Abstract
Transglutaminase (TGase) is a Ca2+-dependent cross-linking enzyme, which has both enzymatic and nonenzymatic properties. TGase is involved in several cellular activities, including adhesion, migration, survival, apoptosis, and extracellular matrix (ECM) organization. In this study, we focused on the role of the TGase enzyme in controlling hematopoiesis in the crayfish, Pacifastacus leniusculus. We hypothesized that a high TGase activity could mediate an interaction of progenitor cells with the ECM to maintain cells in an undifferentiated stage in the hematopoietic tissue (HPT). We found here that the reversible inhibitor cystamine decreases the enzymatic activity of TGase from crayfish HPT, as well as from guinea pig, in a concentration-dependent manner. Cystamine injection decreased TGase activity in HPT without affecting production of reactive oxygen species. Moreover, the decrease in TGase activity in the HPT increased the number of circulating hemocytes. Interestingly the cystamine-mediated TGase inhibition reduced aggressive behavior and movement in crayfish. In conclusion, we show that cystamine-mediated TGase inhibition directly releases HPT progenitor cells from the HPT into the peripheral circulation in the hemolymph and strongly reduces aggressive behavior in crayfish.
Keywords: hematopoietic stem cells, hematopoiesis, extracellular matrix, transglutaminase, invertebrate, crustacean, blood cell, cystamine, arthropod, hemocyte, hemolymph
Introduction
Transglutaminase (TGase)2 is a multifunctional enzyme that participates in controlling a wide range of cellular activities (1). Dysfunction of TGase enzymes is proposed to have roles in the development of many diseases, such as Alzheimer's disease, Parkinson's disease, and Huntington's disease (2). Nine isoforms of TGase have been identified in the human genome, and these isoforms play different roles depending on their tissue distribution, subcellular localization, and substrate specificity. For example TGases are involved in blood coagulation, skin formation, and signal transduction (3, 4). The catalytic activity of TGase is a Ca2+-dependent posttranslational modification of proteins by generating covalent bonds between free amine groups to form an ϵ-(γ-glutamyl)-lysine isopeptide bond (1). In addition to this Ca2+-dependent reaction, Ca2+-independent enzymatic as well as nonenzymatic activities of TGase have been described. Tissue TGase or TGase2 is a well-studied enzyme of this family. The broad substrate specificity and interactions with many genes or proteins by TGase2 explain its multiple biological functions including cell adhesion, migration, growth, survival, apoptosis, differentiation, and extracellular matrix organization (4). The nonenzymatic functions are based on the noncovalent interactions of TGase acting as an adapter protein and in this way mediating adhesion/signaling at the cell surface (5).
In invertebrates, TGase was first identified in the horseshoe crab (Tachypleus tridentatus) (6) and then reported in many species such as crayfish (Pacifastacus leniusculus) (7), shrimp (Litopenaeus vannamei) (8), and the fruit fly (Drosophila melanogaster) (9, 10). However, the invertebrate TGases described so far are mostly involved in coagulation or clot formation, which is the same function as human factor XIIIa or plasma TGase (7, 11). Additionally, the sequence of invertebrate TGase shows a high similarity with human factor XIIIa. Human factor XIIIa catalyzes the formation of ϵ-(γ-glutamyl)-lysine isopeptide bonds between fibrin– strands to stabilize the fibrin clot during coagulation (12). In crustaceans, clot formation is directly induced by the cross-linking activity of TGase on a clotting protein (CP), first described in crayfish (13). In response to wounding or infection, TGase is released from hemocytes or the hematopoietic tissue (HPT), and then CP molecules are cross-linked by TGase into large aggregates (13). Recently, CP was also found to function as an extracellular matrix (ECM) component in crayfish HPT (14). CP functions together with collagen, TGase, and the cytokine astakine 1 (Ast1) in the regulation of HPT progenitor cell behavior (14). The important roles of TGase in hematopoietic regulation have been studied in detail in crayfish, and high extracellular TGase activity mediates the interaction of progenitor cells with the environment to maintain cells in an undifferentiated stage in crayfish HPT (15, 16). Furthermore, the crayfish TGase regulatory promoter region contains GATA binding motifs, as also found in the human factor XIIIa gene (16). GATA is a transcription factor that plays a crucial role during hematopoietic development. GATA family proteins function in lineage specification and differentiation of hematopoietic progenitor cells (17). The presence of the GATA motif in crayfish TGase genes highlights the potential roles of TGase in hematopoietic regulation like that occurring in mammals (16).
In this study, we focused on the roles of the TGase enzyme in controlling hematopoiesis in the crayfish, P. leniusculus. Cystamine, a small disulfide-containing molecule, was used to inhibit TGase activity in both in vivo and in vitro experiments.
Results
Cystamine inhibits TGase enzymatic activity
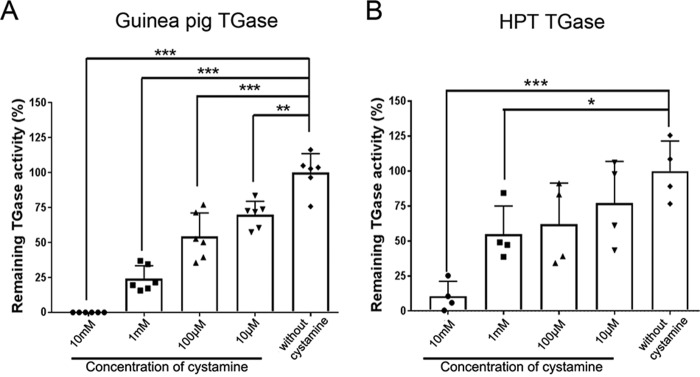
To examine the important role of TGase activity on hematopoiesis, we used cystamine, a reversible TGase inhibitor, to inhibit TGase activity. First, we investigated whether cystamine could inhibit TGase enzyme activity from crayfish HPT lysate. Cystamine at different final concentrations of 10 μm, 100 μm, 1 mm, and 10 mm were tested. The inhibitory efficiency of cystamine on crayfish TGase was compared with the inhibition of a purified commercial guinea pig liver TGase (Fig. 1). By using a nonradioactive microtiter plate assay, which measured TGase-mediated BPHN2 incorporation, the highest concentration of cystamine at 10 mm significantly reduced (p < 0.001) TGase activity from guinea pig compared with control (without cystamine) (Fig. 1A). At the same concentration of cystamine (p < 0.001), the remaining activity was 10% for TGase from HPT compared with the control (Fig. 1B). The remaining activity of both TGase from guinea pig and TGase from HPT was decreased in a dose-dependent manner according to the concentration of cystamine (Fig. 1, A and B). These results indicate that cystamine is efficient in inactivating crayfish TGase as well as guinea pig TGase.
