either the HCD or METH (data not shown).
5-HT2CR effects on reversal learning measures after METH or HCD exposure
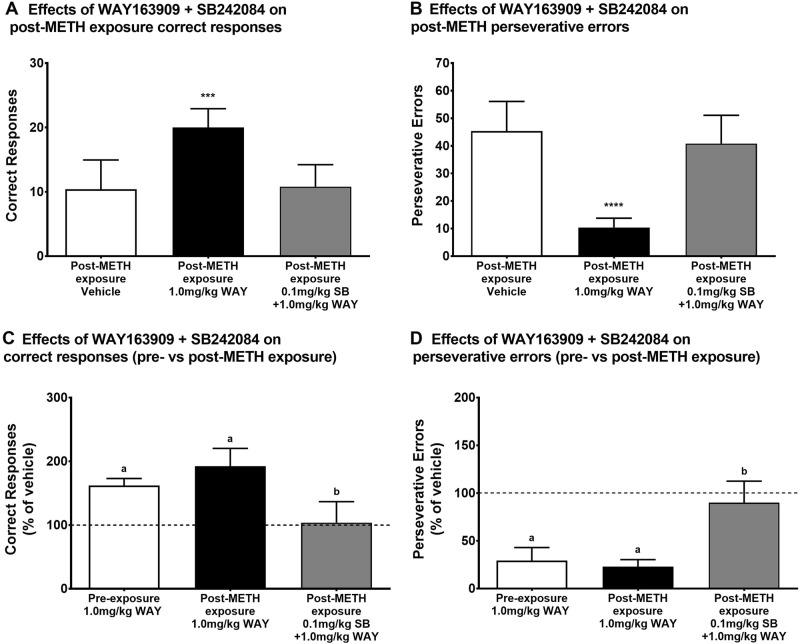
Following 6 months of METH SA, the highest dose of WAY163909 (1.0 mg/kg) increased correct responses [F(2,8) = 30.46, p = 0.0002] (Fig. 3A) and decreased perseverative errors [F(2,8) = 50.22, p < 0.0001] (Fig. 2B) compared to vehicle. When a dose of the antagonist SB242084 (0.1 mg/kg) was given in combination with the high dose of WAY163909, these effects of WAY163909 were blocked. When normalized to vehicle performance (% of vehicle), there was no difference between the effects of 1.0 mg/kg WAY163909 on pre- vs post-METH exposure correct responses (Fig. 3C) or perseverative errors (Fig. 3D), but the combined 0.1 mg/kg SB242084 + 1.0 mg/kg WAY163909 pretreatment produced a much smaller increase in post-METH exposure correct responses [(F(2,8) = 21.89, p = 0.0006] and much small decrease in perseverative errors [(F(2,8) = 30.02, p = 0.0002], than the high WAY163909 dose alone. Random errors (data not shown) were not affected by the high dose of WAY163909 or the combined SB242084 + WAY163909 pretreatment [F(2,8) = 3.79, p = 0.0695] and there was no difference in acquisition latency (data not shown) for any of the pretreatments [F(2,8) = 2.646, p = 0.1312].
Fig. 3.
