Abstract
An association between vitamin D and attention deficit hyperactivity disorder (ADHD) has been proposed by several researchers in recent years; however, the investigations have led to inconsistent results. The present study was conducted to summarize the published observational data on the relation between vitamin D status and the likelihood of ADHD. Online databases, including PubMed, the ISI Web of Science, Google Scholar, and Scopus, were checked up to June 2017 for relevant observational studies. A random-effects model was incorporated to summarize the study results. Out of 2770 retrieved articles, 13 observational studies (9 case-control or cross-sectional studies and 4 prospective studies) were eligible for inclusion in the systematic review and meta-analysis. Analysis of the 10,334 children and adolescents who attended the 9 case-control or cross-sectional studies revealed that children with ADHD have lower serum concentrations of 25-hydroxyvitamin D than do healthy children (weighted mean difference: −6.75 ng/mL; 95% CI: −9.73, −3.77 ng/mL; I2 = 94.9%]. Five case-control studies reported the OR for developing ADHD based on vitamin D status; the meta-analysis of their data revealed that lower vitamin D status is significantly associated with the likelihood of ADHD (OR: 2.57; 95% CI: 1.09, 6.04; I2 = 84.3%). Furthermore, the meta-analysis of prospective studies conducted in 4137 participants indicated that perinatal suboptimal vitamin D concentrations are significantly associated with a higher risk of ADHD in later life (RR: 1.40; 95% CI: 1.09, 1.81; I2 = 0.0%). It should be noted that the association found in prospective studies was sensitive to one of the included investigations. The present review provides evidence supporting the relation between vitamin D deficiency and ADHD. However, the overall effect sizes are small, and therefore the association should be considered equivocal at this time. Further prospective cohort studies and community-based intervention trials are highly recommended to better elucidate the causal association.
Keywords: vitamin D, attention deficit disorder with hyperactivity, children, adolescents, systematic review, meta-analysis
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common behavioral conditions, particularly among children (1). A systematic review and meta-analysis estimated that ∼5.29% of people are affected by ADHD or hyperactivity disorder worldwide (2). ADHD typically starts in childhood and often persists throughout life (3). The symptoms are inattention, hyperactivity, and impulsivity (4). People with ADHD might reach lower education levels than healthy children and their occupational attainment and social level might be lower in adulthood, and this might have an economic burden to societies (5).
The causes of ADHD are not fully understood; however, several environmental (e.g., exposure to certain foods or inhalants) and genetic risk factors have been proposed (6). It has been suggested that dietary interventions such as ω-3 FA (7) and vitamin and mineral supplementation (8), restriction diets, and the avoidance of synthetic food color additives (9) might affect ADHD symptoms (10). Recently a number of studies have proposed that vitamin D might play a role in ADHD pathogenesis. The mechanisms by which vitamin D might affect a number of neurological diseases, including ADHD, are not clear. Nevertheless, there is evidence demonstrating the widespread presence of vitamin D receptors and 1α-hydroxylase (the enzyme responsible for the formation of the active vitamin) in the human brain; therefore, it is suggested that vitamin D might have neurohormonal properties in the human brain (11). Furthermore, a recent study proposed that vitamin D directly upregulates expression of tyrosine hydroxylase (a rate-limiting enzyme in dopamine synthesis) by binding to the nuclear vitamin D receptor (12). It is also suggested that this vitamin is involved in the synthesis of serotonin in the brain (13).
Several observational studies have examined the association between vitamin D status and ADHD in children and adolescents in recent years; however, they have achieved inconsistent results (14–17). The majority of case-control studies have revealed that serum vitamin D concentrations are lower in ADHD children than in healthy controls (15, 16, 18–20). However, a study done by Celik et al. (14) could not show the same association. Moreover, in a cohort study, Gustafsson et al. (17) reported that there is no difference in cord blood vitamin D concentrations at birth between children with ADHD and healthy controls. In contrast, another cohort study conducted by Morales et al. (21) revealed that higher maternal circulating concentrations of 25-hydroxyvitamin D [25(OH)D] in pregnancy might be associated with a lower risk of developing ADHD-like symptoms in offsprings.
Although the findings of a connection between vitamin D status and ADHD have been controversial, we are aware of no systematic literature review that summarizes the evidence. The present systematic review was therefore designed to summarize all of the relevant observational studies. We also aimed to perform a meta-analysis on the data provided by the relevant publications, to provide overall estimates and find the potential sources of heterogeneity between the results.
Methods
The present systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol was registered in the international prospective register of systematic reviews (PROSPERO) database with the registration number of CRD42016038469.
Search Strategy and Study Selection
A literature search was conducted using PubMed, the ISI Web of Science, Google Scholar, and Scopus up to June 2017. We used 2 sets of keywords selected from Medical Subject Headings (Mesh) and also text words that might be used in the title and/or abstract of the relevant papers: 1) “vitamin D,” “cholecalciferol,” “ergocalciferol,” “calcitriol,” “calcifediol,” “25-hydroxyvitamin D 2,” “25-hydroxyvitamin D,” “1–25-dihydroxy-23,23-difluorovitamin D3,” “25(OH)D,” “1,25(OH)(2)D,” “1,25(OH)D,” “1,25-(OH)(2) D(3),” “25 hydroxyvitamin D,” “25-(OH)D(3),” and “25-(OH)D(2)” in combination with 2) “ADHD,” “hyperactivity,” “inattention,” “impulsivity,” “attention deficit hyperactivity disorder,” “minimal brain dysfunction,” “attention deficit and disruptive behavior disorder,” “hyperkinesia,” “attention deficit disorder with hyperactivity,” “attention deficit,” “hyperactivity disorder,” and “attention.” No restrictions were applied regarding the date or original language of the publications. The reference lists of the relevant articles were checked for any additional investigations. Publications found were compared and reviewed for their relevance with the use of the pre-specified inclusion and exclusion criteria by 2 independent authors (YK and AS-A) and disagreements were resolved by group discussion; if any disagreement still remained, it was resolved by discussion with the third author (RB).
Eligibility Criteria
We included any original observational study that tried to assess the relation between current or perinatal vitamin D status and ADHD in childhood and/or adolescence (Table 1). No restriction in terms of the biomarker selected for vitamin D status was considered. Studies that were conducted on children with other neurological disorders or that only considered hyperactivity disorder as the outcome variable, review articles, and duplicate reports of the same works were excluded from the systematic review.
TABLE 1.