Figure 1.
Inhibition by cystamine on TGase activity in vitro. A, inhibition of commercial TGase from guinea pig liver by different concentrations of cystamine. B, inhibition of TGase activity from HPT lysate. Each symbol represents an individual crayfish. The columns represent the mean from four to six crayfish, and the error bar represents the S.D. value. *, p < 0.05, **, p < 0.01, and ***, p < 0.001 indicate a significant difference compared with the control.
Cystamine injection has a direct inhibitory effect on TGase activity in the HPT in vivo
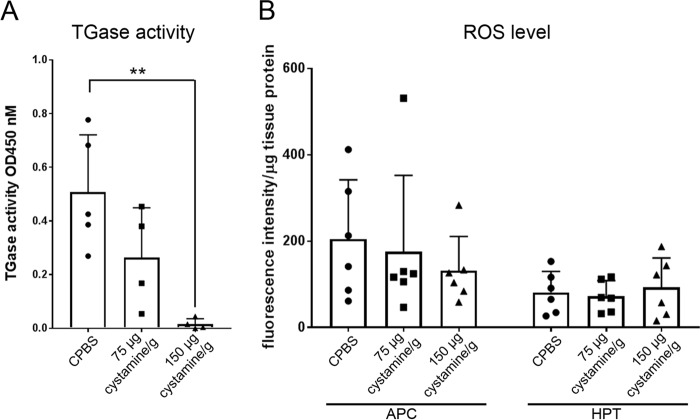
To investigate whether cystamine could inhibit TGase activity in HPT tissue in vivo, TGase activity in HPT tissue at 3 h post-cystamine injection was determined (Fig. 2A). A significant decrease in TGase activity in the HPT was observed (p < 0.01) in animals injected with 150 μg cystamine/g crayfish compared with those injected with crayfish phosphate-buffered saline (CPBS) (Fig. 2A). However, cystamine at a concentration of 75 μg cystamine/g only slightly inhibited TGase activity when compared with CPBS-injected animals (Fig. 2A). It is known that cystamine may have multiple mechanisms and has been reported to increase GSH production and then reduce oxidative stress–induced cell death (18). We showed previously that low ROS level induced by N-acetylcysteine injection could prolong the decrease in hemocyte numbers by increasing extracellular TGase activity, thereby maintaining the cells inside the HPT (15). Therefore, we examined the effect of cystamine on ROS production after TGase inhibition by cystamine injection. However, cystamine injection had no effect on the ROS level at 1 or 3 h (Fig. 2B). Thus, it seems that cystamine has a direct effect on TGase activity without interfering with ROS production.
Figure 2.
Effect of cystamine on in vivo TGase activity in HPT and ROS production in vivo. A, TGase activity in HPT at 3 h after injection of cystamine (75 μg cystamine/g or 150 μg cystamine/g) or CPBS as a control. The columns represent the mean from four to five crayfish, and the error bar represents the S.D. value. Each symbol represents an individual crayfish. **, p < 0.01 indicates a significant difference compared with the control. B, ROS production at 3 h post-injection of cystamine (75 μg cystamine/g and 150 μg cystamine/g) or CPBS as a control. The ROS level was calculated per microgram of tissue protein for each individual crayfish. Six crayfish were used in each experimental group. The columns represent the mean from six crayfish, and the error bar represents the S.D. value. Each symbol represents individual crayfish.
TGase activity inhibition is involved in new hemocyte synthesis
A high level of extracellular TGase activity was found in less differentiated progenitor cells residing in the crayfish HPT compared with cells migrating out of the tissue (16). These results suggest the involvement of TGase activity in the regulation of hematopoiesis. Because cystamine significantly reduced TGase activity, and to verify whether TGase inhibition caused by cystamine could affect new hemocyte production, the differential hemocyte number of granular cells (GC) and semigranular cells (SGC) was determined as well as the total number of circulating hemocyte cells (THC) after inhibition of TGase activity by cystamine injection. CPBS-injected animals served as controls (Fig. 3). Two different doses of cystamine were injected into crayfish (75 μg cystamine/g and 150 μg cystamine/g), and at the higher concentration of cystamine the hemocyte numbers increased significantly at 1 h (p < 0.05 for GC; p < 0.001 for SGC and THC). The hemocyte numbers, which increased gradually from 1 to 6 h, were restored to a normal level at 24 h (Fig. 3). Injection of cystamine at 75 μg cystamine/g similarly increased the circulating hemocyte number but to a lesser extent at later time points compared with cystamine at 150 μg cystamine/g. The hemocyte numbers (GC, SGC, and THC) increased significantly at 6 h (p < 0.01 for GC and SGC; p < 0.05 for THC) and were restored to normal levels after 24 h (Fig. 3). The inhibitory effect of cystamine on TGase activity in HPT was concentration-dependent. In addition, TGase activity in HPT was not significantly different when the number of circulating hemocytes was restored to normal level at 24 h (data not shown). The results of this experiment suggest that inhibition of TGase by cystamine may stimulate the differentiation of hematopoietic progenitor cells in the HPT and then promote the release of these cells into the peripheral circulation.
Figure 3.
