Abstract
Butyrate, a four-carbon short-chain fatty acid, is produced through microbial fermentation of dietary fibers in the lower intestinal tract. Endogenous butyrate production, delivery, and absorption by colonocytes have been well documented. Butyrate exerts its functions by acting as a histone deacetylase (HDAC) inhibitor or signaling through several G protein–coupled receptors (GPCRs). Recently, butyrate has received particular attention for its beneficial effects on intestinal homeostasis and energy metabolism. With anti-inflammatory properties, butyrate enhances intestinal barrier function and mucosal immunity. However, the role of butyrate in obesity remains controversial. Growing evidence has highlighted the impact of butyrate on the gut-brain axis. In this review, we summarize the present knowledge on the properties of butyrate, especially its potential effects and mechanisms involved in intestinal health and obesity.
Keywords: butyrate, G protein–coupled receptors, gut-brain axis, histone deacetylase, inflammation, intestinal barrier, intestinal microbiota, obesity
Introduction
SCFAs, primarily acetate, propionate, and butyrate, are organic acids produced in the intestinal lumen by bacterial fermentation of mainly undigested dietary carbohydrates, specifically resistant starch and dietary fiber and, to a lesser extent, dietary and endogenous proteins (1, 2). Most micro-organisms prefer to ferment carbohydrates over proteins, so the concentrations of SCFAs are highest in the proximal colon, where most substrates for fermentation are available, and decline towards the distal colon (3). It has been estimated that SCFAs contribute to ∼60–70% of the energy requirements of colonic epithelial cells and 5–15% of the total caloric requirements of humans (4).
Among SCFAs, butyrate has received particular attention for its beneficial effects on both cellular energy metabolism and intestinal homeostasis (5). Although it is the least abundant SCFA produced (∼60% acetate, 25% propionate, and 15% butyrate in humans) (6, 7), butyrate is the major energy source for colonocytes (8, 9). Butyrate modulates biological responses of host gastrointestinal health by acting as a histone deacetylase (HDAC) inhibitor and binding to several specific G protein–coupled receptors (GPCRs) (10). Numerous in vitro and in vivo studies have shown that butyrate plays an important role in modulating immune and inflammatory responses and intestinal barrier function (11, 12). However, the effect of butyrate on obesity remains controversial, with opposite results also reported (13, 14). Although butyrate is well known to exert a plethora of beneficial effects on the intestinal tract, growing evidence points to the impact of butyrate on the brain via the gut-brain axis. For example, changes in butyrate-producing bacteria can modulate the peripheral and central nervous systems and brain functions, reinforcing the notion for the existence of the microbiota-gut-brain axis (15). Herein, we summarize current knowledge on butyrate, especially its potential effects and possible mechanisms of action in relation to host gastroenteric health and obesity.
Endogenous Butyrate Producers and Production Pathways
A large number of bacteria are present in the human cecum and colon, accounting for ∼1010–1011 CFUs/g wet weight or 1013 CFUs in total of the hindgut (16). Similar estimates have been reported in other omnivores such as pigs (17). More than 50 genera and 400 species of bacteria have been found in human feces (18). The dominant bacteria are anaerobes, including Bacteroides, Bifidobacteria, Eubacteria, Streptococci, and Lactobacilli. Other anaerobes, including Enterobacteria, are usually found in smaller quantities (19).
Among gram-positive anaerobic bacteria, butyrate-producing bacteria are widely distributed. Two of the most important groups are Faecalibacterium prausnitzii in the Clostridium leptum cluster (or Clostridial cluster IV) and Eubacterium rectale/Roseburia spp. in the Clostridium coccoides (or Clostridial cluster XIVa) cluster of Firmicutes (20). Each of these groups typically accounts for ∼5–10% of the total bacteria detectable in fecal samples of healthy adult humans. In addition to these groups, butyrate-producing bacteria are widely distributed across several clusters including clusters IX, XV, XVI, and XVII (21).
Butyrate is produced from dietary fibers through bacterial fermentation via 2 metabolic pathways (Figure 1). In the first pathway, butyryl-CoA is phosphorylated to form butyryl-phosphate and transformed to butyrate via butyrate kinase (22). In the second pathway, the CoA moiety of butyryl-CoA is transferred to acetate via butyryl-CoA:acetate CoA-transferase, leading to the formation of butyrate and acetyl-CoA (23). Analysis of the metagenome data also suggested that butyrate can be synthesized from proteins via the lysine pathway (24).
FIGURE 1.
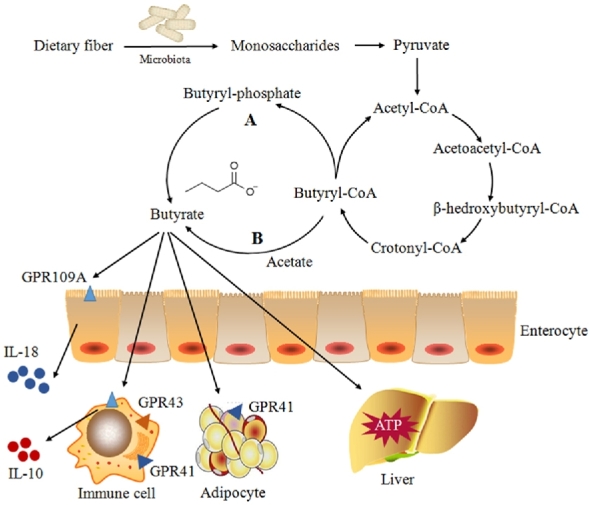
Butyrate biosynthesis and absorption in the large intestine and subsequent metabolism. Two pathways of endogenous butyrate production from butyryl-CoA in bacteria have been reported. The letter “A” indicates that butyryl-CoA is phosphorylated to butyryl-phosphate and converted to butyrate via butyrate kinase. The letter “B” shows that the CoA moiety of butyryl-CoA is transferred to external acetate via butyryl-CoA:acetate CoA transferase, leading to the formation of butyrate and acetyl-CoA. Several receptors for butyrate have been identified, including GPR41, GPR43, and GPR109A. GPR41 is found in adipose tissues and immune cells. The highest expression of GPR43 has been found in immune cells, whereas GPR109A is essential for butyrate-mediated induction of IL-18 in colonic epithelium. A small portion of butyrate is transported to the liver and metabolized to produce ATP. GPR, orphan G protein–coupled receptor.