Population, intervention/exposure, comparison, outcome, and study design criteria for eligible studies
| Criteria | Description |
|---|---|
| Population | Children and adolescents aged <18 y |
| Intervention/exposure | Vitamin D status in children and adolescents |
| Perinatal (neonatal or maternal) vitamin D status | |
| Comparison | Vitamin D sufficiency in children or adolescents |
| Perinatal (neonatal or maternal) vitamin D sufficiency | |
| Outcome | Attention deficit hyperactivity disorder |
| Study design | Case-control studies, cohort studies, nested case-control studies |
Data Extraction
We extracted the following information from the eligible articles: first author's last name; date of publication; study design; research location (latitude); participants’ age and gender; study sample size; biomarkers used to assess vitamin D status and their assessment methods; follow-up time for cohort studies; mean ± SD of serum vitamin D concentrations in participants with ADHD and healthy controls; OR, RR, or HR for ADHD in vitamin D-insufficient or-deficient participants compared with vitamin D-sufficient subjects; and the use of any matching or adjustment for confounding variables in the data analysis. Data extraction from articles, tables and figures was performed by 2 of the authors (YK and RB) independently, and the accuracy of the data entry was double-checked and confirmed by the third author (AS-A).
Quality Assessment
We used the Newcastle-Ottawa scale to assess the quality of studies included in the systematic review (22). This scale was developed to check the quality of non-randomized studies by a collaboration between the University of Newcastle and the University of Ottawa. The scale considers 3 major domains: the selection of the study groups (4 items); the comparability of the study groups (1 item); and the ascertainment of either the exposure or the outcome (3 items) for the case-control and cohort studies, respectively. A study receives a maximum of 1 star (score) for each item of the selection and outcome or exposure domains. However, it receives a maximum of 2 stars for the item designed to assess the comparability. Therefore, a study might receive a total score of 9 using this tool (22). In the present study, studies scoring ≥7 were considered to be high quality, and those with scores between 4 and 6 and <4 were considered as middle and low quality, respectively.
Statistical Analysis
The sample sizes and the mean ± SD for serum 25(OH)D concentrations in participants with and without ADHD were obtained to derive the mean difference ± SD in serum vitamin D concentrations between cases and healthy controls. This was then used as the effect size for the meta-analysis of means. For consistency, if the serum concentrations of 25(OH)D were presented in nanomoles per liter, we converted them to nanograms per milliliter by using the conversion factor 1 ng/mL = 2.5 nmol/L.
Furthermore, a number of case-control investigations reported data on the OR and its 95% CI, comparing the likelihood of ADHD between vitamin D–insufficient or –deficient and vitamin D–sufficient children. Therefore we conducted a separate meta-analysis of studies with data on ORs. Moreover, cohort studies reported the RR of developing ADHD based on perinatal vitamin D status (maternal or cord blood concentrations). Data on RRs and their corresponding 95% CIs were used to conduct the meta-analysis of prospective studies. As ADHD is prevalent in children and adolescents, the ORs and RRs might differ. Therefore, we separately conducted a meta-analysis on the case-control and prospective studies.
We used DerSimonian and Laird's random effects model to conduct all the meta-analyses (23) because this model takes the between-study variability into account. Statistical heterogeneity between studies was evaluated using Cochran's Q test and the I2 statistic (23). Subgroup analysis was incorporated to explore the possible sources of heterogeneity between study results (23). Sensitivity analysis was performed by excluding studies from the meta-analysis one by one. This analysis was done to determine the extent to which a study might affect the overall estimates. Publication bias was assessed by visually checking funnel plots and conducting Egger's regression and Begg's adjusted rank correlation asymmetry tests (24). All analyses were done with the use of STATA, version 11.2 (Stata Corp.). P values <0.05 were considered significant.
Results
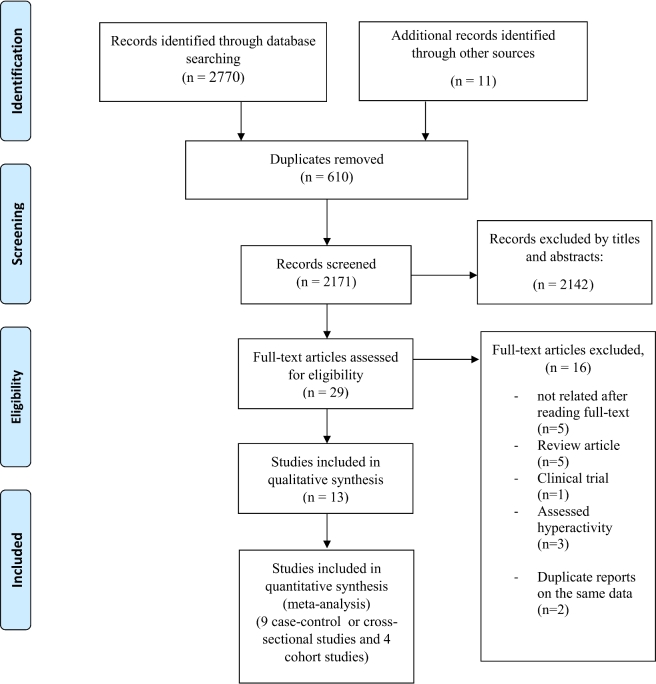
The database search and the hand search led to 2770 and 11 publications, respectively. After removal of duplicates, 2171 articles were checked for potential eligibility. Screening the titles and abstracts led to the exclusion of 2142 publications; the full texts of the remaining 29 articles were carefully checked. At this stage, 5 studies were excluded because they were assessed to not be relevant (25–29), 5 papers were review articles (13, 30–33), 1 study was a clinical trial (34), and 3 papers assessed vitamin D status in association with hyperactivity disorder (35–37). Furthermore, 3 of the studies (16, 20, 38) were based on the same data, so we kept the study conducted by Bener and Kamal (20) because the association between vitamin D status and ADHD was better illustrated and they included a larger sample size in their analysis. The flow of the study selection process is illustrated in Figure 1. In total, 13 relevant investigations [9 retrospective case–control or cross-sectional studies that compared serum vitamin D concentrations between children with ADHD and healthy controls (14, 15, 18–20, 39–42) and 4 prospective studies that assessed the relation between maternal or offsprings’ vitamin D status and ADHD in later life (17, 21, 43, 44)] remained to be included in the current systematic review and meta-analysis.
FIGURE 1.
Flow chart of the number of studies identified and included in the systematic review and meta-analysis.
Table 2 represents the general characteristics of studies entered in the qualitative and quantitative synthesis. In total, 3484 patients with ADHD (2183 from the case-control and cross-sectional studies, 1301 from the prospective studies) and 11,837 healthy children (8151 from the case-control and cross-sectional studies, 3686 from the prospective studies) aged between 5 and 18 y were included. Five studies were conducted in Turkey (14, 19, 40–42), 2 in Denmark (43, 44), and 1 each in China (18), Qatar (20), Germany (39), Iran (15), Spain (21), and Sweden (17).
