Abstract
Polynucleotide kinase/phosphatase (PNKP) and X-ray repair cross-complementing 1 (XRCC1) are key proteins in the single-strand DNA break repair pathway. Phosphorylated XRCC1 stimulates PNKP by binding to its forkhead-associated (FHA) domain, whereas nonphosphorylated XRCC1 stimulates PNKP by interacting with the PNKP catalytic domain. Here, we have further studied the interactions between these two proteins, including two variants of XRCC1 (R194W and R280H) arising from single-nucleotide polymorphisms (SNPs) that have been associated with elevated cancer risk in some reports. We observed that interaction of the PNKP FHA domain with phosphorylated XRCC1 extends beyond the immediate, well-characterized phosphorylated region of XRCC1 (residues 515–526). We also found that an XRCC1 fragment, comprising residues 166–436, binds tightly to PNKP and DNA and efficiently activates PNKP's kinase activity. However, interaction of either of the SNP-derived variants of this fragment with PNKP was considerably weaker, and their stimulation of PNKP was severely reduced, although they still could bind DNA effectively. Laser microirradiation revealed reduced recruitment of PNKP to damaged DNA in cells expressing either XRCC1 variant compared with PNKP recruitment in cells expressing WT XRCC1 even though WT and variant XRCC1s were equally efficient at localizing to the damaged DNA. These findings suggest that the elevated risk of cancer associated with these XRCC1 SNPs reported in some studies may be due in part to the reduced ability of these XRCC1 variants to recruit PNKP to damaged DNA.
Keywords: DNA repair, protein-protein interaction, protein-nucleic acid interaction, circular dichroism (CD), single-nucleotide polymorphism (SNP), fluorescence spectroscopy, laser-microirradiation, polynucleotide kinase/phosphatase, X-ray repair cross-complementing, XRCC1
Introduction
Human polynucleotide kinase/phosphatase (PNKP)5 is required to process unligatable strand break termini generated by many genotoxic agents or as intermediates in repair pathways and thus often participates in DNA single- and double-strand break repair (1–4). In its role in single-strand break repair and in the alternative nonhomologous end-joining pathway, PNKP is associated with the scaffolding protein XRCC1 and DNA ligase III (5, 6). PNKP possesses 5′-DNA kinase and 3′-DNA phosphatase activities (7, 8), both of which can be stimulated by XRCC1 (9–11). It has been suggested that XRCC1 functions in a dual capacity to enhance PNKP kinase activity: first, XRCC1 enhances the capacity of PNKP to discriminate between strand breaks with 5′-OH termini and those with 5′-phosphate termini, and second, XRCC1 stimulates PNKP activity by displacing PNKP from the phosphorylated DNA product (12). Phosphorylation of XRCC1 plays an important role in promoting its interaction with PNKP and proteins with similar forkhead-associated (FHA) domains (5, 10, 13, 14). However, although it is generally considered that interaction between PNKP and XRCC1 is mediated by the binding of CK2-phosphorylated XRCC1 protein to the FHA domain of PNKP (5, 15), it is clear that XRCC1 in its nonphosphorylated form can interact with the catalytic domain of PNKP, thereby stimulating PNKP activity (9, 10, 12). In a recent study, a second PNKP interaction site in XRCC1 was identified that binds PNKP with lower affinity and independently of XRCC1 phosphorylation (16). This low-affinity interaction site requires the highly conserved Rev1-interacting region (RIR) motif in XRCC1 and includes three critical and evolutionarily conserved phenylalanine residues Phe-173, Phe-191, and Phe-192.
To gain further insight into the mechanism of activation of PNKP by XRCC1, we have now examined the interactions between different regions of XRCC1 with PNKP and DNA. For this purpose, we utilized several XRCC1 fragments (Fig. 1) including the following: (i) the extended BRCT2 domain of XRCC1 (EB2) from residues 511 to 633, because this region possesses a cluster of CK2 phosphorylation sites implicated in the interaction with the FHA domain (5); (ii) the BLB (BRCT1-linked BRCT2) region of XRCC1 comprising residues 295–633; and (iii) the extended BRCT1 domain (EB1) comprising the nuclear localization domain and BRCT1 domain (residues 166–436), which is essential for the recruitment of XRCC1 to sites of DNA damage and DNA replication (17). Hanssen-Bauer et al. (17) suggested a key role for this region in mediating DNA repair, but the mechanism by which it confers this property remains unclear. The EB1 domain also retains two of the most common amino acid variants of XRCC1, namely R194W and R280H. These variants have been linked in many (but not all) studies with increased incidence of specific types of cancer and response to chemotherapy in defined populations, although the penetrance of their effects may be influenced by tissue type and ethnicity (18–24). Cells expressing these variants exhibit different repair profiles when treated with methyl methanesulfonate or hydrogen peroxide and reduced DNA repair capacity compared with the WT protein (17). The observed differences in DNA repair profiles of the mutants from the WT protein could be associated with either the affinity with which they bind DNA or their ability to interact with other DNA repair proteins such as PNKP.
Figure 1.

Schematic of human XRCC1. The diagram shows the major identified domains within XRCC1 and the protein fragments used for this study. NTD, N-terminal domain.
The data presented here addresses the binding of phosphorylated XRCC1 to PNKP indicating that the interaction is more extensive than previously envisaged. We also show, by comparing the WT and variant EB1 fragments, that the stimulation of PNKP is primarily due to its direct interaction with XRCC1 rather than XRCC1 competition for substrate DNA. Finally, we provide evidence indicating that the reduced repair capacity associated with the variant XRCC1 species may be, at least in part, attributable to poorer stimulation of PNKP and reduced recruitment of PNKP to damaged DNA.
Results
Overview of the fluorescence-based analytical approach for studying PNKP interaction with XRCC1 fragments
To study the interaction between XRCC1 fragments, DNA substrates, and PNKP, we carried out a series of steady-state fluorescence measurements. Binding of protein to DNA substrates was examined by the change in intrinsic Trp fluorescence. To analyze the binding between XRCC1 fragments and PNKP, PNKPWFX402 (a mutated form of PNKP in which all the tryptophans, except Trp-402, have been replaced with phenylalanine) was labeled with acrylodan (AC), a sulfhydryl-specific covalent label, and the effect of XRCC1 fragments binding to PNKPWFX402-AC was monitored by quenching of AC fluorescence around 490 nm following excitation at 380 nm (25, 26). The AC-labeled PNKP was functionally active when tested for its kinase activity and retained ∼85–90% of its activity compared with unlabeled PNKP. The degree of labeling of PNKP with AC was 1.3 ± 0.2 (mean ± S.E., n = 4) mol of AC/mol of PNKP. PNKPWFX402-AC when excited at 380 nm exhibits an emission maximum around 490 nm, suggesting that the environment of AC in PNKP has considerable hydrophobic character (12, 25).
Interaction between PNKP and the C-terminal domain of XRCC1 (EB2)
The C-terminal domain of XRCC1 (residues 511–633), which is known to contain several CK2 phosphorylation sites (Fig. 1), is considered to contribute significantly to XRCC1 binding to the FHA domain of PNKP (5). We first compared the interaction of full-length PNKP with nonphosphorylated and phosphorylated EB2. Phosphorylated EB2 was produced by co-expression of the XRCC1 fragment together with CK2 in bacterial cells (see under “Methods and materials”). Addition of pEB2 (where “p” denotes CK2 phosphorylated protein) resulted in quenching AC fluorescence at 490 nm (Fig. 2), and fluorescence titration as a function of pEB2 concentration yielded a Kd value of 400 ± 30 nm, whereas npEB2 (where “np” denotes nonphosphorylated peptide) did not induce any significant quenching in AC fluorescence even at 5 μm concentration, indicating that the nonphosphorylated protein does not bind to PNKP (Table 1, top).
Figure 2.

