Abstract
Dopamine D2 receptor occupancy (D2RO) is a key feature of all currently approved antipsychotic medications. However, antipsychotic efficacy associated with high D2RO is often limited by side effects such as motor disturbances and hyperprolactinemia. Lumateperone (ITI-007) is a first-in-class selective and simultaneous modulator of serotonin, dopamine and glutamate in development for the treatment of schizophrenia and other disorders. The primary objective of the present study was to determine D2RO at plasma steady state of 60 mg ITI-007, a dose that previously demonstrated antipsychotic efficacy in a controlled trial, administered orally open-label once daily in the morning for two weeks in patients with schizophrenia (N = 10) and after at least a two-week washout period from standard of care antipsychotics. D2RO was determined using positron emission tomography with 11C-raclopride as the radiotracer. Mean peak dorsal striatal D2RO was 39% at 60 mg ITI-007 occurring 1 h post-dose. Lumateperone was well-tolerated with a favorable safety profile in this study. There were no clinically significant changes in vital signs, ECGs, or clinical chemistry laboratory values, including prolactin levels. There were no adverse event reports of akathisia or other extrapyramidal motor side effects; mean scores on motor function scales indicated no motor disturbances with lumateperone treatment. This level of occupancy is lower than most other antipsychotic drugs at their efficacious doses and likely contributes to the favorable safety and tolerability profile of lumateperone with reduced risk for movement disorders and hyperprolactinemia. If approved, lumateperone may provide a new and safe treatment option for individuals living with schizophrenia.
Subject terms: Schizophrenia, Predictive markers, Drug development, Translational research
Introduction
All antipsychotics approved to date in the USA for the treatment of schizophrenia, have to a varying degree, dopamine D2 receptor occupancy (D2RO) as a key feature of their pharmacology. It has been suggested that D2RO is required for antipsychotic efficacy [1]. Different antipsychotics exhibit different threshold levels of D2RO at efficacious doses. Most antipsychotics are both pre-synaptic and post-synaptic dopamine D2 receptor antagonists and have shown antipsychotic efficacy associated with about 65–80% striatal D2RO [2–5]. The dopamine receptor pre- and postsynaptic partial agonist, aripiprazole, is associated with relatively higher (>85%) D2RO at doses that achieve antipsychotic efficacy [6–8]. However, antipsychotic efficacy by high D2RO drugs is often limited by side effects associated with D2RO. High (>80%) D2RO is commonly associated with motor disturbances such as extrapyramidal side effects (EPS), including akathisia [3, 9]. The threshold for D2RO associated with hyperprolactinemia, is relatively lower, in the range of about 50–73%, and largely associated with D2 receptor antagonism [8, 10, 11]. At therapeutic doses in patients with schizophrenia, clozapine, arguably the most effective antipsychotic, is an exception with relatively low D2RO ≤ 60% [12, 13].
Lumateperone, also known as ITI-007 [14], is a first-in-class selective and simultaneous modulator of serotonin, dopamine and glutamate, three neurotransmitters implicated in serious mental illness. As a dopamine receptor phosphoprotein modulator (DPPM), ITI-007 acts as a presynaptic partial agonist and postsynaptic antagonist at dopamine D2 receptors [15]. This novel action allows for reduced presynaptic release of dopamine and postsynaptic blockade of dopamine for more efficient reduction of dopaminergic signaling than most antipsychotic drugs [15]. ITI-007 also benefits from potent serotonin 5-HT2A receptor antagonism, serotonin transporter (SERT) inhibition, and increased phosphorylation of glutamatergic N-methyl-d-aspartate (NMDA) GluN2B receptors likely downstream of dopamine D1 receptor activation in mesolimbic brain regions [15]. Together, this unique pharmacological profile predicts more efficient dopamine modulation with antipsychotic efficacy at relatively low levels of D2RO.
In a positron emission tomography (PET) study in healthy volunteers, ITI-007 was safe and well tolerated and rapidly entered the brain with long-lasting and dose-related D2RO [16]. At relatively low doses, ITI-007 (10 mg single oral dose) demonstrated high occupancy (>80%) of cortical 5-HT2A receptors and low occupancy of striatal D2 receptors (~12%). D2RO increased with dose and significantly correlated with plasma concentration. At a higher but still moderate dose in healthy volunteers, ITI-007 (40 mg single oral dose) resulted in a mean of 29% D2RO and maximal peak occupancy up to 39% D2RO and up to 33% occupancy of serotonin transporters. Based on these data, the dose projected to achieve a maximal peak occupancy ~50% D2RO was 60 mg ITI-007.
ITI-007 is in late phase clinical development for the treatment of schizophrenia and other psychiatric disorders. A double-blind, placebo-controlled and active-controlled clinical trial met the primary endpoint and demonstrated efficacy for the treatment of schizophrenia with statistically significant superiority over placebo as measured by the Positive and Negative Syndrome Scale total score [17]. A dose of 60 mg ITI-007 demonstrated antipsychotic efficacy in patients with acute schizophrenia without motor side effects, without hyperprolactinemia, and with a favorable metabolic and cardiovascular profile [17].
Because it is rare for a drug to achieve antipsychotic efficacy at D2RO levels lower than 60% and because receptor occupancy in healthy normal brain may or may not accurately predict receptor occupancy in patients with schizophrenia whose brain disease or history of medication may alter dopamine receptors, the primary objective of the present study was to determine the D2RO at plasma steady state of 60 mg ITI-007 (orally once daily in the morning) in patients with schizophrenia (N = 10) and after at least a two-week washout period from their standard of care antipsychotic medications. The study design is shown in Table 1.
Table 1.