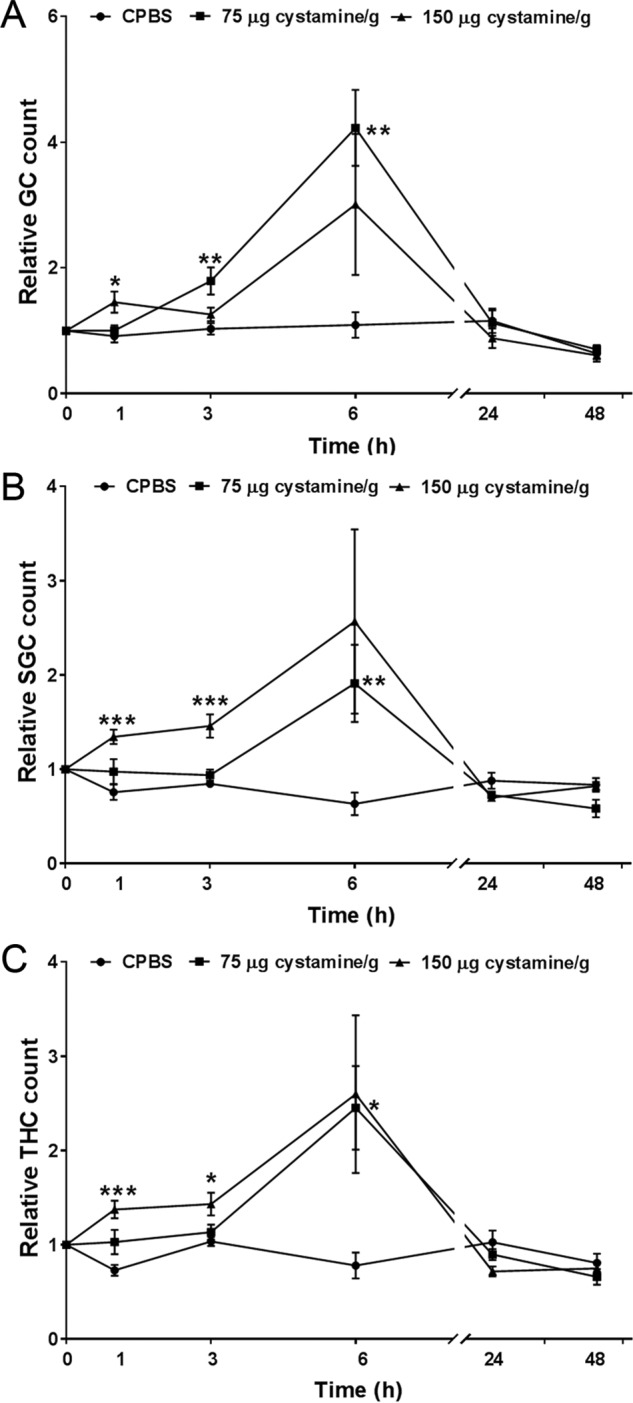
In vivo effect of cystamine injection on the circulating hemocyte number. A, relative GC count, B, relative SGC count, and C, relative THC count at 1, 3, 6, 24, and 48 h post-injection of cystamine (75 μg cystamine/g (■) and 150 μg cystamine/g (▴)) or CPBS (●) as a control. Four to 12 crayfish were used in each experimental group. The data points represent the mean from four to 12 crayfish, and the error bar represents the S.D. value. *, p < 0.05, **, p < 0.01, and ***, p < 0.001 indicate a significant difference compared with the control.
TGase activity mediated release of progenitor cells HPT into the circulation
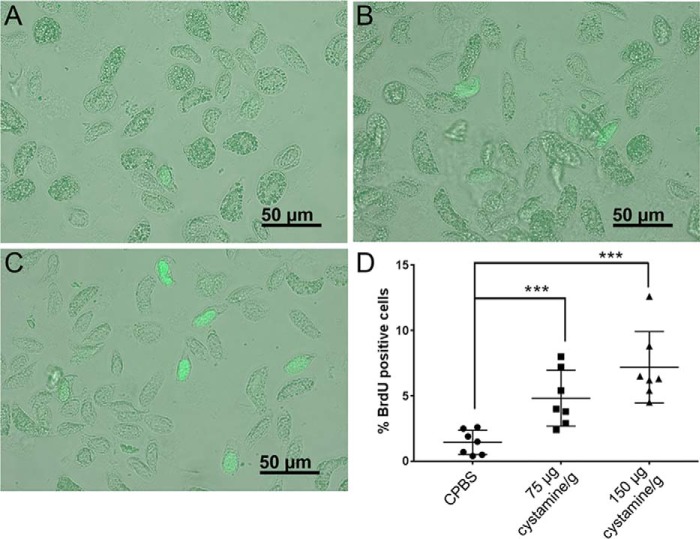
The cross-linking function of TGase was shown to be essential in mediating the interaction between progenitor cells with ECM proteins such as collagen and CP in crayfish (14–16, 19). To determine whether the inhibition of TGase activity has an effect on hematopoietic progenitor cell proliferation, 5-bromo-2′-deoxyuridine (BrdU) incorporation was performed in HPT after cystamine injection. However, there was no clear difference in BrdU-positive cell numbers in HPT after cystamine injection (data not shown), but the number of proliferating cells (BrdU-labeled cells) in hemolymph was significantly increased in the groups injected with 75 μg cystamine/g or 150 μg cystamine/g (Fig. 4). The presence of circulating BrdU-labeled cells may indicate that newly synthesized hemocytes are released into the circulation when TGase in the HPT is inhibited. Because we did not observe a notable increase of BrdU-positive cells in the HPT, our conclusion is that inhibition of TGase activity in HPT affected progenitor cell behavior by stimulating the release of new hemocytes into the hemolymph.
Figure 4.