Fluorescence titration of PNKPWFX402-ACversus pEB2. Labeled protein (40 nm) was excited at 380 nm, and the relative fluorescence (Rel.Fluor.) intensities were monitored at 490 nm (see inset). The fraction bound versus pEB2 concentration is plotted.
Table 1.
Affinities of XRCC1 fragments for PNKPWFX402-AC and PNKPFHA-AC
“p” denotes CK2 phosphorylated protein and np denotes nonphosphorylated peptide.
| Sample | Kda |
|---|---|
| nm | |
| Binding to PNKPWFX402-AC | |
| EB2 (residues 511–633) | 400 ± 30 |
| npEB2 (residues 511–633) | NDb |
| pBLB (residues 295–633) | 80 ± 10 |
| npBLB (residues 295–633) | 550 ± 30 |
| npEB1 (residues 166–436)c | 120 ± 10 |
| npEB1R194W | NDb |
| npEB1R280H | NDb |
| Binding to PNKPFHA-AC | |
| pEB2 (residues 511–633) | 250 ± 20 |
| pBLB (residues 295–633) | 50 ± 5 |
aKd values (mean ± S.E., n = 3) were determined by fluorescence titration.
b ND means not determined.
c SDS gel profiles of the purified EB1 fragment and the two variants, EB1R280H and EB1R194W, are shown in Fig. S1.
We then looked more directly at the interaction with the PNKP FHA domain. To obtain quantitative data for this interaction, we labeled the single Cys residue (Cys-46) in the FHA domain with AC (10, 12). When the labeled protein was excited at 380 nm, the emission maximum occurred around 490 nm, and the addition of the pEB2 fragment resulted in AC fluorescence quenching (Fig. 3A). Titration of PNKPFHA-AC with pEB2 yielded a Kd of 250 ± 20 nm (Table 1, bottom), i.e. slightly tighter binding than to full-length PNKP. Extension of the C-terminal region to include the BRCT1 domain (i.e. the p295–633 fragment, pBLB) significantly increased the binding to either full-length PNKP (Kd 80 ± 10 nm, Table 1, top) or the isolated FHA domain (Kd 50 ± 5 nm, Fig. 3B and Table 1, bottom). Replotting the data from Fig. 3, A and B, as fraction bound versus the log of the ligand concentration to directly compare pEB2 and pBLB interaction with PNKPFHA-AC (Fig. 3C) readily indicates that pBLB exhibits a higher affinity than pEB2. We also examined the binding of the nonphosphorylated BLB fragment to PNKPWFX402-AC and observed a Kd of ∼550 nm. Taken together, these results suggest that regions of XRCC1 other than the CK2-phosphorylated region immediately adjacent to BRCT2 contribute to the binding to PNKP.
Figure 3.
Fluorescence titration of PNKPFHA-ACversus pEB2 and pBLB. Labeled PNKPFHA-AC (40 nm) was excited at 380 nm, and the relative fluorescence (Rel.Fluor.) intensities were monitored at 490 nm (see insets). A, fraction bound versus pEB2 concentration. B, fraction bound versus pBLB. C, replotting the data from A and B as fraction bound versus the log of the ligand (pEB2 and pBLB) concentration.
Interaction between PNKP and the extended BRCT1 domain of XRCC1 (EB1)
Others have shown that the extended BRCT1 domain, EB1 (residues 166–436), is required for the scaffolding function of XRCC1 (17). This fragment is also the location for two common XRCC1 variants, R194W and R280H, resulting from SNPs. We therefore examined the capacity of this XRCC1 fragment and the R194W and R280H variants to bind to PNKP. Although the WT fragment bound PNKPWFX402-AC tightly with a Kd of ∼120 nm (Table 1, top), neither of the variant fragments caused sufficient fluorescence quenching (<6%) even at the saturating concentration of 3 μm with the result that titration could not be carried out with these two variants to measure the Kd values. These results suggest that the region of XRCC1 N-terminal to BRCT1 is involved in PNKP interactions. We also examined the binding of full-length XRCC1 and the two full-length variants to PNKPWFX402-AC. (Purity of the full-length protein preparations is shown in Fig. S1) XRCC1 exhibited tight binding to AC-labeled PNKP resulting in nearly 30% quenching of AC fluorescence, and the Kd value obtained from fluorescence titration was 55 ± 5 nm, whereas the two variants induced only ∼5% AC quenching when the concentrations of PNKPWFX402-AC and the variants were 50 nm and 3 μm, respectively, with the result that no binding affinity could be determined.
DNA binding to the extended BRCT1 domain of XRCC1 (EB1)
We have previously determined the binding affinities of full-length WT XRCC1 for substrates that model DNA strand breaks (27) by monitoring the effect of DNA binding on the intrinsic Trp fluorescence of XRCC1. Because the EB1 domain has been implicated in XRCC1 binding to damaged sites, we examined the affinity of this domain for various substrates that model DNA strand breaks by fluorescence titration (Fig. 4 and Table 2). The rank-order of affinities with which this fragment bound these ligands was 1-nt–gapped DNA > nicked DNA > intact duplex > single-stranded oligonucleotide, which is the same rank order we previously observed with full-length XRCC1 (27). Interestingly, both the EB1 and BLB fragments, like full-length XRCC1, preferentially bound a gapped DNA substrate over a single-stranded substrate (Table 2) with similar Kd values, suggesting that a major DNA-binding domain of XRCC1 lies between residues 295 and 436, which contains the BRCT1 domain.
Figure 4.

Fluorescence titration of EB1 versus duplex DNA with a single nucleotide gap. The protein (0.1 μm) was excited at 295 nm, and the fluorescence intensity was monitored at 340 nm (see inset). The fraction bound (i.e. relative fluorescence quenching) versus ligand concentration is plotted.
Table 2.
Binding of XRCC1 fragments to substrates that model DNA strand breaks
| Sample | Substrate | Kda |
|---|---|---|
| μm | ||
| EB1 (residues 166–436) | 1-nt–gapped DNAb | 0.15 ± 0.01 |
| Nicked DNA | 0.26 ± 0.01 | |
| Duplex (20-mer) | 0.50 ± 0.05 | |
| Single-stranded (24-mer) | 0.75 ± 0.05 | |
| BLB (residues 295–633) | 1-nt–gapped DNA | 0.16 ± 0.01 |
| Single-stranded (24-mer) | 0.65 ± 0.05 | |
| EB1R194W | 1-nt–gapped DNA | 0.37 ± 0.01 |
| Single-stranded (24-mer) | 1.70 ± 0.20 | |
| EB1R280H | 1-nt–gapped DNA | 0.55 ± 0.02 |
| Single-stranded (24-mer) | 3.30 ± 0.30 | |
| EB1W385A | 1-nt–gapped DNA | 0.17 ± 0.01 |
| Single-stranded (24-mer) | 1.10 ± 0.10 |
aKd values (means ± S.E., n = 3) were determined by fluorescence titration.
b nt indicates nucleotide.
In the EB1 fragment there are two Trp residues, at positions 353 and 385. Therefore, to further investigate the XRCC1–DNA interaction, we replaced Trp-385 with alanine (EB1W385A) and observed that this mutant bound the 24-mer ssDNA and the 1-nt–gapped DNA with Kd values of 1.10 and 0.17 μm, respectively. Because these values are highly comparable with those for the WT EB1 fragment (Table 2), it suggests that the observed quenching in Trp fluorescence following the addition of DNA arises mainly from the perturbation of Trp residues at position 353. Interestingly, Trp-353 is positioned near the putative phosphate-binding pocket of the XRCC1 BRCT. It may be that in XRCC1 the BRCT phosphate-binding pocket plays a role in DNA binding, as has been suggested for replication factor C (28).
We also examined the EB1 R194W and R280H variant fragments for their DNA-binding capacity, and we observed that they exhibited moderately lower binding affinities toward ssDNA and 1-nt–gapped DNA than the WT fragment (Table 2).