Study design and treatment assignment
| [C11]-Raclopride | ||||
|---|---|---|---|---|
| Subject demographics: age (years) and sex/race | ITI-007 dose | Start time of post-dose scan(s) | ||
| 46 M/B, 26 M/B, 36 M/B, 42 M/B, 33 M/B, 57 F/B | 60 mg | 1 h | ||
| 36 M/B, 48 M/AS, 31 M/B, 38 M/B | 60 mg | 3 h | 7.5 h | ~24–27 h |
Six subjects received a baseline scan with [C11]-raclopride followed by treatment with 60 mg ITI-007 for approximately 2 weeks and a post-dose scan that started 1 h after the last dose of ITI-007. Four subjects received a baseline scan with [C11]-raclopride followed by treatment with 60 mg ITI-007 for approximately 2 weeks and three post-dose scans that started approximately 3, 7.5, and 24–27 h after the last dose of ITI-007
AS Asian, B Black/African American, M male, F female
Patients and methods
Patients
To be eligible for participation, 18–60-year-old females (post-menopausal or surgically sterile) or males with a clinical diagnosis that met the Diagnostic and Statistical Manual of Mental Disorders, Version IV-TR criteria for schizophrenia were required to be in clinical remission, free from acute exacerbation of their psychosis as operationally defined by no hospitalization for at least 3 months before screening. Participating subjects must have had a Clinical Global Impression scale for Severity of illness (CGI-S; [18]) score less than or equal to 4 (moderately ill), been in generally good health with a body mass index of 19–36 kg/m2 and a minimum weight of 60 kg. Participating subjects were required to have voluntarily stopped their antipsychotic and other psychotropic medication, and remained antipsychotic-free for at least 14 days during inpatient supervision prior to the first dose of ITI-007. Lorazepam was allowed as needed to reduce anxiety, agitation, or insomnia during the course of the study, with no more than 6 mg/day allowed during the screening and washout period and not more than 4 mg/day on 4 days/week during the treatment period. Patients were excluded from the study if they had previous exposure to ITI-007, a sensitivity or likely allergy to psychotropic drugs, recent exposure to ionizing radiation, recently donated blood, or had a medical condition considered in appropriate for safe participation. In order to ensure a drug-free baseline assessment, subjects were excluded if they received antipsychotic or psychotropic medication within 14 days before the first dose or if they received any recent long-acting psychotropic medications (e.g., aripiprazole) within 2 months before Day −15. Recent ingestion of any investigational product or any potent cytochrome P450 3A4 inducer or inhibitor were also excluded. Regular consumption of excessive xanthine-containing products or alcohol were excluded. Additionally, subjects were excluded if they could not provide written informed consent or for any reason were not considered appropriate for the study by the investigator.
This study was conducted at a single recruiting clinical center, CBH Health, and at a single PET imaging site, the Johns Hopkins University (JHU) School of Medicine/Johns Hopkins Hospital (JHH). The protocol and study-related documents were approved by Institutional Review Boards (IRBs) for each institution (Copernicus Group IRB, Durham, NC, USA and JHU School of Medicine: Office of Human Subjects Research, Baltimore, MD, USA). The clinical trial was registered on clinicaltrials.gov: NCT02288845.
Study design
In this open-label study, patients who met inclusion criteria were admitted to the inpatient clinic at CBH Health. Before the first PET scan, patients were fitted with a thermoplastic facemask to facilitate maintaining position of the head for all scans and received a magnetic resonance image (MRI) scan (3D MPRAGE) of the head to identify anatomy of the brain for co-registration with subsequent PET images. After a two-week inpatient drug-free washout period, patients were escorted to JHU/JHH for baseline scan(s). Up to four PET scans were performed in each subject. The exact number of PET scans performed in each subject are shown in Table 1. Baseline scans and baseline safety measures were conducted on Study Day −2 or −1 (two days or one day before the first dose of ITI-007, respectively), to allow for scanner scheduling and patient tolerability of the procedures. After the baseline scan, patients returned to CBH Health to continue their inpatient stay and received lumateperone (60 mg ITI-007 or lumateperone tosylate; equivalent to 42 mg lumateperone, the free base or active moiety) orally once daily as a formulated capsule with food in the morning for two weeks. On Study Days 12–15 (to allow for scanner scheduling), patients were escorted to JHU/JHH again for post-dose scans where patients could have up to 3 post-dose scans for time-course determination. A pharmacokinetic (PK) sample was collected before the start of each post-dose PET scan as close to the start time of the scan as feasible to measure the actual drug plasma level at the time of each scan. Following the last post-dose scan, patients were discontinued from ITI-007 and stabilized on standard-of-care antipsychotic medication for up to 4 days before discharge from the inpatient unit.
For each PK sample, approximately 7 mL blood was collected into polypropylene tubes containing sodium heparin. Plasma was extracted and stored at −70 °C until analysis using HPLC/tandem mass spectrometry (HPLC/MS/MS) bioanalytical assays. The assay was validated at a level of detection of 0.05–50 ng/mL for ITI-007 (parent molecule) and 0.2–100 ng/mL for two active metabolites, IC200131 and IC200161. In vitro receptor binding affinities (Ki) to 5-HT2A receptors are 0.54, 2, and 61 nM and to D2 receptors are 32, 30, and 574 nM, respectively for ITI-007, IC200161, and IC200131 [19].
Safety was monitored throughout the trial and included physical examinations, vital signs, 12-lead electrocardiograms (ECGs), clinical laboratory tests, and adverse events. Measures of mental health included the PANSS [20], Calgary Depression Scale for Schizophrenia (CDSS; [21]) and the CGI-S [18]. Suicidal ideation/behavior was assessed using the Columbia Suicide Severity Rating Scale (C-SSRS; [22]). EPS were assessed using the Simpson Angus Scale (SAS; [23]) and akathisia was assessed using the Barnes Akathisia Rating Scale (BARS; [24]).