Absorption of Butyrate
SCFAs are absorbed in both the small and large intestine by similar mechanisms (25, 26). Different mechanisms of absorbing SCFAs across the apical membrane of the colonocytes are reported, including diffusion of the undissociated form and active transport of the dissociated form by SCFA transporters (27). Two SCFA transporters exist, including monocarboxylate transporter (MCT) isoform 1 (MCT1), which is coupled to a transmembrane H+-gradient (28), and solute carrier (SLC) family 5 member 8 (SLC5A8), which is also known as sodium-coupled monocarboxylate transporter (SMCT) 1 (SMCT1) and is a Na+-coupled co-transporter (11).
A carrier-mediated, HCO3− gradient-dependent anion-butyrate exchange system is present on the basolateral membrane (5). In humans, MCT3 is expressed in low concentrations in the ileum, whereas MCT4 and MCT5 are expressed abundantly in the distal colon (29).
MCTs are also involved in butyrate transport on the apical membrane of colonocytes (30). Butyrate transportation with MCTs is saturated, coupled with H+, and inhibited by several monocarboxylates such as acetate, propionate, pyruvate, lactate, and α-ketobutyrate. The pH for the optimal activity of the colonic butyrate transporters appears to be ∼5.5. In addition, a second class of MCTs, called SMCTs, was identified (31), such as SLC5A8 (SMCT1) and SLC5A12 (SMCT2) (32). Different from MCTs, SMCT transport involves Na+ uptake by the transport cycle and also uses nicotinate and ketone bodies as substrates (33).
Cellular Signaling Pathways of Butyrate
Butyrate functions as signaling molecules of GPCRs
GPCRs are the largest and most diverse family of transmembrane proteins (34). In 2003, orphan G protein–coupled receptor 41 (GPR41) and GPR43 were identified as receptors for SCFAs and thus renamed FFA receptors (FFARs) 3 and 2, respectively (35). However, these receptors show specificities for different SCFAs (36–47) (Table 1). For example, butyrate preferentially binds to GPR41 over GPR43, which has higher affinities for acetate and propionate (30). GPR43 is expressed in a variety of tissues, with the highest expression in immune cells. This includes polymorphonuclear neutrophils, indicating that SCFAs could be involved in the activation of leucocytes (48, 49) (Figure 1). GPR41 is even more widely expressed than GPR43, having been detected in adipose tissues, the pancreas, spleen, lymph nodes, bone marrow, and peripheral blood mononuclear cells (26). Butyrate directly regulates GPR41-mediated sympathetic nervous system activity to control body energy expenditure and maintain metabolic homeostasis (39). Another major GPCR activated by butyrate is GPR109A (50) (Table 1). GPR109A signaling activates the inflammasome pathway in colonic macrophages and dendritic cells, resulting in the differentiation of regulatory T cells and IL-10–producing T cells (46). The secretion of IL-18 is also increased in intestinal epithelial cells via butyrate-stimulated signaling of GPR109A (45). On the other hand, the anti-inflammatory properties of butyrate are also achieved through inhibition of the production of proinflammatory enzymes and cytokines (51).
TABLE 1.
Ligand preference, expression pattern, and functions of 3 major receptors for SCFAs1
| GPCRs | Ligands | Expression sites | Functions | Study, year (reference) |
|---|---|---|---|---|
| GPR41/FFAR3 | Acetate, propionate, butyrate, and pentanoate | Adipocytes, bone marrow, colon, spleen, various immune cells, and enteroendocrine L cells | Increased leptin expression, sympathetic activation increased PYY production; increased Tregs and dendritic cell precursors, hematopoiesis of dendritic cells from bone marrow | De Vadder et al., 2014 (36); Nøhr et al., 2013 (42); Trompette et al., 2014 (38); Kimura et al., 2011 (39) |
| GPR43/FFAR2 | Formate, acetate, propionate, butyrate, and pentanoate | Adipocytes, skeletal muscle, heart, spleen, fetal membrane, various immune cells, enteroendocrine L cells, and gut epithelium | Anorexigenic effects via secretion of PYY and GLP-1, increased insulin sensitivity and energy expenditure; anti-inflammatory, anti-tumorigenic; expansion and differentiation of Tregs, resolution of arthritis and asthma | Kimura et al., 2013 (40); Voltolini et al., 2012 (41); Nøhr et al., 2013 (42); Smith et al., 2013 (43) |
| GPR109A/HCA2 | Nicotinate and butyrate | Adipocytes, various immune cells, intestinal epithelial cells, epidermis in squamous carcinoma, and retinal pigment epithelium | HDL metabolism, cAMP reduction in adipocytes, improved epithelial barrier function, dendritic cell trafficking, anti-inflammatory, increase in Treg generation, IL-10–producing T cells, and antitumorigenic | Ingersoll et al., 2012 (44); Macia et al., 2015 (45); Singh et al., 2014 (46); Bermudez et al., 2011 (47) |
FFAR, free fatty acid receptor; GLP-1, glucagon-like peptide 1; GPCR, G protein–coupled receptor; GPR, orphan G protein–coupled receptor; HCA2, hydroxycarboxylic acid 2; PYY, peptide YY; Treg, regulatory T cell.