CD analysis of the extended BRCT1 domain of XRCC1 and interaction with DNA
Information concerning the secondary structure of EB1 and its two variants was obtained from far-UV–CD data (Fig. 5). All three EB1 fragments exhibited two negative CD bands around 209 and 222 nm, indicating the presence of α-helical organization. Secondary structure analysis suggested that the above two mutations in EB1 did not induce any major conformational change in EB1 (Table 3). However, the effect of DNA binding on these proteins was different, and the maximum change in protein conformation as a result of DNA binding was observed with the WT EB1 peptide (Fig. 5). Addition of 2 μm 1-nt–gapped DNA induced a conformational change in EB1; the molar ellipticity value [θ]M at 209 nm was reduced from −7500 ± 300 to −5400 ± 300 deg cm2 dmol−1, upon binding DNA. The observed changes in [θ]M at 209 nm for EB1R194W and EB1R280H upon binding DNA were −7600 ± 300 to −6600 ± 300 and −7350 ± 300 to −6800 ± 300, respectively. The observed change with EB1R280H was very small, only slightly above the experimental error, and these results correlate very well with the DNA-binding ability of these proteins (Table 3).
Figure 5.

CD analysis of the EB1 WT (A), R194W (B), and R280H (C) fragments. Protein concentration used was 0.5 mg/ml in 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5 mm MgCl2, and 1 mm DTT.
Table 3.
Secondary structural analysis of 166–436, R194W, R280 H, and 166–436-DNA complex
| Protein | α-Helixa | β-Structurea | Randoma |
|---|---|---|---|
| % | % | % | |
| EB1 | 21 | 51 | 28 |
| EB1R194W | 26 | 44 | 30 |
| EB1R280H | 22 | 49 | 29 |
| EB1 + DNA | 16 | 49 | 35 |
aAnalysis of the CD spectra according to the method of Provencher and Glöckner (48).
Effect of XRCC1 and its fragments on the DNA kinase activity of PNKP
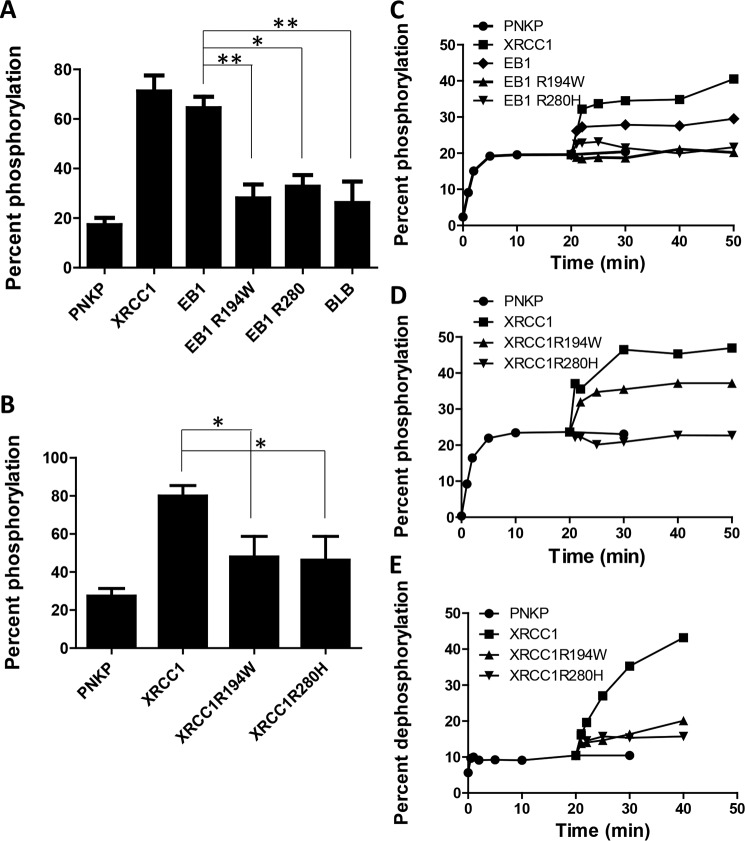
To test whether XRCC1 and its fragments stimulates the 5′-kinase activity of PNKP, a single-stranded oligonucleotide (24-mer) was employed as the DNA substrate. DNA kinase reactions were carried out in the presence of [γ-32P]ATP. Under conditions that allowed for limited 5′-phosphorylation by PNKP when used alone, the kinase activity of PNKP increased ∼4-fold in the presence of full-length XRCC1, and the WT EB1 fragment was almost as effective (Fig. 6A). In contrast, the R194W and R280H mutants of EB1 showed a significantly diminished capacity to stimulate the PNKP kinase activity. The longer XRCC1 nonphosphorylated BLB fragment also had a limited effect (Fig. 6A). Even though the BLB fragment composes a larger portion of XRCC1, it was less effective in activating PNKP compared with the WT EB1 fragment, suggesting that linker 1 in the N-terminal segment of XRCC1 plays an important role in activating PNKP. We also examined the effect of the R194W and R280H variants of full-length XRCC1 on the DNA kinase activity of PNKP (Fig. 6B). These two single-point mutants induced limited activation of the kinase activity of PNKP.
Figure 6.
Activation of PNKP by full-length XRCC1 and XRCC1 fragments. A, stimulation of PNKP kinase activity on ssDNA by co-incubation with full-length XRCC (XRCC1), EB1(166–436), EB1R194W, EB1R280H, and BLB(295–633). The statistical significance in the difference between DNA phosphorylation in the presence of EB1 and EB1R194W, EB1R280H, or BLB is indicated by the asterisks (*, p < 0.05; **, p < 0.01 Student's t test). B, stimulation of PNKP kinase activity by co-incubation with full-length WT and variant XRCC1. C, stimulation of PNKP kinase activity due to enzyme turnover by addition of full-length XRCC1 (XRCC1), EB1(166–436), EB1R194W, and EB1R280H after 20 min of incubation of PNKP with ssDNA. D, stimulation of PNKP kinase activity by full-length WT and variant XRCC1. E, stimulation of PNKP phosphatase activity by full-length WT and variant XRCC1.
Influence of XRCC1 fragments on the turnover of PNKP
Full-length XRCC1 is known to enhance the displacement of PNKP from the product of its reaction, i.e. 5′-phosphorylated or 3′-dephosphorylated strand break termini, thereby increasing the turnover rate of PNKP (10, 12). To examine the influence of WT and variant EB1 on PNKP turnover, the kinase activity of PNKP was monitored using a limited concentration of the enzyme with a 5′-OH bearing a 24-mer single–stranded oligonucleotide and [γ-32P]ATP, followed 20 min later by the addition of full-length XRCC1 or EB1 fragment (Fig. 6C). The rate of product accumulation decreased over the course of the assay and reached a plateau after ∼10 min. Addition of full-length XRCC1 at 20 min (i.e. in this plateau region) resulted in reactivation of PNKP kinase activity, and the percent 32P incorporated nearly doubled. The observed increase in kinase activity was due to PNKP, because XRCC1 has no kinase activity. Addition of WT EB1, although not as active as full-length XRCC1, nonetheless showed a significant level of PNKP kinase reactivation. Neither the R194W nor the R280H EB1 variants when added to PNKP in the plateau region showed any appreciable effect, implying that these two variants were not able to reactivate PNKP's kinase activity. Similarly, the BLB fragment failed to significantly stimulate PNKP turnover (data not shown). The influence of single-point mutants R194W and R280H in full-length XRCC1 on the turnover rate of PNKP is shown in Fig. 6D. Although the full-length R194W mutant was able to stimulate PNKP's kinase activity (albeit to a lower extent than the WT protein), the R280H mutant XRCC1 failed to do so. A similar analysis of the PNKP 3′-phosphatase also indicated that the full-length XRCC1 variants severely impacted this activity (Fig. 6E).