Effects of combined pretreatment with WAY163909 and SB242084 on reversal learning measures after METH exposure. Subjects in the METH group (N = 5) self-administered METH for 6 months. Afterward, post-METH exposure perseverative behavior was measured using a DRL task under the following experimental conditions: vehicle, 1.0 mg/kg WAY163909, and 1.0 mg/kg WAY163909 + 0.1 mg/kg SB242084. Effects of these pretreatments are shown for a correct responses and b perseverative errors. The effects of WAY163909 and SB242084 before and after METH exposure are shown for c correct responses (% of vehicle) and d perseverative errors (% of vehicle). The data are presented as the group mean ± SEM for each experimental condition. ***p < 0.001 for post-METH exposure correct responses compared with vehicle; ****p < 0.0001 for post-METH exposure perseverative errors compared with vehicle; bars with the same letter (a or b) are not statistically different, while bars with different letters denote statistical difference
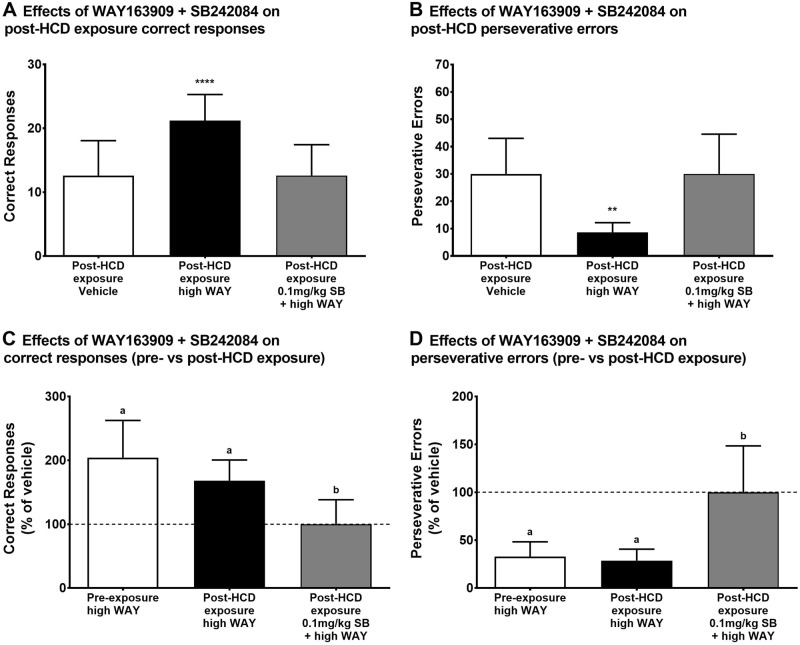
Following 6-month access to the HCD, the high WAY163909 dose increased correct responses [F(2,8) = 43.89, p < 0.0001] (Fig. 4A) and decreased perseverative errors [F(2,8) = 15.15, p = 0.0019] (Fig. 4B) compared to vehicle. SB242084 (0.1 mg/kg) blocked these effects. When normalized to vehicle performance (% of vehicle), there was no difference between the effects of 1.0 mg/kg WAY163909 on pre- vs post-HCD exposure correct responses (Fig. 4C) or perseverative errors (Fig. 4D), but the combined 0.1 mg/kg SB242084 + 1.0 mg/kg WAY163909 pretreatment produced a much smaller increase in post-HCD exposure correct responses [(F(2,8) = 6.434, p = 0.0216] and much small decrease in perseverative errors [(F(2,8) = 10.15, p = 0.0064], than the high WAY163909 dose alone. Random errors (data not shown) were not affected by any of the pretreatments [F(2,8) = 3.253, p = 0.0925] and there was no difference in acquisition latency (data not shown) across these conditions [F(2,8) = 2.341, p = 0.1583].
Fig. 4.
