Abstract
Background
To determine if adiponectin levels are associated with weight loss, low muscle mass, and physical functioning among the elderly and to determine independent associations with incident disability and death.
Methods
Included were 3,044 participants from the Health, Aging and Body Composition Study, who had whole-body dual energy absorptiometry performed to evaluate appendicular lean mass index (ALMI, kg/m2) and fat mass index (FMI, kg/m2), computed tomography measures of thigh muscle density, weight histories, estimates of physical functioning, and adiponectin levels at enrollment. Associations between adiponectin levels and body composition, weight loss, and physical functioning were assessed in multivariable linear regression models. Associations between adiponectin and incident disability and mortality were assessed in mediation analyses, adjusting for other factors.
Results
Greater adiponectin at baseline was independently associated with low FMI Z-score, lower waist circumference, low ALMI Z-score, low muscle density, a history of weight loss, and poor physical functioning (all p < .05). Greater adiponectin levels (per SD) were associated with incident disability [HR: 1.14 (1.08, 1.20), p < .001] and greater mortality [HR: 1.17 (1.10, 1.25), p < .001] in models adjusting for demographic factors, adiposity, and comorbid conditions. The association was completely attenuated and no longer significant (all p > 0.05) when adjusting for body composition, muscle density, weight loss, and physical functioning at baseline.
Conclusions
Greater serum adiponectin levels are associated with historical weight loss, low skeletal muscle mass, low muscle density, and poor physical functioning. High adiponectin is associated with a greater risk of incident disability and death, but not independently of these factors.
Keywords: Adiponectin, Adipokine, Mortality, Body composition
Adiponectin is an adipokine that is produced by adipocytes and myocytes in response to caloric restriction and negative energy balance. It has been termed the “starvation signal,” as it increases with weight loss (1) and is generally found to be higher among thin patients. Accumulation of visceral fat is thought to promote TNF-α production in the viscera, which suppresses the transcription of the adiponectin gene (2,3). Muscle fibers also produce adiponectin and it may act to increase skeletal muscle lipid oxidation (4). Among healthy young individuals, greater adiponectin levels have been shown to correlate with better long-term cardiovascular outcomes (5,6). A direct and causal protective role of adiponectin with cardiovascular disease has not been confirmed.
A number of studies have demonstrated that higher adiponectin levels are paradoxically associated with an increased risk of premature death among chronic inflammatory conditions including chronic heart failure and end-stage renal disease (7–9). A previous study in the Healthy Aging and Body Composition Study demonstrated that higher adiponectin levels were associated with an increased risk of premature death among the elderly independent of other cardiovascular risk factors (10).
In general, adiponectin shows inverse associations with adverse outcomes in healthy middle-aged populations. The opposite is observed in cohorts with prevalent cardiovascular disease, heart failure, or advanced age, whereas higher levels are associated with greater risk (7–9, 11). A recent study among older individuals demonstrated that both the highest and lowest levels are associated with greater cardiovascular risk (12). Cachexia or sarcopenia related to aging, chronic inflammation, and illness may explain these paradoxical epidemiological associations between adiponectin and higher mortality in these populations. In support of this hypothesis, greater adiponectin levels have been observed among individuals with evidence of weight loss, cachexia, and poor physical functioning (13–18).
We hypothesized that high adiponectin levels among the elderly are correlated with low body mass index (BMI), low fat mass and low lean mass, poor muscle quality, a history of weight loss, poor physical functioning, and greater risk of incident disability and death. We evaluated independent associations between adiponectin levels and body composition, physical functioning, incident disability, and mortality in the Healthy Aging and Body Composition Study.
Methods
Study Setting
Participants were enrolled in the Health, Aging and Body Composition (Health ABC) study, a prospective observational study of 3,075 well-functioning, community-dwelling older adults aged 70–79 years. Study participants were recruited from a random sample of White and Black Medicare beneficiaries living in Pittsburgh, PA and Memphis, TN that were within a 1 hour drive of the examination sites. All individuals with adiponectin levels and whole-body x-ray absorptiometry (DXA) results available at enrollment were included in this analysis (N = 2,821).
Measures of body composition
Whole body dual-energy DXA was performed at both the Pittsburgh and the Memphis field centers (Hologic 4500A, version 9.03; Hologic, Inc., Waltham, MA, USA). In addition, bone mineral–free ALM and fat mass were derived from the whole body scan. DXA quality assurance measurements were performed at both study sites to ensure scanner reliability, and identical patient scan protocols were used for all participants. For soft tissue, the CVs were 1.0 and 2.1 per cent for whole-body lean mass and fat mass, respectively. Total fat mass index (FMI) and appendicular lean mass index (ALMI) were determined from whole-body DXA and converted to age-, sex-, and race-specific Z-scores as described previously (19) using published nationally representative reference ranges from the National Health and Nutrition Examination Survey (NHANES) (20).