Cellular interaction of WT and mutant XRCC1 with PNKP
To gain insight into the cellular impact of the R280H and R194W XRCC1 variants on the behavior of PNKP at damaged DNA, we transiently co-expressed mRFP-tagged PNKP with mGFP-tagged full-length WT XRCC1, the R280H and R194W variants, and empty vector. To circumvent the effects of the interactions of mRFP-tagged PNKP with endogenous XRCC1, we used EM9 cells that lack the expression of any XRCC1. Western blotting indicated that the levels of tagged PNKP and XRCC1 were very similar in the transfected cells (Fig. S2). Then we carried out laser micro-irradiation and followed the accumulation of PNKP at sites of DNA damage in real time. Consistent with previous observations, PNKP weakly accumulated at sites of DNA damage in EM9 cells (Fig. 7, A and B) (29). However, co-expressing WT XRCC1 further enhanced the recruitment of PNKP to DNA-damaged sites, which is consistent with previous observations on the interaction between the two proteins. Cells expressing either of the two variants of XRCC1 displayed a significantly reduced accumulation of PNKP to DNA damage sites compared with cells expressing WT XRCC1. To rule out the possibility that this effect might be due to decreased recruitment of the XRCC1 variants compared with the WT protein, we followed and quantified their recruitment to DNA damage sites and observed relatively small differences between the WT XRCC1 and the two variants (Fig. 7C). Our XRCC1 recruitment data are very similar to those of Hanssen-Bauer et al. (17). They did not detect a significantly different pattern of recruitment between the R194W variant and WT XRCC1, whereas the R280H variant showed a small but consistently lower recruitment over time than the WT protein, although in our case the differences were not statistically significantly different.
Figure 7.

Effect of XRCC1 mutants on the recruitment of PNKP to sites of DNA damage. EM9 cells, which lack endogenous XRCC1, were co-transfected with mRFP-PNKP, mGFP (vector only), and mGFP-tagged versions of wildtype (WT) and R194W and R280H mutant XRCC1. Laser micro-irradiation was performed as described under “Materials and methods.” A, examples showing the recruitment of mRFP-PNKP in cells cotransfected with mGFP, the R194W mutant, and WT XRCC1. B, recruitment of mRFP-PNKP in EM9 cells expressing mGFP (+ GFP), WT XRCC1 (+ WT XRCC1), R194W variant (+ R194W), and R280H variant (+R280H). For each data set 12 cells were selected for quantification of mRFP-PNKP. C, recruitment of mGFP-tagged WT XRCC1 (WT) and R194W and R280H variant XRCC1 proteins to sites of DNA damage. For each data set 12 cells were selected for quantification of mGFP-XRCC1. Each line in B and C is based on three independent experiments, n = 12, and error bars represent S.E.
Discussion
XRCC1 is regarded as a scaffolding protein capable of interacting with several proteins participating in single-strand break repair. It forms repair complexes with poly(ADP-ribose) polymerase (30, 31), DNA polymerase-β (32, 33), and DNA ligase III (34). XRCC1 has been shown to interact with the PNKP FHA domain through a peptide sequence in XRCC1 phosphorylated by CK2 and to stimulate the DNA kinase and DNA phosphatase activities at damaged DNA termini (5, 9). We also found that nonphosphorylated XRCC1 can interact with the catalytic domain of PNKP and stimulate PNKP activity as well (10, 12, 16). The purpose of this study was to examine in more detail the interactions between different regions of XRCC1 and PNKP to increase our understanding of the mechanism(s) of PNKP activation and their possible role in DNA repair.
Interactions of XRCC1 fragments with PNKP
Our earlier studies have clearly established a phosphorylation-independent interaction of XRCC1 with PNKP and also revealed that XRCC1 and CK2-phosphorylated XRCC1 (pXRCC1) bind to PNKP at different sites (10, 12, 16).
In XRCC1, the C-terminal domain contains the CK2 phosphorylation sites (residues 518, 519, 523, and 535) (5, 35). The XRCC1 pEB2 fragment (residues 511–633), which encompasses the phosphorylation sites in addition to the BRCT2 domain of XRCC1, was capable of binding to the FHA domain as well as to full-length PNKP. The observed binding affinities of pEB2 to the FHA domain and full-length PNKP were distinctly lower than the affinities of the longer pBLB peptide (residues 295–633), suggesting that other regions of XRCC1, including additional phosphorylated residues in linker 2 between residues 403 and 494 (5, 10, 36) and BRCT1, can contribute to enhancing the interaction between phosphorylated XRCC1 and PNKP. Mass spectral analysis of the phosphorylation of the BLB peptide produced by co-expression with human casein kinase 2α (CK2α) in Escherichia coli (as used for this study) led to the identification of 15 CK2-dependent phosphorylation sites within linker 2 and the BRCT2 domain (36). Others have also come to the conclusion that the interaction between XRCC1 and PNKP involves regions in XRCC1 that extend beyond the C-terminal domain containing the phosphopeptide (residues 515–526) and the FHA domain of PNKP (11). These investigators used a yeast two-hybrid approach to identify mutations in XRCC1 that disrupt interaction with PNKP and observed that converting alanine 482 to threonine significantly reduced binding to PNKP (11). Whether this exchange directly impacts the XRCC1–PNKP interaction interface or causes a conformational change in XRCC1 has yet to be determined. However, the fact that the pBLB fragment bound the FHA domain with a similar affinity as it bound to full-length PNKP suggests that the binding of this component of XRCC1 is confined to the FHA domain of PNKP.
The EB1 domain, comprising residues 166–436, is involved in the recruitment of XRCC1 to sites of damage and DNA replication (17), implying that this region plays a major role in mediating DNA repair. Our data indicate that this region of XRCC1 contributes significantly to the binding of nonphosphorylated XRCC1 to PNKP, whereas the binding of the nonphosphorylated BLB fragment is considerably weaker. Nonetheless, the difference in Kd values for the interaction of full-length XRCC1 and the EB1 fragment with PNKP (43 versus 120 nm) suggests that other residues enhance the binding of full-length nonphosphorylated XRCC1 to PNKP.
The extended BRCT1 domain also harbors two common amino acid variants of XRCC1, namely R194W and R280H. These variants exhibited different repair profiles compared with the WT protein (17). Our data indicate that the amino acid changes have a profound effect on binding to PNKP, which will be discussed in further detail below. In the case of the R280H mutant, this could be related to the altered conformation revealed by CD (Table 3).
Interactions of XRCC1 fragments with DNA
Several lines of evidence indicate that the extended BRCT1 domain plays an important role in XRCC1 binding to DNA. A key observation is that the EB1 fragment, like full-length XRCC1, displays differential binding to various DNA substrates, with the tightest interactions occurring between the fragment and the 1-nt gap and nicked DNA substrates, which mimic DNA single-strand breaks. The extended C-terminal domain BLB bound with similar affinity to DNA as EB1, suggesting that the overlapping region between the two fragments, i.e. residues 295–436, may be responsible for DNA binding.
The variant R194W and R280H EB1 fragments exhibited lower binding affinity for 24-mer ssDNA and 1-nt–gapped DNA compared with WT EB1. The CD data showed that DNA binding to WT EB1 induced a conformational change in the protein fragment, and its effect on the two variants was considerably less; in fact, with EB1R280H the observed change in ellipticity around 210 nm was only slightly above the experimental error. Berquist et al. (37) have also reported reduced DNA-binding ability for this variant. XRCC1 might serve as a strand break sensor in addition to its structural role as a scaffolding protein as it specifically binds gapped and nicked single-strand breaks with high affinities (27, 38). The observed differences in the DNA-binding profile of these XRCC1 mutants may interfere in their ability to take part in DNA repair by affecting the sensor role and in turn their ability to direct the enzyme PNKP to the damaged site. However, it should be noted that the full-length XRCC1 proteins containing either of these mutations were recruited to sites of DNA damage induced by laser micro-irradiation with similar kinetics to the WT protein (Fig. 7C), although the R280H variant has previously been shown to dissociate more rapidly than WT XRCC1 from sites of DNA damage induced by micro-irradiation (39).