BrdU-positive cells in the circulation 3 h after injection of cystamine. A, BrdU-positive cells (green) in the CPBS-injected group; B, BrdU-positive cells in the 75 μg cystamine/g–injected group; and C, BrdU positive-cells in the 150 μg cystamine/g–injected group. D, percentage of BrdU-positive cells relative to total number of hemocytes after cystamine injection at 3 h. Seven crayfish were used in each experimental group. Each symbol represents an individual crayfish. Each symbol represents an individual crayfish, and the data are shown as the mean ± S.D.
Inhibition of TGase activity by cystamine induces changes in crayfish behavior
Cystamine injection clearly suppressed the mobility of the crayfish (Figs. 5 and 6). Normal or CPBS-injected crayfish usually exhibit aggressive behavior (Fig. 5). This aggressive behavior includes attacking, fighting over resources, raising the claw for grabbing, and/or holding the object (Fig. 6 and Movie S1). Injection at a concentration of 75 μg cystamine/g suppressed the frequency of movements and also induced a fear response (Fig. 6 and Movie S2). A fear response is defined as a nonfighting behavior during which the animal tries to escape from resources or objects. This behavior disappeared and returned to normal aggressive behavior after 24 h (Fig. 6 and Movie S4 and S5). Interestingly, cystamine at a concentration of 150 μg/g injection clearly induced a “nonmovement” behavior (Fig. 6 and Movie S3). Crayfish injected with 150 μg cystamine/g were completely motionless or moved very little with any part of the body (Figs. 5 and 6, and Movie S3 and S6). This immobility behavior was observed at 1 to 24 h after injection of 150 μg cystamine/g, with restoration to normal active or aggressive behavior after 96 h (Fig. 6). The behavior that we observed corresponds to TGase activity inhibition and also to the increase in circulating hemocytes after cystamine injection.
Figure 5.
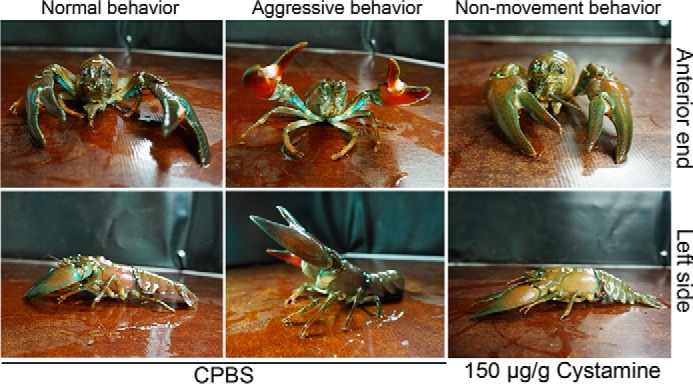
Characteristics of crayfish behavior after cystamine injection. Three h after CPBS injection: anterior and left side views show the resting position of normal behavior or an aggressive behavior. Three h after injection of 150 μg cystamine/g: anterior and left side views show the nonmovement behavior.
Figure 6.

The effect of cystamine on crayfish behavior. After injection, the behavior of the individual crayfish in each group was recorded in a 3-min video at 3, 6, 24, 48, and 96 h. The pictures show in still frames from a video used to determine the different activities of crayfish after cystamine injection. The activities of crayfish in each group were compared with CPBS injected crayfish as a control. The crayfish behavior was analyzed based on their mobility, immobility, aggressiveness, and activities. 20 crayfish were used in each of these experiments.
Discussion
The balance between self-renewal and differentiation of hematopoietic progenitor cells is tightly regulated by a variety of cells and signals, which include the microenvironment or niche (20). In crayfish, the progenitor cells are located in packed lobules or between lobules, which are surrounded by connective tissue and ECM (21). TGase is an abundant protein in HPT and hemocytes, particularly in SGC (16). The co-localization of extracellular TGase with ECM proteins, for example collagen and CP in HPT, has been reported (14, 15). Furthermore, the proliferating cells residing in crayfish HPT have a high extracellular TGase activity compared with differentiated cells or cells migrating out of the tissue (16). These results suggest the involvement of TGase enzyme activity in hematopoietic regulation.
A well-known TGase inhibitor, cystamine, was used to inhibit TGase activity in crayfish HPT. Cystamine inhibits TGase by forming a mixed disulfide with the active-site thiol through a thiol–disulfide interchange mechanism (22). The inhibitory effect of cystamine to TGase has been characterized in many species except crustaceans. In Drosophila, cystamine treatment prevents photoreceptor degeneration and enhances neurodegeneration in a spinocerebellar ataxia (SCA3) mutant (23). In human WI-38 lung cells, a reduction in TGase activity has been observed in a dose-dependent manner by supplementing cystamine into the culture medium (24). In addition, cystamine at a concentration of 10 mm completely reduces TGase activity in these cells (24). As in human WI-38 lung cells, the addition of cystamine completely reduced the TGase activity of commercially purified guinea pig liver TGase and decreased the activity of crayfish TGase from HPT tissue to 10% of the original activity. The inhibitory effect of cystamine on TGase activity was observed in a concentration-dependent manner.