Computed tomography scans of the thighs were obtained at baseline (in Pittsburgh, 9800 Advantage from General Electric, Milwaukee, WI; in Memphis, Somatom Plus 4 from Siemens, Erlangen, Germany, or PQ 2000S, Marconi Medical Systems, Cleveland, OH). A 10-mm-thick axial image (120 kVp, 200–250 mA) was obtained at midfemur. A line was drawn manually along the deep fascial plane surrounding the thigh muscles to distinguish muscle from surrounding subcutaneous adipose tissue, and the femur was segmented out of the muscle. Fat infiltration of muscle was assessed in Hounsfield units (HU, a measure of x-ray attenuation), with lower HU reflecting more fat infiltration. This measurement correlates with muscle triglyceride content determined by histological oil red O staining, and the mean test–retest coefficient of variation in a previous study was 0.51 per cent (21). This analysis used the average muscle density between the two thighs.
Measurement of adiponectin
Samples were drawn at the baseline visit after an overnight fast. Serum samples were frozen at −70°C and stored at McKesson BioServices, Rockville, MD. Adiponectin was assayed in 2002–2003 from frozen serum samples (acquired 7 years prior). Total circulating levels of adiponectin (ng/mL) were measured in duplicate by radio-immuno assay (RAI; Linco Research, St. Charles, MO) with an intra-assay coefficient of variation of 1.8%–3.6%.
Physical performance and disability measures
Health ABC performance battery
(22, 23)Details have been previously described (23). In brief, this battery includes five repeated chair stands, progressively more challenging tests of standing balance, a 6 m walk to determine usual gait speed, and a narrow walk in which participants are instructed to talk between lines of colored tape 20 cm apart at their usual pace. Performance is divided by the maximum possible performance for older adults on each test to create ratio scores that are summed for the four tests to obtain a continuous scale ranging from 0 to 4, with a lower score indicating poorer function.
Long-distance corridor walk (LDCW; 400 M walk)
This is a two-stage, self-paced walking test that was designed to measure cardiorespiratory fitness longitudinally in an initially well-functioning cohort of 70 years old (24). The measure is prognostic of greater disability and mortality (25). The first stage consisted of a 2 minutes warm-up walk, in which distance was recorded and the first 20 m was timed. This stage also served as a stepped-down test for persons unable to walk for a longer period. The second stage consisted of a 400 m walk, which is about the distance an average health older adult can cover in 6 minutes. Participants were asked to walk 400 m as quickly as possible at a pace that they can maintain. Standard encouragement was given throughout the test and time was recorded to the nearest second. A significant proportion of participants were unable to complete the test (24 per cent) at baseline, and therefore, we determined associations with inability to complete the 400 m walk as a measure of mobility disability as defined previously (26).
Grip strength
(27)Isometric grip strength in (kg) was measured using a hand-held dynamometer (JAMAR Technologies, Inc., Hatfield, PA). Two trials were performed for each hand. An average of the trials performed on the strongest hand was used for analyses as has previously been described (27).
Incident disability
(28, 29)We also analyzed adjudicated self-report data on incident physical disability from interviewer-administered questionnaires every 6 months. For incident disability, the outcome of interest was time from baseline to any self-reported disability at a subsequent visit, which was defined as severe difficulty or inability to walk 1/4 mile and/or climb 10 steps, needing equipment to ambulate, or having any difficulty performing activities of daily living (i.e. getting in and out of bed or chairs, bathing or showering, and dressing).
All-cause mortality
Time of death was determined from the adjudicated outcomes data set. All deaths are adjudicated by a central committee for immediate and underlying causes of death as determined by established criteria including review of death certificate, all recent hospital records, and interview with the next of kin.
Assessment of a history of weight loss
At enrollment, study participants were asked about their weight at 50 years of age. This weight was converted to a BMI based on current height and the change in BMI from age 50 to enrollment was calculated. Three categories of per cent weight loss were assessed (none, 5%–10%, or ≥10%) as has been previously described (30). Nearly identical results were obtained in analyses utilizing alternative categories of weight loss based on the change in BMI (Category 1 = change ≥−1 kg/m2; Category 2 = change −1 to −3 kg/m2; Category 3 = change <−3 kg/m2; not shown) (31).