Butyrate functions as an HDAC inhibitor
HDACs are a class of enzymes that remove acetyl groups from ε-N-acetyl lysine on histones, allowing the histones to wrap the DNA more tightly (52). Among the SCFAs, butyrate is the most potent in inhibiting HDAC activities both in vitro and in vivo (53, 54). The mechanism by which butyrate inhibits HDAC activities remains obscure. A model was proposed that butyrate inhibits the recruitment of HDACs to the promoter by transcription factors, specificity protein 1/specificity protein 3 (Sp1/Sp3), leading to histone hyperacetylation (55). Many of the anticancer activities of butyrate have been found to be mediated through HDAC inhibition, which includes inhibition of cell proliferation, induction of cell differentiation or apoptosis, and induction or repression of gene expression (56, 57). In addition to acting as an antitumor agent, butyrate achieves the anti-inflammatory effects partly through HDAC inhibition as well (58, 59). For example, butyrate plays a key role in the downregulation of proinflammatory effectors in lamina propria macrophages (30) as well as regulating cytokine expression in T cells (60). Thus, butyrate-mediated HDAC inhibition and concomitant beneficial health outcomes depend not only on its production amounts but also on which tissue or cell type that it targets.
Butyrate and Host Gastrointestinal Health
Anti-inflammation
Intestinal epithelium maintains a low grade of inflammation in order to prepare for constant immunological challenges on the mucosal surface (48, 61). If the immunological control is disrupted, the enterocytes might suffer from inflammatory and oxidative damages and even cause cancer (62, 63). Many studies have shown that butyrate can act as an anti-inflammatory agent. Several human and animal studies reported that the proinflammatory cytokines IFN-γ, TNF-α, IL-1β, IL-6, and IL-8 are inhibited, whereas IL-10 and TGF-β are upregulated in response to butyrate (25). The mechanism underlying the anti-inflammatory effect of butyrate is at least in part due to inhibition of the activation of a transcription factor known as NF-κB (64). NF-κB is a transcription factor that regulates the expression of a variety of genes involved in inflammation and immunity, such as proinflammatory cytokines and enzymes, adhesion molecules, growth factors, acute-phase proteins, and immune receptors (48, 65). Several studies suggested that butyrate suppresses the NF-κB signaling pathways by rescuing the redox machinery and controlling reactive oxygen species, which mediates NF-κB activation (66). Further studies also showed that butyrate is capable of activating PPAR-γ (67), which is a member of the nuclear hormone receptor family and highly expressed in colonic epithelial cells, and its activation is thought to exert anti-inflammatory effects (68). Apart from the inhibition of NF-κB activation and upregulation of PPAR-γ, butyrate may also exert its anti-inflammatory activities through inhibition of IFN-γ signaling (69).
Butyrate and the intestinal barrier
The barrier function of intestinal epithelial cells is an important first line of defense and ensures appropriate permeability characteristics of the epithelial layer (3, 70). Butyrate is known to repair and enhance barrier function of intestinal epithelial cells (71, 72). A current study by Elamin et al. (73) showed that butyrate exerts a protective effect on intestinal barrier function in Caco-2 cell monolayers. For example, butyrate is capable of upregulating the expression of mucin 2 (MUC2) (74), which is the most prominent mucin on the intestinal mucosal surface and can reinforce the mucous layer, leading to the enhanced protection against luminal pathogens (1, 74). In addition, the expression of trefoil factors (TFFs), which are mucin-associated peptides that contribute to the maintenance and repair of the intestinal mucosa (12), can be increased by butyrate (75). Furthermore, butyrate modulates the expression of tight junction proteins to minimize paracellular permeability (62, 76). One of several mechanisms in which butyrate enhances barrier function is through activation of AMP-activated protein kinase in monolayers (77). Butyrate can also stimulate the production of antimicrobial peptides, such as LL-37 in humans (78). However, on the basis of in vitro models, Huang et al. (79) showed that the effect of butyrate on the intestinal barrier function may be concentration-dependent. Butyrate promotes intestinal barrier function at low concentrations (≤2 mM) (77) but may disrupt intestinal barrier function by inducing apoptosis at high concentrations (5 or 8 mM) (79). On the basis of the physiologic concentration in mammalian gastrointestinal tract, the recommended concentration of butyrate used in in vitro models is currently 0–8 mM (80). However, considering that the majority of butyrate is metabolized as energy substrate by the colonic epithelium (12), the dosages used for treatment may be quite different between in vivo and in vitro models (4). For example, a dose of 100 mM butyrate by rectal administration was commonly used in clinical practice, which is comparable with physiologic concentrations in the colon of humans after the consumption of a high-fiber diet (81).
Butyrate and intestinal mucosal immunity
In addition to anti-inflammatory properties, SCFAs, especially butyrate, can act as modulators of chemotaxis and adhesion of immune cells (61). Butyrate can modulate intestinal epithelial cell–mediated migration of neutrophils to inflammatory sites, and such an effect is concentration-dependent (82, 83). In addition, butyrate plays a role in cell proliferation and apoptosis. Butyrate stimulates cell growth and DNA synthesis and induces growth arrest in the G1 phase of the cell cycle (5, 61). Although low concentrations of butyrate enhance cell proliferation (5), high concentrations of butyrate induce apoptosis (57). Overall, butyrate can influence the immune response by affecting immune cell migration, adhesion, and cellular functions such as proliferation and apoptosis.
Butyrate and Obesity: Inhibition or Promotion?
The abnormalities in glycolipid metabolism are a main reason for obesity, diabetes, and other metabolic syndromes (84). So far, the effect of butyrate on glycolipid metabolism remains controversial. We summarized the experimental studies that evaluated the potential relation between butyrate and obesity (85–89) (Table 2).
TABLE 2.