The importance of TGase in hematopoietic regulation was first studied in crayfish (16) and later reported in other species. In black tiger shrimp, Penaeus monodon, the TGase isoform I (STG I) is expressed mainly in hematopoietic tissue (25) and is not involved in coagulation (26). In addition, a lipopolysaccharide injection stimulates the secretion of STG I and astakine in plasma to induce the HPT cell proliferation (25, 27). In white shrimp, L. vannamei, the knockdown of LvTG II mRNA expression induces an increase in hyaline cell number (28). In crayfish, the knockdown of TGase mRNA transcript promotes HPT cell spreading in vitro (16). We therefore investigated the number of circulating hemocytes after inhibiting TGase activity by cystamine injection. Inhibition of TGase activity by injecting 150 μg cystamine/g resulted in a gradual increase in relative hemocyte numbers (GC, SGC, and THC) from 1 to 6 h, returning to normal levels after 24 h in the circulation. The transient effect of cystamine may be because the cystamine is metabolized after injection. Cystamine has been shown to be quickly metabolized into cysteamine and then converted to cysteine, hypotaurine, and taurine, which are endogenous cellular components commonly used as building blocks for several proteins (29). By using HPLC to measure the level of the metabolized form of cystamine in mice plasma, cysteamine could be detected at 1–3 h after a single injection of 50 and 200 mg cystamine/kg dose, diminishing progressively at 48 h in mice (30). In addition to inhibiting TGase activity, cystamine exhibits antioxidant properties by increasing the l-cysteine level and enhancing the GSH system (31). In crayfish, cystamine injection had an effect on TGase activity in HPT at 3 h, whereas no effect on ROS production in the tissue was observed. A high ROS level has been observed in the anterior proliferation center (APC) before the recovery of hemocyte numbers following a lipopolysaccharide or laminarin injection (32). The APC is a small area in the middle of the anterior HPT and is located close to the brain (32). We also showed that cystamine injection had no influence on ROS production in the APC. We have reported previously that a low ROS level, caused by a N-acetylcysteine injection, induced an increase in extracellular TGase activity and decreased the number of circulating hemocytes (15). Taken together, our results indicate that ROS signaling could affect extracellular TGase activity in controlling hematopoiesis. However, TGase inhibition did not interfere with the ROS level in the APC or the whole HPT.
In an MC3T3 osteoblast cell culture, the inhibition of TGase activity by cystamine treatment results in a decrease of ECM accumulation and stimulates the differentiation of osteoblast cells (33). In crayfish, TGase activity is needed to mediate cell–ECM interactions and to provide a suitable environment for the progenitor cells (14). We hypothesized that TGase inhibition by cystamine injection could interfere with the cell–ECM interaction and promote the differentiation of progenitor cells, thereby inducing cell migration out of the HPT, as we found a significant release of BrdU-labeled cells in the hemolymph at 3 h after cystamine injection.
Cystamine, reported to prevent the formation of Huntingtin protein aggregates, exhibits a neuroprotective effect against cytotoxicity in Parkinson's disease in mice (34). In addition, cystamine rescues the dopaminergic system by increasing the number of dopaminergic neurons; these protective effects result in a prolonged life span and improvement in motor behaviors in an animal model (R6/2 mice) of Parkinson's disease (35). Cystamine injection in crayfish caused a dramatic change in locomotion behavior in a dose-dependent manner. In normal or CPBS-injected crayfish, aggressive and fighting behavior including raising the claw or grabbing the object is usually easily observed. At a high-dose cystamine injection of 150 μg cystamine/g, crayfish displayed a nonmovement behavior remaining entirely motionless or very slow-moving. Cystamine injection at a concentration of 200 mg cystamine/kg can induce signs of hypothermia (shivering) and drowsiness for a period of ∼2 h in mice (36). In crayfish, the impaired movement caused by cystamine injection could be observed from 1 to 24 h (75 μg cystamine/g dose) and from 1 to 48 h (150 μg cystamine/g dose). These changes in behavior of crayfish after cystamine injection corresponded to the inhibitory effect of cystamine on TGase activity and the increase in the numbers of circulating hemocytes. Furthermore, the changed behavior of crayfish had a similarity to the pharmacokinetic properties of cystamine. In adult male Sprague-Dawley rats injected with 250 mg of cysteamine/kg, which is a reduced form of cystamine, the result was tremors in the neck and head and changed behavior (37). Our results indicate a new function for TGase in controlling crayfish movement. However, cystamine injection could have other effects on the nervous system in controlling behavior. Thus, the role of cystamine on TGase activity in the nervous system needs further investigation. In conclusion, we have shown an important role for TGase in the direct regulation of hematopoiesis by mediating interactions between the cells and the ECM in HPT and, in addition, a putative role for TGase in regulating crayfish locomotion.
Experimental procedures
Animals
Freshwater crayfish, P. leniusculus, were from Lake Erken, Sweden. The animals were maintained in aquaria with aeration at 10 °C. Healthy and intermolt male crayfish were used for the experiments.
TGase enzyme preparation
To prepare TGase, HPT was freshly dissected as described previously (17). The tissue was then homogenized in 100 μl of radioimmune precipitation assay buffer (50 mm Tris, 150 mm NaCl, 10 mm EDTA, 1% IGEPAL, and 1% SDS, pH 7.5) containing 10× diluted protease inhibitor mixture (Hoffmann-La Roche) from the recommended stock solution (10×). After centrifugation of the homogenate at 13,000 × g for 15 min at 4 °C, the supernatant was immediately used in a TGase inhibition assay. The protein concentration was determined by using a Coomassie Plus protein assay reagent (Thermo Fisher Scientific). For a commercial TGase, lyophilized guinea pig liver TGase (T5398) powder (Sigma-Aldrich) was dissolved in 1 ml of dH2O as an enzyme stock solution. One unit of commercial purified TGase was freshly diluted at 1:1000 in 0.1 m Tris-HCl, pH 8.5, before immediate use in the experiments.
TGase activity assay
TGase activity was assayed by using a modified nonradioactive microtiter plate assay (38). Briefly, the microtiter plate was coated with 100 μl of N,N′-dimethylcasein (10 mg/ml) (Sigma-Aldrich) at 4 °C overnight. After blocking with blocking solution (0.5% BSA in 0.1 m Tris-HCl, pH 8.5) for 30 min at room temperature, the wells were washed three times with 0.1 m Tris-HCl, pH 8.5. The incubation mixture, which contained 5 mm CaCl2, 10 mm DTT, 0.1 mm BPHN2 (Thermo Fisher Scientific), 5 μg of protein whole-cell lysates, and 0.1 m Tris-HCl, pH 8.5, was added to obtain a total volume of 100 μl/well. After incubation at 37 °C for 30 min, the reaction was stopped by washing twice with 200 mm EDTA followed by washing three times with 0.1 m Tris-HCl, pH 8.5. Streptavidin–horseradish peroxidase conjugate (GE Healthcare) was diluted to 1:1000 in blocking solution before being added to the wells for a 30-min incubation at room temperature. The plate was washed twice with 0.01% Triton X-100,followed by five washes with 0.1 m Tris-HCl, pH 8.5. Then, 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate solution (TMB, Sigma) was added to each well. After incubation for 5 min at room temperature, the reactions were stopped by the addition of 50 μl of 3N HCl to each well. The TGase cross-linking activity was quantified by measuring the absorbance at 450 nm in a plate reader.
