Abstract
Curcumin, from the spice turmeric, exhibits anti-inflammatory, antioxidant, anticancer, antiviral, and neurotrophic activity and therefore holds promise as a therapeutic agent to prevent and treat several disorders. However, a major barrier to curcumin's clinical efficacy is its poor bioavailability. Efforts have therefore been dedicated to developing curcumin formulations with greater bioavailability and systemic tissue distribution. However, it is proposed in this review that curcumin's potential as a therapeutic agent may not solely rely on its bioavailability, but rather its medicinal benefits may also arise from its positive influence on gastrointestinal health and function. In this review, in vitro, animal, and human studies investigating the effects of curcumin on intestinal microbiota, intestinal permeability, gut inflammation and oxidative stress, anaphylactic response, and bacterial, parasitic, and fungal infections are summarized. It is argued that positive changes in these areas can have wide-ranging influences on both intestinal and extraintestinal diseases, and therefore presents as a possible mechanism behind curcumin's therapeutic efficacy.
Keywords: curcumin, turmeric, herbal medicine, intestinal health, intestinal microbiota, microbiome, polyphenols
Introduction
Curcumin, a constituent from the rhizome of the herb Curcuma Longa (turmeric), has received widespread attention over the past decade. Excitement originated after encouraging in vitro discoveries showed its influence on many biological mechanisms associated with several diseases. In particular, in vitro and animal models confirmed that curcumin has anti-inflammatory, antioxidant, anticancer, antiviral, and neurotrophic effects, just to name a few (1). Investigations also indicated curcumin's potential to treat several cancers, gastrointestinal diseases, metabolic diseases, dermatologic conditions, cardiovascular diseases, neurodegenerative diseases, autoimmune conditions, and psychiatric disorders (2, 3). Unfortunately, these encouraging initial findings have not been consistently supported through human clinical trials. Presently, some of the strongest evidence for the therapeutic efficacy of curcumin (confirmed by meta-analytic analyses of randomized controlled trials) is for the treatment of arthritis (8 studies) (4), pain and analgesia (8 studies) (5), and major depressive disorder (6 studies) (6). From a molecular standpoint, meta-analyses have also supported curcumin's ability to lower concentrations of C-reactive protein (CRP) (6 studies) (7), TNF-α (8 studies) (8), and IL-6 (9 studies) (9). Although curcumin may have beneficial effects on other diseases, an increasing body of support is required before definitive conclusions can be made.
Despite these positive effects, a major criticism of curcumin is its poor bioavailability. In fact, some researchers have argued that given its unstable, reactive, and nonbioavailable nature, further clinical trials on curcumin are unwarranted (10). Curcumin has been confirmed to exhibit very poor bioavailability, with many studies showing very low, or even undetectable, concentrations in blood and extraintestinal tissue. Major reasons postulated are due to its poor absorption, rapid metabolism, chemical instability, and rapid systemic elimination (11). The majority of oral curcumin is excreted in the feces (≤90%), as determined in several animal studies (12). To overcome this problem, numerous methods have been undertaken to increase its bioavailability. These include the use of adjuvants such as piperine, formulating liposomal curcumin, curcumin nanoparticles, curcumin phospholipid complexes, and the use of structural analogs of curcumin such as turmeric oil (11, 13). Such efforts have met with some success, as increased blood concentrations have been shown. However, investigations comparing the therapeutic potencies and pharmacodynamic response of these more bioavailable forms with standard curcumin have yet to be extensively conducted in human clinical trials. Moreover, serum concentrations necessary to obtain a given clinical or biological effect have not yet been identified.
It is proposed in this review that curcumin's potential as a therapeutic agent may not necessarily rely on its bioavailability, but rather, its medicinal benefits may also arise from its positive influence on gastrointestinal health and function. The importance of our gastrointestinal system has long been recognized. In fact, in 400 bc, Hippocrates was quoted as saying “death sits in the bowels” and “bad digestion is the root of all evil” (14). It is increasingly acknowledged that gut health is not only essential for our gastrointestinal system but is also important for overall human health. This is particularly highlighted by the high comorbidity of most gastrointestinal disorders with other medical conditions, and the significant adverse effects of gastrointestinal diseases and symptoms on quality of life (15–17). Therefore, approaches to improve gut function can improve overall health and well-being. Curcumin presents as a natural agent to support gastrointestinal function, and the evidence of its gastrointestinal influences is reviewed in this article. In particular, animal and human studies, along with in vitro models investigating its effects on intestinal microbiota, intestinal permeability, gut inflammation and oxidative stress, antianaphylactic effects, and its influence on bacterial, parasitic, and fungal infections, are summarized. Although these mechanisms are discussed separately in this review, it is acknowledged that they are strongly connected, with substantial influences on each other.
Methods
Information for this review was compiled by searching PubMed (https://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (https://scholar.google.com.au), PsycINFO (https://search.proquest.com/psycinfo), Scopus (https://www.scopus.com), and The Cochrane Library databases (http://www.cochranelibrary.com), and by examining reference lists of relevant articles to locate additional studies that were not identified by the database searches. Databases were scanned from all years of study until May 2017 for animal, in vitro, or human studies. A systematic search of databases with the use of the terms “curcumin,” “turmeric,” “intestinal,” “gastrointestinal,” “gut,” “digestion,” “microbiome,” and “microbiota” was completed.
Intestinal Microbiota and Overall Health
The human body is inhabited by a vast number of bacteria, archaea, viruses, and unicellular eukaryotes, collectively known as our microbiota. It is estimated that the human microbiota contains as many as 1014 bacterial cells. This is 10 times more than the number of human cells present in our body (18). The microbiota colonizes virtually every surface of the human body that is exposed to the external environment, including our skin and in the genitourinary, gastrointestinal, and respiratory tracts. However, our gastrointestinal tract is by far the most heavily colonized organ, with the colon estimated to contain >70% of all the microbes in the human body (19, 20).
The microbiota has several important roles in normal human physiology. Some of its functions include immune defense and protection against pathogens, maintenance of barriers to the external environment (e.g., epidermal or dermal structures and intestinal mucosa), lipid metabolism, extraction of nutrients, production of vitamins, metabolism of xenobiotics, and production of SCFAs such as acetate, propionate, and butyrate (21). SCFAs are an important energy source for intestinal mucosa and critical for modulating immune responses and tumorigenesis in the gut (22). The microbiota also regulates and manufactures neurotransmitters such as serotonin and γ-aminobutyric acid (23). Moreover, the microbiota has a crucial role in the bidirectional gut-brain communication and evidence suggests that it influences core neurological processes, including neurogenesis, synaptic plasticity, neurotransmitter signaling, neurodevelopment, and neuroinflammation (24).