DNA ligase III is another key binding partner of XRCC1 (34). Because the interaction between XRCC1 and DNA ligase III is mediated through the XRCC1 BRCT2 domain and a region of the linker immediately N-terminal to the BRCT2 domain (40, 41), it is not clear if either R194W or R280H would directly affect the binding of XRCC1 to the ligase. However, the reduced DNA binding of the XRCC1 variants may also impact DNA ligation, although DNA ligase III itself can bind tightly to nicked DNA with a Kd of ∼ 30 nm (42).
Effect of XRCC1 fragments on the kinase activity of PNKP
Full-length XRCC1 and its WT-extended BRCT1 fragment EB1 were effective in markedly activating the kinase activity of PNKP (Fig. 6A) and stimulating PNKP turnover (Fig. 6C). However, the nonphosphorylated BLB peptide had minimal effect on either the kinase activity or turnover of PNKP, clearly establishing the important role of linker 1 in the N-terminal region of XRCC1 in its stimulatory interaction with PNKP. The R194W and R280H variants of EB1 also had a limited effect on the kinase activity or turnover of PNKP. However, because the nonphosphorylated BLB fragment, as well as the variant EB1 fragments, bound DNA reasonably tightly, we infer that the stimulation of PNKP activity depends on XRCC1 interaction with PNKP rather than displacement of PNKP due to competitive binding to DNA. Related to this, we found that the full-length R194W and R280H XRCC1 variants were recruited to laser-induced DNA damage with similar kinetics as WT XRCC1, but showed a significantly reduced capacity to enhance PNKP recruitment (Fig. 7). This raises the important possibility that the observed difference in DNA repair profiles of the SNP-derived variants compared with the WT protein (14) could be due to their poorer ability to bind and activate PNKP. Because full-length nonphosphorylated XRCC1 binds only to the catalytic domain of PNKP and not to the FHA domain (10), it would imply that EB1 also binds to the catalytic domain rather than the FHA domain. Therefore, the observation that the R194W and R280H XRCC1 variants have diminished capacity to recruit PNKP strongly suggests that interaction of XRCC1 with the catalytic domain of PNKP is required for the efficient recruitment of PNKP to DNA strand breaks in the nucleus.
Our present observations regarding the important role of linker 1 in the interaction and stimulation of PNKP by XRCC1 concur with the recent identification of a region just downstream of the N-terminal domain in XRCC1 that mediates a low-affinity interaction with PNKP independently of XRCC1 phosphorylation (16). This region contains a putative RIR motif (PGALFFSR) in which Arg-194 constitutes the C-terminal amino acid. The RIR is a protein interaction domain present in several translesion DNA polymerases (43). Mutation of the two phenylalanine residues in the putative RIR as well as Phe-173 resulted in the disruption of XRCC1 interaction with PNKP and prevented the stimulation of the kinase and phosphatase activities of PNKP in vitro (16). The mutation of the RIR motif also significantly reduced the single-strand break repair and enhanced the sensitivity of cells exposed to hydrogen peroxide (16).
In summary, we have shown that the linker 1 in the N-terminal region of XRCC1 plays an important role in its interaction with PNKP. Phosphorylation of XRCC1 at its C-terminal end only strengthens its interaction with PNKP by virtue of its ability to bind to the FHA domain. Polymorphic variations, R194W and R280H, in the extended central BRCT domain disrupted its ability to interact with PNKP, and our data suggest that this is potentially responsible for altering the DNA repair capability of cells carrying these changes to XRCC1.
Materials and methods
Bacterial PNKP expression and purification
His-tagged human PNKP, PNKPWFX402, and the FHA domain of PNKP were expressed in E. coli BL21(DE3)pLysS (Millipore, Etobicoke, Ontario, Canada), purified as described previously (10, 44, 45), and kept in storage buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 5 mm MgCl2, and 1 mm dithiothreitol) at −80 °C.
Bacterial XRCC1 expression and purification
The XRCC1 cDNA encoding residues 511–633 (EB2) and 295–633 (BLB) incorporating a C-terminal hexahistidine tag were cloned into the pET28a vector (Millipore) using a restriction-free cloning procedure (46). Briefly, in the first step a regular PCR amplification was performed employing a template vector of human XRCC1, which was kindly provided by Dr. Tom Ellenberger (Washington University, St Louis). In the second step, further PCR amplification was carried out using primers designed to have a 3-bp overlap with the sites of integration in the destination vector. The amplified products then served as mega-primers for insertion into the vector. The parental plasmid DNA was removed by DpnI treatment, and then DNA was directly transformed into competent E. coli DH5α cells. The selected colonies were checked by DNA sequence verification. The cDNA encoding full-length XRCC1 and residues 166–436 was amplified by PCR and inserted into the bacterial expression plasmid pEX-N-His (OriGene, Rockville, MD) using AscI–RsrII restriction sites. Point mutations encoding R280H and R194W were introduced into WT XRCC1 using overlap extension PCR techniques with the following forward and reverse primers for the R194W mutant: 5′-GGGCTCTCTTCTTCAGCTGGATCAACAAGACATCC-3′ and 5′-GGATGTCTTGTTGATCCAGCTGAAGAAGAGAGCCC-3′; and for the R280H mutant: 5′-GCCAGCTCCAACTCATACCCCAGCCACAG-3′ and 5′-CTGTGGCTGGGGTATGAGTTGGAGCTGGC-3′. All generated plasmids were sequence-verified.
His-tagged proteins were expressed in E. coli BL21 Gold transformed with bacterial expression plasmids after reaching A600 0.6 followed by induction with 1 mm IPTG at 37 °C for 3 h. Subsequently, proteins were purified using Pro-Bond nickel chelating resin (Life Technologies, Inc.) following the manufacturer's protocol for lysis and purification. The purified proteins were dialyzed against 50 mm Tris, pH 7.4, 100 mm NaCl, and 5 mm MgCl2.
In vivo phosphorylation of XRCC1 fragments and purification
The XRCC1 cDNAs encoding residues 511–633 (EB2) and 295–633 (BLB) inserted in the pET28a vector were transformed into BL21(DE3) E. coli cells and grown in LB-kanamycin media. Starting culture (2 ml) was transferred to 100 ml of TYM media (2% bacto-tryptone, 0.2% yeast extract, 0.1 m NaCl and 0.01 m MgCl2). After reaching A600 of 0.4, the cells were harvested at 3000 rpm for 10 min, and the cells pellets were resuspended in 20 ml of TFB1 buffer (30 mm KAc, 50 mm MnCl2, 10 mm CaCl2, 100 mm KCl, and 15% glycerol, pH 5.8). Following incubation for 2 h on ice, the cells were harvested at 3000 rpm for 10 min again, and the cells pellets were resuspended in 4 ml of TFB2 (10 mm Na-MOPS, pH 7.0, 75 mm CaCl2, 10 mm KCl, 15% glycerol). The cells were divided into aliquots of 50 μl and stored at −80 °C. Phosphorylated XRCC1 fragments were prepared by co-expression with human CK2α (the cDNA for which was cloned in the pGEX-3x vector) in E. coli Rosetta cells, as described previously (36) (kindly provided by Dr. Tom Ellenberger, Washington University School of Medicine, St. Louis, MO).
After growth to A600 0.4, the culture was cooled to 18 °C, and protein expression was induced with 1 mm IPTG for 18 h. The cells were harvested by centrifugation (6000 × g for 20 min) and resuspended in lysis buffer (20 mm imidazole, 20 mm Tris, pH 8.0, 300 mm NaCl, 1 mm EDTA, and 5 mm β-mercaptoethanol), and protease inhibitor mixture was added before lysis by sonication. A cleared lysate was prepared by centrifugation, and proteins were purified by immobilized metal-affinity chromatography followed by gel filtration. The soluble fraction was added to Ni-NTA–agarose resin (Amersham Biosciences) and mixed gently by shaking at 4 °C for 2 h. The protein solution/Ni-NTA–agarose mixture was loaded into a column and washed with 300 ml of lysis buffer and 100 ml of lysis buffer containing 25 mm imidazole and eluted with 10 ml of lysis buffer containing 300 mm imidazole. The buffer was then exchanged for gel-filtration buffer (20 mm Tris-HCl, pH 8, 150 mm NaCl, 1 mm EDTA, 5 mm MgCl2, and 5 mm β-mercaptoethanol), and the protein was further purified on a Superdex 75 26/60 (Amersham Biosciences) gel-filtration column.