TGase inhibition assay
The inhibition experiments were performed using a modified nonradioactive microtiter plate assay as described above. The reaction mixture consisted of four different concentrations of cystamine at final concentrations of 10 mm, 1 mm, 100 μm, or 10 μm in the presence of a fixed amount of a commercial guinea pig TGase (1 unit of stock commercial guinea pig TGase diluted 1:1000 in 0.1 m Tris-HCl, pH 8.5) or 5 μg of the HPT lysate protein (an endogenous TGase from HPT). The optical density was quantified by measuring the absorbance at 450 nm in a plate reader.
ROS detection
To detect the ROS levels in the tissues, the HPT was dissected and washed twice with PBS (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4 and 2 mm KH2PO4, pH 7.4). A stock solution of 5 mg/ml 2′,7′-dichlorofluorescin diacetate (DCF-DA, Sigma-Aldrich), freshly diluted with PBS (1:1000), was added to the tissues and incubated for 15 min in the dark. After washing three times with PBS, the ROS level was determined using a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The fluorescence intensity was calculated as the fluorescence intensity of the sample minus the fluorescence intensity of PBS without tissue (serving as a baseline value). The results were reported as fluorescence intensity/μg of tissue protein. The protein concentration was determined using a Coomassie Plus protein assay reagent (Thermo Fisher Scientific).
Circulating hemocyte count after cystamine injection
The experiments were performed using 4–12 crayfish (25–30 g) in each experimental group. Prior to the injection of cystamine, one drop of hemolymph was collected and immediately fixed in 10% formalin. The hemocyte number prior to injection was counted and served as the baseline value. After baseline bleeding, the animals were allowed to rest for 48 h. Subsequently, the animals were injected in the base of the fourth walking leg with 1) CPBS as a control, 2) cystamine (75 μg cystamine/g crayfish), or 3) cystamine (150 μg cystamine/g crayfish). The hemolymph concentration of cystamine after injection was estimated with a dye dilution assay using amaranth (Sigma-Aldrich). A working solution at 5 mg/ml was then diluted to a final concentration of 0.625, 1.25, 2.5, 5, 12.5, or 25 μg/ml with crayfish plasma, and absorbance was measured at 520 nm for a standard curve. Then 100 μl of amaranth at a concentration of 5 mg/ml was injected into a walking leg of crayfish. After 2 h the hemolymph was withdrawn and processed to remove hemocyte by centrifugation (800 × g for 10 min at 4 °C). The resulting supernatant was diluted with ice-cold anticoagulant buffer 1:1 (v/v) (16), and absorbance was analyzed at 520 nm. The concentration of dye in the hemolymph sample was examined relative to the standard curve, and the hemolymph volume was calculated using Equation 1,
| (Eq. 1) |
where V is the hemolymph volume, a is the injected volume, C1 is the injected concentration of amaranth, and C2 is the final concentration of amaranth. The average hemolymph volume was estimated to 38 ± 5% (n = 4) relative to body weight. The cystamine injection would then result in an approximate hemolymph concentration of 1.5 or 3 mm after injection of 75 μg cystamine/g crayfish or 150 μg cystamine/g crayfish, respectively.
At 1, 3, 6, 24, and 48 h post-injection, the hemolymph was collected and fixed in 10% formalin. The total hemocyte and differential hemocyte (granular and semigranular) numbers after injection were subsequently counted and reported as relative hemocyte count (hemocyte number after injection divided by the hemocyte number prior to injection).
Cell proliferation detected by BrdU incorporation
To detect the proliferating cells, crayfish were injected with 10 μl/g fresh weight of 50 mm BrdU (Sigma-Aldrich) in CPBS for 24 h before the animals were injected with CPBS or cystamine (75 and 150 μg cystamine/g crayfish). At 3 h post-CPBS and -cystamine injections, the hemocytes were collected and immediately fixed with 4% paraformaldehyde in PBS for 1 h. The fixed hemocytes were treated with 2N HCl for 30 min at room temperature and washed five times, for 15 min each time, with PBST (0.5% Tween 20 in PBS buffer) before overnight incubation with mouse anti-BrdU (1:50) (BD Biosciences) in PBST at 4 °C. Subsequently, the primary antibodies were removed, and the samples were washed 5 times with PBST and incubated for 1 h with FITC-conjugated anti-mouse IgG (1:300) (Life Technologies) and Hoechst 33258 dye at a concentration of 1 μg/ml to stain the nuclei. After washing 5 times with PBST, the number of BrdU-incorporating cells was observed under a fluorescence microscope. The number of BrdU-labeled cells was calculated as positive BrdU cell/total number of cells.
Crayfish behavior after cystamine injection
The crayfish were randomly collected from the holding tanks, separated into groups of 3–5 individuals, and assigned to the control or test group. The crayfish were allowed to adjust to the new surroundings for 48 h before being injected with 1) CPBS as a control, 2) 75 μg cystamine/g crayfish, or 3) 150 μg cystamine/g crayfish. The behavior of individual animals in each experimental group was recorded in a 3-min video at 1, 3, 6, 24, 48, and 96 h. The activities of the crayfish were analyzed based on their mobility or immobility. We focused on a specific movement and nonmovement behavior at the time of data collection. Immobility was defined as the animal remaining completely unmoving, with no movement of any part of the body.
Statistical analysis
The ROS levels and differential hemocyte counts are shown as the mean ± S.D., and statistical analysis was performed using one-way analysis of variance followed by Duncan's new multiple range test and Tukey's test. For comparisons between two groups, a t test was used, and statistical significance was considered at p < 0.05.