Radiopharmaceuticals and positron emission tomography
Carbon-11 radiolabeled compounds were synthesized at JHH Nuclear Medicine Radiochemistry PET Facility according to the Center’s Standard Operating Procedures and in compliance with United States Pharmacopoeia (USP) < 823 > , “radiopharmaceuticals for positron emission tomography: compounding”. Each patient received a new batch of radiotracer manufactured and administered just before each PET scan using the HRRT (high resolution research tomograph, Siemens). The injected activity of any radiopharmaceutical administered was approximately 20 mCi for each PET scan. Carbon-11-Raclopride ([11C]-RAC) is a selective dopamine D2/D3 receptor antagonist with a high affinity for imaging D2 dopamine receptors [25, 26]. The effective dose radiation exposure to patients was approximately 0.46 rem from [11C]-RAC from each PET scan. As per local guidelines, occupationally exposed individuals are permitted to receive effective dose exposures of up to 5 rem per year. For this study the cumulative effective dose exposure to each patient did not exceed 2 rem, with no more than 4 scans per patient. Each radiopharmaceutical administration was followed by a 90-min dynamic PET scan. The dynamic PET scans were acquired in full 3D list mode and reconstructed with high resolution span-3 modality for 30-frames over 90-min scan (4 × 15 s, 4 × 30 s, 3 × 60 s, 2 × 120 s, 5 × 240 s, 12 × 300 s). The OP-OSEM iterative algorithm using 16 subsets 6 iterations followed by 2 mm 3D Gaussian post-smoothing was used for PET image reconstruction. The attenuation, dead time, and decay corrections were applied for generation of quantitative dynamic images (image volume: 256 × 256 × 207; voxel size: 1.22 × 1.22 × 1.22 mm3) of the intrinsic spatial resolution of 2.5 mm full-width at half-maximum at the center of the field of view in x, y, and z directions.
Data analysis
The means of 90-min dynamic PET images were used for MRI to PET co-registration using Statistical Parametric Mapping software (SPM8, https://www.fil.ion.ucl.ac.uk/spm/). Regions of interest (ROIs) were manually drawn on the coregistered MRI for PET data with caudate and putamen used to calculate dorsal striatal D2RO, nucleus accumbens used to calculate ventral striatal D2RO, and cerebellar gray used as a reference region. PMOD (https://www.pmod.com/) was used for MRI-to-PET coregisteration and ROI drawing. A simplified reference tissue model (SRTM; [27]) was used to estimate BPND. Parametric maps of the BPND was generated by using the SRTM with a linear regression and spatial constraint algorithm [28]. ROI BPND value was obtained by applying ROIs to BPND images. Target occupancy was expressed as percent change in the ratio of bound and free components of each radiotracer obtained before and after ITI-007 administration. Occupancy(%) = 100 × (BPND(baseline)−BPND(blocking))/BPND(baseline).
Individual patient ITI-007 and active metabolite plasma concentration data were correlated with the individual levels of D2RO. Safety data were analyzed using descriptive statistics and shifts from baseline.
Results
Patient disposition is shown in the CONSORT diagram in Fig. 1. Ten patients received ITI-007 60 mg and all completed the study. Participating patients had a mean age of 39.3 years (standard deviation, SD, 9.1; range 26–57). Of the 10 patients, 9 (90%) were Black/African American and 1 (10%) was Asian, 9 (90%) were male and 1 (10%) was female. Patients were moderately ill, but stable, at baseline with a mean PANSS total score of 72.7 (±7.39 SD) and mean CGI-S score of 3.8 (±0.63). All of the patients had taken at least one prior antipsychotic medication before the washout period began, though dose and treatment duration varied. The most common antipsychotics taken were quetiapine (8 patients, 80%) and risperidone (3 subjects, 30%). Other prior antipsychotics included haloperidol, lurasidone, olanzapine, and paliperidone; some patients took more than one. No patient took an antipsychotic during the washout period or the study treatment period. The only concomitant psychotropic medication was lorazepam (9 patients, 90%).
Fig. 1.
CONSORT Diagram
The main outcome variables in this study were non-displaceable binding potential (BPND) for [11C]-RAC and D2RO in caudate and putamen (occupancy in the two regions were reported separately and then averaged to report mean dorsal striatal D2RO) after two weeks of treatment with 60 mg ITI-007 (N = 10). The range of baseline BPND in these subjects was 2.9–4.2. Based on previous studies, peak plasma levels (of the ITI-007 parent molecule) and peak brain occupancies were anticipated to occur 1–3 h post-dose, therefore the [11C]-RAC scans for the initial patients (N = 6) started 1 h post-dose on Study Day 12 or 15 (90 min scans lasted from 1 to 2.5 h post-dose). Additional patients (N = 4) received post-dose [11C]-RAC scans starting approximately 3–4, 7.5, and 24–27 h after the last dose of 60 mg ITI-007 (after two weeks of once daily administration), to investigate the time course of receptor occupancy. Data from individual patients in [11C]-RAC scans are shown in Table 2 and the time course is shown in Fig. 2.
Table 2.