Statistical analysis
Adiponectin levels were log-transformed to fit a normal distribution and then standardized so that associations demonstrated represent the effect of one standard deviation greater level. Univariate associations between adiponectin and prehypothesized factors were assessed using Spearman and Pearson correlations. Factors hypothesized to be important included demographics, comorbid conditions (hypertension, congestive heart failure, diabetes, history of heart attack, and history of cancer), FMI Z-score, ALMI Z-score, muscle density, weight change since age 50, grip strength, the Health ABC performance score, and completion of the LDCW. Multivariable linear regression was utilized to identify independent associations between factors identified in univariate analysis and adiponectin levels.
Independent associations between adiponectin and muscle outcomes were also assessed using multivariable linear regression with adjustment for prehypothesized factors, including total FMI Z-score, waist circumference, demographics, smoking status, and comorbid conditions. Independent associations between adiponectin and physical functioning measures were assessed in successive models to assess the impact of adjustment for body composition. Similarly, successive multivariable Cox proportional hazards models assessed the impact of adjustment for body composition on the association between adiponectin and incident disability and overall mortality.
In all analyses, testing for effect modification by sex was performed by testing the significance of multiplicative interaction terms in multivariable models. Stratified analyses by sex are provided in Supplementary Tables 1–6.
Results
The details of the study population have been previously published. We included 2,821 individuals (1,374 men and 1,447 women) out of the total 3,075 who had whole-body DXA and adiponectin values recorded. The basic characteristics of the population are presented in Table 1.
Table 1.
Basic Characteristics of the Health ABC Study Sample, 1997–1998
| N | 2,821 |
|---|---|
| Age | 74.1 (2.87) |
| Female | 51.2% |
| Race (% African-American) | 41.6% |
| BMI (kg/m2) | 27.3 (4.72) |
| Body composition | |
| ALMI (kg/m2) | 7.65 (1.36) |
| ALMI Z-score | −0.049 (0.89) |
| FMI (kg/m2) | 9.71 (3.44) |
| FMI Z-score | −0.27 (0.92) |
| Thigh muscle density (HU) | 35.7 (6.8) |
| Mean Δ BMI since 50 | +1.43 (3.70) |
| Physical functioning | |
| Health ABC score | 2.20 (0.53) |
| Completed LDCW, N(%) | 2162 (76.6%) |
| Grip strength (kg) | 32.3 (10.5) |
Note: ALMI = Appendicular lean mass index; BMI = Body mass index; FMI = Fat mass index; Health ABC = Health Aging and Body Composition; HU = Hounsfield units; LDCW = Long-distance corridor walk.
Factors Associated With Adiponectin Levels
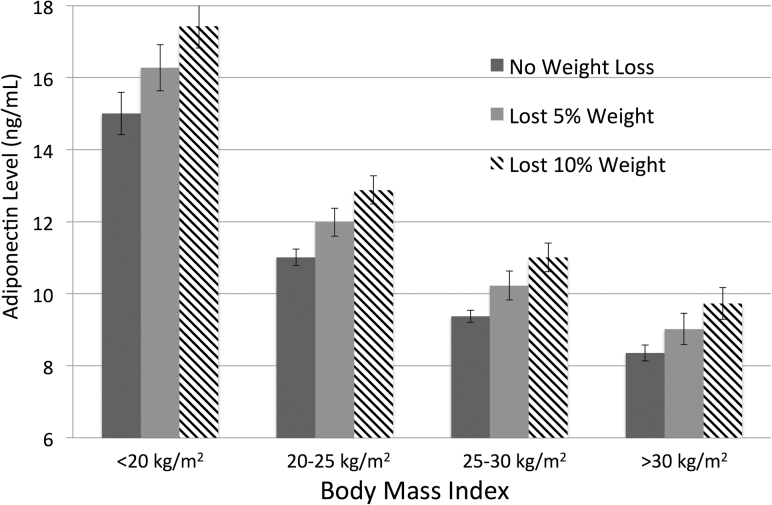
Associations between baseline factors and log-transformed adiponectin levels (per SD) are presented in Table 2. Greater FMI Z-score, waist circumference, ALMI Z-score, and muscle density were all independently associated with lower adiponectin levels. Better physical functioning was also independently associated with lower adiponectin levels. An increase in BMI (per 1 kg/m2) since age 50 was associated with lower adiponectin levels independent of current body composition. Per cent weight loss (5 or 10 per cent) was associated with higher adiponectin levels across all weight categories in models adjusting for age, sex, and race (Figure 1).
Table 2.