Author contributions
K. J., K. S., and I. S. conceptualization; K. J. data curation; K. J. formal analysis; K. J. validation; K. J. investigation; K. J. visualization; K. J., K. S., and I. S. methodology; K. J. writing-original draft; K. J., K. S., and I. S. writing-review and editing; K. S. and I. S. resources; K. S. and I. S. supervision; K. S. and I. S. funding acquisition; K. S. and I. S. project administration.
Supplementary Material
Acknowledgment
We thank Chadanat Noonin for comments on the manuscript.
This work was supported by Svenska Forskningsrådet Formas (Swedish Research Council Formas) Grant 2011-601 (to K. S.) and Vetenskapsrådet (VR) Grants 621-2011-4797 (to I. S.) and 621-2012-2418 (to K. S.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Movies S1–S6.
- TGase
- transglutaminase
- ROS
- reactive oxygen species
- ECM
- extracellular matrix
- HPT
- hematopoietic tissue
- CP
- clotting protein
- GC
- granular cells
- SGC
- semigranular cells
- THC
- total hemocyte cells
- CPBS
- crayfish-buffered saline
- APC
- anterior proliferation center
- BrdU
- 5-bromo-2′-deoxyuridine
- BPHN2
- 5-(biotinamido)pentylamine substrate.
References
- 1. Eckert R. L., Kaartinen M. T., Nurminskaya M., Belkin A. M., Colak G., Johnson G. V., and Mehta K. (2014) Transglutaminase regulation of cell function. Physiol. Rev. 94, 383–417 10.1152/physrev.00019.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martin A., De Vivo G., and Gentile V. (2011) Possible role of the transglutaminases in the pathogenesis of Alzheimer's disease and other neurodegenerative diseases. Int. J. Alzheimers Dis. 2011, 865432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klöck C., and Khosla C. (2012) Regulation of the activities of the mammalian transglutaminase family of enzymes. Protein Sci. 21, 1781–1791 10.1002/pro.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odii B. O., and Coussons P. (2014) Biological functionalities of transglutaminase 2 and the possibility of its compensation by other members of the transglutaminase family. Scientific World Journal 2014, 714561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nurminskaya M. V., and Belkin A. M. (2012) Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 294, 1–97 10.1016/B978-0-12-394305-7.00001-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tokunaga F., Muta T., Iwanaga S., Ichinose A., Davie E. W., Kuma K., and Miyata T. (1993) Limulus hemocyte transglutaminase cDNA cloning, amino acid sequence, and tissue localization. J. Biol. Chem. 268, 262–268 [PubMed] [Google Scholar]
- 7. Wang R., Liang Z., Hall M., and Söderhall K. (2001) A transglutaminase involved in the coagulation system of the freshwater crayfish, Pacifastacus leniusculus. Tissue localisation and cDNA cloning. Fish Shellfish Immunol. 11, 623–637 10.1006/fsim.2001.0341 [DOI] [PubMed] [Google Scholar]
- 8. Yeh M. S., Liu C. H., Hung C. W., and Cheng W. (2009) cDNA cloning, identification, tissue localisation, and transcription profile of a transglutaminase from white shrimp, Litopenaeus vannamei, after infection by Vibrio alginolyticus. Fish Shellfish Immunol. 27, 748–756 10.1016/j.fsi.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 9. Iklé J., Elwell J. A., Bryantsev A. L., and Cripps R. M. (2008) Cardiac expression of the Drosophila transglutaminase (CG7356) gene is directly controlled by myocyte enhancer factor-2. Dev. Dyn. 237, 2090–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shibata T., and Kawabata S. (2015) Transglutaminase in invertebrates. In: Transglutaminases (Hitomi K., Kojima S., Fesus L., eds), pp. 117–127, Springer, Tokyo [Google Scholar]
- 11. Cerenius L., and Söderhäll K. (2011) Coagulation in invertebrates. J. Innate Immun. 3, 3–8 10.1159/000322066 [DOI] [PubMed] [Google Scholar]
- 12. Nikolajsen C. L., Dyrlund T. F., Poulsen E. T., Enghild J. J., and Scavenius C. (2014) Coagulation factor XIIIa substrates in human plasma. J. Biol. Chem. 289, 6526–6534 10.1074/jbc.M113.517904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall M., Wang R., van Antwerpen R., Sottrup-Jensen L., and Söderhäll K. (1999) The crayfish plasma clotting protein: A vitellogenin-related protein responsible for clot formation in crustacean blood. Proc. Natl. Acad. Sci. U.S.A. 96, 1965–1970 10.1073/pnas.96.5.1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Junkunlo K., Söderhäll K., and Söderhäll I. (2018) Clotting protein: An extracellular matrix (ECM) protein involved in crustacean hematopoiesis. Dev. Comp. Immunol. 78, 132–140 10.1016/j.dci.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 15. Junkunlo K., Söderhäll K., Söderhäll I., and Noonin C. (2016) Reactive oxygen species affect transglutaminase activity and regulate hematopoiesis in a crustacean. J. Biol. Chem. 291, 17593–17601 10.1074/jbc.M116.741348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin X., Söderhäll K., and Söderhäll I. (2008) Transglutaminase activity in the hematopoietic tissue of a crustacean, Pacifastacus leniusculus, importance in hemocyte homeostasis. BMC Immunol. 9, 58 10.1186/1471-2172-9-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao J., Chen Y. H., and Peterson L. C. (2015) GATA family transcriptional factors: Emerging suspects in hematologic disorders. Exp. Hematol. Oncol. 4, 28 10.1186/s40164-015-0024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Siegel M., and Khosla C. (2007) Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol. Ther. 115, 232–245 10.1016/j.pharmthera.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Junkunlo K., Söderhäll K., Noonin C., and Söderhäll I. (2017) PDGF/VEGF-related receptor affects transglutaminase activity to control cell migration during crustacean hematopoiesis. Stem Cells Dev. 26, 1449–1459 10.1089/scd.2017.0086 [DOI] [PubMed] [Google Scholar]
