Abstract
Background
Blacks experience greater multisystem physiological dysregulation, or cumulative biological risk, which is associated with poor cardiometabolic health and mortality. In this study, we assess race differences in change in risk over 4 years among older whites, blacks, and Hispanics.
Method
We examined race differences in 4-year change in individual biomarkers and a cumulative measure of risk—cardiometabolic risk (CMR)—using data for each respondent from two waves of the Health and Retirement Study’s biomarker assessment (n = 5,512). CMR is a count of high-risk cardiovascular and metabolic biomarkers. We estimated mean CMR at baseline and follow-up by race/ethnicity, and used logistic regression to determine whether race differences exist in 4-year transitions between high- and low-risk states for individual biomarkers.
Results
Blacks had higher baseline CMR than whites and Hispanics and experienced an increase in risk over 4 years; conversely, CMR decreased among whites and Hispanics. Blacks were more likely to develop high-risk pulse pressure and high-risk hemoglobin A1c, which contributed to increases in CMR. Whites and Hispanics were more likely to become low-risk on C-reactive protein and high-density lipoprotein cholesterol which contributed to declines in CMR. Race differences in transitions between risk states remained after controlling for social, behavioral, and health care-related factors. However, the racial patterning of these differences was influenced by disease diagnosis and medication use.
Conclusions
We show that the cardiometabolic health of older blacks worsens as they age both absolutely and relative to that of whites and Hispanics because of poor blood pressure control and diabetes prevention.
Keywords: Biomarkers, Cardiovascular, Health Disparities, Minority Aging
Introduction
Racial and ethnic differences in various health conditions have been documented extensively (1,2). Non-Hispanic blacks experience an earlier onset, greater severity, and earlier age of death due to cardiovascular diseases (2). We have yet to fully understand the nature and determinants of differences in physiological changes that lead to these disparities. Cumulative measures of biological risk use multiple biomarkers to capture multisystem, physiological dysregulation (3) and are associated with numerous diseases (4). At nearly all ages of adulthood, blacks have worse biological risk profiles than whites and Hispanics (5,6). Race differences in health trajectories (7,8) and age patterns of risk show a widening black-white difference during middle adulthood that narrows in old age (9,10). This narrowing, however, is likely due to selective mortality (10)—the earlier death of sicker, disadvantaged populations—and obscures the true nature of individual change in risk and physiological functioning underlying these disparities.
Prospective studies are needed to better understand the physiological processes leading to systematic differences in cardiovascular morbidity and mortality (10). Examining transitions between high- and low-risk states of individual biomarkers can identify the physiological systems driving worsening or improving cumulative risk and evaluate the effectiveness of medical treatment in preventing the onset of high-risk values. Current research among older populations is predominately cross-sectional (11,12) and does not address the underlying processes of change. Moreover, most studies examining change in risk used international samples (13), geographically limited U.S. samples (14) or samples under-representing racial/ethnic minorities (15). Thus, the question remains—do U.S. whites, blacks, and Hispanics experience similar changes in risk as they age, and, if not, which biomarkers contribute to these differences?
The current study examines race differences in 4-year change in cumulative and individual risk measures among U.S. older adults. We use a measure of cardiometabolic risk (CMR) that includes biomarkers associated with physiological processes implicated in the pathophysiology of (16) and risk for (17,18) cardiovascular and metabolic diseases. Cumulative risk measures are informative of mortality risk stratification (19) and are therefore useful in elucidating how physiological changes confer risk for death and disease. We hypothesize that blacks will have the highest CMR and experience the greatest increases in risk as they age, and that worsening CMR among blacks will be driven by the disproportionate onset of high-risk biomarker values, particularly among older blacks whose chronic conditions are not effectively controlled.
