Abstract
Purpose
We compared oncologic outcomes of patients with upper tract urothelial carcinoma (UTUC) who underwent open nephroureterectomy (ONU) or laparoscopic nephroureterectomy (LNU).
Materials and Methods
Consecutive cases of ONU and LNU between 2000 and 2012 at five participating institutions were included in this retrospective analysis. Clinical characteristics and pathologic outcomes were compared between the two surgical approaches. The influence of the type of surgical approach on intravesical recurrence-free survival (IVRFS), progression-free survival (PFS), cancer-specific survival (CSS), and overall survival (OS) was analyzed using the Kaplan-Meier method and differences were assessed with the log-rank test. Predictors of IVRFS, PFS, CSS, and OS were also analyzed with a multivariable Cox regression model.
Results
A total of 1,521 patients with UTUC were eligible for the present study (ONU, 906; LNU, 615). The estimated 5-year IVRFS (57.8 vs. 51.0%, p=0.010), CSS (80.4 vs. 76.4%, p=0.032), and OS (75.8 vs. 71.4%, p=0.026) rates were significantly different between the two groups in favor of LNU. Moreover, in patients with locally advanced disease (pT3/pT4), the LNU group showed better 5-year IVRFS (62.9 vs. 54.1%, p=0.038), CSS (64.3 vs. 56.9%, p=0.022), and OS (60.4 vs. 53.1%, p=0.018) rates than the ONU group. Multivariable Cox regression analyses showed that type of surgical approach was independently associated with IVRFS, but was not related to PFS, CSS, and OS.
Conclusion
Our findings indicate that LNU provided better oncologic control of IVRFS, CSS, and OS compared with ONU for the management of patients with UTUC.
Keywords: Transitional cell cancer, Laparoscopy, Malignant disease, Survival
Introduction
Upper tract urothelial carcinoma (UTUC) arising from the urothelium that lines the ureter and renal pelvis is a rare malignancy and accounts for only 5% of all urothelial carcinomas [1,2]. Although kidney-sparing surgery can be carried out in selected patients with low-risk UTUC, radical nephroureterectomy (RNU) with bladder cuff excision is considered the current standard of management for the majority of non-metastatic UTUCs [3]. The conventional surgical approach for RNU has been open nephroureterectomy (ONU). Recently, a shift toward minimally invasive treatments has emerged, and laparoscopic nephroureterectomy (LNU) has increasingly been used as an accepted alternative to ONU. LNU has been associated with reduced blood loss, faster recovery, and shorter hospital stay compared with ONU [4]. However, there remain some concerns about oncologic safety following LNU because of a higher risk of recurrence due to the high-pressure environment of the pneumoperitoneum, and the oncologic outcomes between ONU and LNU remain controversial.
In recent years, numerous studies have been conducted to compare the oncologic efficacy of ONU and LNU in patients with UTUC [5-13]. The comparative outcomes of these studies were various, without a definitely accepted conclusion on which surgical approach was more beneficial for patients with UTUC. Although a large number of studies in patients with UTUC undergoing RNU have shown no difference in recurrence-free survival (RFS), cancer-specific survival (CSS), or overall survival (OS) based on the type of surgical approach [5-10], some studies have shown an association of LNU with inferior CSS and OS in locally advanced UTUC [5,13]. In contrast, other studies reported that LNU could improve the CSS [11,12,14]. Thus, it is not fully established whether LNU is an effective and safe substitute for ONU in the treatment of UTUC. Furthermore, the results of previous retrospective studies were limited by the small number of patients and single-institution experience.
Knowledge of the efficacy of the two different surgical approaches is essential not only to guide patient counseling and clinical decision making, but also to develop clinical practice guidelines. The aim of this study was to compare the oncologic outcomes between ONU and LNU approaches in a large population obtained from a multicenter collaborative group. We also evaluated predictive factors associated with oncologic outcomes.