The composition of the microbiota can be influenced by a multitude of environmental and lifestyle factors. For example, its composition varies between infants born via cesarian delivery and by natural birth, and between breastfed infants and formula-fed infants (25). The microbiome can also be influenced by diet, exposure to antibiotics, stress, and sleep patterns (21, 26). Research also confirms an influence of exercise on the gut microbiota (27). Disruption in the composition of the gut microbiome, termed “dysbiosis,” is associated with several intestinal and extraintestinal diseases. Common intestinal disorders associated with disturbed microbial composition include irritable bowel syndrome and inflammatory bowel disease (28). Intestinal dysbiosis has also been implicated in metabolic conditions such as diabetes and obesity (29, 30); several disorders of the nervous system such as multiple sclerosis, autism, Parkinson disease, and Alzheimer disease (31–33); pain-related conditions such as osteoarthritis and myalgic encephalomyelitis (34, 35); certain cancers (36); and psychiatric disorders such as depression, bipolar disorder, and schizophrenia (18, 37–39). Although correlation does not confirm causation, it is speculated that, given the wide-ranging physiologic roles of the gut microbiota, disturbances in its composition (i.e., dysbiosis) may at least have a contributory role in disease progression.
Curcumin and Its Influence on the Microbiome
In several animal studies, curcumin has been shown to influence the diversity of our microbiota. For example, feeding rats a high-fat diet changed the composition of the gut microbiota. However, when curcumin was added to the high-fat diet, the composition of the gut microbiota shifted toward that of the lean comparison rats fed a normal diet. (40). In another study, mice received intraperitoneal injections of the mutagenic agent azoxymethane to induce colonotropic carcinogenicity. A curcumin-supplemented diet entirely eliminated tumor burden, increased bacterial richness, prevented age-related decreases in α diversity, increased the relative abundance of Lactobacillales, and decreased Coriobacterales order (41). In a murine model of hyperacute, T-helper 1 ileitis after peroral infection with Toxoplasma gondii, curcumin-supplemented animals showed fewer proinflammatory enterobacteria and enterococci, and higher anti-inflammatory lactobacilli and bifidobacteria loads (42). Finally, commercial broiler breeds were fed from hatch with a diet supplemented with capsicum and Curcuma longa oleoresins, and co-infected with Eimeria maxima and Clostridium perfringens to induce necrotic enteritis. Oleoresin supplementation was associated with a normalization of gut Lactobacillus, Selenihalanaerobacter, Clostridium, Calothrix, and Geitlerinema induced by necrotic enteritis (43).
One study was identified that examined the effects of turmeric in human participants. In this study, 8 healthy participants fasted for 12 h and ingested curry and rice with or without turmeric. Breath-hydrogen concentrations were analyzed every 15 min for 6 h. Curry with turmeric significantly increased the AUC of breath hydrogen compared with turmeric-free curry, suggesting that dietary turmeric activated carbohydrate colonic fermentation (44).
Intestinal Permeability and Overall Health
The intestinal barrier consists of a single layer of epithelial cells that form the main physical barrier between the lumen and mucosal tissues. This barrier has several functions, including the regulation of nutrients, electrolytes, and water absorption from the lumen into systemic circulation, and the prevention and entry of pathogenic micro-organisms and toxic luminal substances (38, 45). This regulation of molecules influences the balance between tolerance and immunity to self and non-self antigens (38, 46).
The paracellular space is sealed by tight junctions, which regulate the flow of water ions and small molecules through the composition of protein structures that consist of transmembrane proteins such as claudin, occludin, and tricullin (45). These tight junctions and therefore the permeability of the intestinal barrier can be influenced by several factors. One primary regulator of this epithelial barrier is the composition of the intestinal microbiota. Consequently, factors that influence the microbiota have the potential to affect intestinal permeability. A state of dysbiosis can lead to increased intestinal permeability (often termed intestinal hyperpermeability or “leaky gut”) (47). In animal studies, high-fat diets have been associated with increased intestinal permeability (48, 49). The epithelial tight junctions can also be altered by several viruses and pathogens (e.g., Helicobacter pylori, Enterococcus coli, Salmonella typhimurium), excess alcohol intake, and pharmaceutical medications such as proton pump inhibitors and nonsteroidal anti-inflammatory drugs (50, 51). Stress can also influence the intestinal barrier and has been associated with increased gut permeability (52, 53).
A hyperpermeable intestinal barrier is associated with a proinflammatory state, although the sequence of this process is still not understood. Increased gut permeability results in increased translocation of molecules, which triggers a proinflammatory response. However, systemic inflammation can also increase intestinal barrier permeability and thus allow the translocation of commensal bacterial, further contributing to inflammation (38). Investigations into intestinal permeability have confirmed that it is a frequent phenomenon in a series of diseases and health conditions. These include both intestinal (e.g., infectious diarrhea, irritable bowel syndrome, inflammatory bowel disease, and celiac disease) and extraintestinal (e.g., allergies, arthritis, autism, obesity, diabetes, and depression) diseases (45, 51, 54).
Curcumin and Its Influence on Intestinal Permeability
In an in vitro study, treatment with apical leptin deteriorated tight junction function in Caco-2 cells. This effect was blunted by pretreatment with 20 μm curcumin added to a basolateral compartment for 30 min. Curcumin was found to inhibit the leptin receptor–dependent signaling pathway and the induction of gene expression encoding tight junction–associated proteins and proinflammatory cytokines (55). Caco-2 cells are recognized as in vitro models of human intestinal epithelial cells due to the similarity in morphologic and biochemical characteristics of intestinal cells and are widely used to investigate the intestinal uptake of drugs, nutrients, and phytochemicals. However, these in vitro models of human intestinal cells have limitations in representing the complexity of structural and digestive nature of the human small intestine (56). Therefore, replication via in vivo studies is important to help decipher the applicability of this finding in clinical populations. In another cell-based study, curcumin attenuated hydrogen peroxide–induced disruption of paracellular permeability in human intestinal epithelial cells. Curcumin also restored hydrogen peroxide–induced changes in occludin and zonula occludens 1 (ZO-1) proteins (57). Moreover, pretreatment with curcumin significantly attenuated LPS-induced secretion of IL-1β from intestinal epithelial cells and intestinal macrophages. Intestinal epithelial cell inflammation can contribute to greater intestinal permeability. Curcumin also protected against LPS-induced intestinal permeability by reducing the disorganization of tight junction proteins, ZO-1, claudin-1, claudin-7, and actin filaments (58). Finally, by using a Caco-2 cell line, IL-1α induced greater permeability of intestinal epithelial barrier, which curcumin was able to inhibit (59).