Method
The Health and Retirement Study (HRS) is a nationally representative, prospective cohort study of U.S. adults age 51 and older. In 2006, a random half-sample of HRS households were selected for a face-to-face interview that included the collection of anthropometric and blood-based biomarker data; data were collected from the other half-sample in 2008 (20). Assessments were repeated on survivors 4 years later. Of the 9,237 individuals with complete biomarker data at baseline (ie, 2006/2008), 6,334 were present at follow-up (ie 2010/2012; 985 (11%) died, 1,496 (16%) did not complete a second assessment; 422 (4%) were lost to follow-up), and 5,859 had complete biomarker data. We limited our analyses to 5,724 blacks, whites and Hispanics because other racial groups represented a small (<3%), heterogeneous population. After excluding 212 individuals missing on other study variables (4%), our final analytic sample consisted of 5,512 individuals with complete data at baseline and follow-up. This sample reflects a loss of approximately 30% of baseline eligible respondents who died, were lost to follow-up or did not complete all or part of the follow-up physical and biomarker assessments. Although individuals who died between baseline and follow-up had significantly higher baseline CMR (sample = 1.8, deceased = 2.1; p <.0001), the proportion who died during this period did not differ significantly by race/ethnicity (percent deceased: whites = 15%, blacks = 16%, Hispanics = 12%; p = .122). However, blacks (76%) and Hispanics (75%) were less likely to participate in the follow-up assessments than whites (80%; p <.0001); and individuals who did not participate had higher baseline CMR (sample = 1.8, did not participate=2.0; p < .0001).
Seven biomarkers measured CMR: pulse pressure, resting heart rate, C-reactive protein (CRP), waist circumference, glycosylated hemoglobin A1c (HbA1c), and total and high-density lipoprotein cholesterol (HDL-C). Blood pressure and heart rate were measured using an automatic blood pressure monitor. Three measurements were taken and averaged to determine systolic and diastolic blood pressure; pulse pressure is the difference between systolic and diastolic blood pressure. Waist circumference was measured by wrapping a standard measuring tape around an individual’s waist at the navel. Dried blood spots (DBS), which involves collecting blood droplets on filter paper (21), were assayed for CRP, HbA1c, total cholesterol, and HDL-C (20). We used the NHANES-equivalent HRS values because DBS values and venous values may differ (22). Additional biomarker details are available elsewhere (20). CMR is a count of high-risk biomarkers (9) (Supplementary Table 1 delineates cut-points for low- and high-risk). It ranges from 0 to 7 and is calculated at baseline and follow-up.
All other variables, including race/ethnicity, were self-reported and assessed at baseline. Analyses compared non-Hispanic whites (“whites”) to non-Hispanic blacks (“blacks”) and Hispanics. We included variables related to the diagnosis and treatment of chronic conditions because they may influence biomarker levels. Moreover, changes in risk among healthy individuals or those with undiagnosed, controlled, or uncontrolled conditions are informative of prevention and treatment effectiveness. We, therefore, created disease state measures for chronic conditions that have clinical guidelines for diagnosis and treatment (eg, hypertension and diabetes). For these conditions, healthy was defined as a low-risk measured value and no reported diagnosis or medication use for the biomarker-associated condition. Controlled individuals had a low-risk measured value and either self-reported diagnosis or medication use for the condition. Undiagnosed was defined as a high-risk measured value and no self-reported condition or medication use; and uncontrolled was defined as a high-risk measured value and either a self-reported condition or medication use.
Covariates include age, gender, and foreign-born status. To address alternative explanations for race differences in CMR changes, we included variables for education, smoking, obesity, health insurance, and foregone medications. Individuals completing less than high school were compared to those with a high school degree, some college, or a college degree or higher. Non-smokers were compared to former and current smokers. Body mass index (BMI) is informative of physical activity levels and diet; we included an indicator for class II obesity (ie, BMI ≥ 35) to capture these lifestyle factors. Uninsured individuals were compared to those with health insurance. Foregone medication captures fiscal barriers to consistent medication use. We compared individuals who reported foregoing their medications at baseline or follow-up to those who did not.
Statistical Analysis
Sample characteristics were compared across race/ethnicity using an F-test. Poisson regression was used to estimate baseline and follow-up CMR counts for each group, adjusting for covariates. For the individual biomarkers, we used logistic regression to assess race differences in 4-year transitions between low- to high-risk states. We then calculated the predicted probability of being high-risk at follow-up, among individuals low-risk at baseline, by race/ethnicity adjusting for covariates; this onset of high-risk status represented worsening physiological functioning. We repeated this analysis for transitions from high- to low-risk. For each biomarker demonstrating differential change by race/ethnicity, we estimated a fully adjusted logistic regression model to determine if race differences exist after accounting for alternative explanations. These models were stratified by relevant disease states—either healthy and controlled, or undiagnosed and uncontrolled—when applicable (ie, when biomarker-specific guidelines exist for the diagnosis and treatment of a condition). Biomarkers demonstrating differential change from low- to high-risk were stratified by the healthy and controlled disease states; biomarkers demonstrating differential change from high- to low-risk were stratified by the undiagnosed and uncontrolled disease states. Analyses used Stata 14 and sampling weights were applied to account for the complex sample design of the HRS and differential nonresponse.