Factors Associated With Adiponectin Levels at Baseline in Unviariate and Multivariable Analyses
| Adiponectin Per 1 SD Univariate |
p-Value | Adiponectin Per 1 SD Multivariable*–R2 = 0.34 |
||
|---|---|---|---|---|
| β (95% CI) | β (95% CI) | p-Value | ||
| Age (per 10 y) | 0.37 (0.25, 0.50) | <.001 | 0.15 (0.036, 0.26) | .01 |
| Female | 0.57 (0.51, 0.64) | <.001 | 0.48 (0.41, 0.55) | <.001 |
| Black | −0.53 (−0.60, −0.46) | <.001 | −0.60 (−0.66, −0.53) | <.001 |
| Hypertension | −0.097 (−0.14, −0.054) | <.001 | −0.042 (−0.08, −0.00) | .045 |
| Diabetes | −0.54 (−0.62, −0.45) | <.001 | −0.48 (−0.58, −0.38) | <.001 |
| Cancer | 0.039 (−0.027, 0.10) | .25 | — | — |
| Heart attack | −0.027 (−0.069, 0.014) | .20 | — | — |
| CHF | −0.020 (−0.057, 0.017) | .90 | — | — |
| Current smoking | −0.13 (−0.25, −0.0075) | .04 | — | — |
| Former smoking | −0.20 (−0.27, −0.12) | <.001 | — | — |
| Total/abdominal adiposity | — | — | ||
| FMI Z-score | −0.26 (−0.30, −0.23) | <.001 | −0.21 (−0.28, −0.14) | <.001 |
| Waist circumference (cm) | −0.022 (−0.025, −0.019) | <.001 | −0.0083 (−0.01, −0.004) | .001 |
| Muscle outcomes | ||||
| ALMI Z-score | −0.29 (−0.33, −0.25) | <.001 | −0.065 (−0.11, −0.018) | .007 |
| Thigh muscle density (per SD) | −0.14 (−0.17, −0.010) | <.001 | −0.25 (−0.29, −0.21) | <.001 |
| Weight change since 50 (kg/m2) | −0.044 (−0.053, −0.034) | <.001 | −0.023 (−0.038, −0.014) | <.001 |
| Physical function | ||||
| Health ABC performance | −0.098 (−0.16, −0.031) | .004 | −0.11 (−0.19, −0.036) | .003 |
| Grip strength (kg) | −0.028 (−0.031, −0.024) | <.001 | — | — |
| Completed LDCW | −0.048 (−0.13, 0.034) | .25 | — | — |
Notes: *After stepwise deletion of nonsignificant variables (smoking and grip strength).
ALMI = Appendicular lean mass index; CHF = Congestive heart failure; CI = Confidence interval; FMI = Fat Mass Index; Health ABC = Healthy Aging and Body Composition; LDCW = Long-distance Corridor Walk; SD = Standard deviation.
Figure 1.
Adiponectin levels among patients who have lost weight across BMI categories. Values are adjusted from regression models including age, sex, and race.
Association of Adiponectin With Physical Functioning
Adiponectin was inversely associated with physical functioning as measured by the Health ABC performance score, ability to complete the 400 m walk, and grip strength independent of demographics and comorbid conditions (Table 3, Model 1). These associations were substantially attenuated for all three outcomes with adjustment for FMI Z-score and waist circumference (Table 3, Model 2). Further adjustment for ALMI Z-score, muscle density, and a history of weight loss further attenuated these associations. After adjustment for these factors, adiponectin remained modestly associated only with the Health ABC performance score (Table 3, Model 3), whereas other associations were no longer significant. There were no significant interactions by sex (all p for interactions > 0.08).
Table 3.
Correlations Between Adiponectin and Physical Functioning in Sequential Multivariable Regression Models Adjusting for Body Composition and Muscle Density
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| B (95% CI) | B (95% CI) | B (95% CI) | |
| Health ABC performance | |||
| Adiponectin (per SD) | −0.068*** (−0.088, −0.048) |
−0.036** (−0.058, −0.016) |
−0.030** (−0.051, −0.009) |
| Grip strength | |||
| Adiponectin (per SD) | −0.55*** (−0.85, −0.24) |
−0.27 (−0.60, −0.049) |
−0.23 (−0.56, 0.091) |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Completion of LDCW | |||
| Adiponectin (per SD) | 0.82** (0.74, 0.91) |
0.88* (0.79, 0.99) |
0.90 (0.81, 1.01) |
Model 1: Adjusted for age, sex, black race, BMI category, diabetes, HTN, CHF, cancer, MI, smoking, FMI Z-score and waist circumference.