TABLE 2.
Characteristics of studies investigating the association between vitamin D concentration and the risk of ADHD1
| Study authors (year) (ref) | Study design | Sample size | Participants (age) | Follow-up period | Country (city) | Sample | Serum vitamin D concentration, ng/mL | Adjusted variable | Risk estimate (95% CI) | Latitude | Newcastle-Ottawa score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Garipardic et al. (2017) (42) | Case-control | Cases: 36 Controls: 25 | Children and adolescents(ADHD: 7.67 ± 3.132 y, Controls: 9.90 ± 4.13 y) | — | Turkey (Van) | Serum | ADHD: 18.50 ± 8.53Control: 29.42 ± 9.07 | Age and gender | — | 38°30'N | 6 |
| Avcil et al. (2017) (41) | Case-control | Cases: 105 Controls: 95 | Children and adolescents(6–16 y) | — | Turkey (Aydin) | Serum | ADHD: 16.92 ± 4.72Control: 22.50 ± 6.32 | Age and gender, season of sampling | 5.98 (3.24, 11.06)Reference group: serum vitamin D ≥20 ng/mL | 37°50'N | 7 |
| Çelık et al. (2016) (14) | Case-control | Cases: 33 Controls: 20 | Children (ADHD: 9.5 ± 2.5 y, Controls: 11.7 ± 5.2 y) | — | Turkey (Adana) | Serum | ADHD: 16.6 ± 9.9Control: 21.5 ± 10.2 | — | 0.07 (0.01, 0.63)Reference group: serum vitamin D >20 ng/mL | 37°00'N | 3 |
| Meyer et al. (2016) (39) | Cross-sectional | Cases: 430 Controls: 6492 | Adolescents (ADHD: 14.4 ± 2 y, Controls: 14.6 ± 2 y) | — | Germany (National) | Serum | ADHD: 16.42 ± 8.01Control: 17.94 ± 11.21 | — | — | 51°17'N | 5 |
| Bala et al. (2016) (40) | Case-control | Cases: 34 Controls: 27 | Children and adolescents (ADHD: 7.68 ± 3.20 y, Controls: 9.80 ± 4.01 y) | — | Turkey (Van) | Serum | ADHD: 19.49 ± 8.53Control: 28.73 ± 9.04 | Age and gender | — | 38°30'N | 6 |
| Mossin et al. (2016) (44) | Cohort | 1233 | Toddlers (2.7 ± 0.6 y) | 4 y | Denmark (Odense) | Cord blood | — | — | 1.60 (1.03, 2.46)Reference group: maternal serum vitamin D≥50 nmol/L | 55°22'N | 8 |
| Sharif et al. (2015) (15) | Case-control | Cases: 37 Controls:37 | Children (6–12 y) | — | Iran (Isfahan) | Serum | ADHD: 19.11 ± 10.10Control: 28.67 ± 13.76 | — | 3.43 (1.25, 9.47)Reference group: serum vitamin D ≥30 ng/mL | 32°50'N | 3 |
| Shang-Guan and Zhao (2015) (18) | Case-control | Cases: 97 Controls: 97 | Children (ADHD: 8.7 ± 1.7 y, Controls: 8.5 ± 1.5 y) | — | China (Shengjin) | Serum | ADHD: 17 ± 7 Control: 23 ± 8 | — | 6.05 (2.20, 16.63)Reference group: serum vitamin D ≥30 ng/mL | 41°40'N | 5 |
| (Continued) | |||||||||||
| Morales et al. (2015) (21) | Cohort | 1650 | Children (4.8 y) | 5 y | Spain (Menorca, Granada, Valencia, Sabadell, Asturias, Gipuzkoa) | Maternal plasma | — | — | 1.39 (0.90, 2.14)Reference group: maternal serum vitamin D ≥30 ng/mL | 37°10'N to 43°31'N | 7 |
| Gustafsson et al. (2015) (17) | Prospective case-control | ADHD: 202Controls: 202 | Children (not reported) | Not reported | Sweden (Malmö) | Cord blood | — | Year and country of birth; Reference group: cord blood vitamin D >9.17 ng/mL | 1.13 (0.69, 1.84) | 55°36'N | 6 |
| Bener et al. (2014) (38) | Case-control | Cases: 1331 Controls: 1331 | Children and adolescents (5–18 y) | — | Qatar (Doha) | Serum | ADHD: 16.6 ± 7.8Control: 23.5 ± 9 | Age and nationality | 1.89 (1.47, 2.42)Reference group: serum vitamin D ≥30 ng/mL | 25°15'N | 8 |
| Goksugur et al. (2014) (19) | Case-control | Cases: 60 Controls: 30 | Children and adolescents (7–18 y) | — | Turkey (Bol) | Serum | ADHD: 20.9 ± 19.4Control: 34.9 ± 15.4 | — | — | 40°45'N | 5 |
| Strøm et al. (2014) (43) | Cohort | 850 | Adolescents (7.2–21 y) | ≤22 y | Denmark (Aarhus) | Maternal serum | — | Parity, maternal age, maternal prepregnancy BMI, maternal smoking during pregnancy, maternal education, offspring sex, and season of birth | 2.45 (0.63, 9.51)Reference group: Maternal serum vitamin D 50–75 nmol/L | 56°08'N | 9 |
1ADHD: Attention Deficit Hyperactivity Disorder; ref, reference.
2Mean ± SD (all such values).
Of the 9 case-control and cross-sectional studies, 3 (14, 15, 18) were conducted entirely on children (6–12 y) and 1 on adolescents (aged 12–16.6 y) (39), and the others reported the association for both children and adolescents (5–18 y) (19, 20, 40–42). The majority of included studies tried to adjust for age or gender as the principal confounding variables by using matching or statistical methods (17, 18, 38, 40–43). However, other studies did not control for possible confounding factors. Only 2 studies matched the case and control groups for nationality and country of birth (17, 38). There were also 2 studies that adjusted for season of vitamin D sampling (41, 43). The study conducted by Strøm et al. (43) incorporated the greatest number of variables, including parity, maternal age, maternal prepregnancy, BMI, maternal smoking during pregnancy, maternal education, offspring sex, and season of birth. Strøm et al.’s model assessed the association between maternal serum vitamin D and risk of ADHD in adolescence.