Paradoxical effect of butyrate on obesity1
| Viewpoints | Models | Design | Conclusions | Study, year (reference) |
|---|---|---|---|---|
| Inhibition | Specific pathogen–free, male C57BL/6J mice | High fat diet–induced obese mice were gavaged with sodium butyrate, whereas the control group received vehicle | Short-term oral administration of sodium butyrate alleviates diet-induced obesity and insulin resistance through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle | Hong et al., 2016 (13) |
| Male C57J/B6 mice and male Lepob/ob mice | Two groups were fed a low-fat diet with or without VSL#3 (Tau Sigma, Gaithersburg, MD), and 2 groups were continued on a high-fat diet with or without VSL#3 | Butyrate stimulates the release of GLP-1 from intestinal L cells, thereby providing a plausible mechanism for VSL#3 action | Yadav et al., 2013 (85) | |
| Human L cells (NCI-h716 cell line) | Stimulation with specific TLR-agonists and butyrate | Butyrate increases PYY expression through stimulating TLR expression | Larraufie et al., 2017 (86) | |
| Rat pituitary cell line | Rat pituitary cell lines were transiently transfected with wt-GH and treated with 10 nM GHRH, 5 mM butyrate, or both | Butyrate stimulates GH secretion from rat anterior pituitary cells via GPR41 and GPR43 | Miletta et al., 2014 (87) | |
| C57Bl/6J mice; PPAR-γ Lox/Lox mice | The experimental groups were fed a semisynthetic high-fat diet incorporated with SCFAs at 5%, whereas the control groups were fed a normal-fat diet | SCFAs protect against high fat diet–induced obesity via a PPAR-γ–dependent switch from lipogenesis to fat oxidation | den Besten et al., 2015 (88) | |
| Promotion | Female Sprague-Dawley rats | Pregnant rats were randomly assigned to either a control or butyrate diet | Maternal butyrate supplementation induces insulin resistance associated with enhanced intramuscular fat deposition in the offspring | Huang et al., 2017 (14) |
| Shrimp | — | Dietary supplementation with propionate and butyrate in different dietary concentrations modify the intestinal microbiota and improve the growth of Litopenaeus vannamei | da Silva et al., 2016 (89) |
GH, growth hormone; GHRH, growth hormone–releasing hormone; GLP-1, glucagon-like peptide 1; GPR, orphan G protein–coupled receptor; Lepob/ob, leptin-deficient; Lox/Lox, lipoxygenase/lipoxygenase; PYY, peptide YY; TLR, Toll-like receptor; wt, wild-type.
Alleviating obesity
The involvement of butyrate in diet-induced obesity and insulin resistance has been studied (90). Butyrate has been reported to improve glucose homeostasis in rodents (36). A recent study by Hong et al. (13) showed that butyrate alleviates diet-induced obesity and insulin resistance in mice. Another study in mice also showed that dietary butyrate supplementation prevented and reversed high-fat-diet–induced obesity by downregulating the expression and activity of PPAR-γ, promoting a change from lipogenesis to lipid oxidation (88). Consequently, the expression of mitochondrial uncoupling protein 2 and the AMP-to-ATP ratio were increased, thereby stimulating the oxidative metabolism in the liver and adipose tissue (88, 91).
Nevertheless, different mechanisms have been proposed to explain the effects of butyrate on alleviating obesity. The stimulation of gut hormones and inhibition of food intake by butyrate may represent a novel mechanism by which the gut microbiota regulates host metabolism (92). In vitro and in vivo studies have shown that butyrate enhances the secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) (85, 93) (Figure 2). GLP-1 is a gastrointestinal hormone that is secreted mainly by enteroendocrine L cells in the distal gut (94). It exerts multiple biological effects, including a glucose-dependent insulinotropic effect on pancreatic B cells, reduction in appetite, and inhibition of gastric emptying (95). These properties can be extended to patients with obesity. By using a cell culture system, Yadav et al. (85) showed that butyrate stimulated the release of GLP-1 from intestinal L cells. However, several studies in FFAR3-deficient mice showed that FFAR3 plays a minor role in butyrate stimulation of GLP-1 (92). Thus, these effects indicated the involvement of additional mechanisms in butyrate-mediated stimulation of GLP-1 (92).
FIGURE 2.
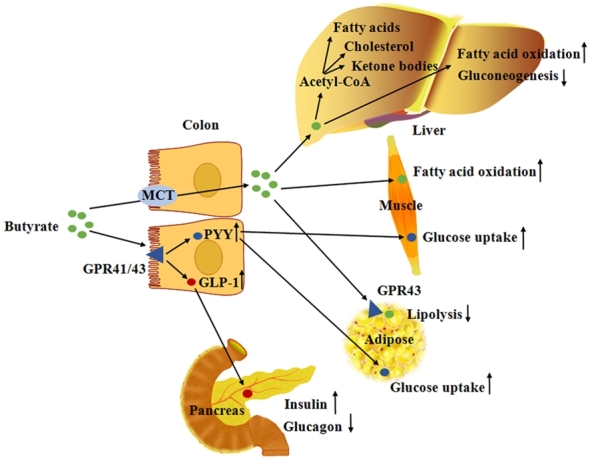
Schematic overview of the mechanisms by which butyrate affects glucose and lipid metabolism. MCTs are involved in butyrate transport in colonic luminal membrane. For glucose metabolism, butyrate increases PYY and GLP-1 expression in the colon via GPR41 and GPR43. GLP-1 increases insulin and decreases glucagon production in the pancreas, and PYY increases glucose uptake in the muscle and adipose tissue. Meanwhile, butyrate decreases hepatic gluconeogenesis. For lipid metabolism, butyrate increases FA oxidation in the muscle and decreases lipolysis via the GPR43 pathway in white adipose tissue. In addition, butyrate is converted to FAs, cholesterol, and ketone bodies in the liver. GLP-1, glucagon-like peptide 1; GPR, orphan G protein–coupled receptor; MCT, monocarboxylate transporter; PYY, peptide YY.