Model 2: Model 1 plus adjustment for ALMI Z-score and muscle density.
Model 3: Model 2 plus adjustment for % weight loss since age 50.
ALMI = Appendicular lean mass index; BMI = Body mass index; CHF = Congestive heart failure; FMI = Fat mass index; Health ABC = Healthy Aging and Body Composition; HTN = Hypertension; LDCW = Long-distance Corridor Walk; OR = Odds ratio.
*p < .05; **p < .01; ***p < .001.
Association of Adiponectin With Incident Disability and Death
Incident disability occurred in 2,296 participants among whom the median time-to-event was 3.0 years. Higher adiponectin level was associated with a greater risk of incident disability after adjustment for demographic factors, comorbidities, FMI Z-score, and waist circumference (Table 4, Model 1). This association was substantially attenuated when further adjusting for ALMI Z-score, muscle density, and history of weight loss at baseline (Table 4, Model 2). Adjustment for physical functioning at baseline also further attenuated the already tenuous relationship (Table 4, Model 3). The association between adiponectin and incident disability was not different between men and women (p for interaction = .64 in adjusted models).
Table 4.
Multivariable Cox Proportional Hazards Models Assessing Associations Between Adiponectin Levels and Incident Disability and Mortality
| Incident disability | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Adiponectin (per SD) | 1.13 (1.08, 1.19)** | 1.05 (0.99, 1.12) | 1.05 (0.98, 1.11) |
| FMI Z-score | 1.24 (1.16, 1.33)*** | 1.11 (1.02, 1.21) | 1.00 (0.90, 1.10) |
| Waist circumference (cm) | 1.01 (1.00, 1.01)*** | 1.01 (1.00, 1.01)** | 1.01 (1.00, 1.01) |
| ALMI Z-score | — | 0.98 (0.91, 1.04) | 1.10 (1.02, 1.19)* |
| Muscle density | — | 0.96 (0.96, 0.97)*** | 0.97 (0.96, 0.98)*** |
| Weight loss (v. none) | — | ||
| 5% | — | 1.07 (0.90, 1.26) | 0.97 (0.80, 1.16) |
| 10% | — | 1.34 (1.14, 1.58)*** | 1.19 (0.99, 1.42) |
| Grip strength (kg) | — | — | 0.98 (0.98, 0.99)*** |
| Health ABC performance | — | — | 0.51 (0.46, 0.57)*** |
| Completed LDCW | — | — | 0.73 (0.65, 0.83)*** |
| Mortality | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Adiponectin (per SD) | 1.17 (1.10, 1.25)*** | 1.08 (1.01, 1.16)* | 1.05 (0.97, 1.13) |
| FMI Z-score | 0.88 (0.80, 0.96)** | 0.89 (0.79, 0.99)* | 0.83 (0.72, 0.94)** |
| Waist circumference (cm) | 1.01 (1.00, 1.01)* | 1.01 (1.00, 1.02)** | 1.01 (1.00, 1.01) |
| ALMI Z-score | — | 0.88 (0.81, 0.95)** | 0.92 (0.84, 1.01) |
| Muscle density | — | 0.98 (0.97, 0.99)** | 0.99 (0.98, 1.00) |
| Weight loss (v. none) | — | ||
| 5% | — | 1.38 (1.13, 1.67)** | 1.19 (0.96, 1.48) |
| 10% | — | 1.58 (1.32, 1.90)*** | 1.34 (1.15, 1.73)** |
| Grip strength (kg) | — | — | 0.98 (0.97, 0.99)** |
| Health ABC performance | — | — | 0.66 (0.58, 0.76)*** |
| Completed LDCW | — | — | 0.71 (0.61, 0.82)*** |
All models adjusted for age, sex, black race, study site, HTN, CHF, history of any cancer, diabetes, MI, and smoking status.
ALMI = Appendicular lean mass index; FMI = Fat mass index; Health ABC = Healthy Aging and Body Composition; HR = Hazard ratio; LDCW = Long-distance Corridor Walk.
*p < .05; **p < .01; ***p < .001.