Effects of combined pretreatment with WAY163909 and SB242084 on reversal learning measures after HCD exposure. Subjects in the HCD group (N = 5) were given access to both a LCD and a palatable HCD for 6 months. Post-HCD exposure perseverative behavior was measured under the following conditions: vehicle, high WAY163909, and high WAY163909 + 0.1 mg/kg SB242084. Effects of these pretreatments are shown for a correct responses and b perseverative errors. The effects of WAY163909 and SB242084 before and after HCD exposure are shown for c correct responses (% of vehicle) and d perseverative errors (% of vehicle). The data are presented as the group mean ± SEM for each experimental condition. **p < 0.01 for post-HCD exposure perseverative errors compared with vehicle; ****p < 0.0001 for post-HCD exposure correct responses compared with vehicle; bars with the same letter (a or b) are not statistically different, while bars with different letters denote statistical difference
5-HT2CR effects on food consumption after HCD exposure
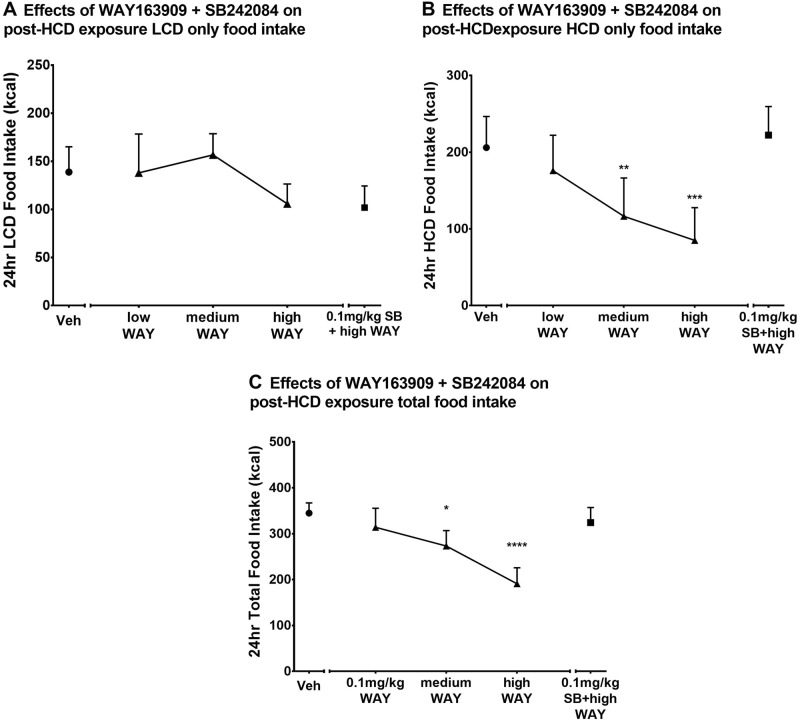
RM ANOVA revealed no change in 24-h LCD calorie intake (Fig. 5A) across any of the pretreatments tested [F(4,16) = 0.8625, p = 0.5073]. However, WAY163909 did have an effect on HCD only intake (Fig. 5B), with the medium and high doses decreasing HCD only 24-h food intake [F(4,16) = 12.63, p < 0.0001] compared to vehicle. SB242084 (0.1 mg/kg) again blocked this effect. WAY163909 also had an effect on total food intake (Fig. 5C), with the medium and high WAY163909 doses decreasing total 24-h food intake [F(4,16) = 12.14, p < 0.0001] compared to vehicle.
Fig. 5.
Effects of combined pretreatment with WAY163909 and SB242084 on food intake after HCD exposure. Subjects in the HCD group (N = 5) were given access to both a LCD and a palatable HCD for 6 months. Immediately after the 6-month period, a dose-effect curve for WAY163909 (low, medium, and high doses) was established for the HCD group for 24-h a LCD only food intake, b HCD only food intake, and c total food intake. Intake was also measured when a high dose of WAY163909 + SB242084 dose (0.1 mg/kg) combination pretreatment was given. The data are presented as the group mean ± SEM for each experimental condition. *p < 0.05 for post-HCD exposure 24 h total food intake compared with vehicle; **p < 0.01 for post-HCD exposure 24 h HCD food intake compared with vehicle; ***p < 0.001 for post-HCD exposure 24 h HCD food intake compared to vehicle; ****p < 0.0001 for post-HCD exposure 24 h total food intake compared with vehicle
Discussion
The present study examined the effects of long-term HCD or METH intake and the 5-HT2CR on perseverative behavior and food intake. Extended METH or HCD intake increased perseveration in all subjects. WAY163909 decreased perseveration at baseline and following this extended period of HCD or METH intake. WAY163909 also reduced HCD, but not LCD, intake. SB242084 blocked the effects of WAY163909 on reversal learning and HCD intake following 6 months in the dietary choice condition.