Animal models have also confirmed an effect of curcumin on intestinal permeability. In rats fed a high-fat diet for 16 wk, curcumin intervention resulted in improvements in the structure of tight junctions of the intestinal mucosa, intercellular space, edema in the mitochondria, and dilation of the endoplasmic reticulum. It also reduced serum concentrations of aspartate aminotransferase, alanine aminotransferase, diamine oxidase, TNF-α, and LPS and upregulated the expression of occludin in the intestinal mucosa (60). In a similar study, mice were fed a Western diet for 16 wk to increase intestinal permeability. Supplementation with curcumin significantly attenuated the diet-induced increase in plasma LPS concentrations and improved intestinal barrier function at multiple levels by restoring intestinal alkaline phosphatase activity and expression of tight junction proteins, ZO-1, and claudin-1 (61). Curcumin supplementation was also found to be beneficial in rats exposed to intestinal ischemia-reperfusion injury, because pretreatment with curcumin reduced induced changes in intestinal TNF-α, improved the histologic structure of intestinal mucosa, and dramatically attenuated the downregulated expression of ZO-1 (62). Finally, damage to the intestinal mucosa barrier of rats exposed to methotrexate was also reduced by the administration of curcumin. Curcumin also decreased the concentrations of d-lactate, diamine oxidase, myeloperoxidase, intercellular adhesion molecule-1, IL-1β, and TNF-α but increased the concentrations of IL-10 and superoxide dismutase (63).
Other Mechanisms of Curcumin's Gastrointestinal-Enhancing Effects
Anti-inflammatory and immune effects in the intestine
Curcumin is widely recognized as an anti-inflammatory and antioxidant, and it is believed that these mechanisms account significantly for its health-enhancing capacity. In Table 1, in vitro and animal studies examining the effects of curcumin on procedures specifically designed to disturb gastrointestinal function are summarized. In particular, the effects of curcumin on markers associated with immune defense, inflammation, and oxidative stress in the gastrointestinal tract are detailed. As shown in Table 1, curcumin has wide-ranging beneficial effects on markers associated with inflammation, antioxidant defenses, and oxidative stress. The procedures used to elicit gastrointestinal damage and corresponding immune response were wide-ranging, comprising surgical procedures and infection with substances to promote inflammation. Many of these procedures are designed to elicit dysfunction associated with several gastrointestinal diseases such as colitis, Crohn disease, and colon cancer.
TABLE 1.
In vitro and animal studies examining anti-inflammatory, immune-modulating, and antioxidant effects of curcumin in gastrointestinal induction procedures1
| Induction procedure | Effect of curcumin administration on measured markers | Reference |
|---|---|---|
| Anti-inflammatory and immune effects | ||
| Trinitrobenzene sulfonic acid to induce intestinal inflammation | In rats, a dietary dose of 0.1% curcumin for 10 wk suppressed the high expression of proinflammatory cytokine IL-1β mRNA and increased the low expression of IL-10 mRNA in the colonic mucosa. Curcumin intake was associated with a 70% suppression in intestinal tumor formation. | (64) |
| Intestinal microvascular endothelial cells stimulated with VEGF to induce angiogenesis | HIMECs pretreated with curcumin inhibited COX-2 protein expression at 1 µM, with increasing effect at 10 µM curcumin, whereas 20 µM curcumin completely abolished COX-2 expression after VEGF activation. In addition, immunofluorescence staining of HIMECs pretreated with 10 μM curcumin showed inhibition of COX-2 expression after VEGF activation. | (65) |
| Mice infected with Toxoplasma gondii | Compared with placebo, oral administration of curcumin (100 mg/kg body weight) for 7 d increased numbers of regulatory T cells by 20–30% and augmented intestinal epithelial cell proliferation/regeneration; reduced mucosal T lymphocyte and neutrophilic granulocyte numbers; increased concentrations of the anti-inflammatory cytokine IL-10 in ileum, mesenteric lymph nodes, and spleen; and decreased proinflammatory cytokine expression (IL-23p19, IFN-γ, TNF-α, IL-6, MCP-1) in the ileum by 25–30%. | (42) |
| Rats fed a high-fat diet | Rats fed a dietary dose of 0.5% curcumin for 3 wk showed increased IgA concentrations in feces by 120% and in colon contents by 80%. However, no change occurred in rats fed a low-fat diet. | (66) |
| Mouse model of colon cancer | Oral intake of 2% curcumin diet from 4 to 18 wk of age blunted increases in mRNA expression of IL-1β and completely blocked the increased expression of IL-6, TNF-α, and CCL2; and blunted increases in protein concentrations of IL-1β and CCL2. | (67) |
| Human and mouse colonocytes stimulated with IFN-γ | Cells treated with 50 μM curcumin inhibited the Jak/Stat activation pathway by modulating Stat1 phosphorylation, nuclear translocation, DNA binding, and transcription of IFN-γ–inducible major histocompatibility complex II genes and T cell chemokines. | (68) |
| Intestinal subepithelial myofibroblasts isolated from the colon of Crohn disease patients and human colonic cell line of myofibroblasts were stimulated with TNF-α | Cells treated with 20 µM curcumin normalized matrix metalloproteinase 3 concentrations in TNF-α–stimulated cells. | (69) |
| Induction of colitis by intracolonic administration of 4% acetic acid | Oral treatment with curcumin (50 mg · kg−1 · d−1) for 14 d decreased serum lactate dehydrogenase, colonic alkaline phosphatase, IL-1β, TNF-α , colonic myeloperoxidase, and lipid peroxide concentrations and increased colonic PGE2 and IL-10 concentrations. | (70) |
| Gastrointestinal mucosal lesions induced by the administration of reserpine | Co-administration of reserpine and curcumin (at doses of 100 and 200 mg/kg) decreased inflammatory response and balanced the expression of vasoactive intestinal peptide and gastrin in the reserpine-treated rats; and high-dose (200 mg/kg) curcumin inhibited increases in NF-κB, phosphorylated-IκB-α, Bax, and cleaved caspase 3. | (71) |
| Experimental colitis induced by administering 2,4,6-trinitrobenzene sulfonic acid/ethanol solution to rats | Intragastric administration of curcumin for 7 d at a dose of 200 mg/kg alleviated inflammatory injury of the colonic mucosa, increased the number of Treg cells and inhibited the secretion of TNF-α, IL-2, IL-6, IL-12 p40, IL-17, and IL-21 and the expression of costimulatory molecules [CD205, CD54, TLR4, CD252, CD256, and CD254] of dendritic cells. | (72) |
| DSS-induced colitis | Curcumin-containing ETOs (doses from 5 to 50 mg/kg) and standard curcumin (doses from 5 to 50 mg/kg) provided protection against DSS-induced inflammation. ETO-curcumin improved disease activity index dose-dependently, whereas the anti-inflammatory efficacy of standard curcumin remained constant. Gene expression analysis showed that anti-inflammatory cytokines including IL-10 and IL-11 as well as FOXP3 were upregulated in the colon by ETO-curcumin. | (73) |
| Effects on antioxidant defense and oxidative stress | ||