- 20. Hoffman C. M., and Calvi L. M. (2014) Minireview: Complexity of hematopoietic stem cell regulation in the bone marrow microenvironment. Mol. Endocrinol. 28, 1592–1601 10.1210/me.2014-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaga O., Söderhäll K., and Lignell M. (1995) The haemopoietic cells of the freshwater crayfish, Pacifastacus leniusculus. Anim. Biol. 4, 50–70 [Google Scholar]
- 22. Lorand L., and Conrad S. M. (1984) Transglutaminases. Mol. Cell. Biochem. 58, 9–35 10.1007/BF00240602 [DOI] [PubMed] [Google Scholar]
- 23. Lin Y., He H., Luo Y., Zhu T., and Duan R. (2015) Inhibition of transglutaminase exacerbates polyglutamine-induced neurotoxicity by increasing the aggregation of mutant ataxin-3 in an SCA3 Drosophila model. Neurotox. Res. 27, 259–267 10.1007/s12640-014-9506-8 [DOI] [PubMed] [Google Scholar]
- 24. Birckbichler P. J., Orr G. R., Patterson M. K. Jr., Conway E., and Carter H. A. (1981) Increase in proliferative markers after inhibition of transglutaminase. Proc. Natl. Acad. Sci. U.S.A. 78, 5005–5008 10.1073/pnas.78.8.5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang C. C., Sritunyalucksana K., Söderhäll K., and Song Y. L. (2004) Molecular cloning and characterization of tiger shrimp (Penaeus monodon) transglutaminase. Dev. Comp. Immunol. 28, 279–294 10.1016/j.dci.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 26. Chen M. Y., Hu K. Y., Huang C. C., and Song Y. L. (2005) More than one type of transglutaminase in invertebrates?: A second type of transglutaminase is involved in shrimp coagulation. Dev. Comp. Immunol. 29, 1003–1016 10.1016/j.dci.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 27. Chang Y. T., Lin C. Y., Tsai C. Y., Siva V. S., Chu C. Y., Tsai H. J., and Song Y. L. (2013) The new face of the old Molecules: Crustin Pm4 and transglutaminase type I serving as RNPs down-regulate astakine-mediated hematopoiesis. PLoS One 8, e72793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y., Chen W. C., and Cheng W. (2014) The second type of transglutaminase regulates immune and stress responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 37, 30–37 10.1016/j.fsi.2014.01.010 [DOI] [PubMed] [Google Scholar]
- 29. Gibrat C., and Cicchetti F. (2011) Potential of cystamine and cysteamine in the treatment of neurodegenerative diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry. 35, 380–389 10.1016/j.pnpbp.2010.11.023 [DOI] [PubMed] [Google Scholar]
- 30. Bousquet M., Gibrat C., Ouellet M., Rouillard C., Calon F., and Cicchetti F. (2010) Cystamine metabolism and brain transport properties: Clinical implications for neurodegenerative diseases. J. Neurochem. 114, 1651–1658 10.1111/j.1471-4159.2010.06874.x [DOI] [PubMed] [Google Scholar]
- 31. Borrell-Pagès M., Canals J. M., Cordelières F. P., Parker J. A., Pineda J. R., Grange G., Bryson E. A., Guillermier M., Hirsch E., Hantraye P., Cheetham M. E., Néri C., Alberch J., Brouillet E., Saudou F., and Humbert S. (2006) Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J. Clin. Invest. 116, 1410–1424 10.1172/JCI27607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noonin C., Lin X., Jiravanichpaisal P., Söderhäll K., and Söderhäll I. (2012) Invertebrate hematopoiesis: An anterior proliferation center as a link between the hematopoietic tissue and the brain. Stem Cells Dev. 21, 3173–3186 10.1089/scd.2012.0077 [DOI] [PubMed] [Google Scholar]
- 33. Al-Jallad H. F., Nakano Y., Chen J. L., McMillan E., Lefebvre C., and Kaartinen M. T. (2006) Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MC3T3-E1 osteoblast cultures. Matrix Biol. 25, 135–148 10.1016/j.matbio.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 34. Min B., and Chung K. C. (2018) New insight into transglutaminase 2 and link to neurodegenerative diseases. BMB Rep. 51, 5–13 10.5483/BMBRep.2018.51.1.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cisbani G., Drouin-Ouellet J., Gibrat C., Saint-Pierre M., Lagacé M., Badrinarayanan S., Lavallée-Bourget M. H., Charest J., Chabrat A., Boivin L., Lebel M., Bousquet M., Lévesque M., and Cicchetti F. (2015) Cystamine/cysteamine rescues the dopaminergic system and shows neurorestorative properties in an animal model of Parkinson's disease. Neurobiol. Dis. 82, 430–444 10.1016/j.nbd.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 36. Gibrat C., Bousquet M., Saint-Pierre M., Lévesque D., Calon F., Rouillard C., and Cicchetti F. (2010) Cystamine prevents MPTP-induced toxicity in young adult mice via the up-regulation of the brain-derived neurotrophic factor. Prog. Neuropsychopharmacol. Biol. Psychiatry. 34, 193–203 10.1016/j.pnpbp.2009.11.005 [DOI] [PubMed] [Google Scholar]
- 37. Haroutunian V., Mantin R., Campbell G. A., Tsuboyama G. K., and Davis K. L. (1987) Cysteamine-induced depletion of central somatostatin-like immunoactivity: Effects on behavior, learning, memory and brain neurochemistry. Brain Res. 403, 234–242 10.1016/0006-8993(87)90060-6 [DOI] [PubMed] [Google Scholar]
- 38. Slaughter T. F., Achyuthan K. E., Lai T. S., and Greenberg C. S. (1992) A microtiter plate transglutaminase assay utilizing 5-(biotinamido) pentylamine as substrate. Anal. Biochem. 205, 166–171 10.1016/0003-2697(92)90594-W [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.