All studies included in the systematic review and meta-analysis measured serum 25(OH)D as the marker for vitamin D status. The marker was measured by using immunochemical assays (18, 39), radioimmunoassay (20), liquid chromatography tandem mass spectrometry (17, 44), HPLC (21), ELISA (15, 19), and chemiluminescent microparticle immunoassay methods (41) in the case-control and cross-sectional studies. Serum concentrations of vitamin D in 3 of the prospective studies were measured by LC-MS/MS (17, 43, 44) and in the other one, vitamin D was measured by HPLC (21). Two prospective studies assessed serum vitamin D derived from cord blood (17, 44) and the other 2 studies measured this marker in maternal serum (21, 43).
The quality score of studies ranged from 3 to 9, based on the Newcastle-Ottawa scale. Overall, 2 studies were assessed to be low quality (14, 15), 6 were of middle quality (17–19, 39, 40, 42) and the other studies were high quality (20, 21, 41, 43, 44) (Table 2).
Meta-analysis of Mean Serum Vitamin D Concentrations
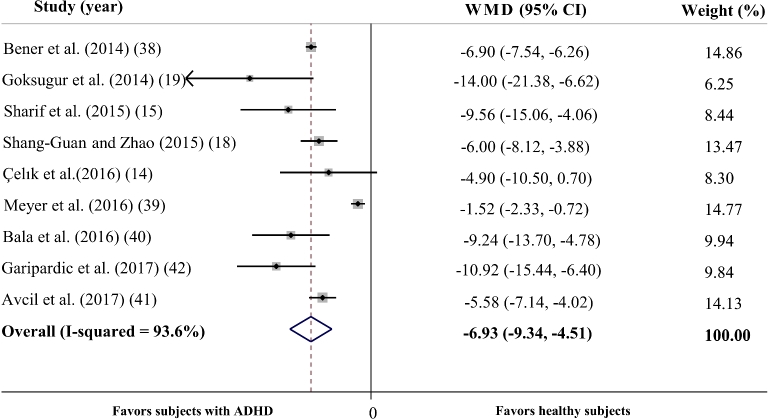
In total, 9 studies reported the mean ± SD vitamin D concentrations in subjects with and without ADHD (14, 15, 18–20, 39–42) and the meta-analysis of their results showed that children with ADHD had 6.93 ng/mL lower serum vitamin D concentrations compared with healthy controls (95% CI: −9.34, −4.51 ng/mL; P < 0.001) (Figure 2). However, we found a significant between-study heterogeneity (Cochran's Q test, P < 0.001; I2 = 93.6%). To explore the possible sources of heterogeneity, we conducted a subgroup analysis based on the variables adjusted for the association. The analysis revealed a significant difference in serum vitamin D concentrations between cases and controls [weighted mean difference (WMD): –6.3 ng/mL; 95% CI: –10.11, –2.49 ng/mL; P = 0.001) in studies with no adjustment or matching for confounding variables (14, 15, 18, 19); however, the heterogeneity was still significant (Cochran's Q test, P < 0.001; I2 = 87.7%). Serum vitamin D concentrations were also lower in children and adolescents with ADHD in studies which addressed ≥1 confounding variable (20, 40–42) (WMD: −7.05 ng/mL; 95% CI: −8.55, −5.54 ng/mL; P < 0.001) and the heterogeneity was slightly lower (Cochran's Q test, P = 0.079; I2 = 55.8%). The subgroup analysis based on the quality of included studies also revealed that the difference is significant in low- (14, 15) (WMD: −7.26 ng/mL; 95% CI: −11.83, −2.70 ng/mL; P for effect = 0.002; Cochran's Q test, P for heterogeneity = 0.245; I2 = 26.1%), middle- (18, 19, 39, 40, 42) (WMD: −7.61 ng/mL; 95% CI: −11.86, −3.36 ng/mL; P for effect < 0.001; Cochran's Q test, P for heterogeneity < 0.001; I2 = 91.6%) and high-quality studies (20, 41) (WMD: −6.44 ng/mL; 95% CI: −7.67, −5.21 ng/mL; P for effect <0.001; Cochran's Q test, P for heterogeneity = 0.125; I2 = 57.6%). We also checked if the heterogeneity is because of one or a number of studies using sensitivity analysis. When we removed the study by Meyer et al. (39), the heterogeneity was considerably less (Cochran's Q test, P = 0.088; I2 = 43.6%), whereas the WMD was still significant (WMD: –7.04 ng/mL; 95% CI: −8.27, −5.81 ng/mL; P <0.001). We found that the study by Meyer et al. (39) was the only cross-sectional study included in the present review and was conducted in West European countries; the other studies were conducted in East Asian countries and the Middle East (14, 15, 18–20, 40–42). The Meyer et al. study (39) was also conducted at the latitude of 51°17′N, whereas the other studies were carried out at lower latitudes (25°15′N–41°40′N).
FIGURE 2.
Forest plot illustrating the WMD in serum vitamin D concentrations between participants with ADHD and healthy control participants. The analysis reveals that children and adolescents with ADHD have 6.93 ng/dL lower vitamin D concentrations on average. The analysis was conducted using a random effects model. ADHD, attention deficit hyperactivity disorder; WMD, weighted mean difference.
Sensitivity analysis showed that removing each study included in the meta-analysis did not substantially change the overall estimate. Although a slight asymmetry was observed in Begg's funnel plot, the asymmetry tests demonstrated that there is no evidence of publication bias in the meta-analysis on serum vitamin D concentrations (Begg's test, P = 0.854; Egger's test, P = 0.466).
Meta-analysis of Odds Ratios Derived from Case-Control Studies
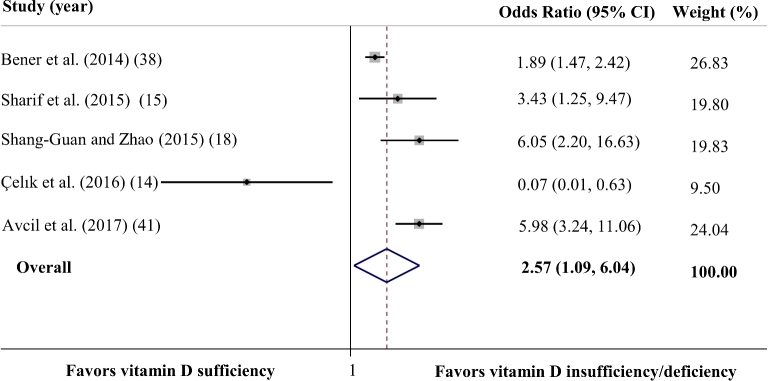
In total, 5 studies provided data on the ORs for developing ADHD in children and adolescents with vitamin D deficiency or insufficiency compared with those with sufficient serum vitamin D concentrations (14, 15, 18, 20, 41). Two studies from Turkey defined vitamin D insufficiency or deficiency as serum 25(OH)D <20 ng/mL (14, 41) and 3 other studies categorized subjects with serum 25(OH)D <30 ng/mL as vitamin D insufficient or deficient.