| Rats exposed to intestinal ischemia reperfusion | Curcumin (100 mg/kg) administered by gastric gavage for 3 d before intestinal ischemia reperfusion decreased elevations in tissue MDA concentrations by 30% and attenuated reductions in superoxide dismutase and glutathione peroxidase enzyme activities in intestinal tissues samples. | (74) |
| Rats exposed to intestinal ischemia reperfusion | Curcumin (200 mg/kg; single dose) delivered via oral gavage 15 min before the injury insult lowered serum MDA concentrations by 27% but had no significant effect on total antioxidant capacity levels. | (75) |
| Rats exposed to intestinal ischemia reperfusion | Curcumin delivered orally (200 mg/kg) for 20 d lowered MDA concentrations in intestinal (12% reduction) and heart (17% reduction) tissue and increased superoxide dismutase activity in the intestine (186% increase) and lung tissue (245% increase). | (76) |
| Rats treated with methotrexate to induce intestinal damage | Turmeric extract (at 200 mg/kg but not 100 mg/kg) delivered orally for 30 d increased blood concentrations of antioxidant enzymes superoxide dismutase (50% increase), glutathione peroxidase (39% increase), and catalase (56% increase); increased total antioxidant status (122% increase); and decreased a marker of lipid peroxidation (MDA; 24% decrease). | (77) |
| Bile duct ligation in rats | Curcumin (100 mg/kg) given orally for 14 d decreased mucosal injury by 60%, lowered elevations in tissue MDA concentrations and myeloperoxidase activity, increased reduced glutathione concentrations in intestinal tissues, and inhibited apoptosis and cell proliferation. | (78) |
1CCL2, chemokine ligand 2; COX-2, cyclo-oxygenase 2; DSS, dextran sodium sulfate; ETO, essential turmeric oil; FOXP3, forkhead box P; HIMEC, human intestinal microvascular endothelial cell; Jak/Stat, Janus tyrosine Kinase/Signal transducer and activator of transcription; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; Stat1, signal transducer and activator of transcription 1; TLR4, Toll-like receptor 4; Treg, T-regulatory; VEGF, vascular endothelial growth factor.
Effects on bacterial, parasitic, and fungal infections
Bacterial and parasitic infections are commonly associated with several digestive symptoms, including nausea, diarrhea, and abdominal pain. However, their effects can be systemic. For example, their presence has been found in autoimmune diseases (79), dementia (80, 81), and psychiatric illnesses such as depression, schizophrenia, and autism (82, 83). Although an association does not confirm a causative effect, such infections may exacerbate symptom severity, reduce treatment efficacy, or contribute to other symptomatic presentations.
In vitro, animal, and some human studies have confirmed curcumin's antibacterial, antiparasitic, and antifungal activity (84–86) and therefore presents as another mechanism associated with its potential health-enhancing effects. A selection of investigations into curcumin's activity on some common gastrointestinal infections is briefly summarized below. This is not an exhaustive list and readers are encouraged to see other reviews such as Haddad et al. (85) and Moghadamtousi et al. (84).
H. pylori
H. pylori is one of the most common bacterial infections that invades the mucosal lining of the stomach and is the cause of ≤95% of duodenal ulcers and ≤75% of gastric ulcers (87). On the basis of several in vitro, early cell culture, animal research, and a few preclinical trials, curcumin has been projected as a potential therapeutic candidate against H. pylori–mediated gastric pathogenesis. However, efficacy from clinical trials has been inconsistent (88). In a randomized, double-blind, placebo-controlled trial, adjunctive curcumin administration to triple therapy with clarithromycin, amoxicillin, and pantoprazole was associated with a greater improvement in dyspepsia symptoms but had no enhancing effect on the eradication of H. pylori infection (89). In another trial, curcumin had a minimal antibactericidal effect on H. pylori, and on the production of inflammatory cytokines (90). However, in this study, turmeric rather than curcumin tablets were used, which contained a relatively small quantity of 40 mg curcumin/tablet (delivered 3 times/d). The therapeutic potency of a higher dose of curcumin is therefore unknown.
Candida
Candida is a genus of yeasts and is the most common cause of fungal infections worldwide. Candida albicans is the most commonly isolated species and can cause infections (candidiasis or thrush) in humans (91). An in vitro study of the antifungal activity of curcumin against 14 strains of Candida confirmed curcumin's antifungal properties against all tested Candida strains, with minimum inhibitory concentrations varying from 250 to 2000 µg/mL. However, on the basis of concentration, efficacy of curcumin was significantly less than that of the standard antifungal fluconazole (92). In another in vitro study, the antifungal activity of curcumin was evaluated against 23 fungi strains, along with its in vitro inhibitory effect on the adhesion of Candida species to human buccal epithelial cells. Curcumin exhibited minimum inhibitory concentrations of 0.5 to 256 mg/L against most tested human-pathogenic fungi. In fact, curcumin was found to be more efficient than fluconazole in inhibiting the adhesion of many Candida species to human buccal epithelial cells, particularly those strains isolated from the buccal mucosa of AIDS patients (93). In another study, suspensions of Candida were treated with 9 curcumin concentrations and exposed to light-emitting diode for the inactivation of C. albicans. Curcumin significantly reduced C. albicans viability after photodynamic therapy, for both planktonic and biofilm cultures. Complete inactivation of the planktonic form was observed with curcumin at 7.4 mg/L with the use of low fluences (94).
Giardia (Giardiosis)
Giardiosis is an infection of the bowel, caused by a parasite called Giardia. Giardiosis is one of the common parasite-induced causes of diarrhea in humans, especially in children, worldwide (95). In mice infected with Giardia, curcumin reduced fecal cyst and intestinal trophozoite counts and improved the intestinal mucosal damage induced by Giardia infection (96).
E. coli
E. coli are a diverse group of bacteria normally habitating in the intestinal tract. Most E. coli are harmless and may be important for a healthy human intestinal tract. However, some are pathogenic, causing diarrhea, stomach pain, and vomiting (97). In an in vitro study, turmeric extracts inhibited the growth of E. coli (98). Curcumin has also been shown to induce an apoptosis-like response in E. coli (99).
T. gondii
T. gondii is one of the most common parasites in developed countries, affecting ∼30–50% people (100). Oral treatment with curcumin ameliorated acute small intestinal inflammation in mice infected with T. gondii (42). In an in vitro study, curcumin inhibited the propagation of cultured T. gondii and the enzymatic activity of glyoxalase 1. Glyoxalase 1 is involved in the production of cytotoxic methylglyoxal, which is produced during T. gondii infection (101).