Similarly, PYY is also synthesized and released from endocrine L cells throughout the intestinal tract (96, 97) and is implicated in the regulation of food intake, gut motility, and insulin secretion (98, 99). As a gut hormone, PYY can also contribute to alleviating obesity in obese people (100). Numerous studies have shown the close relation between butyrate and PYY expression (86, 101). In in vitro models, Larraufie et al. (86) showed that butyrate can increase PYY expression through upregulation of Toll-like receptor–dependent microbial sensing. In addition to gastrointestinal hormones, butyrate also has positive effects on the secretion and metabolic actions of growth hormone (GH) (102), which is a type of somatotropin hormone secreted from the pituitary gland in a pulsatile manner (87). GH plays a potent role in controlling energy homeostasis by stimulating lipolysis and protein retention (103, 104). By using a rat pituitary tumor cell line, Miletta et al. (87) reported that butyrate can stimulate GH synthesis and promote basal and GH-releasing hormone-induced GH secretion, indicating an improved lipolysis and oxidative metabolism.
Inducing obesity
The findings that the total amount of SCFAs is higher in obese humans than in lean individuals (105) and that treated obese individuals showed reduced fecal SCFAs (106) suggest that SCFAs are rapidly assimilated into host carbohydrates and lipids and could contribute to the obese phenotype by providing ∼10% of our daily energy requirements (107, 108). Several in vitro studies have shown that intestinal epithelial cells, especially colonocytes, have adapted to the use of butyrate as their primary source of energy, accounting for ∼70% of ATP produced (109, 110). Through FA oxidation, colonic cells exhibit a great capacity to rapidly oxidize butyrate into carbon dioxide (111). Furthermore, butyrate is able to increase lipid synthesis from acetyl-CoA or ketone bodies via the β-hydroxy-β-methylglutaryl-CoA pathway, which potentially contributes to obesity (112).
A small fraction of butyrate could be transported via the portal vein and reach the liver, where it is involved in lipid biosynthesis and influences glycolipid metabolism (109). First, butyrate metabolism yields acetyl-CoA in the liver, similar to colonocytes that enter into the citric acid cycle (113). Second, butyrate is shown to be metabolized to produce FAs, cholesterol, and ketone bodies via acetyl-CoA, thereby providing specific substrates for lipid biosynthesis (5). Butyrate plays a role in obesity not only through providing the substrate for energy expenditure but also by engaging in signaling pathways involved in glycolipid metabolism. Consistently, maternal butyrate supplementation induces mRNA and protein expression of lipogenic genes and decreases the amount of lipolytic enzymes in the offspring, indicating insulin resistance and impaired glucose tolerance (14).
In conclusion, although a large body of evidence has suggested the effect of butyrate on alleviating high fat diet–induced obesity and insulin resistance, a few studies showed an opposite effect. Therefore, additional investigations are warranted to understand the apparently paradoxical effects of butyrate on obesity (34, 114).
Butyrate Maintains Homeostasis through the Gut-Brain Axis
A growing body of evidence indicates extensive communications between the brain and the gut via the gut-brain axis (115, 116). The gut-brain axis is composed of the central nervous system, enteric nervous system, and different types of afferent and efferent neurons that are involved in signal transduction between the brain and gut (15, 117). The bidirectional communication between the gut and the brain occurs through various pathways, including the vagus nerve, neuroimmune pathways, and neuroendocrine pathways (118, 119). As a microbial metabolite, butyrate is capable of exerting its effects on host metabolism indirectly by acting through the gut-brain axis (114, 120). For instance, butyrate can enhance the proportion of cholinergic enteric neurons via epigenetic mechanisms (121). Moreover, with an ability to cross the blood-brain barrier, butyrate activates the vagus nerve and hypothalamus, thus indirectly affecting host appetite and eating behavior (122, 123). Some of the beneficial metabolic effects of butyrate are mediated through gluconeogenesis from the gut epithelium and through a gut-brain neural circuit to increase insulin sensitivity and glucose tolerance (124, 125). For example, butyrate binds to its receptor in the intestinal cells and signals to the brain through the cAMP signaling pathway (126, 127). More studies are needed to explore the impact of butyrate on glycolipid metabolism abnormalities and disease via the gut-brain axis.
Conclusions
Microbe-derived butyrate plays an important role in both gut health and obesity of the host. New mechanisms are being revealed. The reason behind the paradoxical effect of butyrate on glucose and lipid metabolism, especially with regard to its role in obesity, remains elusive. The effect of endogenous butyrate on the gut-brain axis warrants further investigations. A better understanding of the mechanism of action of butyrate in intestinal physiology and lipid metabolism will facilitate the application of butyrate and HDAC inhibitors in gut health improvement and control and the prevention of metabolic diseases.
Acknowledgments
The authors’ responsibilities were as follows—XM: conceived and designed the review; HL and JW: collected and analyzed the literature and drafted the manuscript; TH, XM, SB, and GZ: edited the manuscript; DL: provided advice and consultation; and all authors: read and approved the final manuscript.
Supported by the National Key R&D Program of China (2017YFD0500501), the National Basic Research Program of China (973 Program, 2013CB117301), the National Natural Science Foundation of China (31722054, 31472101, and 31528018), the 111 Project (B16044), and the National Department Public Benefit Research Foundation (201403047).
Author disclosures: HL, JW, TH, SB, GZ, DL, and XM, no conflicts of interest.
HL and JW contributed equally to this work.