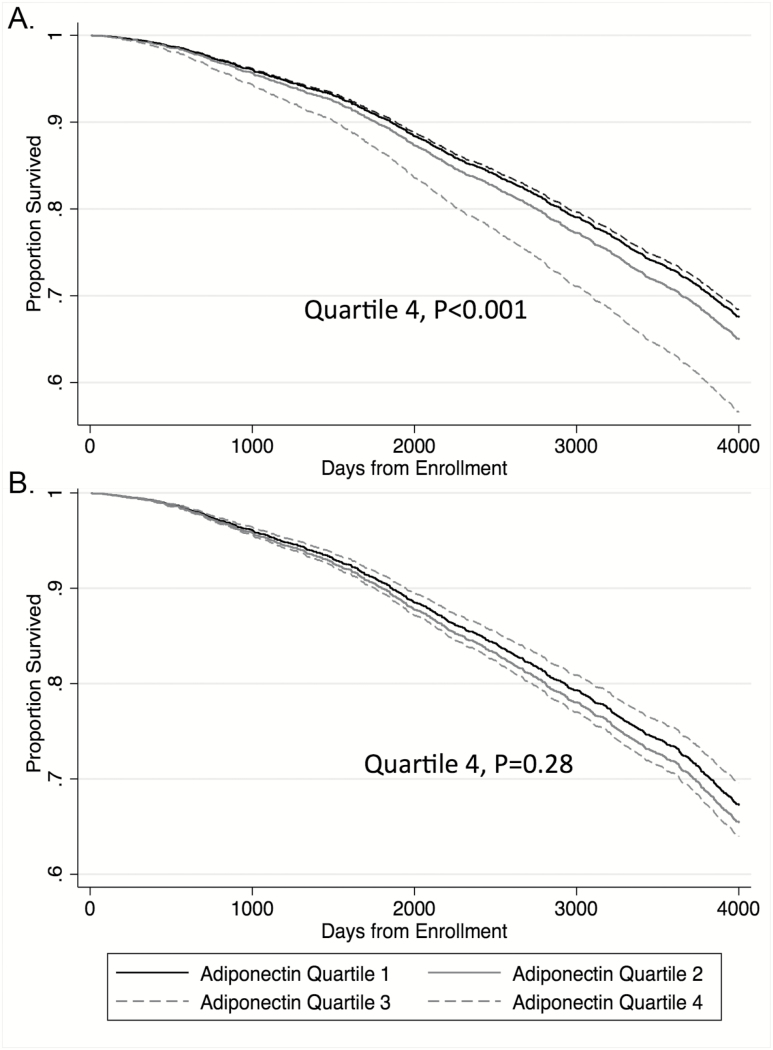
There were 1,370 deaths among whom the median time-to-event was 7.5 years. Greater adiponectin levels (per SD) were associated with greater mortality in models adjusting for FMI Z-scores, waist circumference, comorbid conditions, demographic variables, and smoking [HR 1.17 (1.10, 1.25), p < .001; Table 4, Model 1]. Similar to models evaluating associations with incident disability, the association between adiponectin and mortality was attenuated and no longer significant in sequential models adjusting for body composition, muscle density, weight loss, and physical functioning at baseline [HR 1.05 (0.97, 1.13) p = .26; Table 4, Model 3]. The predicted survival was shorter for individuals in the highest adiponectin quartile when adjusting for FMI Z-score and waist circumference only (Figure 2A). In contrast, there was no apparent difference in predicted survival by adiponectin quartile after full adjustment for all mediators, including lean mass, muscle density, weight loss, strength, and physical functioning (Figure 2B). The association between adiponectin and mortality was not different between men and women (p for interaction = .46 in adjusted models).
Figure 2.
Survival plots based on multivariable Cox proportional hazards models by adiponectin quartile for (A) partially adjusted model and (B) fully adjusted model. (A) Partially adjusted for age, sex, black race, study site, HTN, CHF, history of any cancer, diabetes, MI, smoking status, FMI Z-score, and waist circumference. (B) Fully adjusted: further adjusted for ALMI Z-score, muscle density, weight change since age 50, Health ABC performance, grip strength, and completion of the LDCW.
Discussion
We found a significant relationship between serum levels of adiponectin and historical weight loss, low muscle mass, and low muscle density. Thus, the data presented here support the hypothesis that adiponectin is a biomarker of adverse body composition in the context of aging. Weight loss and loss of muscle mass and quality with aging and chronic illness may explain previously noted epidemiological associations observed between adiponectin, poor physical functioning, and death among the elderly. Thus, these data suggest that, while adiponectin is not likely to play a causal role, it may be an important biomarker of these processes, which themselves affect the long-term risk of disability and mortality.