Results
Table 1 presents sample characteristics by race/ethnicity. Whites were older than blacks and Hispanics, more likely to be male and to have a college degree. Half of Hispanics were foreign-born and blacks were more likely to be smokers and obese compared to whites. Only 76% of Hispanics were insured compared to 92% of whites. Foregoing medications was most prevalent among blacks (19%), followed by Hispanics (15%), and whites (8%).
Table 1.
Weighted Baseline Characteristics by Race/Ethnicity; Health and Retirement Study
| White (n = 4,404) | Black (n = 644) | Hispanic (n = 464) | p-value | |
|---|---|---|---|---|
| Mean (SE) or % | Mean (SE) or % | Mean (SE) or % | ||
| Age | 64.8 (0.3) | 63.2 (0.4) | 62.4 (0.7) | <.001 |
| Female | 52.9 | 60.9 | 58.6 | <.01 |
| Less than HS | 8.9 | 26.4 | 46.5 | <.0001 |
| HS/GED | 35.4 | 34.9 | 27.7 | |
| Some college | 25.4 | 23.6 | 17.0 | |
| College | 30.3 | 15.1 | 8.8 | |
| Foreign-born | 3.7 | 4.0 | 52.6 | <.0001 |
| Never smoked | 43.9 | 41.9 | 48.0 | <.001 |
| Former smoker | 43.2 | 37.2 | 39.5 | |
| Current smoker | 12.9 | 20.9 | 12.5 | |
| Obese | 14.2 | 24.1 | 14.0 | <.0001 |
| Has health insurance | 91.7 | 88.2 | 76.3 | <.0001 |
| Foregone medication | 8.3 | 19.2 | 14.9 | <.0001 |
Note: SE = standard error. n = 5,512.
p-values test race difference in the proportion of each characteristic.
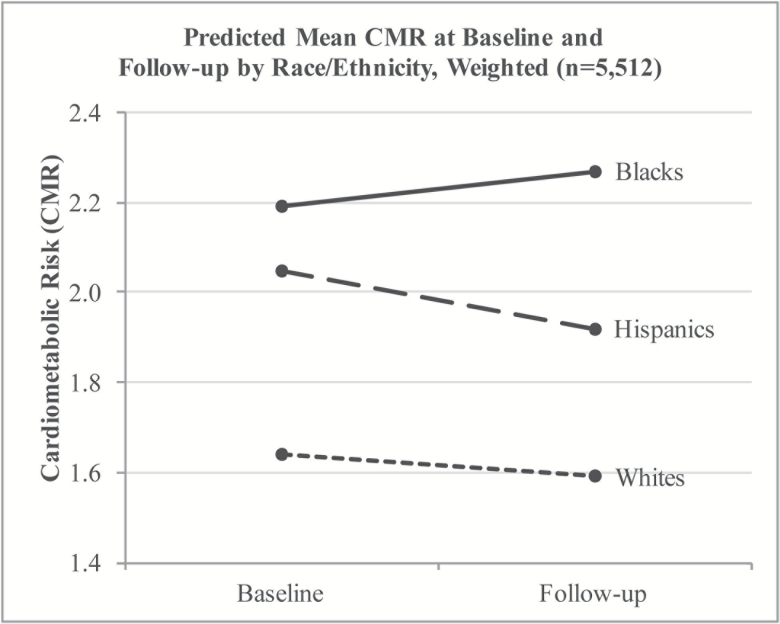
Figure 1 shows predicted CMR at baseline and follow-up. At baseline, blacks had the highest CMR followed by Hispanics and whites. The rank-order of the groups remained the same at follow-up, however risk increased for blacks during the 4-year period, but decreased for Hispanics and whites.
Figure 1.
Predicted Mean CMR at Baseline and Follow-up by Race/Ethnicity, Weighted (n = 5,512). Note. Cardiometabolic risk (CMR) at baseline and follow-up by race/ethnicity. Values adjusted for age, gender and foreign-born status.