Individual subject data for [11C]-RAC measuring striatal D2RO
| Subject age sex/race/prior AP | ITI-007 Dose (mg) | Scan start time (h)a | Baseline BPND | %D2RO | Plasma Level (ng/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caudate | Putamen | V-Str | Caudate | Putamen | D-Str mean | V-Str | Parent | Metabolite IC200131 | Metabolite IC200161 | Parent + Metabolites | |||
| 46 M/B/Ris, Que | 60 | 1 | 3.040 | 3.149 | 2.446 | 17.9 | 18 | 18 | 18.9 | 0.811b | 2.75 | 0.664 | 4.225 |
| 26 M/B/Que | 60 | 1 | 4.033 | 5.234 | 3.346 | 43.1 | 39.2 | 41 | 42.8 | 20.1 | 21.3 | 15.6 | 57 |
| 36 M/B/Hal, Que | 60 | 1 | 4.216 | 5.519 | 3.534 | 35.5 | 34 | 35 | 37.8 | 25.5 | 12.9 | 8.86 | 47.26 |
| 42 M/B/Pal | 60 | 1 | 3.399 | 4.556 | 2.190 | 49.8 | 47.5 | 49 | 25.0 | 9.34 | 19.9 | 36.3 | 65.54 |
| 33 M/B/Ola | 60 | 1 | 3.641 | 4.619 | 3.026 | 52.3 | 50 | 51 | 42.1 | 44.6 | 23.9 | 28.4 | 96.9 |
| 57 F/B/Que | 60 | 1 | 3.684 | 4.074 | 2.714 | 41.0 | 34.1 | 38 | 17.3 | 9.2 | 2.43 | 4.51 | 16.14 |
| 36 M/B/Lur, Que | 60 | 3 | 3.872 | 4.476 | 3.440 | 36.8 | 36.1 | 36 | 36.4 | 20.4 | 33.2 | 17.0 | 70.6 |
| 48 M/AS/Ris, Que | 60 | 3 | 2.939 | 3.419 | 2.909 | 24.1 | 21.5 | 23 | 23.9 | 9.96 | 19.3 | 6.98 | 36.24 |
| 31 M/B/Que | 60 | 3 | 3.965 | 4.600 | 3.595 | 35.7 | 36 | 36 | 38.2 | 16.6 | 18.3 | 5.59 | 40.49 |
| 38 M/B/Ris, Que | 60 | 3 | 3.079 | 3.653 | 3.229 | 41.8 | 40.3 | 41 | 42.0 | 12.8 | 24.3 | 21.1 | 58.2 |
| 36 M/B/Lur, Que | 60 | 7.5 | 3.872 | 4.476 | 3.440 | 16.0 | 16.1 | 16 | 14.0 | 3.23 | 19.1 | 2.87 | 25.2 |
| 48 M/AS/Ris, Que | 60 | 7.5 | 2.939 | 3.419 | 2.909 | 12.8 | 10.8 | 12 | 14.4 | 2.46 | 11.0 | 1.08 | 14.54 |
| 31 M/B/Que | 60 | 7.5 | 3.965 | 4.600 | 3.595 | 13.8 | 16 | 15 | 19.9 | 2.69 | 10.8 | 0.744 | 14.234 |
| 38 M/B/Ris, Que | 60 | 7.5 | 3.079 | 3.653 | 3.229 | 36.0 | 31.2 | 34 | 34.5 | NA | – | – | – |
| 36 M/B/Lur, Que | 60 | 24–27 | 3.872 | 4.476 | 3.440 | 4.7 | 5.9 | 5 | −0.5 | <0.500 | 4.73 | <0.500 | 4.73 |
| 48 M/AS/Ris, Que | 60 | 24–27 | 2.939 | 3.419 | 2.909 | 0.5 | –3.6 | −2 | 1.7 | <0.500 | 1.42 | <0.500 | 1.42 |
| 31 M/B/Que | 60 | 24–27 | 3.965 | 4.600 | 3.595 | 9.6 | 12.5 | 11 | 15.1 | <0.500 | 3.37 | <0.500 | 3.37 |
| 38 M/B/Ris, Que | 60 | 24–27 | 3.079 | 3.653 | 3.229 | 12.9 | 14.7 | 14 | 17.8 | <0.500 | 3.59 | <0.500 | 3.59 |
Striatal D2RO and associated steady state plasma levels are shown for individual subjects treated with ITI-007 once daily for two weeks. Subject demographics and prior antipsychotics (AP) taken before washout are provided; prior antipsychotics were washed out at least 14 days before baseline
[11C]-RAC Raclopride radiopharmaceutical, AP antipsychotic, AS Asian, B Black/African American, BPND nondisplaceable binding potential, D2RO dopamine D2-receptor occupancy, F female, h hour, Hal haloperidol, Lur lurasidone, M male, NA not applicable, Ola olanzapine, Pal paliperidone, PET positron emission tomography, Que quetiapine, Ris risperidone, V Str ventral striatum
aThese values are the targeted postdose scan start times. The actual postdose scan start time for each subject may have varied
bSteady state plasma levels measured around the time of expected maximal concentrations (1–3 h post-dose) of less than 1 ng/mL of parent indicate poor exposure; therefore, data should be interpreted with caution
Fig. 2.
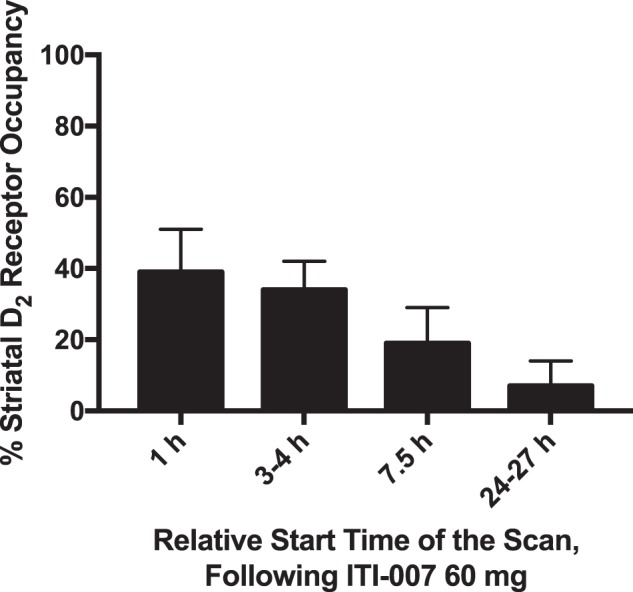
Striatal D2 receptor occupancy time course following the last dose of ITI-007 at plasma steady state. Percent occupancy is shown as a function of the relative start time of the PET scan following ITI-007 60 mg oral administration. Mean and standard deviation are shown
Peak dorsal striatal D2RO at 60 mg ITI-007 occurred 1 h post-dose with a mean of 39% (±12% SD; 39% median; N = 6). The PK sample collected from one patient (46 M) just before the start of the post-dose scan indicated low exposure to ITI-007 (<1 ng/mL of ITI-007 parent), suggesting a trough plasma level, rather than peak, so that drug may not have been ingested or absorbed the day of the scan in this patient. Correspondingly, this patient also had the lowest D2RO. To be sure that data interpretations were not confounded by a low outlier, the data were reanalyzed without patient 46 M. This re-analysis confirmed the initial analysis result with mean D2RO approximately 40% with 60 mg ITI-007, but, as would be expected, with less variability (43 ± 7%; 41% median; N = 5). The time course data showed progressively decreasing mean D2RO of 34% (±8%; 36% median) with scans starting 3–4 h post-dose, 19% (±10%; 15% median) at 7.5 h post-dose, and 7% (±7%; 8% median) at 24–27 h post-dose (N = 4).