Steady-state fluorescence spectroscopy
Labeling of PNKPWFX402 and the PNKP FHA domain with AC was carried out as described in our earlier studies (12). The interaction between phosphorylated and nonphosphorylated XRCC1 fragments and PNKP was studied using acrylodan-labeled PNKPWFX402 and the FHA domain as described previously (12, 27). The AC-labeled proteins were excited at 380 nm, and the changes in AC fluorescence at the emission maximum (490 nm) were monitored. The interaction between XRCC1 fragments and DNA substrates was studied using the intrinsic fluorescence due to tryptophan residues as detailed in our earlier works (27). All fluorescence and CD measurements were carried out in 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5 mm MgCl2, and 1 mm DTT. GraphPad Prism software (GraphPad Software Inc., La Jolla, CA) was used for the analysis of binding data.
CD spectroscopy
Far-UV CD measurements were performed with an Olis DSM 17 CD spectropolarimeter (Bogart, GA) as described previously (27). Protein concentrations used for each determination are presented in the corresponding figure legends. The CD spectra were analyzed according to the method of Chen et al. (47).
Kinase activity assay
PNKP (0.5 μg, 9 pmol) was premixed with 60 pmol of either XRCC1 or XRCC1 WT or mutant fragment in 5 μl and incubated at 37 °C for 5 min. The volume was increased to 20 μl with addition of kinase buffer (final concentration: 80 mm succinic acid, pH 5.5, 10 mm MgCl2, and 1 mm DTT), 0.2 nmol of 24-mer DNA substrate (Integrated DNA Technologies, Coralville, IA), and 3.3 pmol of [γ-32P]ATP (PerkinElmer Life Sciences, Waltham, MA) and incubated for 5 more min. 4-μl samples were mixed with 2 μl of 3× sequencing gel loading dye (ThermoFisher Scientific, Ottawa, Ontario, Canada), boiled for 10 min, and run on a 12% polyacrylamide, 7 m urea sequencing gel at 200 V. Gels were scanned with a Typhoon 9400 variable mode imager (GE Healthcare, Bucks, UK), and the resulting bands were quantified using ImageQuant 5.2 (GE Healthcare).
Turnover of PNKP
To follow the turnover of PNKP kinase activity, reaction mixtures (50 μl) containing kinase buffer (80 mm succinic acid, pH 5.5, 10 mm MgCl2, and 1 mm DTT), 0.2 nmol of 24-mer DNA substrate, 0.4 nmol of unlabeled ATP, 3.3 pmol of [γ-32P]ATP, and 0.9 pmol of PNKP were incubated at 37 °C. From one of the reaction mixtures, 4-μl samples were taken at 0, 1, 2, 5, 10, 20, and 30 min. To the other reaction mixtures, 60 pmol of full-length XRCC1 or XRCC1 fragment was added after 20 min of initial incubation, and 4-μl samples were taken after 1, 2, 5, 10, 20, and 30 min of further incubation. The samples were mixed with 2 μl of 3× sequencing gel loading dye (ThermoFisher Scientific), boiled for 10 min, and run on a 12% polyacrylamide, 7 m urea sequencing gel at 200 V. Gels were scanned on a Typhoon 9400 variable mode imager, and the resulting bands were quantified using ImageQuant 5.2. To monitor the phosphatase activity, we followed a previously published protocol (16), except that we used a single-stranded 24-mer substrate.
Mammalian expression plasmids
Mammalian expression plasmids expressing WT XRCC1 and PNKP were generated by inserting cDNA encoding these proteins using AscI–RsrII (XRCC1 and mutants) and AsiSI–MluI (PNKP) restriction sites in pCMV6-AN–mGFP and pCMV6-AN–mRFP mammalian expression plasmids (Origene), respectively). The following primers were used to generate the XRCC1 cDNA: forward 5′-AGCAGCGCGGCGCGCCAATGCCGGAGATCCGCCT-3′ and reverse 5′-AGCAGCGCCGGTCCGTCAGGCTTGCGCCACCA-3′. The primers used to generate PNKP cDNA were forward 5′-AGCAGCGCGCGATCGCCATGGGCGAGGTGGAG-3′ and reverse 5′-AGCAGCGCACGCGTTCAGCCCTCGGAGAA-3′. Point mutations encoding for the changes to amino acids R280H and R194W were introduced into WT XRCC1 using the overlap extension PCR technique as described above. All generated plasmids were sequence-verified.
Cell culture and transfection
The Chinese hamster ovary cell line, EM9, was kindly provided by Dr. Keith Caldecott (University of Sussex, United Kingdom) and cultured in Dulbecco's modified Eagle's medium/F-12 supplemented with 10% fetal calf serum. For transfection, cells were grown on 35-mm glass bottom dishes (MatTek Corp., Ashland, MA), and the following day cells were transfected with DNA constructs of interest using Turbofectin 8.0 (OriGene, Rockville, MD, catalog no. TF81001) according to the manufacturer's protocol. Cells were used for live cell imaging 24–48 h post-transfection.
Laser micro-irradiation
For two-photon laser micro-irradiation, cells were grown on 35-mm glass bottom dishes. Before laser micro-irradiation, cells were incubated in medium containing Hoechst 33258 (Sigma, catalog no. 94403) to a final concentration of 1 μg/ml for 20 min, which was then replaced with fresh medium for 10 min. Subsequently, cells were placed on a 37 °C heated stage of a Zeiss LSM510 NLO laser-scanning confocal microscope. Micro-irradiation was carried out using a near IR titanium sapphire laser. To introduce damage within nuclei of individual cells, a 1.2-μm-wide region was pre-defined and subsequently micro-irradiated with 10 iterations of a 750-nm laser line at 10% power using a Plan-Neofluar ×40/1.3 NA oil immersion objective. For time-lapse experiments of mRFP-tagged proteins, the fluorescent signal was recorded using excitation with a 543-nm He-Ne laser and a 559–634-nm bandpass emission filter. Similarly, for mGFP and enhanced GFP-tagged proteins, the signal was recorded after excitation with a 488-nm argon laser and a 515–540-nm bandpass emission filter. Cells with low to medium expression levels of fluorescent proteins were selected, and accumulation of fluorescently tagged protein in micro-irradiated areas was quantified and compared with that in unirradiated regions. The intensity was normalized so that the total cell intensity remained constant throughout the experiment. This process compensates for photobleaching during acquisition. Images were then realigned using ImageJ software, and subsequently fluorescence signals of the exported Tiff images were quantified using Metamorph software 6.0 (Molecular Devices). Results of plotted recruitment curves represent averages of at least two independent experiments. Typically 12 cells were monitored for each data point.
Author contributions
R. S. M., I. M., M. J. H., J. N. M. G., and M. W. conceptualization; R. S. M., I. M., I. A., and M. F. data curation; R. S. M., I. M., I. A., and M. F. investigation; R. S. M., I. M., I. A., and M. F. methodology; R. S. M., I. A., and J. N. M. G. writing-original draft; M. J. H., J. N. M. G., and M. W. supervision; M. J. H. and M. W. writing-review and editing; J. N. M. G. and M. W. project administration; M. W. formal analysis; M. W. funding acquisition.
Supplementary Material
Acknowledgments
We thank Dr. Tom Ellenberger (Washington University School of Medicine, St. Louis, MO) and Dr. Keith Caldecott (University of Sussex, United Kingdom) for providing reagents and cells.