To determine which biomarkers were driving differential change in CMR, we calculated the predicted probabilities of transitioning between low- and high-risk states for each race group and examined absolute differences in these probabilities (Table 2). Compared to whites, blacks were more likely to develop high-risk pulse pressure (difference = 0.120, p < .001) and HbA1c (difference = 0.128, p < .001) and less likely to become low-risk on CRP (difference = −0.163, p < .0001) and HDL-C (difference = −0.174, p = .017). Hispanics were more likely to develop high-risk HbA1c compared to whites (difference = 0.052, p = .006). These findings show that race differences in CMR changes stem from race differences in the onset of high- and low-risk states for pulse pressure, HbA1c, CRP, and HDL-C.
Table 2.
Race Differences in the Predicted Probabilitya of Becoming High-risk or Low-risk by Follow-up: Health and Retirement Study
| White | Black | Hispanic | Black-White Difference | Hispanic-White Difference | # Low-risk at Baseline | |
|---|---|---|---|---|---|---|
| Predicted probability of transitioning from low- to high-risk | ||||||
| CRP | 0.157 | 0.163 | 0.157 | 0.006 | 0.000 | 3,494 |
| PP | 0.125 | 0.245 | 0.169 | 0.120*** | 0.044+ | 4,121 |
| HR | 0.035 | 0.046 | 0.044 | 0.011 | 0.009 | 5,256 |
| HbA1c | 0.071 | 0.200 | 0.123 | 0.129*** | 0.052** | 4,868 |
| Low HDL-C | 0.138 | 0.169 | 0.181 | 0.031 | 0.043 | 4,509 |
| TC | 0.099 | 0.119 | 0.116 | 0.020 | 0.017 | 4,409 |
| Waist | 0.235 | 0.292 | 0.332 | 0.057 | 0.097+ | 2,044 |
| Predicted probability of transitioning from high- to low-risk | ||||||
| White | Black | Hispanic | Black-White Difference | Hispanic-White Difference | # High-risk at Baseline | |
| CRP | 0.437 | 0.274 | 0.458 | −0.163*** | 0.021 | 2,018 |
| PP | 0.401 | 0.314 | 0.392 | −0.087+ | −0.009 | 1,391 |
| HR | 0.766 | 0.803 | 0.905 | 0.037 | 0.139+ | 256 |
| HbA1c | 0.281 | 0.271 | 0.267 | −0.010 | −0.014 | 644 |
| Low HDL-C | 0.709 | 0.535 | 0.724 | −0.174* | 0.015 | 1,003 |
| TC | 0.723 | 0.704 | 0.744 | −0.019 | 0.021 | 1,103 |
| Waist | 0.087 | 0.088 | 0.132 | 0.001 | 0.045 | 3,468 |
Note: CRP, C-reactive protein; HR, heart rate; HbA1c, hemoglobin A1c; HDL-C, high density lipoprotein cholesterol; PP, pulse pressure; TC, total cholesterol; Waist, waist circumference. n = 5,512.
aPredicted probabilities come from separate logistic regression models estimating the odds of becoming high-risk among those low-risk at baseline (top panel) and the odds of becoming low-risk among individuals high-risk at baseline (bottom panel). Models adjusted for age, gender and foreign-born status.
+ p < .10, *p < 0.05, **p < .01, ***p < .001.
Because change in pulse pressure reflects changes in both systolic blood pressure (SBP) and diastolic blood pressure (DBP), we conducted sensitivity analyses that separately examined 4-year transitions between low- and high-risk states for SBP and DBP (see Supplementary Table 3). Compared to whites, blacks were more likely to transition into a high-risk state for SBP (difference = 0.117, p < .001) and less likely to transition into a low-risk state (difference = −0.006, p < .01). Blacks also experienced a greater onset of high-risk DBP than whites but this finding was not significant (difference = 0.044, p < .10).
To determine why blacks were more likely to develop high-risk pulse pressure and HbA1c, we estimated the relative odds of becoming high-risk among individuals initially at low-risk. For each biomarker, we stratified by healthy and controlled and estimated one model adjusted for covariates and another adjusted for all study variables. Table 3 presents findings for pulse pressure (see Supplementary Table 4 for complete models). Among healthy individuals, the odds of becoming high-risk did not differ significantly between blacks and whites (Model 1: OR = 1.22, 95% CI = 0.56, 2.65). Healthy Hispanics, however, had more than twice the odds as whites of becoming high-risk by follow-up (Model 1: OR = 2.30, 95% CI = 1.22, 4.35). A significant Hispanic-white difference remained after accounting for explanatory factors (Model 2: OR = 2.01, 95% CI = 1.09, 3.69). Among individuals who initially had controlled blood pressure, blacks had more than twice the odds of whites of developing high-risk pulse pressure (Model 3: OR = 2.31, 95% CI = 1.60, 3.33). This difference remained after adjusting for the other variables (Model 4: OR = 2.11, 95% CI = 1.46, 3.05).