The highest plasma levels associated with 60 mg ITI-007 occurred at 1–3 h post-dose (Table 2). The plasma levels of the parent and IC200161 were slightly higher at 1 h (N = 6, 18.3 ng/mL for parent, 15.8 ng/mL for IC200161) than at 3 h (N = 4, 14.9 ng/mL for parent, 12.7 ng/mL for IC200161), whereas the plasma level of IC200131 was higher at 3 h (23.8 ng/mL) than at 1 h (13.9 ng/mL). The total plasma level (parent plus the two active metabolites, IC200161 and IC200131) was similar at 1 h and 3 h post-dose with a mean of 47.8 ng/mL at 1 h and a mean of 51.4 ng/mL at 3 h. Combining the data from the ten subjects evaluated with 60 mg ITI-007 at 1 h or 3 h, the mean D2RO was 37% and was associated with a mean total plasma level of 49.3 ng/mL (parent plus the two active metabolites, IC200161 and IC200131) with a statistically significant correlation between D2RO and total plasma level (r2 = 0.6; p = 0.0077). If the data from the time course assessment at 7.5 and 24–27 h are added to the analysis, the occupancy and the plasma levels decrease with time and the correlation between D2RO and total plasma level is even more robust (r2 = 0.77; p < 0.001), though the within-subject design at the 3, 7.5, and 24–27 h time points should be noted as these are not independent measures. When subject 46 M with lower than expected plasma levels and low occupancy was excluded from the correlation analysis, the correlation remained high (r2 = 0.78; p < 0.0001).
Ventral striatal D2RO was also determined using the high resolution of the HRRT PET, and the values are shown in Table 2. Ventral striatal D2RO values were similar to dorsal striatal D2RO values in individual subjects, except when ventral striatal BPND baseline levels were low (<3), which resulted in relatively lower occupancy. In the three subjects with BPND values greater than 3, mean ventral striatal D2RO was 40.9% (Table 2).
ITI-007 was well-tolerated with a favorable safety profile in this study. There were no clinically significant changes in vital signs, ECGs, or clinical chemistry laboratory values, including no elevation of prolactin. The most frequent adverse events (occurring in more than two patients) that were reported to be at least possibly related to ITI-007 were mild to moderate headache (4/10, 40%) and mild sedation (4/10, 40%). There were no adverse event reports of akathisia or other EPS; mean scores of motor function as measured by BARS and SAS indicated no motor disturbances with ITI-007 treatment. There were no adverse event reports of agitation or induction of mania. There was no increase in suicidal ideation or behavior as measured by the C-SSRS. Mean levels of depression as measured by CDSS were low at baseline and remained low at Day 11. The stability of symptoms of psychosis was maintained throughout the ITI-007 treatment period as demonstrated by a mean PANSS total score of 72.7 (±7.39 SD) at baseline and 73.2 (±9.93) at Day 11 (tested before the scheduled study days with PET scans) and corresponding mean CGI-S score were 3.8 (±0.63) at baseline and 3.9 (±0.57) at Day 11. There was individual variability in symptoms of schizophrenia, however, and even within the relatively narrow range of D2RO up to 51% and treatment duration of only approximately 2 weeks with ITI-007, a post-hoc analysis revealed a statistically significant negative correlation (r2 = 0.4987, p = 0.0225) of D2RO for 60 mg ITI-007 with mean change from baseline in the PANSS total score such that higher occupancy was associated with larger decreases or improvements in schizophrenia symptoms. The patient with the highest D2RO of 51% experienced the greatest improvement from baseline in schizophrenia symptoms as reflected by a decrease of 11 points in the PANSS total score.
Discussion
The present study demonstrates mean peak D2RO of about 40% at 60 mg ITI-007. Efficacy at this dose was previously demonstrated in acute exacerbated schizophrenia [17]. This level of occupancy is lower than most other atypical antipsychotic drugs at their efficacious doses (see Table 3) and likely contributes to the favorable safety and tolerability profile of ITI-007 with placebo-like levels of EPS, including akathisia, and blood prolactin levels [17]. In the present study conducted in patients with stable schizophrenia at baseline, symptoms of schizophrenia remained stable on the average with short-term (approximately 2 weeks) administration of ITI-007. However, within the limited range of occupancy observed in the present study up to approximately 50%, there was a significant correlation observed with greater levels of D2RO associated with greater improvement in schizophrenia symptoms.
Table 3.