Abbreviations
- FFAR
free fatty acid receptor
- GH
growth hormone
- GLP-1
glucagon-like peptide 1
- GPCR
G protein–coupled receptor
- GPR
orphan G protein–coupled receptor
- HDAC
histone deacetylase
- MCT
monocarboxylate transporter
- PYY
peptide YY
- SLC
solute carrier
- SMCT
sodium-coupled monocarboxylate transporter
References
- 1. Canani RB, Di Costanzo M, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extrainitestinal diseases. World J Gastroenterol 2011;17:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fan P, Li L, Rezaei A, Eslamfam S, Che D, Ma X. Metabolites of dietary protein and peptides by intestinal microbes and their impacts on gut. Curr Protein Pept Sci 2015;16:646–54. [DOI] [PubMed] [Google Scholar]
- 3. Ma N, Tian Y, Wu Y, Ma X. Contributions of the interaction between dietary protein and gut microbiota to intestinal health. Curr Protein Pept Sci 2017;18:795–808. [DOI] [PubMed] [Google Scholar]
- 4. Brahe LK, Astrup A, Larsen LH. Is butyrate the link between diet, intestinal microbiota and obesity‐related metabolic diseases? Obes Rev 2013;14:950–9. [DOI] [PubMed] [Google Scholar]
- 5. Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev 2010;23:366–84. [DOI] [PubMed] [Google Scholar]
- 6. Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients 2013;5:1417–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conlon MA, Bird AR. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2014;7:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen J, Li Y, Tian Y, Huang C, Li D, Zhong Q, Ma X. Interaction between microbes and host intestinal health: modulation by dietary nutrients and gut-brain-endocrine-immune axis. Curr Protein Pept Sci 2015;16:592–603. [DOI] [PubMed] [Google Scholar]
- 9. Jacobi SK, Odle J. Nutritional factors influencing intestinal health of the neonate. Adv Nutr 2012;3:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Clercq NC, Groen AK, Romijn JA, Nieuwdorp M. Gut microbiota in obesity and undernutrition. Adv Nutr 2016;7:1080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014:91–119. [DOI] [PubMed] [Google Scholar]
- 12. Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008;27:104–19. [DOI] [PubMed] [Google Scholar]
- 13. Hong J, Jia Y, Pan S, Jia L, Li H, Han Z, Cai D, Zhao R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016;7:56071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang YP, Gao SX, Chen JL, Albrecht E, Zhao RQ, Yang XJ. Maternal butyrate supplementation induces insulin resistance associated with enhanced intramuscular fat deposition in the offspring. Oncotarget 2017;8:13073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Eslamfam S, Fang L, Qiao S, Ma X. Maintenance of gastrointestinal glucose homeostasis by the gut-brain axis. Curr Protein Pept Sci 2017;18:541–7. [DOI] [PubMed] [Google Scholar]
- 16. van der Waaij LA, Harmsen HJ, Madjipour M, Kroese FG, Zwiers M, van Dullemen H, de Boer N, Welling G, Jansen PL. Bacterial population analysis of human colon and terminal ileum biopsies with 16S rRNA‐based fluorescent probes: commensal bacteria live in suspension and have no direct contact with epithelial cells. Inflamm Bowel Dis 2005;11:865–71. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Han M, Zhang G, Qiao S, Li D, Ma X. The signal pathway of antibiotic alternatives on intestinal microbiota and immune function. Curr Protein Pept Sci 2016;17:785–96. [DOI] [PubMed] [Google Scholar]
- 18. Lagier JC, Hugon P, Khelaifia S, Fournier PE, La Scola B, Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev 2015;28:237–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson M. The human microbiota: an historical perspective. In: Henderson B, Nibali L. (eds).. The human microbiota and chronic disease: dysbiosis as a cause of human pathology. Hoboken (NJ): Academic Press; 2016, 3–36. [Google Scholar]
- 20. Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr 2017;8:240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haenen D, Zhang J, da Silva CS, Bosch G, van der Meer IM, van Arkel J, van den Borne JJ, Gutiérrez OP, Smidt H, Kemp B. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr 2013;143:274–83. [DOI] [PubMed] [Google Scholar]
- 22. Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol 2017;19:29–41. [DOI] [PubMed] [Google Scholar]
- 23. Trachsel J, Bayles DO, Looft T, Levine UY, Allen HK. Function and phylogeny of bacterial butyryl coenzyme a: acetate transferases and their diversity in the proximal colon of swine. Appl Environ Microbiol 2016;82:6788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio 2014;5:e00889–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol 2014;14:667–85. [DOI] [PubMed] [Google Scholar]
- 26. Byrne C, Chambers E, Morrison D, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond) 2015;39:1331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar A, Alrefai WA, Borthakur A, Dudeja PK. Lactobacillus acidophilus counteracts enteropathogenic E. coli-induced inhibition of butyrate uptake in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2015;309:G602–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Counillon L, Bouret Y, Marchiq I, Pouysségur J. Na+/H+ antiporter (NHE1) and lactate/H+ symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim Biophys Acta 2016;1863:2465–80. [DOI] [PubMed] [Google Scholar]
- 29. Wang H, Yang C, Doherty JR, Roush WR, Cleveland JL, Bannister TD. Synthesis and structure–activity relationships of pteridine dione and trione monocarboxylate transporter 1 inhibitors. J Med Chem 2014;57:7317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 2014;111:2247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schug ZT, Voorde JV, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer 2016;16:708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plata C, Gavi-Maza M, Vázquez N, Romero M, Gamba G. Aspirin and salicylate are solutes of both sodium monocarboxylate transporters (SMCT1/Slc5a8 and SMCT2/Slc5a12)(896.9). FASEB J 2014;28(Suppl 1):896.9. [Google Scholar]
- 33. Singh V, Yang J, Chen T-e, Zachos NC, Kovbasnjuk O, Verkman AS, Donowitz M. Translating molecular physiology of intestinal transport into pharmacologic treatment of diarrhea: stimulation of Na+ absorption. Clin Gastroenterol Hepatol 2014;12:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016;165:1332–45. [DOI] [PubMed] [Google Scholar]
- 35. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ. et al. The orphan G protein–coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003;278:11312–9. [DOI] [PubMed] [Google Scholar]
- 36. De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014;156:84–96. [DOI] [PubMed] [Google Scholar]
- 37. Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Moller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015;290:126–37. [DOI] [PubMed] [Google Scholar]
- 38. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014;20:159–66. [DOI] [PubMed] [Google Scholar]