This work was supported by Grant MOP 15385 from the Canadian Institutes of Health Research (to M. W. and M. J. H.), Grant PO1CA092584 from the National Institutes of Health (to J. N. M. G.), and Alberta Cancer Foundation Graduate Studentship Grant ACF GSA 26283 (to I. A.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S2.
- PNKP
- polynucleotide kinase/phosphatase
- XRCC1
- X-ray repair cross-complementing 1
- ssDNA
- single-stranded DNA
- SNP
- single-nucleotide polymorphism
- FHA
- forkhead-associated
- RIR
- Rev1-interacting region
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- nt
- nucleotide
- AC
- 6-acryloyl-2-diaminonaphthalene (acrylodan)
- mRFP
- monomeric red fluorescent protein
- mGFP
- monomeric green fluorescent protein
- Ni-NTA
- nickel-nitrilotriacetic acid.
References
- 1. Bernstein N. K., Karimi-Busheri F., Rasouli-Nia A., Mani R., Dianov G., Glover J. N., and Weinfeld M. (2008) Polynucleotide kinase as a potential target for enhancing cytotoxicity by ionizing radiation and topoisomerase I inhibitors. Anticancer Agents Med. Chem. 8, 358–367 10.2174/187152008784220311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allinson S. L. (2010) DNA end-processing enzyme polynucleotide kinase as a potential target in the treatment of cancer. Future Oncol. 6, 1031–1042 10.2217/fon.10.40 [DOI] [PubMed] [Google Scholar]
- 3. Weinfeld M., Mani R. S., Abdou I., Aceytuno R. D., and Glover J. N. (2011) Tidying up loose ends: the role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem. Sci. 36, 262–271 10.1016/j.tibs.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiederhold L., Leppard J. B., Kedar P., Karimi-Busheri F., Rasouli-Nia A., Weinfeld M., Tomkinson A. E., Izumi T., Prasad R., Wilson S. H., Mitra S., and Hazra T. K. (2004) AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 15, 209–220 10.1016/j.molcel.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 5. Loizou J. I., El-Khamisy S. F., Zlatanou A., Moore D. J., Chan D. W., Qin J., Sarno S., Meggio F., Pinna L. A., and Caldecott K. W. (2004) The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell 117, 17–28 10.1016/S0092-8674(04)00206-5 [DOI] [PubMed] [Google Scholar]
- 6. Audebert M., Salles B., Weinfeld M., and Calsou P. (2006) Involvement of polynucleotide kinase in a poly(ADP-ribose) polymerase-1-dependent DNA double-strand breaks rejoining pathway. J. Mol. Biol. 356, 257–265 10.1016/j.jmb.2005.11.028 [DOI] [PubMed] [Google Scholar]
- 7. Jilani A., Ramotar D., Slack C., Ong C., Yang X. M., Scherer S. W., and Lasko D. D. (1999) Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3′-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. J. Biol. Chem. 274, 24176–24186 10.1074/jbc.274.34.24176 [DOI] [PubMed] [Google Scholar]
- 8. Karimi-Busheri F., Daly G., Robins P., Canas B., Pappin D. J., Sgouros J., Miller G. G., Fakhrai H., Davis E. M., Le Beau M. M., and Weinfeld M. (1999) Molecular characterization of a human DNA kinase. J. Biol. Chem. 274, 24187–24194 10.1074/jbc.274.34.24187 [DOI] [PubMed] [Google Scholar]
- 9. Whitehouse C. J., Taylor R. M., Thistlethwaite A., Zhang H., Karimi-Busheri F., Lasko D. D., Weinfeld M., and Caldecott K. W. (2001) XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104, 107–117 10.1016/S0092-8674(01)00195-7 [DOI] [PubMed] [Google Scholar]
- 10. Lu M., Mani R. S., Karimi-Busheri F., Fanta M., Wang H., Litchfeld D. W., and Weinfeld M. (2010) Independent mechanisms of stimulation of polynucleotide kinase/phosphatase by phosphorylated and non-phosphorylated XRCC1. Nucleic Acids Res. 38, 510–521 10.1093/nar/gkp1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Della-Maria J., Hegde M. L., McNeill D. R., Matsumoto Y., Tsai M. S., Ellenberger T., Wilson D. M. 3rd, Mitra S., and Tomkinson A. E. (2012) The interaction between polynucleotide kinase phosphatase and the DNA repair protein XRCC1 is critical for repair of DNA alkylation damage and stable association at DNA damage sites. J. Biol. Chem. 287, 39233–39244 10.1074/jbc.M112.369975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mani R. S., Fanta M., Karimi-Busheri F., Silver E., Virgen C. A., Caldecott K. W., Cass C. E., and Weinfeld M. (2007) XRCC1 stimulates polynucleotide kinase by enhancing its damage discrimination and displacement from DNA repair intermediates. J. Biol. Chem. 282, 28004–28013 10.1074/jbc.M704867200 [DOI] [PubMed] [Google Scholar]
- 13. Cherry A. L., Nott T. J., Kelly G., Rulten S. L., Caldecott K. W., and Smerdon S. J. (2015) Versatility in phospho-dependent molecular recognition of the XRCC1 and XRCC4 DNA-damage scaffolds by aprataxin-family FHA domains. DNA Repair 35, 116–125 10.1016/j.dnarep.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horton J. K., Stefanick D. F., Çağlayan M., Zhao M. L., Janoshazi A. K., Prasad R., Gassman N. R., and Wilson S. H. (2018) XRCC1 phosphorylation affects aprataxin recruitment and DNA deadenylation activity. DNA Repair 64, 26–33 10.1016/j.dnarep.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breslin C., and Caldecott K. W. (2009) DNA 3′-phosphatase activity is critical for rapid global rates of single-strand break repair following oxidative stress. Mol. Cell. Biol. 29, 4653–4662 10.1128/MCB.00677-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breslin C., Mani R. S., Fanta M., Hoch N., Weinfeld M., and Caldecott K. W. (2017) The Rev1 interacting region (RIR) motif in the scaffold protein XRCC1 mediates a low-affinity interaction with polynucleotide kinase/phosphatase (PNKP) during DNA single-strand break repair. J. Biol. Chem. 292, 16024–16031 10.1074/jbc.M117.806638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanssen-Bauer A., Solvang-Garten K., Gilljam K. M., Torseth K., Wilson D. M. 3rd., Akbari M., and Otterlei M. (2012) The region of XRCC1, which harbours the three most common nonsynonymous polymorphic variants, is essential for the scaffolding function of XRCC1. DNA Repair 11, 357–366 10.1016/j.dnarep.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fang Z., Chen F., Wang X., Yi S., Chen W., and Ye G. (2013) XRCC1 R194W and R280H polymorphisms increase bladder cancer risk in Asian population: evidence from a meta-analysis. PLoS ONE 8, e64001 10.1371/journal.pone.0064001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y., Wang Y., Wu J., and Li L. J. (2013) XRCC1 R194W polymorphism is associated with oral cancer risk: evidence from a meta-analysis. Tumour Biol. 34, 2321–2327 10.1007/s13277-013-0779-y [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y., Wang M., Gu D., Wu D., Zhang X., Gong W., Tan Y., Zhou J., Wu X., Tang C., Zhang Z., and Chen J. (2013) Association of XRCC1 gene polymorphisms with the survival and clinicopathological characteristics of gastric cancer. DNA Cell Biol. 32, 111–118 10.1089/dna.2012.1840 [DOI] [PubMed] [Google Scholar]
- 21. Chen J., Wang H., and Li Z. (2017) Association between polymorphisms of X-ray repair cross complementing group 1 gene and pancreatic cancer risk: a systematic review with meta-analysis. Pathol. Oncol. Res. 2017, 10.1007/s12253-017-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang L., Qian J., Ying C., Zhuang Y., Shang X., and Xu F. (2016) X-ray cross-complementing groups 1 rs1799782 C>T polymorphisms and colorectal cancer susceptibility: A meta-analysis based on Chinese Han population. J. Cancer Res. Ther. 12, C264–C267 [DOI] [PubMed] [Google Scholar]