Adiponectin may represent biomarker of adverse catabolic processes related to features of frailty. Although the analyses presented here do not suggest that adiponectin plays a directly causal role in promoting disability and premature death, the role of adiponectin as a biomarker and prognostic tool is clarified. Previous studies have demonstrated that adiponectin is associated strongly with weight loss. For example, patients undergoing bariatric surgery have dramatic increases in adiponectin. The greater levels observed in this group are substantially higher than controls who may weigh less, but have not lost weight (1). The current study supports the hypothesis that adiponectin levels are influenced by both weight and weight loss. Among the elderly, weight loss that occurs is more likely to be unintentional (32–34). Therefore, adiponectin may identify unintentional weight loss and catabolic processes associated with the development of sarcopenia or cachexia. Other studies have shown correlations between adiponectin and cachexia in chronic conditions (congestive heart failure, kidney disease, rheumatoid arthritis) (8, 9, 16). Previous studies have also identified associations between adiponectin and mortality in chronic conditions (7–9).
Our study is one of few studies to evaluate associations between adiponectin and physical functioning and muscle strength among the elderly. One previous study demonstrated that adiponectin tracked with functional decline among the elderly (18). The current study does not suggest a causal relationship between adiponectin and physical functioning among the elderly. However, these data do support the hypothesis that high adiponectin levels are a marker of body composition changes that may, themselves, be associated with poor physical functioning. Adiponectin might be a useful and inexpensive clinical biomarker to help us identify elderly patients with weight loss, altered body composition, disability, and greater mortality risk.
Adiponectin plays a role in coordinating energy utilization, particularly in the setting of starvation, as it is thought to be important in promoting lipid oxidation and regulating glucose metabolism (4, 35). In the setting of unintentional weight loss due to aging or cachexia, observed elevations in adiponectin are likely to represent normal compensatory response to low energy availability as in the setting of starvation. This will explain why higher adiponectin levels may be paradoxically associated with either better or more adverse outcomes in different clinical contexts.
Limitations of the current study include the lack of longitudinal measures of adiponectin which might clarify the nature of the relationship weight, body composition changes, and changes in the adipokine. Adiponectin exists in a number of forms with different physiological functions. Because this study only included total adiponectin levels, we were unable to characterize relationships between these different forms. In addition, other adipokines may also be important and were not assessed here. Although this study focused on an at-risk group of elderly patients, this study was unable to evaluate differences in association across the age range. Finally, although we studied a number of hypothesized confounders, unmeasured confounding may still be present. For example, it was out of the scope of the current analysis to evaluate the effects of medications on adiponectin. Strengths of the current study include the large sample size in an at-risk population, highly validated estimates of body composition, assessment of weight histories, and comprehensive assessment of physical functioning and grip strength.
In summary, high adiponectin levels in the elderly are associated with incident disability and early death. However, this relationship is explained as an association between adiponectin and low muscle mass and quality, weight loss, and baseline physical function. Adiponectin may therefore represent a biomarker of adverse catabolic processes among the elderly (i.e. cachexia and sarcopenia).
Funding
This research was supported by National Institute on Aging (NIA) Contracts (N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106); NIA grant (R01-AG028050), and National Institute of Nursing Research grant (R01-NR012459). J.F.B. is supported by a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). D.R.W. was supported by National Institutes of Health grant (K12HD068373).
Conflicts of Interest
The authors would like to disclose that A.B.N. is the Editor in Chief of the Journal and T.H. serves on the Editorial Board.