Materials and Methods
1. Study population
In this institutional review board–approved study, medical records of patients with non-metastatic UTUC undergoing ONU or LNU at five tertiary medical centers in the Urothelial Cancer-Advanced Research and Treatment Study Group between 2000 and 2012 were retrospectively reviewed. Patients who had previous or concomitant radical cystectomy, a bilateral tumor, and those who were treated with neoadjuvant chemotherapy were excluded from this study. Clinicopathologic variables recorded included age at surgery, sex, body mass index, American Society of Anesthesiologists score, previous bladder cancer, concomitant bladder cancer, tumor location, pathological tumor stage, tumor grade, the presence of lymphovascular invasion (LVI) or concomitant carcinoma in situ (CIS), lymph node status, receipt of adjuvant chemotherapy, follow-up, and oncologic outcomes. Tumor staging was assessed according to the 2010 American Joint Committee on Cancer/Union Internationale Contre le Cancer (Tumor-Node-Metastasis classification) [15]. Tumor grading was determined based on the 1998 World Health Organization/International Society of Urologic Pathology consensus classification [16]. LVI was defined as the presence of tumor cells within an endothelium-lined space without underlying muscular walls [17].
2. Surgical procedures
The indications for ONU or LNU were mainly based on the surgeon’s discretion and the patient's informed consent after counseling regarding the procedures. If LNU was converted to ONU, the patients were only included in ONU group. The techniques of ONU and LNU have previously been reported [13,18]. ONU was performed according to standard criteria through a flank incision combined with a lower abdominal incision (Gibson, Pfannenstiel, or median) for the distal ureter and the bladder. A bladder cuff excision was performed through either an intravesical or extravesical approach. LNU was also performed according to standard criteria using either the transperitoneal or retroperitoneal approach. Regional or extended lymphadenectomy was performed in patients with suspicious lymphadenopathies on preoperative imaging or intraoperative examination regardless of the open or laparoscopic method.
3. Follow-up regimen
Although postoperative follow-up was not standardized because of the retrospective nature of this study, patients were generally followed up every 3-4 months during the first 2 years after surgery, every 6 months for the next 2-3 years, and annually thereafter. Patients underwent physical examinations with laboratory tests, urinary cytology, cystoscopy, chest radiography, and computed tomography scans for abdomen and pelvis at each visit. Bone scintigraphy scan or chest computed tomography was performed when clinically indicated. The intravesical recurrence-free survival (IVRFS) was defined as time from RNU to tumor relapse in the bladder. The progression-free survival (PFS) was defined as time from RNU to local recurrence (tumor relapse in operative field) or distant metastasis. The CSS and OS were defined as time from RNU to death due to UTUC and due to any cause, respectively.
4. Statistical analyses
Median and interquartile range (IQR) were used to describe quantitative variables, and frequency and percentage were used for qualitative variables. Clinical characteristics and pathological outcomes were compared between two surgical approaches (ONU vs. LNU). The Shapiro-Wilk normality test was used to investigate the normal distribution of continuous variables. Continuous variables were compared using the Mann-Whitney U test whereas categorical variables were compared using the chi-square test. The influence of the type of surgical approach on IVRFS, PFS, CSS, and OS in the entire study group and pathological T stage subgroups was analyzed using the Kaplan-Meier method and differences were assessed with the log-rank test. Multivariable Cox proportional hazard models were used to evaluate the associations between risk factors of interest and intravesical tumor recurrence, progression, death from UTUC, and death from all causes. Statistical significance in this study was set at p < 0.05. All reported p-values are twosided. Statistical analyses were performed with SPSS for Windows, ver. 21.0 (IBM Corp., Armonk, NY).
5. Ethical statement
The institutional review board of each study site approved the study protocol. The study protocol was conducted according to the ethical guidelines of the World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects. The requirement for written informed consent was waived by the institutional review board.
Results
Overall, 1,521 patients were included in the present study. Of these, 906 (59.6%) underwent ONU and 615 (40.4%) underwent LNU. Median age was 65 years (IQR, 57 to 72) and 74.1% (1,127/1,521) of the patients were male. Median follow-up duration was 54.9 months (IQR, 32.7 to 89.7). The clinical and pathological details for each of the groups are described in Table 1. Relative to the ONU group, patients in the LNU group had significantly higher body mass index (p=0.003), higher American Society of Anesthesiologists score (p=0.017), less LVI (p=0.010), were less likely to have lymph node metastases (p < 0.001), and had a shorter followup duration (p < 0.001).