Antianaphylactic effects
The antianaphylactic effect of curcumin was examined in a murine model, in which intestinal anaphylaxis was induced by exposure to ovalbumin (OVA), the main protein found in egg white (102). The frequent ingestion of curcumin during oral OVA exposure inhibited the development of mastocytosis and intestinal anaphylaxis in OVA-challenged allergic mice. Intragastric exposure to OVA in sensitized mice induced a robust IgE-mediated response accompanied by enhanced OVA-IgE concentrations, intestinal mastocytosis, elevated serum monocyte chemoattractant protein 1, and acute diarrhea. However, mice exposed to oral curcumin did not exhibit intense allergic diarrhea or a significant enhancement of OVA-IgE and intestinal mast cell expansion and activation. Moreover, allergic diarrhea, mast cell activation and expansion, and T-helper 2 responses were also suppressed in mice exposed to curcumin during the OVA-challenge phase, despite the presence of elevated concentrations of OVA-IgE. The suppression of intestinal anaphylaxis by curcumin was directly linked to the inhibition of NF-κB activation in curcumin-treated allergic mice. Although, to our knowledge, this is the only study conducted to date, and further replication and extension of this study is required to elaborate on this finding, it has potential implications for people suffering from food allergies, because curcumin may influence allergenic responses to certain foods. Food allergies and sensitivities, particularly those associated with gluten, have been implicated in several diseases, including gastrointestinal disorders (103, 104), rheumatic diseases (105), and even neurological and neuropsychiatric conditions such as depression (106), schizophrenia (107), autism (108, 109), and dementia (110). However, the research is limited and often inconsistent.
Conclusions and Directions for Future Research
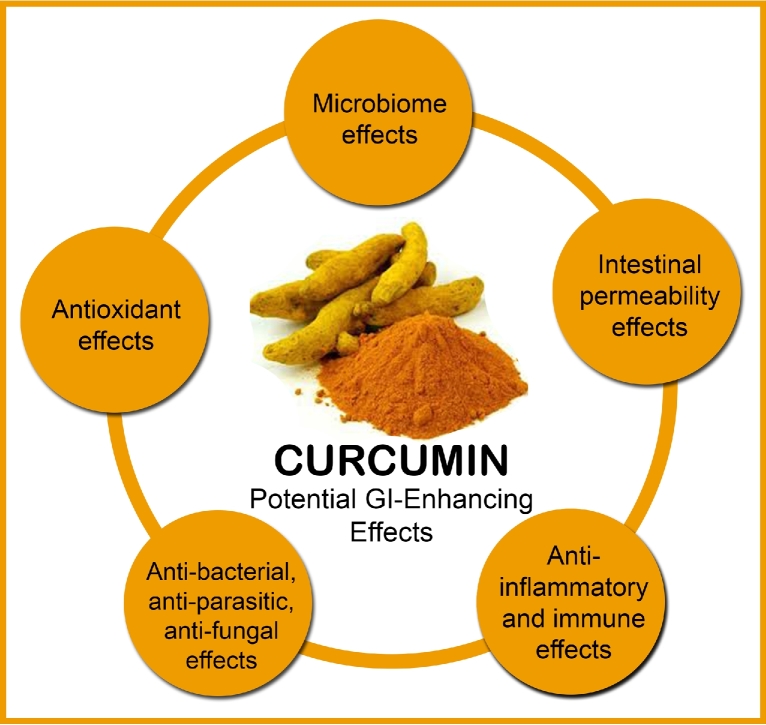
Although research is still in its infancy, evidence from in vitro and in vivo studies suggest that curcumin is a natural agent with positive influences on gastrointestinal health and function. In particular, and as depicted in Figure 1, curcumin seems to influence the composition of our gut microbiota, modulates intestinal permeability, reduces inflammation and oxidative stress in the gastrointestinal tract, and has an influence on bacterial, parasitic, and fungal infections. Moreover, curcumin was also shown in one animal study to reduce the anaphylactic response from oral allergenic food exposure (102). Although these processes are discussed separately in this article, they are strongly interconnected and therefore likely have a significant influence on each other.
FIGURE 1.
Potential GI effects of curcumin that may contribute to its systemic health effects. GI, gastrointestinal.
Because the integrity of our gastrointestinal tract has wide-reaching influences on the health and function of our whole body, it is argued that curcumin's health-enhancing benefits may at least be partly derived via its positive gastrointestinal effect. This helps explain the encouraging medicinal and physiologic benefits of curcumin, despite its poor bioavailability and systemic tissue distribution. However, further research is essential through the completion of clinical trials to determine if changes indeed occur in the human gastrointestinal system from oral curcumin ingestion and whether such changes influence symptomatic and biological processes in the assessed disease states. As has already been discussed, gastrointestinal disturbances are common in a range of extraintestinal diseases (e.g., metabolic disorders, autoimmune conditions, dermatologic conditions, certain cancers, psychiatric disorders, and neurological disorders), although their causative or perpetuating influence on disease progression remains to be determined for most of these conditions.
Although intensive efforts are underway to enhance curcumin's bioavailability and therefore potential clinical efficacy, attention on its gastrointestinal influence may also be prudent. This holds particular promise given increasing interest in probiotics and prebiotics, which are also purported to have systemic health effects via their positive influence on the gastrointestinal system (111). Even though in this review the effects of curcumin have specifically been critiqued, some of the studies reviewed used whole turmeric, rather than curcumin alone. It is therefore plausible (and likely) that turmeric contains a range of compounds that may have therapeutic benefits. For example, in an animal model of dextran sodium sulfate–induced colitis, a curcumin preparation containing essential turmeric oils provided superior anti-inflammatory effects compared with curcumin alone (73). Turmerones contained in turmeric oils can also induce neural stem cell proliferation (112) and have antidepressant-like activity (113). Commercial curcumin is also regularly delivered as a curcuminoid mixture, which includes demethoxycurcumin and bisdemethoxycurcumin, albeit at much lower amounts than curcumin. There are also various metabolites of curcumin, including dihydrocurcumin, testrahydrocurcumin, hexahydrocurcumin, octahydrocurcumin, curcumin glucouronide, and curcumin sulfate (114, 115). These analogs and derivatives have pharmacologic activity and therefore may contribute to the gastrointestinal impact from curcumin supplementation. The influence of these components delivered either individually or in combination therefore requires investigation.
In conclusion, with the increasing burden of environmental, dietary, and lifestyle factors on our gastrointestinal system and the influence our gut has on the health of our overall body, interventions to protect and improve its function are imperative. Curcumin presents as one option that requires further examination.
Acknowledgments
I thank Stephen J Smith for his help with the proofing of this article.