The analysis of ORs showed a significant association between vitamin D concentration and the likelihood of ADHD (OR: 2.57; 95% CI: 1.09, 6.04; P = 0.03) (Figure 3). The heterogeneity between studies was significant (Cochran's Q test, P < 0.001; I2 = 84.3%). The subgroup analysis showed that vitamin D status is not associated with ADHD (OR: –1.51; 95% CI: 0.22, 10.12; P = 0.647) in studies with no adjustment or matching for confounding variables (14, 15, 18). However, the heterogeneity was still significant (Cochran's Q test, P < 0.002; I2 = 84.6%). Analysis of studies which considered ≥1 confounding variable (20, 41) revealed that participants with vitamin D insufficiency or deficiency had a greater chance of developing ADHD than did those who were vitamin D sufficient (OR: 3.24; 95% CI: 1.05, 10.03; P < 0.041), and this heterogeneity was significant (Cochran's Q test, P = 0.001; I2 = 91.4%). The association was not significant in studies which were categorized as low quality (14, 15) (OR: 0.55; 95% CI: 0.1, 25.77; P for effect = 0.758; Cochran's Q test, P for heterogeneity = 0.002; I2 = 82.9%). However, lower serum vitamin D concentrations were related to a greater chance of developing ADHD in studies of medium (18) (OR: 6.05 ng/mL; 95% CI: 2.2, 16.63 ng/mL; P < 0.001) or high quality (20, 41) (OR: 3.24; 95% CI: 1.05, 10.03; P for effect = 0.03; Cochran's Q test, P for heterogeneity = 0.001; I2 = 91.4%). When we conducted the sensitivity analysis, the heterogeneity was decreased but remained significant (Cochran's Q test, P = 0.001; I2 = 81.0%) by removing a study done by Çelık et al. (14) from the analysis. In that study, the authors did not examine the association between vitamin D status and ADHD as the primary outcome, and the sample size was very low. Even after this exclusion, the results still suggested that those with lower concentrations of vitamin D have a greater chance of developing ADHD (OR: 3.70; 95% CI: 1.76, 7.75). The sensitivity analysis showed that none of the studies could influence the overall effect very much.
FIGURE 3.
Forest plot summarizing the association between vitamin D status and the likelihood of ADHD in case-control studies. The analysis shows that vitamin D-insufficient or -deficient children have an ∼2.57-times greater chance of developing ADHD than those with sufficient vitamin D concentrations. The analysis was conducted using a random effects model. ADHD, attention deficit hyperactivity disorder.
Meta-analysis of Prospective/Cohort Studies
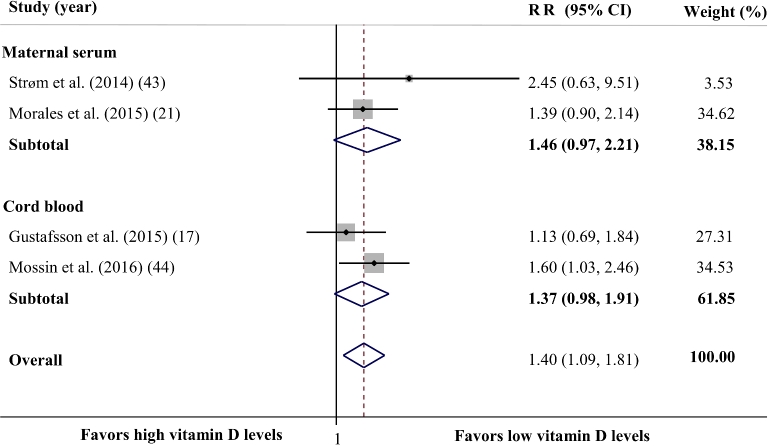
The analysis of prospective studies (17, 21, 43, 44) showed that lower maternal or cord serum vitamin D concentrations increase the risk of developing ADHD in childhood or adolescence by 40% (RR: 1.40; 95% CI: 1.09, 1.81; P = 0.009) (Figure 4). There was no evidence of between-study heterogeneity (Cochran's Q test, P = 0.538; I2 = 0.0%). Subgroup analysis based on the source of vitamin D samples demonstrated a marginally significant association between maternal serum vitamin D concentration and the risk of ADHD (RR: 1.46; 95% CI: 0.97, 2.21; P = 0.070), and the heterogeneity between studies was not significant (Cochran's Q test, P = 0.435; I2 = 0.0%). There was also a reverse but nonsignificant association between cord blood vitamin D concentrations and risk of ADHD (RR: 1.37; 95% CI: 0.98, 1.91; P = 0.067). The between-study heterogeneity was not significant in this subgroup (Cochran's Q test, P = 0.301; I2 = 6.7%).
FIGURE 4.
Forest plot illustrating the overall RR for comparison of the ADHD risk between perinatal (as well as maternal and cord blood) vitamin D status and the risk of developing ADHD in childhood and adolescence. Lower perinatal vitamin D concentrations were associated with a 40% higher risk of ADHD in later life. The analysis was conducted using a random effects model. ADHD, attention deficit hyperactivity disorder.
Sensitivity analysis revealed that excluding the study conducted by Mossin et al. (44), which assessed the association between cord blood vitamin D concentrations and childhood ADHD, rendered the overall effect nonsignificant (RR: 1.31; 95% CI: 0.96, 1.80; P = 0.089).
Discussion
The present systematic review and meta-analyses found that children and adolescents with ADHD have lower mean concentrations of serum 25(OH)D than do healthy controls. The studies which reported ORs also showed a significant association between lower vitamin D status and the likelihood of ADHD. Furthermore, the meta-analysis of prospective studies indicated that perinatal vitamin D status is significantly associated with risk of ADHD in childhood or adolescence. It should be noted that this association was sensitive because the association vanished after excluding the study conducted by Mossin et al. (44).