Supplementary Material
Acknowledgments
Dr. Baker would like to acknowledge the support of a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government. This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
References
- 1. Wroblewski E, Swidnicka-Siergiejko A, Hady HR et al. Variation in blood levels of hormones in obese patients following weight reduction induced by endoscopic and surgical bariatric therapies. Cytokine. 2016;77:56–62. doi: 10.1016/j.cyto.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 2. Zappalà G, Rechler MM. IGFBP-3, hypoxia and TNF-alpha inhibit adiponectin transcription. Biochem Biophys Res Commun. 2009;382:785–789. doi: 10.1016/j.bbrc.2009.03.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barth N, Langmann T, Schölmerich J, Schmitz G, Schäffler A. Identification of regulatory elements in the human adipose most abundant gene transcript-1 (apM-1) promoter: role of SP1/SP3 and TNF-alpha as regulatory pathways. Diabetologia. 2002;45:1425–1433. doi: 10.1007/s00125-002-0895-5. [DOI] [PubMed] [Google Scholar]
- 4. Dridi S, Taouis M. Adiponectin and energy homeostasis: consensus and controversy. J Nutr Biochem. 2009;20:831–839. doi: 10.1016/j.jnutbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 5. Pischon T, Hu FB, Girman CJ et al. Plasma total and high molecular weight adiponectin levels and risk of coronary heart disease in women. Atherosclerosis. 2011;219:322–329. doi: 10.1016/j.atherosclerosis.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 7. Kistorp C, Faber J, Galatius S et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 8. Rhee CM, Nguyen DV, Moradi H et al. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66:313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szabó T, Scherbakov N, Sandek A et al. Plasma adiponectin in heart failure with and without cachexia: catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr Metab Cardiovasc Dis. 2014;24:50–56. doi: 10.1016/j.numecd.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 10. Poehls J, Wassel CL, Harris TB et al. ; Health ABC Study Association of adiponectin with mortality in older adults: the Health, Aging, and Body Composition Study. Diabetologia. 2009;52:591–595. doi: 10.1007/s00125-009-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Behre CJ. Adiponectin: saving the starved and the overfed. Med Hypotheses. 2007;69:1290–1292. doi: 10.1016/j.mehy.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 12. Kizer JR, Benkeser D, Arnold AM et al. Associations of total and high-molecular-weight adiponectin with all-cause and cardiovascular mortality in older persons: the Cardiovascular Health Study. Circulation. 2012;126:2951–2961. doi: 10.1161/CIRCULATIONAHA.112.135202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kizer JR, Arnold AM, Strotmeyer ES et al. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci. 2010;65:1208–1214. doi: 10.1093/gerona/glq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hozawa A, Sugawara Y, Tomata Y et al. Relationship between serum adiponectin levels and disability-free survival among community-dwelling elderly individuals: The Tsurugaya Project. J Gerontol A Biol Sci Med Sci. 2012;67:530–536. doi: 10.1093/gerona/glr191. [DOI] [PubMed] [Google Scholar]
- 15. Van Berendoncks AM, Garnier A, Beckers P et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010;3:185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 16. Baker JF, Von Feldt JM, Mostoufi-Moab S, Kim W, Taratuta E, Leonard MB. Insulin-like growth factor 1 and adiponectin and associations with muscle deficits, disease characteristics, and treatments in rheumatoid arthritis. J Rheumatol. 2015;42:2038–2045. doi: 10.3899/jrheum.150280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai JS, Wu CH, Chen SC et al. Plasma adiponectin levels correlate positively with an increasing number of components of frailty in male elders. PLoS One. 2013;8:e56250. doi: 10.1371/journal.pone.0056250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newman AB, Sanders JL, Kizer JR et al. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol. 2016;45:1135–1145. doi: 10.1093/ije/dyw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker JF, Long J, Ibrahim S, Leonard MB, Katz P. Are men at greater risk of lean mass deficits in rheumatoid arthritis?Arthritis Care Res (Hoboken). 2015;67:112–119. doi: 10.1002/acr.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly TL, Wilson KE, Heymsfield SB. Dual energy x-ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985). 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 22. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group The association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004;52:502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 23. Simonsick EM, Newman AB, Nevitt MC et al. ; Health ABC Study Group Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 24. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x [DOI] [PubMed] [Google Scholar]
- 25. Newman AB, Simonsick EM, Naydeck BL et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 26. Vestergaard S, Patel KV, Walkup MP et al. Stopping to rest during a 400-meter walk and incident mobility disability in older persons with functional limitations. J Am Geriatr Soc. 2009;57:260–265. doi: 10.1111/j.1532-5415.2008.02097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klepin HD, Geiger AM, Tooze JA et al. ; Health, Aging and Body Composition Study Physical performance and subsequent disability and survival in older adults with malignancy: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2010;58:76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen DS, Betz J, Yaffe K et al. ; Health ABC Study Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci. 2015;70:654–661. doi: 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanders JL, Boudreau RM, Penninx BW et al. ; Health ABC Study Association of a Modified Physiologic Index with mortality and incident disability: the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2012;67:1439–1446. doi: 10.1093/gerona/gls123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring). 2013;21:1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baker JF, Billig E, Michaud K et al. Weight loss, the obesity paradox, and the risk of death in rheumatoid arthritis. Arthritis Rheumatol. 2015;67:1711–1717. doi: 10.1002/art.39136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JS, Kritchevsky SB, Harris TB, Tylavsky F, Rubin SM, Newman AB. Short-term weight changes in community-dwelling older adults: the Health, Aging, and Body Composition Weight Change Substudy. Am J Clin Nutr. 2005;82:644–650. [DOI] [PubMed] [Google Scholar]
- 33. Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165:1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 34. Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician. 2014;89:718–722. [PubMed] [Google Scholar]
- 35. Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/51043-2760(01)00524-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.