Table 3.
Odds Ratios for Developing High-risk Pulse Pressure by Follow-up, Weighted: Health and Retirement Study
| Healthy (n = 1,991) | Controlled (n = 2,130) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Blacka | 1.22 | (0.56, 2.65) | 1.08 | (0.50, 2.33) | 2.31 | (1.60, 3.33) | 2.11 | (1.46, 3.05) |
| Hispanica | 2.30 | (1.22, 4.35) | 2.01 | (1.09, 3.69) | 0.84 | (0.44, 1.59) | 0.77 | (0.38, 1.57) |
Note: Model 1 controls for age, gender, and foreign-born status; Model 2 additionally controls for education, smoking behavior, body mass index, health insurance, and foregone medications.
aref = white.
Table 4 presents findings for HbA1c (see Supplementary Table 5 for complete models). Healthy blacks had four times the odds of becoming high-risk compared to healthy whites (Model 1: OR = 4.06, 95% CI = 2.79, 5.89). This difference was reduced to 3.29 in the fully-adjusted model (Model 2: 95% CI = 2.27, 4.75). There were no significant race differences in the odds of becoming high-risk among individuals with controlled HbA1c levels (Models 3 and 4).
Table 4.
Odds Ratios for Developing High-risk Hemoglobin A1c (HbA1c) by Follow-up, Weighted: Health and Retirement Study
| Healthy (n = 4,345) | Controlled (n = 523) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Blacka | 4.06 | (2.79, 5.89) | 3.29 | (2.27, 4.75) | 1.57 | (0.88, 2.78) | 1.54 | (0.81, 2.95) |
| Hispanica | 1.32 | (0.58, 3.01) | 1.06 | (0.44, 2.57) | 1.58 | (0.74, 3.37) | 1.80 | (0.79, 4.09) |
Note: Model 1 adjusts for age, gender, and foreign-born status; Model 2 additional adjusts for education, smoking behavior, body mass index, health insurance, and foregone medications.
aref = white.
For CRP and HDL-C, we followed a similar modeling procedure but estimated the relative odds of becoming low-risk among individuals high-risk at baseline. We did not stratify by disease state because there are no diagnosis or treatment guidelines for high CRP or low HDL-C; thus, designations of undiagnosed and uncontrolled are not appropriate. Blacks had lower odds than whites of becoming low-risk for CRP (Table 5, Model 2: OR = 0.52, 95% CI = 0.35, 0.78) and HDL-C (Model 4: OR = 0.43, 95% CI =0.23, 0.80). In supplemental analyses, we ran an additional model for CRP and HDL-C accounting for the use of lipid-lowering drugs because initiating the use of these drugs may affect CRP and HDL-C levels (see Supplementary Table 6 for CRP and Table 7 for HDL-C). Findings regarding race differences in the transition between disease states were unchanged: blacks were still less like to become low-risk on both biomarkers.
Table 5.
Odds Ratios for Becoming Low-risk on C-reactive Protein and HDL Cholesterol by Follow-up, Weighted: Health and Retirement Study
| High-risk C-reactive Protein at Baseline (n = 2,018) | High-risk HDL Cholesterol at Baseline (n = 1,003) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Blacka | 0.49 | (0.34, 0.70) | 0.52 | (0.35, 0.78) | 0.47 | (0.26, 0.86) | 0.43 | (0.23, 0.80) |
| Hispanica | 1.09 | (0.74, 1.61) | 1.24 | (0.82, 1.90) | 1.08 | (0.54, 2.14) | 1.16 | (0.51, 2.61) |
Note: Model 1 controls for age, gender, and foreign-born status; Model 2 additionally controls for education, smoking behavior, body mass index, health insurance, and foregone medications.
aref = white.