To the best of our knowledge, this study is the first systematic review and meta-analysis about the association between vitamin D status and ADHD. However, a number of literature reviews have mentioned the vitamin D deficiency as a risk factor for diseases of the central nervous system. In a recent narrative review, Föcker et al. (45) attempted to summarize published studies concerning the association between vitamin D status and a variety of neurological and mental disorders in children and adolescents, including autism spectrum disorder, depression, alcohol use disorders, nocturnal enuresis, psychiatric distress and disorders, and ADHD. However, they did not assess the quality of the studies included, nor did they conduct a meta-analysis. In their study, Föcker et al. used a brief range of keywords and only searched the PubMed database; therefore, they did not find 6 case-control studies (15, 16, 19, 20, 38, 39) [3 of which were based on the same data (16, 20, 38)] or 2 prospective studies (17, 21) on the association between vitamin D status and ADHD. In the present study, we used a wide range of keywords to search PubMed, Scopus, Google Scholar, and the ISI Web of Science, and found 11 case-control or cross-sectional (14–16, 18–20, 38–42) and 4 prospective studies (17, 21, 43, 44). As 3 of the case-control studies (16, 20, 38) were based on the same data, we considered only one of them (20). In total, 13 relevant investigations [9 case–control or cross-sectional studies (14, 15, 18–20, 39–42) and 4 prospective studies (17, 21, 43, 44)] were included in the present systematic review and meta-analysis. Conducting the meta-analysis on the maximum number of studies enabled us to summarize the effects and also to explore the possible sources of difference in findings of the included studies.
It is found that ADHD prevalence is higher in areas with mostly sunny weather, though it has been suggested that sunlight might protect children from the disease (46). Phototherapy and sunray therapy have been considered as strategies for treatment (47) because vitamin D is produced from sunlight on the skin (46). The role of vitamin D in ADHD and other neurological diseases is not fully understood. However, studies have demonstrated the role of vitamin D in regulating the development and function of nerve cells (27). Vitamin D is involved in the functioning of the central nervous system, and this is supported by the presence of the enzyme 25(OH)D3–1α-hydroxylase (which is responsible for the formation of the active vitamin D) as well as vitamin D receptors in the brain, mainly in the hypothalamus and dopaminergic neurons of the substantia nigra (11). These parts of the brain have been associated with the cause of ADHD (48, 49).
Vitamin D as a hormone has also been mentioned as a key regulator of the synthesis of serotonin (13), a neurotransmitter that plays critical roles in brain functions (50). It is proposed that adolescents with ADHD and reduced brain serotonin concentrations experience increased aggressive behavior (51).
At the molecular level, it has been revealed that polymorphisms in the tryptophan hydroxylase 2 (TPH2) gene and other genes related to serotonin synthesis are associated with increased susceptibility to ADHD (52). The TPH2 gene is activated by vitamin D hormone through its vitamin D response element (53). It is suggested that vitamin D status might interact with polymorphisms in the TPH2 gene, as the mice with the polymorphism are more sensitive to vitamin D deficiency in adulthood. These mice showed intense defects in cognitive function and behavior when their vitamin D intake was restricted (54, 55).
The timing of the vitamin D deficiency is also proposed to be important regarding its effect on cognitive and behavioral functions. Insufficient vitamin D concentrations during early life might accelerate the risk of cognitive dysfunction and brain morphology defects (13). Studies have also revealed that vitamin D deficiency during pregnancy leads to a 28% increase in the size of brain lateral ventricles in neonates (56). One of the clinical factors observed in ADHD is the enlargement of the lateral ventricles (57).
Vitamin D status might also affect the dopamine system through its effect on the expression of tyrosine hydroxylase, the rate-limiting enzyme for synthesis of dopamine (12). The polymorphism in this enzyme's gene has been associated with ADHD in animal models (58). Dopamine, as well as other neurotransmitters, including serotonin and opioids, is involved in the controlling of mood (59).
A number of points should be considered when interpreting our results. First, we found that serum vitamin D concentrations in children and adolescents with ADHD were ∼6.93 ng/mL lower than in the healthy controls. However, although the difference was statistically significant, it might not be of great clinical importance. Furthermore, our analysis of both case-control and cross-sectional studies revealed that lower vitamin D concentrations are associated with ADHD. A causal association cannot be inferred from these types of studies because the deficiency might occur as a result of lifestyle change experienced by children with ADHD. It should be noted that the majority of case-control studies had selected their participants from clinics; therefore, their results cannot be extrapolated to the general population. In fact, only 2 studies tried to recruit their participants from the general population (38, 39). In the present review, we could include only 4 prospective studies, which were conducted in Europe, East Asia, and the Middle East; therefore, it is not clear if the association exists in other regions.
The meta-analysis of prospective studies also showed an inverse association between vitamin D concentration and ADHD; however, the summary estimate was sensitive to the investigation done by Mossin et al. (44). Therefore, this result should be interpreted with caution. In addition, although the majority of the included studies adjusted the association for age and/or gender as confounding variables (17, 18, 38, 40–43), the other variables, like race or skin color, season of vitamin D sampling, sunlight exposure, latitude, dietary habits, and socioeconomic status, were proposed to affect the serum vitamin D concentration (60) and therefore should be considered while examining the association. Not adjusting for the other possible confounders might affect the overall associations because the difference in vitamin D concentrations found between children with and without ADHD might be because of their difference in race, the season of blood sampling or differences in lifestyle habits. In the present study, we conducted a subgroup analysis to determine if the association is different in studies with and without adjustment for possible confounding variables because it might be an important source of between-study heterogeneity in meta-analyses of observational studies (61). It should also be considered that the included case-control studies used different cut-offs to define suboptimal concentrations of vitamin D. Furthermore, vitamin D deficiency and insufficiency are currently diagnosed using criteria developed based on studies examining vitamin D status in association with bone health (62); therefore, the cut-off values might be different when considering vitamin D in association with mental health.
Based on the points mentioned above, future prospective cohort studies with larger sample sizes and from different populations, and even population-based intervention studies that consider the maximum number of possible confounders, might help to better elucidate the causal association between vitamin D status and the risk of developing ADHD.
In conclusion, in the present systematic review and meta-analysis, we found modest but significant lower serum vitamin D concentrations in children and adolescents with ADHD compared with healthy control subjects. Moreover, lower perinatal and childhood vitamin D status were both associated with a higher likelihood of developing ADHD. It should be noted that the former relation should be considered with caution because it was sensitive to one of the prospective studies included in the analysis. Future cohort studies and population-based trials are highly recommended to confirm our results. Because vitamin D deficiency is highly prevalent in children (62), based on the current findings, community-based interventions trying to increase sun exposure and vitamin D intake in early life should be considered with high priority.
Acknowledgments
All authors read and approved the final manuscript.
Supported by the Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences.