Reversal learning after METH or HCD exposure
Long-term intake of METH or a HCD increased perseverative behavior and this increase appears to be specific, as no effect was seen in other DRL task measures, including correct responses and random errors. The increase in perseveration was likely not driven by a general disruption of learning, or increased training during acquisition, because discrimination acquisition was not affected by the HCD or METH intake period. Reversal learning performance at baseline in this study was poor compared to what others have noted in monkeys [41]. This may be due to the use of forced corrections, which are not commonly employed.
Effects of WAY163909 on reversal learning
WAY163909 decreased perseverative errors, but not random errors, at baseline and following long-term METH or HCD intake, suggesting the effects of WAY163909 on perseveration were specific. Acquisition latency was not affected, indicating that the decrease in perseveration produced by WAY163909 was not due to a decrease in object discrimination exposure or training. These results agree with previous findings that Ro 60-0175 [36] and CP 809.101 [37] decreased perseverative responding, but conflict with another study reporting that the SB242084 had the same effect [38]. Differences in the compounds used, the specifics of the task, and model organisms may account for these disparate results. WAY163909 improved reversal learning performance by increasing reversal correct responses at baseline and after prolonged HCD or METH intake. These results suggest that WAY163909 improves flexible decision-making within the context of changing reinforcement contingencies, regardless of food or drug intake history, by both increasing newly reinforced responses and decreasing responses that are no longer correct.
Effects of WAY163909 on food intake
WAY163909 had no effect on baseline consumption of a LCD. Under the dietary choice condition, WAY163909 reduced total caloric intake by attenuating HCD, but not LCD, intake. Thus, 5-HT2CR modulation of food intake appears to be diet-specific, suggesting that these effects are not due to general decreases in motor function or appetitive behavior, but are specific to highly reinforcing foods [8, 9]. In agreement with the findings reported here, previous studies using the less selective 5-HT2CR agonist lorcaserin found that this compound reduced intake of a high fat diet and body weight in rats [46] and humans [47].
Agonists at the 5-HT2CR exhibit efficacy at decreasing intake of drugs and foods that have known effects on reward circuitry. The 5-HT2CR agonist Ro 60-0175 reduced cocaine intake in squirrel monkeys [19], and lorcaserin reduced nicotine [25] and alcohol [26] intake in rats. In a previous study, the same doses of WAY163909 used here reduced drug intake and drug seeking of METH and cocaine [21] in rhesus monkeys. One study reported that 7-day treatment with lorcaserin, which is less selective for the 5-HT2CR and exhibits more 5-HT2AR agonist activity than WAY163909 [40], did not attenuate cocaine vs food choice [48]. This study evaluated only male rhesus monkeys; whereas, the study using WAY163909 [21] used males and females. Moreover, the drug self-administration paradigms and actual compounds used were different, which may also account for the opposing results. Overall, these studies suggest that the effects of 5-HT2C agonists may be generalizable to behaviors that show characteristics of addiction given their impact on the reward system.
The same doses of WAY163909 decreased HCD intake and perseverative responding, and the same dose of SB242084 blocked the effects of WAY163909 on both behaviors. Similarly, the same doses of WAY163909 that reduced perseverative behavior in the current study attenuated METH intake and drug seeking of METH [21]. These data suggest that the reduction in HCD or METH intake [21] induced by activation of 5-HT2CRs is accompanied by a parallel decrease in perseverative behavior as assessed by the DRL task. It is unknown at this time how activation of 5-HT2CRs affects other domains of decision-making. However, activation of 5-HT2CRs may be simultaneously modulating both perseverative behavior and HCD or METH intake through related neurochemical mechanisms.
Effects of SB242084 + WAY163909 on reversal learning after METH or HCD exposure, and food intake after HCD exposure
The present findings suggest that the 5-HT2CR is necessary for the effects of WAY163909, as SB242084 blocked them. WAY163909 increased correct responses, and decreased perseverative responding and HCD intake, and these effects were attenuated when SB242084 was given in combination with WAY163909. These results conflict with a previous study showing that SB242084 decreases perseveration [38] in rodents, but they align with other findings demonstrating that 5-HT2C receptor agonists, rather than antagonists, decrease perseverative responding [36, 37]. The present results also agree with studies in rhesus monkeys showing that SB242084 blocks the decreases in cocaine self-administration induced by 5-HT2CR agonists [19] and can itself recapitulate some of the abuse-related effects of psychostimulants [20].