Discussion
This is the first study, to our knowledge, to document differential change in CMR by race/ethnicity and to identify the specific biomarkers driving these differences. Using two waves of data from a nationally representative sample of community-dwelling older adults, we found that blacks had higher CMR than whites and Hispanics and experienced increased risk as they aged. Higher biological risk among blacks has been documented in prior studies (5,6), but these studies used cross-sectional data and could not examine race differences in change in risk. We build upon this existing research by demonstrating an increase among blacks over a 4-year period that is driven by the disproportionate onset of high-risk pulse pressure and HbA1c. In contrast, improvements in CRP and HDL-C drove declines in risk among whites and Hispanics, who were more likely than blacks to transition from high- to low-risk states. The overall result was a diverging pattern of change in CMR and a widening of the black-white and black-Hispanic disparities in risk.
Although cross-sectional studies suggest that biological risk increases with age (9), this is not true for all population subgroups; an increase in risk was only evident among blacks in this study and warrants explanation. We provide support for the hypothesis that blacks experience more rapid physiological dysregulation as they age (6,23) and improve our understanding of why this disadvantage occurs. Lifestyle and health care factors partially accounted for black-white differences in the onset of high-risk pulse pressure and high-risk HbA1c, but a considerable proportion of the differences remained unexplained. Race differences in treatment effectiveness may contribute to persistent and widening disparities in risk. The black-white difference in the onset of high-risk pulse pressure was solely observed among individuals who, at baseline, had successfully controlled their blood pressure. This lack of sustained blood pressure control among hypertensive blacks signifies a failure in chronic disease management. Despite recent national trends showing increased blood pressure control across races, blacks are still less likely to maintain control (24); they are also more likely to use multiple medications, which can contribute to medication nonadherence (25), a major driver of differences in blood pressure control (26). Similar to findings observed in a sample of Medicare beneficiaries (27), a larger proportion of blacks in our study reported inconsistent medication use due to costs. Foregone medication was associated with a higher likelihood of developing high-risk pulse pressure, and this association was also limited to individuals who initially had controlled blood pressure (See Supplementary Table 5). Thus, economic hardships may hinder consistent medication use among older hypertensive blacks and contribute to poor blood pressure control and increasing CMR.
Shortcomings in primary disease prevention may also contribute to increasing CMR. Among individuals considered healthy at baseline, older blacks had four times the likelihood of developing high-risk HbA1c, an indicator of diabetes, and older Hispanics had twice the likelihood of developing high-risk pulse pressure. These differences did not exist among individuals with controlled diabetes or hypertension, which suggests that once they receive medical care, older diabetic blacks, and hypertensive Hispanics remain low-risk, at least in the short-term. Thus, the issue at hand when examining race differences in CMR is whether current diabetes and hypertension prevention efforts are effective in older minority populations. Recent estimates of the incidence of diabetes and hypertension show that rates have increased for blacks and Hispanics, despite declines seen in the total population (28,29), which supports our claim of poor primary prevention among older minorities.
Short-term improvements in cumulative risk are less evident. One study documented declines over 2.5 years, but included a high-functioning, predominately white sample of adults age 70–79 and did not examine whether changes in individual biomarkers contributed to changes in cumulative risk (15). Declines in CMR among whites and Hispanics were due to declines in CRP and increases in HDL-C levels. These changes may be related to the use of lipid-lowering drugs (eg, statins). Statins may improve CRP and HDL-C levels and usage rates are similar for blacks and whites (30); their efficacy, however, varies by race/ethnicity with whites and Hispanics experiencing greater improvements compared to blacks (31). Reasons for efficacy differences are unclear but may be related to race differences in medication regimens (32). Supplemental analyses showed that individuals who started using lipid-lowering drugs during the study period, or used them at both time points, had greater odds of becoming low-risk on CRP by follow-up. Race differences in this transition, however, remained substantively and statistically the same.
Diverging patterns of change in risk may also be related to structural factors that undermine the health of racially marginalized populations. Maintaining ideal biomarker levels is more difficult for minority populations that have encountered systematic discrimination and barriers to quality health care (33). The adverse effects of discrimination on blood pressure and other physiological outcomes is well-documented (34,35) and may explain why older blacks, a population disproportionately exposed to discrimination (36), are less likely to maintain blood pressure and glucose control and less likely to achieve ideal CRP and HDL-C levels. The social and economic adversities older blacks have faced throughout their lives and the earlier onset of chronic conditions may also hinder their ability to improve CMR, leading to declining physiological functioning over time.
There are caveats to this study. First, a venous blood draw is the standard method for collecting blood-based biomarkers and DBS values may not be as reliable or valid as values based on venous blood (22). However, DBS are ideal for large population-based surveys like the HRS. Additionally, we used NHANES-equivalent biomarker data, provided by HRS, to align the DBS values to venous values.