- 39. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein–coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 2011;108:8030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013;4:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Voltolini C, Battersby S, Etherington SL, Petraglia F, Norman JE, Jabbour HN. A novel antiinflammatory role for the short-chain fatty acids in human labor. Endocrinology 2012;153:395–403. [DOI] [PubMed] [Google Scholar]
- 42. Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier Poulsen S, Han S. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013;154:3552–64. [DOI] [PubMed] [Google Scholar]
- 43. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341:569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ingersoll SA, Ayyadurai S, Charania MA, Laroui H, Yan YT, Merlin D. The role and pathophysiological relevance of membrane transporter PepT1 in intestinal inflammation and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 2012;302:G484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, McKenzie CI, Hijikata A, Wong C. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 2015;6:6734. [DOI] [PubMed] [Google Scholar]
- 46. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014;40:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bermudez Y, Benavente CA, Meyer RG, Coyle WR, Jacobson MK, Jacobson EL. Nicotinic acid receptor abnormalities in human skin cancer: implications for a role in epidermal differentiation. PLoS One 2011;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 2011;22:849–55. [DOI] [PubMed] [Google Scholar]
- 49. Wang W, Yang Q, Sun Z, Chen X, Yang C, Ma X. Advance of interactions between exogenous natural bioactive peptides and intestinal barrier and immune responses. Curr Protein Pept Sci 2015;16:574–5. [DOI] [PubMed] [Google Scholar]
- 50. Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA. et al. Gpr109a is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 2009;69:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, Li SN, Huang BX, Lv QK, Wang W. et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation 2015;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 2009;325:834–40. [DOI] [PubMed] [Google Scholar]
- 53. Turner ND, Lupton JR. Dietary fiber. Adv Nutr 2011;2:151–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012;9:577–89. [DOI] [PubMed] [Google Scholar]
- 55. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133(Suppl):2485S–93S. [DOI] [PubMed] [Google Scholar]
- 56. Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 2012;48:612–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Canani RB, Di Costanzo M, Leone L. The epigenetic effects of butyrate: potential therapeutic implications for clinical practice. Clin Epigenetics 2012;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 2015;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 2010;285:27601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi LZ, Wang RN, Huang GH, Vogel P, Neale G, Green DR, Chi HB. HIF1 alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of T(H)17 and T-reg cells. J Exp Med 2011;208:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care 2010;13:715–21. [DOI] [PubMed] [Google Scholar]
- 62. Ma X, Fan P, Li L, Qiao S, Zhang G, Li D. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci 2012;90:266–8. [DOI] [PubMed] [Google Scholar]
- 63. He L, Han M, Farrar S, Ma X. Impacts and regulation of dietary nutrients on gut microbiome and immunity. Protein Pept Lett 2017;24:380–1. [DOI] [PubMed] [Google Scholar]
- 64. Aguilar E, Leonel A, Teixeira L, Silva A, Silva J, Pelaez J, Capettini L, Lemos V, Santos R, Alvarez-Leite J. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr Meta Cardiovasc Dis 2014;24:606–13. [DOI] [PubMed] [Google Scholar]
- 65. Venkatraman A, Ramakrishna B, Shaji R, Kumar NN, Pulimood A, Patra S. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am J Physiol Gastrointest Liver Physiol 2003;285:G177–84. [DOI] [PubMed] [Google Scholar]
- 66. Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn's mucosa through modulation of antioxidant defense machinery. PLoS One 2012;7:e32841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67. Raso GM, Simeoli R, Russo R, Iacono A, Santoro A, Paciello O, Ferrante MC, Canani RB, Calignano A, Meli R. Effects of sodium butyrate and its synthetic amide derivative on liver inflammation and glucose tolerance in an animal model of steatosis induced by high fat diet. PLoS One 2013;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PPARγ as a new therapeutic target in inflammatory bowel diseases. Gut 2006;55:1341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schröder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NFκB signalling. Mol Immunol 2007;44:3625–32. [DOI] [PubMed] [Google Scholar]
- 70. Liu T, Li J, Liu Y, Xiao N, Suo H, Xie K, Yang C, Wu C. Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264. 7 cells. Inflammation 2012;35:1676–84. [DOI] [PubMed] [Google Scholar]
- 71. Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr 2015;145:2774–80. [DOI] [PubMed] [Google Scholar]
- 72. Ma N, Wu Y, Xie F, Du K, Wang Y, Shi L, Ji L, Liu T, Ma X. Dimethyl fumarate reduces the risk of mycotoxins via improving intestinal barrier and microbiota. Oncotarget 2017;8:44625–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Elamin EE, Masclee AA, Dekker J, Pieters H-J, Jonkers DM. Short-chain fatty acids activate AMP-activated protein kinase and ameliorate ethanol-induced intestinal barrier dysfunction in Caco-2 cell monolayers. J Nutr 2013;143:1872–81. [DOI] [PubMed] [Google Scholar]
- 74. Willemsen L, Koetsier M, Van Deventer S, Van Tol E. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E1 and E2 production by intestinal myofibroblasts. Gut 2003;52:1442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leonel AJ, Alvarez-Leite JI. Butyrate: implications for intestinal function. Curr Opin Clin Nutr Metab Care 2012;15:474–9. [DOI] [PubMed] [Google Scholar]
- 76. Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol 2005;17:453–8. [DOI] [PubMed] [Google Scholar]
- 77. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr 2009;139:1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Lührs H, Gudmundsson G. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 2003;52:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Huang X, Li Z, Zhu L, Huang H, Hou L, Lin J. Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in a Caco-2 cell monolayer model. J Pediatr Gastroenterol Nutr 2014;59:264–9. [DOI] [PubMed] [Google Scholar]
- 80. Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci 2012;57:3126–35. [DOI] [PubMed] [Google Scholar]
- 81. Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJM. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr 2009;28:88–93. [DOI] [PubMed] [Google Scholar]
- 82. Vinolo MA, Rodrigues HG, Hatanaka E, Hebeda CB, Farsky SH, Curi R. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin Sci 2009;117:331–8. [DOI] [PubMed] [Google Scholar]