Limitations and future directions
There were two significant limitations to this study. First, the effects of the 5-HT2CR antagonist alone on reversal performance were not evaluated. This would have provided important within-subjects information and clarified the previously discussed inconsistent effects of the 5-HT2CR on reversal learning. Additionally, it would have been useful to show that other receptor antagonists would not reverse the effects WAY163909. Future studies could greatly clarify and improve upon the findings reported here by addressing these limitations in a separate experiment.
Acknowledgments
Funding
This work was supported by the National Institutes of Health grants DA10344 (L.L.H.), DA31246 (L.L.H.), DK096983 (M.E.W.), and ODP51011132 (YNPRC).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wickizer TM. State-level estimates of the economic costs of alcohol and drug abuse. J Health Care Financ. 2013;39:71–84. [PubMed] [Google Scholar]
- 2.Black DW. Compulsive buying disorder: a review of the evidence. CNS Spectr. 2007;12:124–32. doi: 10.1017/S1092852900020630. [DOI] [PubMed] [Google Scholar]
- 3.Blum K, Werner T, Carnes S, Carnes P, Bowirrat A, Giordano J. Sex, drugs, and rock’n’ roll: hypothesizing common mesolimbic activation as a function of reward gene polymorphisms. J Psychoact Drugs. 2012;44:38–55. doi: 10.1080/02791072.2012.662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neural circuits in addiction. Curr Opin Neurobiol. 2013;23:639–48. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richter L, Foster SE. Effectively addressing addiction requires changing the language of addiction. J Public Health Policy. 2014;35:60–64. doi: 10.1057/jphp.2013.44. [DOI] [PubMed] [Google Scholar]
- 6.Shaffer HJ, LaPlante DA, LaBrie RA, Kidman RC, Donato AN, Stanton MV. Toward a syndrome model of addiction: Multiple expressions, common etiology. Harv Rev Psychiatry. 2004;12:367–74. doi: 10.1080/10673220490905705. [DOI] [PubMed] [Google Scholar]
- 7.Veeneman MMJ, Broekhoven MH, Damsteegt R, Vanderschuren LJMJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37:487–98. doi: 10.1038/npp.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norgren R, Hajnal A, Mungarndee SS. Gustatory reward and the nucleus accumbens. Physiol Behav. 2006;89:531–5. doi: 10.1016/j.physbeh.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–15. doi: 10.1016/S1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 10.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–42. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–65. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- 14.Howell LL, Negus SS. Monoamine transporter inhibitors and substrates as treatments for stimulant abuse. Adv Pharmacol. 2014;69:129–76. doi: 10.1016/B978-0-12-420118-7.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76:460–78. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–46. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- 17.Clemett DA, Punhani T, Duxon MS, Blackburn TP, Fone KC. Immunohistochemical localization of the 5-HT2C receptor protein in the rat CNS. Neuropharmacology. 2000;39:123–32. doi: 10.1016/S0028-3908(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin 2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–88. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manvich DF, Kimmel HL, Cooper DA, Howell LL. Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2012;341:424–34. doi: 10.1124/jpet.111.186981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manvich DF, Kimmel HL, Cooper DA, Howell LL. The serotonin 2C receptor antagonist SB242084 exhibits abuse-related effects typical of stimulants in squirrel monkeys. J Pharmacol Exp Ther. 2012;342:761–9. doi: 10.1124/jpet.112.195156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berro* LF, Perez Diaz* M, Maltbie E, Howell LL. Effects of the serotonin 2C receptor agonist WAY163909 on the abuse-related effects and mesolimbic dopamine neurochemistry induced by abused stimulants in Rhesus monkeys. Psychopharmacology. 2017 doi: 10.1007/s00213-017-4653-2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdallah L, Bonasera SJ, Hopf FW, O’Dell L, Giorgetti M, Jongsma M, et al. Impact of serotonin 2C receptor null mutation on physiology and behavior associated with nigrostriatal dopamine pathway function. J Neurosci. 2009;29:8156–65. doi: 10.1523/JNEUROSCI.3905-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hess R, Cross LB. The safety and efficacy of lorcaserin in the management of obesity. Postgrad Med. 2013;125:62–72. doi: 10.3810/pgm.2013.11.2713. [DOI] [PubMed] [Google Scholar]