Abbreviations
- ADHD
attention deficit hyperactivity disorder
- TPH2
tryptophan hydroxylase 2 gene
- WMD
weighted mean difference
- 25(OH)D
25-hydroxyvitamin D
References
- 1. Subcommittee on Attention-Deficit/Hyperactivity D, Steering Committee on Quality I, Management , Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG. et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128(5):1007–22. doi: 10.1542/peds.2011-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiat 2007. [DOI] [PubMed] [Google Scholar]
- 3. Swanson J, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, Vitiello B, Jensen P, Steinhoff K, Lerner M. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of Children with ADHD (MTA) Part II: Supporting details. J Atten Disord 2008;12(1):15–43. [DOI] [PubMed] [Google Scholar]
- 4. ATTENTION-DEFICIT SO ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011:peds 2011–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein RG, Mannuzza S, Olazagasti MAR, Roizen E, Hutchison JA, Lashua EC, Castellanos FX. Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Arch Gen Psychiat 2012;69(12):1295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005;57(11):1215–20. [DOI] [PubMed] [Google Scholar]
- 7. Hawkey E, Nigg JT. Omega-3 fatty acid and ADHD: blood level analysis and meta-analytic extension of supplementation trials. Clin Psychol Rev 2014;34(6):496–505. doi: 10.1016/j.cpr.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rucklidge JJ, Frampton CM, Gorman B, Boggis A. Vitamin-mineral treatment of attention-deficit hyperactivity disorder in adults: double-blind randomised placebo-controlled trial. Br J Psychiatry 2014;204:306–15. doi: 10.1192/bjp.bp.113.132126. [DOI] [PubMed] [Google Scholar]
- 9. Nigg JT, Lewis K, Edinger T, Falk M. Meta-analysis of attention-deficit/hyperactivity disorder or attention-deficit/hyperactivity disorder symptoms, restriction diet, and synthetic food color additives. J Am Acad Child Adolesc Psychiatry 2012;51(1):86–97. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heilskov Rytter MJ, Andersen LBB, Houmann T, Bilenberg N, Hvolby A, Mølgaard C, Michaelsen KF, Lauritzen L. Diet in the treatment of ADHD in children—a systematic review of the literature. Nord J Psychiatry 2015;69(1):1–18. [DOI] [PubMed] [Google Scholar]
- 11. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat 2005;29(1):21–30. [DOI] [PubMed] [Google Scholar]
- 12. Cui X, Pertile R, Liu P, Eyles D. Vitamin D regulates tyrosine hydroxylase expression: N-cadherin a possible mediator. Neuroscience 2015;304:90–100. [DOI] [PubMed] [Google Scholar]
- 13. Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J 2015;29(6):2207–22. doi: 10.1096/fj.14-268342. [DOI] [PubMed] [Google Scholar]
- 14. Çelık G, Taş D, Tahıroğlu A, Avci A, Yüksel B, Çam P. Vitamin D deficiency in obsessive-compulsive disorder patients with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: a case control study. Noro Psikiyatr Ars 2016;53(1):31–4. doi: 10.5152/npa.2015.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharif MR, Madani M, Tabatabaei F, Tabatabaee Z. The relationship between serum vitamin D level and attention deficit hyperactivity disorder. Iran J Child Neurol 2015;9(4):48–53. [PMC free article] [PubMed] [Google Scholar]
- 16. Kamal M, Bener A, Ehlayel MS. Is high prevalence of vitamin D deficiency a correlate for attention deficit hyperactivity disorder? Atten Defic Hyperact Disord 2014;6(2):73–8. doi: 10.1007/s12402-014-0130-5. [DOI] [PubMed] [Google Scholar]
- 17. Gustafsson P, Rylander L, Lindh CH, Jönsson BAG, Ode A, Olofsson P, Ivarsson SA, Rignell-Hydbom A, Haglund N, Källén K. Vitamin D status at birth and future risk of attention deficit/hyperactivity disorder (ADHD). PloS One 2015;10(10). doi: 10.1371/journal.pone.0140164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shang-Guan LL, Zhao YR. [Serum levels of 25-hydroxyvitamin D in children with attention deficit hyperactivity disorder]. Zhongguo Dang Dai Er Ke Za Zhi 2015;17(8):837–40. [PubMed] [Google Scholar]
- 19. Goksugur SB, Tufan AE, Semiz M, Gunes C, Bekdas M, Tosun M, Demircioglu F. Vitamin D status in children with attention-deficit-hyperactivity disorder. Pediatr Int 2014;56(4):515–9. doi: 10.1111/ped.12286. [DOI] [PubMed] [Google Scholar]
- 20. Bener A, Kamal M. Predict attention deficit hyperactivity disorder? Evidence-based medicine. Glob J Health Sci 2014;6(2):47–57. doi: 10.5539/gjhs.v6n2p47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morales E, Julvez J, Torrent M, Ballester F, Rodríguez-Bernal CL, Andiarena A, Vegas O, Castilla AM, Rodriguez-Dehli C, Tardón A. et al. Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology 2015;26(4):458–65. doi: 10.1097/EDE.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 22. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. Internet: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm(acc-essed June2017.