Second, CMR included pulse pressure rather than systolic and diastolic blood pressure. Pulse pressure is a measure of arterial stiffness (37) and, compared to its components, it is considered a better assessment of cardiovascular functioning among older adults (38,39). In sensitivity analyses using systolic and diastolic blood pressure instead of pulse pressure, the main conclusions of this study remained the same: blacks had the worst risk profiles and did not improve their CMR over time. Thus, in this study, change in systolic and diastolic blood pressure and changes in pulse pressure have similar effects on change in CMR.
An additional study limitation is its focus on short-term as opposed to longer-term changes. However, short-term longitudinal studies provide insights into the physiological process of change by isolating biomarker-specific changes subsequently leading to changes in risk. Therefore, our study on the nature and determinants of short-term CMR change facilitates better understanding of the morbidity process. Few studies exist with repeated biological measurements and none for a large, nationally representative and racially diverse sample of older adults. Therefore, the HRS was ideal for addressing our research questions.
The study’s strengths and contributions outweigh its limitations. We prospectively examined change in cumulative risk across race/ethnicity in a nationally representative sample of older adults. Longitudinal analyses minimize issues of reverse causality because the temporal ordering of predictors and outcomes is clearer. Additionally, our findings generalize to the larger population of older Americans. Past research on race differences in biological risk spanned broad age ranges, but factors contributing to disparities at younger ages are qualitatively different from those influencing CMR at ages when chronic disease risk peaks. Thus, focusing on disparities occurring during midlife and later is most relevant for the health of aging racial/ethnic minorities and can inform targeted interventions for this population. To the extent that change in CMR is associated with mortality (15), primary and secondary interventions aimed at lowering risk among blacks can improve their longevity and reduce racial disparities in life expectancy.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (Grants T32-AG000037, P30-AG043073, R24-AG045061 to U.A.M.; Grants R00-AG039528 to J.A.A.; and Grants P30-AG17265 to E.M.C.).
Conflict of Interest
The authors have no conflicts of interest to report.
Supplementary Material
References
- 1. National Center for Health Statistics (US). Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 2. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. doi:10.1111/j.1749-6632.2009.05339.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seeman TE, Crimmins E, Huang MH et al. . Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58:1985–1997. doi:10.1016/S0277-9536(03)00402-7 [DOI] [PubMed] [Google Scholar]
- 4. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14:311–346. doi:10.1177/1099800412455688 [DOI] [PubMed] [Google Scholar]
- 5. Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007;97:1305–1310. doi:10.2105/AJPH.2006.091892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi:10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown TH, O’Rand AM, Adkins DE. Race-ethnicity and health trajectories: tests of three hypotheses across multiple groups and health outcomes. J Health Soc Behav. 2012;53:359–377. doi:10.1177/0022146512455333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quiñones AR, Liang J, Bennett JM, Xu X, Ye W. How does the trajectory of multimorbidity vary across Black, White, and Mexican Americans in middle and old age?J Gerontol B Psychol Sci Soc Sci. 2011;66:739–749. doi:10.1093/geronb/gbr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol. 2003;38:731–734. [DOI] [PubMed] [Google Scholar]
- 10. Crimmins E, Kim JK, Vasunilashorn S. Biodemography: new approaches to understanding trends and differences in population health and mortality. Demography. 2010;47:S41–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goldman N, Turra CM, Glei DA, Lin YH, Weinstein M. Physiological dysregulation and changes in health in an older population. Exp Gerontol. 2006;41:862–870. doi:10.1016/j.exger.2006.06.050 [DOI] [PubMed] [Google Scholar]
- 12. Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009;57:1525–1531. doi:10.1111/j.1532-5415.2009.02389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glei DA, Goldman N, Lin YH, Weinstein M. Age-related changes in biomarkers: longitudinal data from a population-based sample. Res Aging. 2011;33:312–326. doi:10.1177/0164027511399105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merkin SS, Karlamangla A, Roux AV, Shrager S, Seeman TE. Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: multi-ethnic study of atherosclerosis. Am J Public Health. 2014;104:e48–e55. doi:10.2105/AJPH.2013.301841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006;68:500–507. doi:10.1097/01.psy.0000221270.93985.82 [DOI] [PubMed] [Google Scholar]