- 24.Hoy SM. Lorcaserin: a review of its use in chronic weight management. Drugs. 2013;73:463–73. doi: 10.1007/s40265-013-0035-1. [DOI] [PubMed] [Google Scholar]
- 25.Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, et al. Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther. 2011;338:890–6. doi: 10.1124/jpet.111.183525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rezvani AH, Cauley MC, Levin ED. Lorcaserin, a selective 5-HT(2C) receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacol Biochem Behav. 2014;125:8–14. doi: 10.1016/j.pbb.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Serrano MJ, Perales JC, Moreno-Lopez L, Perez-Garcia M, Verdejo-Garcia A. Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology. 2011;219:673–83. doi: 10.1007/s00213-011-2485-z. [DOI] [PubMed] [Google Scholar]
- 28.Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology. 2012;219:469–90. doi: 10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 30.Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 31.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–23. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lloyd SA, Oltean C, Pass H, Phillips B, Staton K, Robertson CL, et al. Prenatal exposure to psychostimulants increases impulsivity, compulsivity, and motivation for rewards in adult mice. Physiol Behav. 2013;119:43–51. doi: 10.1016/j.physbeh.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Son JH, Kuhn J, Keefe KA. Perseverative behavior in rats with methamphetamine-induced neurotoxicity. Neuropharmacology. 2013;67:95–103. doi: 10.1016/j.neuropharm.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–80. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 35.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 36.Agnoli L, Carli M. Dorsal-striatal 5-HT2A and 5-HT2C receptors control impulsivity and perseverative responding in the 5-choice serial reaction time task. Psychopharmacology. 2012;219:633–45. doi: 10.1007/s00213-011-2581-0. [DOI] [PubMed] [Google Scholar]
- 37.Del’Guidice T, Lemay F, Lemasson M, Levasseur-Moreau J, Manta S, Etievant A, et al. Stimulation of 5-HT2C receptors improves cognitive deficits induced by human tryptophan hydroxylase 2 loss of function mutation. Neuropsychopharmacology. 2014;39:1125–34. doi: 10.1038/npp.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. J Neurosci. 2010;30:930–8. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm Behav. 2012;62:389–99. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev. 2015;67:176–97. doi: 10.1124/pr.114.009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izquierdo AD, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology. 2011;219:607–20. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- 43.Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012;37:1479–90. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Fleur SE, Luijendijk MCM, van der Zwaal EM, Brans MAD, Adan RAH. The snacking rat as a model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int J Obes. 2014;38:643–9. doi: 10.1038/ijo.2013.159. [DOI] [PubMed] [Google Scholar]
- 45.Rolls BJ, Van Duijvenvoorde PM, Rowe EA. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol Behav. 1983;31:21–27. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–87. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- 47.Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, et al. Lorcaserin, a 5-HT2C receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab. 2011;96:837–45. doi: 10.1210/jc.2010-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banks ML, Negus SS. Repeated 7-day treatment with the 5-HT2C agonist lorcaserin or the 5-HT2A antagonist pimavanserin alone or in combination fails to reduce cocaine vs food choice in male rhesus monkeys. Neuropsychopharmacology. 2017;42:1082–92. doi: 10.1038/npp.2016.259. [DOI] [PMC free article] [PubMed] [Google Scholar]