Mean D2RO in Caudate & Putamen at antipsychotic oral doses as measured by displacement of [11C]-raclopride
| Drug | Dose range | Mean D2RO in Caudate and Putamen | Reference |
|---|---|---|---|
| ITI-007 | 60 mg/day | ~ 40% | Present results |
| Clozapine | 75–900 mg/day | 48–61% | Farde et al. [2]; Kapur et al. [39]; Tauscher et al. [31] |
| Quetiapine | 150–750 mg/day | 30–62% | Gefvert et al. [30]; Kapur et al. [32]; Tauscher et al. [31]; Tauscher-Wisniewski et al. [33] |
| Ziprasidone | 40–160 mg/day | 56 to >59% | Mamo et al. [43]; Vernaleken et al. [44] |
| Risperidone | 4 mg/day | 72–81% | Kapur et al. [38, 39]; Nyberg et al. [3]; Tauscher et al. [31] |
| Olanzapine | 5–60 mg/day | 61–80% | Nyberg et al. [45]; Kapur et al. [39, 46]; Tauscher et al. [31] |
| Lurasidone | 40–80 mg | >65% | Wong et al. [5] |
| Cariprazine | 1.5–3 mg/day | 69 to >90% | Reviewed by Citrome [42] |
| Aripiprazole | 10–30 mg | 88–90% | Yokoi et al. [6]; Mamo et al. [7] |
The present data in patients with schizophrenia after two weeks of treatment with ITI-007 extend previous findings of dose-related D2RO after a single dose across a lower dose range of ITI-007 measured in healthy volunteers [16]. More specifically, the previously observed mean D2RO of 12, 19, 27, and 29% at ITI-007 doses of 10, 20, 30, and 40 mg, respectively, fit along the same occupancy curve with 40% at 60 mg even though the current study evaluated subchronic dosing and the previous study evaluated acute doses. Based on plasma concentration occupancy curves from the previous study, it was estimated that a dose of 60 mg would have approximately 50% D2RO, which was found at the high end of the range in the present study. These data are consistent with linear kinetics combined with the observed negligible trough occupancy and blood drug levels. It should be noted, however, that there is a possibility that receptor upregulation may occur during chronic dosing resulting in slightly lower observed occupancy than with acute dosing at 60 mg ITI-007. The potential for receptor upregulation is a technical issue for all chronic occupancy studies, although there is not likely to be a major upregulation in patients already chronically treated with antipsychotics. Slight differences in results between the healthy volunteer study and the present study may also be due to individual variability with overlap in plasma concentrations with doses of 40 mg evaluated previously and 60 mg ITI-007 evaluated in the present study. There appeared to be no systematic differences in occupancy due to prior antipsychotic medication, in terms of specific medication, dose or treatment duration.
The majority of antipsychotic drugs exhibit efficacy at relatively high D2RO (>60%) and it is rare to have antipsychotic efficacy with relatively low D2RO (<50%), as is the case with clozapine, demonstrated with as low as 40% D2RO at 300 mg/day [29]. Although initial reports with quetiapine suggested relatively low occupancy [30, 31], transiently high D2RO up to 62% has been reported with quetiapine [32, 33]. In the present study, oral ITI-007 showed D2RO that peaked at 1 h post-dose and returned to low, but measurable trough levels within 24 h of oral dose administration. Taken together with the previously reported clinical data, these data suggest that sustained high levels of D2RO are not required to achieve or maintain antipsychotic efficacy with ITI-007.
The importance of achieving antipsychotic efficacy with reduced D2RO is the reduced safety risk for induction of EPS, including akathisia, and the reduced risk for hyperprolactinemia. Clozapine treatment is associated with a relatively low risk of hyperprolactinemia and motor side effects, though clozapine use is associated with other safety issues unrelated to D2 receptor occupancy. The addition of haloperidol, a potent and relatively selective D2 receptor antagonist, to a clozapine treatment regimen increased D2RO, prolactin levels, and caused the emergence of akathisia and other EPS [34], consistent with high D2RO modulating these adverse effects. Potent 5-HT2A receptor antagonism may also contribute to the enhanced antipsychotic efficacy of clozapine while reducing motoric side effects and risk for hyperprolactinemia [35]. Of interest, ITI-007 has a wider separation (60-fold) between its affinity for 5-HT2A receptors and D2 receptors than other antipsychotic drugs, including clozapine, gaining the full benefit of 5-HT2A receptor occupancy at relatively low levels of D2RO [15]. Additionally, in preclinical models, unlike many antipsychotics, ITI-007 did not increase the phosphorylation of the serine 40 residue on striatal tyrosine hydroxylase, a site essential for dopamine biosynthesis, and did not increase striatal dopamine turnover [15]. In contrast, ITI-007 preferentially increased dopamine efflux in the medial prefrontal cortex compared with the striatum. Pre-synaptic partial agonism and post-synaptic antagonism at D2 receptors together with mesolimbic/mesocortical functional selectivity may provide a unique approach to the regulation of dopamine neurotransmission. Whereas low receptor occupancy with rapid dissociation is a possible alternative explanation for the lack of effects of ITI-007 on tyrosine hydroxylase phosphorylation, a recent article by Zhang and Hendrick [36] suggested that direct receptor interactions at pre-synaptic and post-synaptic D2 receptors may not account for the functional differences. Zhang and Hendrick demonstrated that ITI-007 behaves as an antagonist in cells expressing either the D2L (mostly localized post-synaptically) or D2S (mostly localized pre-synaptically). While novel receptor binding interactions as a differentiating event cannot be entirely excluded, the differences between the pre- and post-synaptic actions of ITI-007 likely lie beyond direct D2 receptor interactions and may reflect differing intracellular signaling of cells expressing D2 receptors in their in vivo milieu which contribute to different functional outcomes. Moreover, ITI-007 indirectly modulates glutamate neurotransmission via a dopamine D1-mediated intracellular pathway following an increase in phosphorylation of GluN2B subunits on N-methyl-d-aspartate (NMDA) receptors [15]. Taken together, these aspects of the unique pharmacological profile of ITI-007 may contribute to its efficient dopamine modulation at relatively low levels of D2RO to enable antipsychotic efficacy with a favorable side effect profile.