- 23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Smith GD, Altman D. Systematic reviews in health care: meta-analysis in context. New York: John Wiley & Sons; 2008. [Google Scholar]
- 25. Wrzosek M, Lukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piatkiewicz P, Radziwon-Zaleska M, Wojnar M, Nowicka G. Vitamin D and the central nervous system. Pharmacol Rep 2013;65(2):271–8. [DOI] [PubMed] [Google Scholar]
- 26. Le Roy O C Rebollo G MJ, Moraga M F, Díaz Sm X, Castillo-Durán C. Nutrition of children with selected neurological illnesses. An update. Rev Chil Pediatr 2010;81(2):103–13. doi: 10.4067/S0370-41062010000200002. [Google Scholar]
- 27. Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, McGrath JJ, Burne THJ. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 2009;34(Suppl 1):S247–57. doi: 10.1016/j.psyneuen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 28. Mikirova NA, Casciari JJ, Hunninghake RE. The orthomolecular correction of metabolic imbalances found in attention deficit hyperactivity disorder: a retrospective analysis in an outpatient clinic. J Orthomol Med 2013;28(3):101–10. [Google Scholar]
- 29. Humble MB, Gustafsson S, Bejerot S. Low serum levels of 25-hydroxyvitamin D (25-OHD) among psychiatric out-patients in Sweden: relations with season, age, ethnic origin and psychiatric diagnosis. J Steroid Biochem Mol Biol 2010;121(1–2):467–70. doi: 10.1016/j.jsbmb.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 30. Villagomez A, Ramtekkar U. Iron, magnesium, vitamin D, and zinc deficiencies in children presenting with symptoms of attention-deficit/hyperactivity disorder. Children (Basel) 2014;1(3):261–79. doi: 10.3390/children1030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rylander M, Verhulst S. Vitamin D insufficiency in psychiatric inpatients. J Psychiatr Prac 2013;19(4):296–300. doi: 10.1097/01.pra.0000432599.24761.c1. [DOI] [PubMed] [Google Scholar]
- 32. Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis 2013;37(1):147–71. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 33. McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 2008;22(4):982–1001. doi: 10.1096/fj.07–9326rev. [DOI] [PubMed] [Google Scholar]
- 34. Rucklidge JJ, Johnstone J, Gorman B, Boggis A, Frampton CM. Moderators of treatment response in adults with ADHD treated with a vitamin-mineral supplement. Prog Neuropsychopharmacol Biol Psychiatry 2014;50:163–71. doi: 10.1016/j.pnpbp.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 35. Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25 (OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinatal Epidemiol 2014;28(5):434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008;62(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tolppanen AM, Sayers A, Fraser WD, Lewis G, Zammit S, Lawlor DA. The association of 25-hydroxyvitamin D3 and D2 with behavioural problems in childhood. PloS One 2012;7(7). doi: 10.1371/journal.pone.0040097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bener A, Kamal M, Bener H, Bhugra D. Higher prevalence of iron deficiency as strong predictor of attention deficit hyperactivity disorder in children. Ann Med Health Sci Res 2014;4(3):291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meyer T, Becker A, Sundermann J, Rothenberger A, Herrmann-Lingen C. Attention deficit-hyperactivity disorder is associated with reduced blood pressure and serum vitamin D levels: results from the nationwide German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Eur Child Adolesc Psychiatry 2016:1–11. [DOI] [PubMed] [Google Scholar]
- 40. Bala KA, Doğan M, Kaba S, Mutluer T, Aslan O, Doğan SZ. Hormone disorder and vitamin deficiency in attention deficit hyperactivity disorder (ADHD) and autism spectrum disorders (ASDs). J Pediatr Endocrinol Metab 2016;29(9):1077–82. [DOI] [PubMed] [Google Scholar]
- 41. Avcil S, Uysal P, Yilmaz M, Erge D, Demirkaya SK, Eren E. Vitamin D Deficiency and a blunted parathyroid hormone response in children with attention-deficit/hyperactivity disorder. Clin Lab 2017;63(3):435. [DOI] [PubMed] [Google Scholar]
- 42. Garipardic M, Doğan M, Bala KA, Mutluer T, Kaba S, Aslan O, Üstyol L. Association of attention deficit hyperactivity disorder and autism spectrum disorders with mean platelet volume and vitamin D. Med Sci Monit 2017;23:1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strøm M, Halldorsson TI, Hansen S, Granström C, Maslova E, Petersen SB, Cohen AS, Olsen SF. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab 2014;64(3–4):254–61. [DOI] [PubMed] [Google Scholar]
- 44. Mossin MH, Aaby JB, Dalgård C, Lykkedegn S, Christesen HT, Bilenberg N. Inverse associations between cord vitamin D and attention deficit hyperactivity disorder symptoms: a child cohort study. Austr N Z J Psychiatry 2016:0004867416670013. [DOI] [PubMed] [Google Scholar]
- 45. Föcker M, Antel J, Ring S, Hahn D, Ö Kanal, Öztürk D, Hebebrand J, Libuda L. Vitamin D and mental health in children and adolescents. Eur Child Adolesc Psychiatry 2017:1–24. [DOI] [PubMed] [Google Scholar]
- 46. Arns M, van der Heijden KB, Arnold LE, Kenemans JL. Geographic variation in the prevalence of attention-deficit/hyperactivity disorder: the sunny perspective. Biol Psychiatry 2013;74(8):585–90. doi: 10.1016/j.biopsych.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47. Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD. An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry 2006;67(10):1527–35. [DOI] [PubMed] [Google Scholar]
- 48. Romanos M, Weise D, Schliesser M, Schecklmann M, Loffler J, Warnke A, Gerlach M, Classen J, Mehler-Wex C. Structural abnormality of the substantia nigra in children with attention-deficit hyperactivity disorder. J Psychiatry Neurosci 2010;35(1):55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ma L, Chen YH, Chen H, Liu YY, Wang YX. The function of hypothalamus-pituitary-adrenal axis in children with ADHD. Brain Res 2011;1368:159–62. doi: 10.1016/j.brainres.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 50. Lesch KP, Araragi N, Waider J, van den Hove D, Gutknecht L. Targeting brain serotonin synthesis: insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos Trans R Soc Lond B Biol Sci 2012;367(1601):2426–43. doi: 10.1098/rstb.2012.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zepf FD, Stadler C, Demisch L, Schmitt M, Landgraf M, Poustka F. Serotonergic functioning and trait-impulsivity in attention-deficit/hyperactivity-disordered boys (ADHD): influence of rapid tryptophan depletion. Hum Psychopharmacol 2008;23(1):43–51. [DOI] [PubMed] [Google Scholar]
- 52. Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin N Am 2010;33(1):159–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J 2014;28(6):2398–413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- 54. Zhang X, Beaulieu J-M, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004;305(5681):217. [DOI] [PubMed] [Google Scholar]
- 55. Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res 2013;241:120–31. [DOI] [PubMed] [Google Scholar]
- 56. Annweiler C, Montero-Odasso M, Hachinski V, Seshadri S, Bartha R, Beauchet O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol Nutr Food Res 2013;57(2):267–76. [DOI] [PubMed] [Google Scholar]
- 57. Gilmore JH, Smith LC, Wolfe HM, Hertzberg BS, Smith JK, Chescheir NC, Evans DD, Kang C, Hamer RM, Lin W. et al. Prenatal mild ventriculomegaly predicts abnormal development of the neonatal brain. Biol Psychiatry 2008;64(12):1069–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kubinyi E, Vas J, Hejjas K, Ronai Z, Bruder I, Turcsan B, Sasvari-Szekely M, Miklosi A. Polymorphism in the tyrosine hydroxylase (TH) gene is associated with activity-impulsivity in German shepherd dogs. PloS One 2012;7(1):e30271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blum K, Chen AL, Braverman ER, Comings DE, Chen TJ, Arcuri V, Blum SH, Downs BW, Waite RL, Notaro A. et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr Dis Treat 2008;4(5):893–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, El-Hajj Fuleihan G, Josse RG, Lips P, Morales-Torres J. et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20(11):1807–20. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 61. Voils CI, Crandell JL, Chang Y, Leeman J, Sandelowski M. Combining adjusted and unadjusted findings in mixed research synthesis. J Eval Clin Pract 2011;17(3):429–34. doi: 10.1111/j.1365-2753.2010.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug, Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine S Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122(2):398–417. doi: 10.1542/peds.2007-1894. [DOI] [PubMed] [Google Scholar]