References
- 1. Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, Aggarwal BB. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol 2017;174:1325–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kunwar A, Priyadarsini KI. Curcumin and its role in chronic diseases. Adv Exp Med Biol 2016;928:1–25. [DOI] [PubMed] [Google Scholar]
- 3. Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules 2016;21:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food 2016;19:717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sahebkar A, Henrotin Y. Analgesic efficacy and safety of curcuminoids in clinical practice: a systematic review and meta-analysis of randomized controlled trials. Pain Med 2015. [DOI] [PubMed] [Google Scholar]
- 6. Al-Karawi D, Al Mamoori DA, Tayyar Y. The role of curcumin administration in patients with major depressive disorder: mini meta-analysis of clinical trials. Phytother Res 2015;30:175–83. [DOI] [PubMed] [Google Scholar]
- 7. Sahebkar A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother Res 2014;28:633–42. [DOI] [PubMed] [Google Scholar]
- 8. Sahebkar A, Cicero AF, Simental-Mendia LE, Aggarwal BB, Gupta SC. Curcumin downregulates human tumor necrosis factor-alpha levels: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2016;107:234–42. [DOI] [PubMed] [Google Scholar]
- 9. Derosa G, Maffioli P, Simental-Mendia LE, Bo S, Sahebkar A. Effect of curcumin on circulating interleukin-6 concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res 2016;111:394–404. [DOI] [PubMed] [Google Scholar]
- 10. Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin. J Med Chem 2017;60:1620–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807–18. [DOI] [PubMed] [Google Scholar]
- 12. Metzler M, Pfeiffer E, Schulz SI, Dempe JS. Curcumin uptake and metabolism. Biofactors 2013;39:14–20. [DOI] [PubMed] [Google Scholar]
- 13. Siviero A, Gallo E, Maggini V, Gori L, Mugelli A, Firenzuoli F, Vannacci A. Curcumin, a golden spice with a low bioavailability. J Herb Med 2015;5;57–70. [Google Scholar]
- 14. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 15. Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther 2004;20:31–9. [DOI] [PubMed] [Google Scholar]
- 16. Nellesen D, Chawla A, Oh DL, Weissman T, Lavins BJ, Murray CW. Comorbidities in patients with irritable bowel syndrome with constipation or chronic idiopathic constipation: a review of the literature from the past decade. Postgrad Med 2013;125:40–50. [DOI] [PubMed] [Google Scholar]
- 17. Roman AL, Munoz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol 2011;17:2723–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 19. Vuong HE, Yano JM, Fung TC, Hsiao EY. The microbiome and host behavior. Annu Rev Neurosci 2017;40:21–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 2011;6:209–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Logan AC, Jacka FN, Prescott SL. Immune-microbiota interactions: dysbiosis as a global health issue. Curr Allergy Asthma Rep 2016;16:13. [DOI] [PubMed] [Google Scholar]
- 22. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dinan TG, Cryan JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 2017;46:77–89. [DOI] [PubMed] [Google Scholar]
- 24. Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry 2016;21:738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nuriel-Ohayon M, Neuman H, Koren O. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 2016;7:1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles-Hillel A, Qiao Z, Hubert N, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep 2016;6:35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Monda V, Villano I, Messina A, Valenzano A, Esposito T, Moscatelli F, Viggiano A, Cibelli G, Chieffi S, Monda M, et al. Exercise modifies the gut microbiota with positive health effects. Oxid Med Cell Longev 2017;2017:3831972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sundin J, Ohman L, Simren M. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom Med 2017;79:857–67. [DOI] [PubMed] [Google Scholar]
- 29. Brahe LK, Astrup A, Larsen LH. Can we prevent obesity-related metabolic diseases by dietary modulation of the gut microbiota? Adv Nutr 2016;7:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karlsson F, Tremaroli V, Nielsen J, Backhed F. Assessing the human gut microbiota in metabolic diseases. Diabetes 2013;62:3341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun 2014;38:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mulak A, Bonaz B. Brain-gut-microbiota axis in Parkinson's disease. World J Gastroenterol 2015;21:10609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Sci Rep 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y, Luo W, Deng Z, Lei G. Diet-intestinal microbiota axis in osteoarthritis: a possible role. Mediators Inflamm 2016;2016:3495173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navaneetharaja N, Griffiths V, Wileman T, Carding SR. A role for the intestinal microbiota and virome in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)? J Clin Med 2016;5:E55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rajagopala SV, Vashee S, Oldfield LM, Suzuki Y, Venter JC, Telenti A, Nelson KE. The human microbiome and cancer. Cancer Prev Res (Phila) 2017;10:226–34. [DOI] [PubMed] [Google Scholar]
- 37. Mangiola F, Ianiro G, Franceschi F, Fagiuoli S, Gasbarrini G, Gasbarrini A. Gut microbiota in autism and mood disorders. World J Gastroenterol 2016;22:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 2015;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dickerson F, Severance E, Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun 2017;62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Feng W, Wang H, Zhang P, Gao C, Tao J, Ge Z, Zhu D, Bi Y. Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats. Biochim Biophys Acta 2017;1861:1801–12. [DOI] [PubMed] [Google Scholar]
- 41. McFadden RM, Larmonier CB, Shehab KW, Midura-Kiela M, Ramalingam R, Harrison CA, Besselsen DG, Chase JH, Caporaso JG, Jobin C, et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm Bowel Dis 2015;21:2483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bereswill S, Munoz M, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Loddenkemper C, Gobel UB, Heimesaat MM. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS One 2010;5:e15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim JE, Lillehoj HS, Hong YH, Kim GB, Lee SH, Lillehoj EP, Bravo DM. Dietary capsicum and Curcuma longa oleoresins increase intestinal microbiome and necrotic enteritis in three commercial broiler breeds. Res Vet Sci 2015;102:150–8. [DOI] [PubMed] [Google Scholar]
- 44. Shimouchi A, Nose K, Takaoka M, Hayashi H, Kondo T. Effect of dietary turmeric on breath hydrogen. Dig Dis Sci 2009;54:1725–9. [DOI] [PubMed] [Google Scholar]
- 45. Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bengmark S. Gut microbiota, immune development and function. Pharmacol Res 2013;69:87–113. [DOI] [PubMed] [Google Scholar]