The present data must be interpreted in the context of the clinical design and may not be directly comparable to receptor occupancy of other antipsychotics using different radiopharmaceuticals, different methods for calculating occupancy, or different subject populations. Often, studies estimating receptor occupancy of antipsychotics in patients with schizophrenia apply a mathematical model when a drug-free baseline is not available and different mathematical models have been compared [37]; i.e., using historical baseline values for matched controls is assuming no baseline differences in BPND with patients with schizophrenia. The accuracy of measuring receptor occupancy increases when it is possible to directly measure a drug-free baseline for within-subject comparison to post-dose displacement of the radiopharmaceutical, as in the present study. To the extent that previous antipsychotic or other psychotropic medication may have influenced the present results, data analyzed with and without subjects with relatively low baseline binding potentials led to similar conclusions of about 40% D2RO at 60 mg of ITI-007. No specific previous antipsychotic (drug, dose, or duration) appeared to contribute to relatively low baseline binding potential. Differences in baseline BPND may have resulted from individual variability, co-registration error and/or motion artifact on some of the scans. Differences in BPND did not correspond to clinical differences in schizophrenia symptoms nor were they predictive of D2RO. It should also be noted that a minimum baseline BPND of 3 is an arbitrary cut-off to aid in interpretation, but all of the individual data regardless of baseline BPND values are shown and the varying level of baseline BPND did not appear to affect the interpretation of the results. Additionally, with the exception of recent alcohol and/or drug dependence or abuse, other psychiatric conditions were not excluded from eligibility for the present study as along as patients met the primary diagnosis of schizophrenia and were able to withdraw safely from psychotropic medications. Co-morbid psychiatric diagnoses were not systematically analyzed and may have influenced the variability and/or generalizability of the results. It should also be noted that the majority of the subjects in the present study were Black/African American males; whereas race or sex is not thought to influence receptor occupancy or plasma concentrations of ITI-007, it could affect generalizability to other studies conducted in other populations.
Despite caveats of making direct D2RO comparisons of antipsychotics across studies with different methodologies, Table 3 illustrates the results with ITI-007 in the context of other atypical antipsychotic drugs that were tested using [11C]-RAC as the radiopharmaceutical to image striatal D2RO. While risperidone across its effective dose range of 4 to 12 mg/day is associated with mean D2RO 72–81% [3, 31, 38–40], at its mean modal effective dose of 4 mg [41], risperidone was associated with a mean of 73% D2RO [38]. Higher doses of risperidone are associated with higher levels of D2RO and higher levels of EPS [3]. D2RO associated with clinically effective doses of other antipsychotics are shown for comparison. Cariprazine, a dopamine D3 and D2 receptor partial agonist, was associated with dose-related increases in displacement of [11C]-RAC with 69% displacement at 1.5 mg and >90% at a dose of 3 mg; akathisia and EPS were reported among the most common side effects experienced at the effective doses of cariprazine [42]. It should be noted that raclopride binds to both D2 and D3 receptors. Cariprazine has affinity for both receptor subtypes and, therefore, displacement of [11C]-RAC is mediated by interactions at both D2 and D3 receptors with cariprazine. ITI-007 lacks potent interaction with D3 receptors [15]; therefore displacement of [11C]-RAC by ITI-007 reflects only D2 receptor interaction.
Although all of the above-mentioned antipsychotics are able to control the positive symptoms of schizophrenia, such as the hallucinations and delusions, each has a unique risk-benefit profile with regards to its side effects, safety and tolerability which necessitates the need for newer, safer treatment options. Lumateperone exhibits antipsychotic efficacy at apparently relatively low levels of striatal D2RO and with reduced risk for movement disorders and hyperprolactinemia relatively to other antipsychotic drugs. If approved, lumateperone may provide a new and safe treatment option for individuals living with schizophrenia.
Acknowledgements
The authors thank Ayon Nandi, William Willis, Robert Dannals, Hayden Ravert, Daniel Holt, Bill Mathews, and the PET technologists at Johns Hopkins University for their help on this study. The authors also thank the team at CBH Health for their help with clinical conduct and Marc Laruelle and Cedric O’Gorman for helpful comments on study design. Funding: This study was funded by Intra-Cellular Therapies, Inc. (ITI). At the time the study was conducted Dr. Vanover, Ms. Saillard, Dr. Weingart, and Dr. Mates were full time employees of ITI and Dr. Davis was a paid consultant to ITI. Dr. Davis is currently a full time employee of ITI. Drs. Wong, Litman, Brašić, Ye and Zhou and Ms. Gapasin declare that they have no conflict of interest.
Contributor Information
Kimberly E. Vanover, Phone: +917-297-2966, Email: kvanover@intracellulartherapies.com
Dean F. Wong, Phone: +410-955-8433, Email: dfwong@jhmi.edu
References
- 1.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15:2550–9. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–44. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 3.Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiatry. 1999;156:869–75. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- 4.Nordstrom AL, Farde L, Wiesel FA, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry. 1993;33:227–35. doi: 10.1016/0006-3223(93)90288-O. [DOI] [PubMed] [Google Scholar]
- 5.Wong DF, Kuwabara H, Brasic JR et al. Determination of dopamine D receptor occupancy by lurasidone using positron emission tomography in healthy male subjects. Psychopharmacology. 2013.229:245-52 [DOI] [PubMed]
- 6.Yokoi F, Grunder G, Biziere K, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology. 2002;27:248–59. doi: 10.1016/S0893-133X(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 7.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D(2), 5-HT(2), and 5-HT(1A) receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164:1411–7. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 8.Grunder G, Carlsson A, Wong DF. Mechanism of new antipsychotic medications: occupancy is not just antagonism. Arch Gen Psychiatry. 2003;60:974–7. doi: 10.1001/archpsyc.60.10.974. [DOI] [PubMed] [Google Scholar]
- 9.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi T, Bies RR, Suzuki T, et al. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Prog Neuropsychopharmacol. 2013;45:178–82. doi: 10.1016/j.pnpbp.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Nordstrom AL, Farde L. Plasma prolactin and central D2 receptor occupancy in antipsychotic drug-treated patients. J Clin Psychopharmacol. 1998;18:305–10. doi: 10.1097/00004714-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–9. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- 13.Farde L, Nordstrom AL. PET analysis indicates atypical central dopamine receptor occupancy in clozapine-treated patients. Br J Psychiatry. 1992:17:30–33. [PubMed]
- 14.Li P, Zhang Q, Robichaud AJ, et al. Discovery of a tetracyclic quinoxaline derivative as a potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J Med Chem. 2014;57:2670–82. doi: 10.1021/jm401958n. [DOI] [PubMed] [Google Scholar]
- 15.Snyder GL, Vanover KE, Zhu H, et al. Functional profile of a novel modulator of serotonin, dopamine, and glutamate neurotransmission. Psychopharmacology. 2015;232:605–21. doi: 10.1007/s00213-014-3704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis RE, Vanover KE, Zhou Y, et al. ITI-007 demonstrates brain occupancy at serotonin 5-HT2A and dopamine D2 receptors and serotonin transporters using positron emission tomography in healthy volunteers. Psychopharmacology. 2015;232:2863–72. doi: 10.1007/s00213-015-3922-1. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman JA, Davis RE, Correll CU et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016.15;79:952-61 [DOI] [PubMed]
- 18.Guy W. ECDEU assessment manual for psychopharmacology: Revised. In: US Department of Health EaW, Public Health Service, 1976:537–537.