- 23. Lau T. P., Lian L. H., Cheah P. L., Looi L. M., Roslani A. C., Goh K. L., Lee P. C., and Chua K. H. (2017) Lack of correlation between X-ray repair cross-complementing group 1 gene polymorphisms and the susceptibility to colorectal cancer in a Malaysian cohort. Eur. J. Cancer Prev. 26, 506–510 10.1097/CEJ.0000000000000336 [DOI] [PubMed] [Google Scholar]
- 24. Palmirotta R., Carella C., Silvestris E., Cives M., Stucci S. L., Tucci M., Lovero D., and Silvestris F. (2018) SNPs in predicting clinical efficacy and toxicity of chemotherapy: walking through the quicksand. Oncotarget 9, 25355–25382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prendergast F. G., Meyer M., Carlson G. L., Iida S., and Potter J. D. (1983) Synthesis, spectral properties, and use of 6-acryloyl-2-dimethylaminonaphthalene (Acrylodan). A thiol-selective, polarity-sensitive fluorescent probe. J. Biol. Chem. 258, 7541–7544 [PubMed] [Google Scholar]
- 26. Mani R. S., and Kay C. M. (1993) Calcium-dependent regulation of the caldesmon-heavy meromyosin interaction by caltropin. Biochemistry 32, 11217–11223 10.1021/bi00092a035 [DOI] [PubMed] [Google Scholar]
- 27. Mani R. S., Karimi-Busheri F., Fanta M., Caldecott K. W., Cass C. E., and Weinfeld M. (2004) Biophysical characterization of human XRCC1 and its binding to damaged and undamaged DNA. Biochemistry 43, 16505–16514 10.1021/bi048615m [DOI] [PubMed] [Google Scholar]
- 28. Kobayashi M., Ab E., Bonvin A. M., and Siegal G. (2010) Structure of the DNA-bound BRCA1 C-terminal region from human replication factor C p140 and model of the protein–DNA complex. J. Biol. Chem. 285, 10087–10097 10.1074/jbc.M109.054106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanssen-Bauer A., Solvang-Garten K., Sundheim O., Peña-Diaz J., Andersen S., Slupphaug G., Krokan H. E., Wilson D. M. 3rd., Akbari M., and Otterlei M. (2011) XRCC1 coordinates disparate responses and multiprotein repair complexes depending on the nature and context of the DNA damage. Environ. Mol. Mutagen 52, 623–635 10.1002/em.20663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Masson M., Niedergang C., Schreiber V., Muller S., Menissier-de Murcia J., and de Murcia G. (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell. Biol. 18, 3563–3571 10.1128/MCB.18.6.3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El-Khamisy S. F., Masutani M., Suzuki H., and Caldecott K. W. (2003) A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 31, 5526–5533 10.1093/nar/gkg761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caldecott K. W., Aoufouchi S., Johnson P., and Shall S. (1996) XRCC1 polypeptide interacts with DNA polymerase β and possibly poly(ADP-ribose) polymerase, and DNA ligase III is a novel molecular “nick-sensor” in vitro. Nucleic Acids Res. 24, 4387–4394 10.1093/nar/24.22.4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kubota Y., Nash R. A., Klungland A., Schär P., Barnes D. E., and Lindahl T. (1996) Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase β and the XRCC1 protein. EMBO J. 15, 6662–6670 10.1002/j.1460-2075.1996.tb01056.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Caldecott K. W., McKeown C. K., Tucker J. D., Ljungquist S., and Thompson L. H. (1994) An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol. Cell. Biol. 14, 68–76 10.1128/MCB.14.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ali A. A., Jukes R. M., Pearl L. H., and Oliver A. W. (2009) Specific recognition of a multiply phosphorylated motif in the DNA repair scaffold XRCC1 by the FHA domain of human PNK. Nucleic Acids Res. 37, 1701–1712 10.1093/nar/gkn1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim I. K., Stegeman R. A., Brosey C. A., and Ellenberger T. (2015) A quantitative assay reveals ligand specificity of the DNA scaffold repair protein XRCC1 and efficient disassembly of complexes of XRCC1 and the poly(ADP-ribose) polymerase 1 by poly(ADP-ribose) glycohydrolase. J. Biol. Chem. 290, 3775–3783 10.1074/jbc.M114.624718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berquist B. R., Singh D. K., Fan J., Kim D., Gillenwater E., Kulkarni A., Bohr V. A., Ackerman E. J., Tomkinson A. E., and Wilson D. M. 3rd. (2010) Functional capacity of XRCC1 protein variants identified in DNA repair-deficient Chinese hamster ovary cell lines and the human population. Nucleic Acids Res. 38, 5023–5035 10.1093/nar/gkq193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marintchev A., Mullen M. A., Maciejewski M. W., Pan B., Gryk M. R., and Mullen G. P. (1999) Solution structure of the single-strand break repair protein XRCC1 N-terminal domain. Nat. Struct. Biol. 6, 884–893 10.1038/12347 [DOI] [PubMed] [Google Scholar]
- 39. Sizova D. V., Keh A., Taylor B. F., and Sweasy J. B. (2015) The R280H X-ray cross-complementing 1 germline variant induces genomic instability and cellular transformation. DNA Repair 31, 73–79 10.1016/j.dnarep.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Taylor R. M., Wickstead B., Cronin S., and Caldecott K. W. (1998) Role of a BRCT domain in the interaction of DNA ligase III-α with the DNA repair protein XRCC1. Curr. Biol. 8, 877–880 10.1016/S0960-9822(07)00350-8 [DOI] [PubMed] [Google Scholar]
- 41. Cuneo M. J., Gabel S. A., Krahn J. M., Ricker M. A., and London R. E. (2011) The structural basis for partitioning of the XRCC1/DNA ligase III-α BRCT-mediated dimer complexes. Nucleic Acids Res. 39, 7816–7827 10.1093/nar/gkr419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stankova K., Ivanova K., Mladenov E., Rosidi B., Sharma A., Boteva R., and Iliakis G. (2012) Conformational transitions of proteins engaged in DNA double-strand break repair, analysed by tryptophan fluorescence emission and FRET. Biochem. J. 443, 701–709 10.1042/BJ20112151 [DOI] [PubMed] [Google Scholar]
- 43. Gabel S. A., DeRose E. F., and London R. E. (2013) XRCC1 interaction with the REV1 C-terminal domain suggests a role in post replication repair. DNA Repair 12, 1105–1113 10.1016/j.dnarep.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mani R. S., Karimi-Busheri F., Fanta M., Cass C. E., and Weinfeld M. (2003) Spectroscopic studies of DNA and ATP binding to human polynucleotide kinase: evidence for a ternary complex. Biochemistry 42, 12077–12084 10.1021/bi030127b [DOI] [PubMed] [Google Scholar]
- 45. Freschauf G. K., Mani R. S., Mereniuk T. R., Fanta M., Virgen C. A., Dianov G. L., Grassot J. M., Hall D. G., and Weinfeld M. (2010) Mechanism of action of an imidopiperidine inhibitor of human polynucleotide kinase/phosphatase. J. Biol. Chem. 285, 2351–2360 10.1074/jbc.M109.055764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Unger T., Jacobovitch Y., Dantes A., Bernheim R., and Peleg Y. (2010) Applications of the restriction free (RF) cloning procedure for molecular manipulations and protein expression. J. Struct. Biol. 172, 34–44 10.1016/j.jsb.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 47. Chen Y. H., Yang J. T., and Chau K. H. (1974) Determination of the helix and β form of proteins in aqueous solution by circular dichroism. Biochemistry 13, 3350–3359 10.1021/bi00713a027 [DOI] [PubMed] [Google Scholar]
- 48. Provencher S. W., and Glöckner J. (1981) Estimation of globular protein secondary structure from circular dichroism. Biochemistry 20, 33–37 10.1021/bi00504a006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.