- 47. Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017;21:455–66e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 2012;142:1100–1, e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas Rde C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 2012;108:801–9. [DOI] [PubMed] [Google Scholar]
- 50. Bjarnason I, Takeuchi K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J Gastroenterol 2009;44:23–9. [DOI] [PubMed] [Google Scholar]
- 51. Konig J, Wells J, Cani PD, Garcia-Rodenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 2016;7:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soderholm JD, Perdue MH. Stress and gastrointestinal tract. II. Stress and intestinal barrier function. Am J Physiol Gastrointest Liver Physiol 2001;280:G7–13. [DOI] [PubMed] [Google Scholar]
- 53. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tomicronth J, Holvoet L, Farre R, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 2014;63:1293–9. [DOI] [PubMed] [Google Scholar]
- 54. Maes M, Kubera M, Leunis JC, Berk M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord 2012;141:55–62. [DOI] [PubMed] [Google Scholar]
- 55. Kim CY, Kim KH. Curcumin prevents leptin-induced tight junction dysfunction in intestinal Caco-2 BBe cells. J Nutr Biochem 2014;25:26–35. [DOI] [PubMed] [Google Scholar]
- 56. Schweinlin M, Wilhelm S, Schwedhelm I, Hansmann J, Rietscher R, Jurowich C, Walles H, Metzger M. Development of an advanced primary human in vitro model of the small intestine. Tissue Eng Part C Methods 2016;22:873–83. [DOI] [PubMed] [Google Scholar]
- 57. Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, Jiang H. Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci 2012;57:1792–801. [DOI] [PubMed] [Google Scholar]
- 58. Wang J, Ghosh SS, Ghosh S. Curcumin improves intestinal barrier function: modulation of intracellular signaling, and organization of tight junctions. Am J Physiol Cell Physiol 2017;312:C438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim CY. Inhibition of interleukin-1α-induced intestinal epithelial tight junction permeability by curcumin treatment in Caco-2 Cells in Caco-2 cells. J Life Sci 2016;26:1082–7. [Google Scholar]
- 60. Hou HT, Qiu YM, Zhao HW, Li DH, Liu YT, Wang YZ, Su SH. [Effect of curcumin on intestinal mucosal mechanical barrier in rats with non-alcoholic fatty liver disease.] Zhonghua Gan Zang Bing Za Zhi 2017;25:134–8 (in Chinese). [DOI] [PubMed] [Google Scholar]
- 61. Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates Western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice–role of intestinal permeability and macrophage activation. PLoS One 2014;9:e108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tian S, Guo R, Wei S, Kong Y, Wei X, Wang W, Shi X, Jiang H. Curcumin protects against the intestinal ischemia-reperfusion injury: involvement of the tight junction protein ZO-1 and TNF-alpha related mechanism. Korean J Physiol Pharmacol 2016;20:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song WB, Wang YY, Meng FS, Zhang QH, Zeng JY, Xiao LP, Yu XP, Peng DD, Su L, Xiao B, et al. Curcumin protects intestinal mucosal barrier function of rat enteritis via activation of MKP-1 and attenuation of p38 and NF-kappaB activation. PLoS One 2010;5:e12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res 2000;89:169–75. [DOI] [PubMed] [Google Scholar]
- 65. Binion DG, Otterson MF, Rafiee P. Curcumin inhibits VEGF-mediated angiogenesis in human intestinal microvascular endothelial cells through COX-2 and MAPK inhibition. Gut 2008;57:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Okazaki Y, Han Y, Kayahara M, Watanabe T, Arishige H, Kato N. Consumption of curcumin elevates fecal immunoglobulin A, an index of intestinal immune function, in rats fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2010;56:68–71. [DOI] [PubMed] [Google Scholar]
- 67. Murphy EA, Davis JM, McClellan JL, Gordon BT, Carmichael MD. Curcumin's effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J Interferon Cytokine Res 2011;31:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Midura-Kiela MT, Radhakrishnan VM, Larmonier CB, Laubitz D, Ghishan FK, Kiela PR. Curcumin inhibits interferon-gamma signaling in colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2012;302:G85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fontani F, Marcucci T, Picariello L, Tonelli F, Vincenzini MT, Iantomasi T. Redox regulation of MMP-3/TIMP-1 ratio in intestinal myofibroblasts: effect of N-acetylcysteine and curcumin. Exp Cell Res 2014;323:77–86. [DOI] [PubMed] [Google Scholar]
- 70. Gopu B, Dileep R, Rani MU, Kumar CS, Kumar MV, Reddy AG. Protective role of curcumin and flunixin against acetic acid-induced inflammatory bowel disease via modulating inflammatory mediators and cytokine profile in rats. J Environ Pathol Toxicol Oncol 2015;34:309–20. [DOI] [PubMed] [Google Scholar]
- 71. Long L, Wang J, Chen N, Zheng S, Shi L, Xu Y, Luo C, Deng Y. Curcumin ameliorates reserpine-induced gastrointestinal mucosal lesions through inhibiting IkappaB-alpha/NF-kappaB pathway and regulating expression of vasoactive intestinal peptide and gastrin in rats. J Med Food 2016;19:528–34. [DOI] [PubMed] [Google Scholar]
- 72. Zhao HM, Xu R, Huang XY, Cheng SM, Huang MF, Yue HY, Wang X, Zou Y, Lu AP, Liu DY. Curcumin improves regulatory T cells in gut-associated lymphoid tissue of colitis mice. World J Gastroenterol 2016;22:5374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Toden S, Theiss AL, Wang X, Goel A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci Rep 2017;7:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yucel AF, Kanter M, Pergel A, Erboga M, Guzel A. The role of curcumin on intestinal oxidative stress, cell proliferation and apoptosis after ischemia/reperfusion injury in rats. J Mol Histol 2011;42:579–87. [DOI] [PubMed] [Google Scholar]
- 75. Onder A, Kapan M, Gumus M, Yuksel H, Boyuk A, Alp H, Basarili MK, Firat U. The protective effects of curcumin on intestine and remote organs against mesenteric ischemia/reperfusion injury. Turk J Gastroenterol 2012;23:141–7. [DOI] [PubMed] [Google Scholar]
- 76. Okudan N, Belviranli M, Gokbel H, Oz M, Kumak A. Protective effects of curcumin supplementation on intestinal ischemia reperfusion injury. Phytomedicine 2013;20:844–8. [DOI] [PubMed] [Google Scholar]
- 77. Moghadam AR, Mohajeri D, Namvaran-Abbas-Abad A, Manafi H, Shahi D, Mazani M. Protective effect of turmeric extract on ethotrexate-induced intestinal damage and oxidative stress. Chin J Nat Med 2013;11:477–83. [DOI] [PubMed] [Google Scholar]
- 78. Kanter M, Takir M, Mutlu HH, Kanter B, Kostek O, Toprak AE. Protective effects of curcumin on intestinal damage in cholestatic rats. J Invest Surg 2016;29:128–36. [DOI] [PubMed] [Google Scholar]