- 16. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi:10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 17. Selvin E, Steffes MW, Zhu H et al. . Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi:10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Holten TC, Waanders LF, de Groot PG et al. . Circulating biomarkers for predicting cardiovascular disease risk; a systematic review and comprehensive overview of meta-analyses. PLoS One. 2013;8:e62080. doi:10.1371/journal.pone.0062080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasunilashorn S, Best LE, Kim JK, Crimmins EM. Predicting mortality from profiles of biological risk and performance measures of functioning. In: Anson J, Luy M, eds. Mortality in an International Perspective. New York, NY: Springer; 2014:119–135. doi:10.1007/978-3-319-03029-6 [Google Scholar]
- 20. Crimmins EM, Faul J, Kim JK et al. . Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study. Ann Arbor: University of Michigan; 2013. [Google Scholar]
- 21. McDade TW, Williams S, Snodgrass JJ. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 2007;44:899–925. doi:10.1353/dem.2007.0038 [DOI] [PubMed] [Google Scholar]
- 22. Crimmins E, Kim JK, McCreath H, Faul J, Weir D, Seeman T. Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography Soc Biol. 2014;60:38–48. doi:10.1080/19485565.2014.901885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med. 2014;118:27–32. doi:10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu Q, Burt VL, Dillon CF, Yoon S. Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: the National Health and Nutrition Examination Survey, 2001 to 2010. Circulation. 2012;126:2105–2114. doi:10.1161/CIRCULATIONAHA.112.096156 [DOI] [PubMed] [Google Scholar]
- 25. Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5:345–351. doi:10.1016/j.amjopharm. 2007.12.002 [DOI] [PubMed] [Google Scholar]
- 26. Bosworth HB, Powers B, Grubber JM et al. . Racial differences in blood pressure control: potential explanatory factors. J Gen Intern Med. 2008;23:692–698. doi:10.1007/s11606-008-0547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gellad WF, Haas JS, Safran DG. Race/ethnicity and nonadherence to prescription medications among seniors: results of a national study. J Gen Intern Med. 2007;22:1572–1578. doi:10.1007/s11606-007-0385-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quiñones AR, Liang J, Ye W. Racial and ethnic differences in hypertension risk: new diagnoses after age 50. Ethn Dis. 2012;22:175–180. [PMC free article] [PubMed] [Google Scholar]
- 29. Geiss LS, Wang J, Cheng YJ et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA. 2014;312:1218–1226. doi:10.1001/jama.2014.11494 [DOI] [PubMed] [Google Scholar]
- 30. Adedinsewo D, Taka N, Agasthi P, Sachdeva R, Rust G, Onwuanyi A. Prevalence and factors associated with statin use among a nationally representative sample of US adults: National Health and Nutrition Examination Survey, 2011–2012. Clin Cardiol. 2016;39:491–496. doi:10.1002/clc.22577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Albert MA, Glynn RJ, Fonseca FA et al. . Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin (JUPITER) trial. Am Heart J. 2011;162:106–14.e2. doi:10.1016/j.ahj.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 32. Lewey J, Shrank WH, Bowry AD, Kilabuk E, Brennan TA, Choudhry NK. Gender and racial disparities in adherence to statin therapy: a meta-analysis. Am Heart J. 2013;165:665–78, 678.e1. doi:10.1016/j.ahj.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 33. Nelson AR, Stith AY, Smedley BD.. Unequal Treatment: Confronting racial and Ethnic Disparities in Health Care (Full Printed Version). Washington, DC: National Academies Press; 2002. [PubMed] [Google Scholar]
- 34. Lewis TT, Barnes LL, Bienias JL, Lackland DT, Evans DA, Mendes de Leon CF. Perceived discrimination and blood pressure in older African American and white adults. J Gerontol A Biol Sci Med Sci. 2009;64: 1002–1008. doi:10.1093/gerona/glp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams DR, Neighbors H. Racism, discrimination and hypertension: evidence and needed research. Ethn Dis. 2001;11:800–816. [PubMed] [Google Scholar]
- 36. Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40:208–230. doi:10.2307/2676349 [PubMed] [Google Scholar]
- 37. Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–2869. doi:10.1161/01.CIR.0000069826.36125.B4 [DOI] [PubMed] [Google Scholar]
- 38. Blacher J, Staessen JA, Girerd X et al. . Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. doi:10.1001/archinte.160.8.1085 [DOI] [PubMed] [Google Scholar]
- 39. Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur Heart J. 2005;26:2120–2126. doi:10.1093/eurheartj/ehi467 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.