- 83. Böcker U, Nebe T, Herweck F, Holt L, Panja A, Jobin C, Rossol S, Sartor R, Singer M. Butyrate modulates intestinal epithelial cell-mediated neutrophil migration. Clin Exp Immunol 2003;131:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo P, Li Y, Eslamfam S, Ding W, Ma X. Discovery of novel genes mediating glucose and lipid metabolisms. Curr Protein Pept Sci 2017;18:609–18. [DOI] [PubMed] [Google Scholar]
- 85. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem 2013;288:25088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Larraufie P, Doré J, Lapaque N, Blottière HM. TLR ligands and butyrate increase Pyy expression through two distinct but inter‐regulated pathways. Cell Microbiol 2017;19:e12648. [DOI] [PubMed] [Google Scholar]
- 87. Miletta MC, Petkovic V, Eble A, Ammann RA, Fluck CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS One 2014;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ. et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPAR-dependent switch from lipogenesis to fat oxidation. Diabetes 2015;64:2398–408. [DOI] [PubMed] [Google Scholar]
- 89. da Silva BC, Vieira FD, Mourino JLP, Bolivar N, Seiffert WQ. Butyrate and propionate improve the growth performance of Litopenaeus vannamei. Aquacult Res 2016;47:612–23. [Google Scholar]
- 90. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao ZG, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye JP. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009;58:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MFM, Kosinski JR, Hubert JA, Szeto D, Yao XR, Forrest G. et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 2012;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Keim NL, Martin RJ. Dietary whole grain–microbiota interactions: insights into mechanisms for human health. Adv Nutr 2014;5:556–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. DeFronzo RA, Buse JB, Kim T, Burns C, Skare S, Baron A, Fineman M. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: results from two randomised trials. Diabetologia 2016;59:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rondas D, D'Hertog W, Overbergh L, Mathieu C. Glucagon‐like peptide‐1: modulator of β‐cell dysfunction and death. Diabetes Obes Metab 2013;15(Suppl 3):185–92. [DOI] [PubMed] [Google Scholar]
- 96. Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 2006;444:854. [DOI] [PubMed] [Google Scholar]
- 97. Psichas A, Sleeth M, Murphy K, Brooks L, Bewick G, Hanyaloglu A, Ghatei M, Bloom S, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015;39:424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Diamant M, Blaak EE, de Vos WM. Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes Rev 2011;12:272–81. [DOI] [PubMed] [Google Scholar]
- 99. Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care 2014;17:139–44. [DOI] [PubMed] [Google Scholar]
- 100. Steinert RE, Feinle-Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP-1, and PYY (3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev 2017;97:411–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frost GS, Walton GE, Swann JR, Psichas A, Costabile A, Johnson LP, Sponheimer M, Gibson GR, Barraclough TG. Impacts of plant-based foods in ancestral hominin diets on the metabolism and function of gut microbiota in vitro. MBio 2014;5:e00853–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kato SI, Sato K, Chida H, Roh SG, Ohwada S, Sato S, Guilloteau P, Katoh K. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J Endocrinol 2011;211:241–8. [DOI] [PubMed] [Google Scholar]
- 103. Zhang Y, Fang F, Goldstein JL, Brown MS, Zhao T-J. Reduced autophagy in livers of fasted, fat-depleted, ghrelin-deficient mice: reversal by growth hormone. Proc Natl Acad Sci USA 2015;112:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS. Growth hormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab 2013;98:E1072–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010;18:190–5. [DOI] [PubMed] [Google Scholar]
- 106. Knudsen KEB. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv Nutr 2015;6:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- 108. den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Muller M, Groen AK, Hooiveld GJ, Bakker BM. et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol 2013;305:G900–10. [DOI] [PubMed] [Google Scholar]
- 109. Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol 2011;45:S120–S7. [DOI] [PubMed] [Google Scholar]
- 110. Gonçalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab 2013;14:994–1008. [DOI] [PubMed] [Google Scholar]
- 111. Terova G, Díaz N, Rimoldi S, Ceccotti C, Gliozheni E, Piferrer F. Effects of sodium butyrate treatment on histone modifications and the expression of genes related to epigenetic regulatory mechanisms and immune response in European sea bass (Dicentrarchus labrax) fed a plant-based diet. PLoS One 2016;11:e0160332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Birt DF, Boylston T, Hendrich S, Jane J-L, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M. Resistant starch: promise for improving human health. Adv Nutr 2013;4:587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wong DHJ, Beiko RG. Transfer of energy pathway genes in microbial enhanced biological phosphorus removal communities. BMC Genomics 2015;16:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int 2016;99:110–32. [DOI] [PubMed] [Google Scholar]
- 115. Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr 2013;167:374–9. [DOI] [PubMed] [Google Scholar]
- 116. Chen X, D'Souza R, Hong S-T. The role of gut microbiota in the gut-brain axis: current challenges and perspectives. Protein Cell 2013;4:403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 118. Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017;179:223–44. [DOI] [PubMed] [Google Scholar]
- 119. Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther 2016;158:52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Aziz Q, Doré J, Emmanuel A, Guarner F, Quigley E. Gut microbiota and gastrointestinal health: current concepts and future directions. Neurogastroenterol Motil 2013;25:4–15. [DOI] [PubMed] [Google Scholar]
- 121. Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology 2010;138:1772–82, e4. [DOI] [PubMed] [Google Scholar]
- 122. van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J Nutr 2017;147:727–45. [DOI] [PubMed] [Google Scholar]
- 123. Gagliano H, Delgado-Morales R, Sanz-Garcia A, Armario A. High doses of the histone deacetylase inhibitor sodium butyrate trigger a stress-like response. Neuropharmacology 2014;79:75–82. [DOI] [PubMed] [Google Scholar]
- 124. Castanys-Muñoz E, Martin MJ, Vazquez E. Building a beneficial microbiome from birth. Adv Nutr 2016;7:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Selkrig J, Wong P, Zhang X, Pettersson S. Metabolic tinkering by the gut microbiome: Implications for brain development and function. Gut Microbes 2014;5:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour—epigenetic regulation of the gut-brain axis. Genes Brain Behav 2014;13:69–86. [DOI] [PubMed] [Google Scholar]
- 127. Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr 2015;6:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]