- 79. Alam J, Kim YC, Choi Y. Potential role of bacterial infection in autoimmune diseases: a new aspect of molecular mimicry. Immune Netw 2014;14:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xu Y, Wang Q, Liu Y, Cui R, Zhao Y. Is Helicobacter pylori infection a critical risk factor for vascular dementia? Int J Neurosci 2016;126:899–903. [DOI] [PubMed] [Google Scholar]
- 81. Bibi F, Yasir M, Sohrab SS, Azhar EI, Al-Qahtani MH, Abuzenadah AM, Kamal MA, Naseer MI. Link between chronic bacterial inflammation and Alzheimer disease. CNS Neurol Disord Drug Targets 2014;13:1140–7. [DOI] [PubMed] [Google Scholar]
- 82. Bolton DJ, Robertson LJ. Mental health disorders associated with foodborne pathogens. J Food Prot 2016;79:2005–17. [DOI] [PubMed] [Google Scholar]
- 83. Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biol Psychiatry 2017;81:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H, Abubakar S, Zandi K. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed Res Int 2014;2014:186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Haddad M, Sauvain M, Deharo E. Curcuma as a parasiticidal agent: a review. Planta Med 2011;77:672–8. [DOI] [PubMed] [Google Scholar]
- 86. Gunes H, Gulen D, Mutlu R, Gumus A, Tas T, Topkaya AE. Antibacterial effects of curcumin: an in vitro minimum inhibitory concentration study. Toxicol Ind Health 2016;32:246–50. [DOI] [PubMed] [Google Scholar]
- 87. Garza-Gonzalez E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 2014;20:1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sarkar A, De R, Mukhopadhyay AK. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases. World J Gastroenterol 2016;22:2736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Khonche A, Biglarian O, Panahi Y, Valizadegan G, Soflaei SS, Ghamarchehreh ME, Majeed M, Sahebkar A. Adjunctive therapy with curcumin for peptic ulcer: a randomized controlled trial. Drug Res (Stuttg) 2016;66:444–8. [DOI] [PubMed] [Google Scholar]
- 90. Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K, Wipasa J. Investigation of the anti-inflammatory effect of curcuma longa in helicobacter pylori-infected patients. Int Immunopharmacol 2010;10:815–8. [DOI] [PubMed] [Google Scholar]
- 91. Gow NA, van de Veerdonk FL, Brown AJ, Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol 2011;10:112–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA. Curcumin as a promising anticandidal of clinical interest. Can J Microbiol 2011;57:204–10. [DOI] [PubMed] [Google Scholar]
- 93. Martins CV, da Silva DL, Neres AT, Magalhaes TF, Watanabe GA, Modolo LV, Sabino AA, de Fatima A, de Resende MA. Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother 2009;63:337–9. [DOI] [PubMed] [Google Scholar]
- 94. Dovigo LN, Pavarina AC, Ribeiro AP, Brunetti IL, Costa CA, Jacomassi DP, Bagnato VS, Kurachi C. Investigation of the photodynamic effects of curcumin against Candida albicans. Photochem Photobiol 2011;87:895–903. [DOI] [PubMed] [Google Scholar]
- 95. Minetti C, Chalmers RM, Beeching NJ, Probert C, Lamden K. Giardiasis. BMJ 2016;355:i5369. [DOI] [PubMed] [Google Scholar]
- 96. Dyab AK, Yones DA, Ibraheim ZZ, Hassan TM. Anti-giardial therapeutic potential of dichloromethane extracts of Zingiber officinale and Curcuma longa in vitro and in vivo. Parasitol Res 2016;115:2637–45. [DOI] [PubMed] [Google Scholar]
- 97. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004;2:123–40. [DOI] [PubMed] [Google Scholar]
- 98. Gul P, Bakht J. Antimicrobial activity of turmeric extract and its potential use in food industry. J Food Sci Technol 2015;52:2272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yun DG, Lee DG. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl Microbiol Biotechnol 2016;100:5505–14. [DOI] [PubMed] [Google Scholar]
- 100. Saadatnia G, Golkar M. A review on human toxoplasmosis. Scand J Infect Dis 2012;44:805–14. [DOI] [PubMed] [Google Scholar]
- 101. Goo YK, Yamagishi J, Ueno A, Terkawi MA, Aboge GO, Kwak D, Hong Y, Chung DI, Igarashi M, Nishikawa Y, et al. Characterization of Toxoplasma gondii glyoxalase 1 and evaluation of inhibitory effects of curcumin on the enzyme and parasite cultures. Parasit Vectors 2015;8:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kinney SR, Carlson L, Ser-Dolansky J, Thompson C, Shah S, Gambrah A, Xing W, Schneider SS, Mathias CB. Curcumin ingestion inhibits mastocytosis and suppresses intestinal anaphylaxis in a murine model of food allergy. PLoS One 2015;10:e0132467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Volta U, Pinto-Sanchez MI, Boschetti E, Caio G, De Giorgio R, Verdu EF. Dietary triggers in irritable bowel syndrome: is there a role for gluten? J Neurogastroenterol Motil 2016;22:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Casella G, Di Bella C, Salemme M, Villanacci V, Antonelli E, Baldini V, Bassotti G. Celiac disease, non-celiac gluten sensitivity and inflammatory bowel disease. Minerva Gastroenterol Dietol 2015;61:267–71. [PubMed] [Google Scholar]
- 105. Isasi C, Tejerina E, Moran LM. Non-celiac gluten sensitivity and rheumatic diseases. Reumatol Clin 2016;12:4–10. [DOI] [PubMed] [Google Scholar]
- 106. Porcelli B, Verdino V, Bossini L, Terzuoli L, Fagiolini A. Celiac and non-celiac gluten sensitivity: a review on the association with schizophrenia and mood disorders. Auto Immun Highlights 2014;5:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ergun C, Urhan M, Ayer A. A review on the relationship between gluten and schizophrenia: Is gluten the cause? Nutr Neurosci 2017:0. [DOI] [PubMed] [Google Scholar]
- 108. Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr 2016;12:436–42. [DOI] [PubMed] [Google Scholar]
- 109. Elder JH, Kreider CM, Schaefer NM, de Laosa MB. A review of gluten- and casein-free diets for treatment of autism: 2005–2015. Nutr Diet Suppl 2015;7:87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Daulatzai MA. Non-celiac gluten sensitivity triggers gut dysbiosis, neuroinflammation, gut-brain axis dysfunction, and vulnerability for dementia. CNS Neurol Disord Drug Targets 2015;14:110–31. [DOI] [PubMed] [Google Scholar]
- 111. Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics- a review. J Food Sci Technol 2015;52:7577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hucklenbroich J, Klein R, Neumaier B, Graf R, Fink GR, Schroeter M, Rueger MA. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res Ther 2014;5:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liao JC, Tsai JC, Liu CY, Huang HC, Wu LY, Peng WH. Antidepressant-like activity of turmerone in behavioral despair tests in mice. BMC Complement Altern Med 2013;13:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, et al. Biological activities of curcumin and its analogues (congeners) made by man and Mother Nature. Biochem Pharmacol 2008;76:1590–611. [DOI] [PubMed] [Google Scholar]
- 115. Shen L, Ji H. The pharmacology of curcumin: is it the degradation products? Trends Mol Med 2012;18:138–44. [DOI] [PubMed] [Google Scholar]