- 19.Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Exp Rev Neurotherapeutics. 2016;16:601–14. doi: 10.1080/14737175.2016.1174577. [DOI] [PubMed] [Google Scholar]
- 20.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–51. doi: 10.1016/0920-9964(90)90005-R. [DOI] [PubMed] [Google Scholar]
- 22.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 24.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro MJ, Ricard M, Bourgeois S, et al. Biodistribution and radiation dosimetry of [11C]raclopride in healthy volunteers. Eur J Nucl Med Mol Imag. 2005;32:952–8. doi: 10.1007/s00259-005-1783-2. [DOI] [PubMed] [Google Scholar]
- 26.Slifstein M, Hwang DR, Martinez D, et al. Biodistribution and radiation dosimetry of the dopamine D2 ligand 11C-raclopride determined from human whole-body PET. J Nucl Med. 2006;47:313–9. [PubMed] [Google Scholar]
- 27.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Endres CJ, Brasic JR, Huang SC, Wong DF. Linear regression with spatial constraint to generate parametric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. NeuroImage. 2003;18:975–89. doi: 10.1016/S1053-8119(03)00017-X. [DOI] [PubMed] [Google Scholar]
- 29.Farde L, Wiesel FA, Nordstrom AL, Sedvall G. D1- and D2-dopamine receptor occupancy during treatment with conventional and atypical neuroleptics. Psychopharmacology. 1989;99:S28–31. doi: 10.1007/BF00442555. [DOI] [PubMed] [Google Scholar]
- 30.Gefvert O, Lundberg T, Wieselgren IM, et al. D(2) and 5HT(2A) receptor occupancy of different doses of quetiapine in schizophrenia: a PET study. Eur Neuropsychopharmacol. 2001;11:105–10. doi: 10.1016/S0924-977X(00)00133-4. [DOI] [PubMed] [Google Scholar]
- 31.Tauscher J, Hussain T, Agid O, et al. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am J Psychiatry. 2004;161:1620–5. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- 32.Kapur S, Zipursky R, Jones C, Shammi CS, Remington G, Seeman P. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57:553–9. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- 33.Tauscher-Wisniewski S, Kapur S, Tauscher J, et al. Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry. 2002;63:992–7. doi: 10.4088/JCP.v63n1106. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, Roy P, Daskalakis J, Remington G, Zipursky R. Increased dopamine d(2) receptor occupancy and elevated prolactin level associated with addition of haloperidol to clozapine. Am J Psychiatry. 2001;158:311–4. doi: 10.1176/appi.ajp.158.2.311. [DOI] [PubMed] [Google Scholar]
- 35.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. JPET. 1989;251:238–46. [PubMed] [Google Scholar]
- 36.Zhang L, Hendrick JP. The presynaptic D2 partial agonist lumateperone acts as a postsynaptic D2 antagonist. Matters. 2018; 10.19185/matters.201712000006
- 37.Kim E, Howes OD, Yu KS, et al. Calculating occupancy when one does not have baseline: a comparison of different options. J Cereb Blood Flow Metab. 2011;31:1760–7. doi: 10.1038/jcbfm.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapur S, Remington G, Zipursky RB, Wilson AA, Houle S. The D2 dopamine receptor occupancy of risperidone and its relationship to extrapyramidal symptoms: a PET study. Life Sci. 1995;57:PL103–7. doi: 10.1016/0024-3205(95)02037-J. [DOI] [PubMed] [Google Scholar]
- 39.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–93. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 40.Farde L, Nyberg S, Oxenstierna G, Nakashima Y, Halldin C, Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidone-treated schizophrenic patients. J Clin Psychopharmacol. 1995;15:19S–23S. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 41.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. NEJM. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 42.Citrome L. Cariprazine in schizophrenia: clinical efficacy, tolerability, and place in therapy. Adv Ther. 2013;30:114–26. doi: 10.1007/s12325-013-0006-7. [DOI] [PubMed] [Google Scholar]
- 43.Mamo D, Kapur S, Shammi CM, Papatheodorou G, Mann S, Therrien F, Remington GA. PET study of dopamine D2 and serotonin 5-HT2A receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. 2004;161:818–25. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- 44.Vernaleken I, Fellows C, Janouschek H, Brocheler A, Veselinovic T, Landvogt C, et al. Striatal and extrastriatal D2/D3-receptor-binding properties of ziprasidone: a positron emission tomography study with [18F]Fallypride and [11C]raclopride (D2/D3-receptor occupancy of ziprasidone) J Clin Psychopharmacol. 2008;28:608–17. doi: 10.1097/JCP.0b013e31818ba2f6. [DOI] [PubMed] [Google Scholar]
- 45.Nyberg S, Farde L, Halldin CA. PET study of 5-HT2 and D2 dopamine receptor occupancy induced by olanzapine in healthy subjects. Neuropsychopharmacology. 1997;16:1–7. doi: 10.1016/S0893-133X(96)00218-7. [DOI] [PubMed] [Google Scholar]
- 46.Kapur S, Zipursky RB, Remington G, Jones C, DaSilva J, Wilson AA, Houle S. 5-HT2 and D2 receptor occupancy of olanzapine in schizophrenia: a PET investigation. Am J Psychiatry. 1998;155:921–8. doi: 10.1176/ajp.155.7.921. [DOI] [PubMed] [Google Scholar]