Table 1.
Descriptive characteristics of patients treated with either ONU or LNU for upper tract urothelial carcinoma
| Characteristic | All patients (n=1,521) | ONU (n=906, 59.6%) | LNU (n=615, 40.4%) | p-value |
|---|---|---|---|---|
| Age (yr) | 65.0 (57.0-72.0) | 65.0 (57.0-72.0) | 64.0 (57.0-72.0) | 0.627 |
| Male sex | 1,127 (74.1) | 665 (73.4) | 462 (75.1) | 0.452 |
| BMI (kg/m2) | 24.3 (22.2-26.1) | 24.1 (22.0-26.0) | 24.5 (22.7-26.5) | 0.003 |
| ASA score | ||||
| 1 | 388 (25.5) | 254 (28.0) | 134 (21.8) | 0.017 |
| 2 | 1,004 (66.0) | 565 (62.4) | 439 (71.4) | |
| ≥ 3 | 93 (6.1) | 56 (6.2) | 37 (6.0) | |
| Missing/Unknown | 36 (2.4) | 31 (3.4) | 5 (0.8) | |
| Previous bladder cancer | 180 (11.8) | 118 (13.0) | 62 (10.1) | 0.081 |
| Concomitant bladder cancer | 107 (7.0) | 64 (7.1) | 43 (7.0) | 0.957 |
| Tumor laterality | ||||
| Right | 691 (45.4) | 418 (46.1) | 273 (44.4) | 0.502 |
| Left | 830 (54.6) | 488 (53.9) | 342 (55.6) | |
| Tumor location | ||||
| Renal pelvis | 682 (44.8) | 398 (43.9) | 284 (46.2) | 0.073 |
| Ureter | 565 (37.1) | 328 (36.2) | 237 (38.5) | |
| Both renal pelvis and ureter | 274 (18.0) | 180 (19.9) | 94 (15.3) | |
| Pathological T stage | ||||
| pTis/pTa | 235 (15.5) | 143 (15.8) | 92 (15.0) | 0.361 |
| pT1 | 404 (26.6) | 234 (25.8) | 170 (27.6) | |
| pT2 | 255 (16.8) | 143 (15.8) | 112 (18.2) | |
| pT3 | 592 (38.9) | 358 (39.5) | 234 (38.0) | |
| pT4 | 35 (2.3) | 28 (3.1) | 7 (1.1) | |
| Tumor grade | ||||
| Low grade | 485 (31.9) | 279 (30.8) | 206 (33.5) | 0.239 |
| High grade | 993 (65.3) | 603 (66.6) | 390 (63.4) | |
| Missing/Unknown | 43 (2.8) | 24 (2.6) | 19 (3.1) | |
| Concomitant LVI | 332 (21.8) | 218 (24.1) | 114 (18.5) | 0.010 |
| Concomitant CIS | 214 (14.1) | 124 (13.7) | 90 (14.6) | 0.602 |
| Pathological N stage | ||||
| pNx | 773 (50.8) | 490 (54.1) | 283 (46.0) | < 0.001 |
| pN0 | 631 (41.5) | 329 (36.3) | 302 (49.1) | |
| ≥ pN1 | 117 (7.7) | 87 (9.6) | 30 (4.9) | |
| Adjuvant chemotherapy | 340 (22.4) | 211 (23.3) | 129 (21.0) | 0.288 |
| Length of follow-up (mo) | 54.9 (32.7-89.7) | 62.0 (31.3-110.7) | 48.9 (33.5-72.7) | < 0.001 |
Values are presented as number (%). The Shapiro-Wilk normality test was used to investigate the normal distribution of continuous variables. Continuous and non-normally distributed variables are presented as medians with interquartile ranges.
ONU, open nephroureterectomy; LNU, laparoscopic nephroureterectomy; BMI, body mass index; ASA, American Society of Anesthesiologists; LVI, lymphovascular invasion; CIS, carcinoma in situ.
Survival outcomes are summarized in Table 2. During follow-up, there were 631 (41.5%) intravesical recurrences, including 396 (43.7%) in the ONU group and 235 (38.2%) in the LNU group. The 5-year IVRFS estimates were 51.0% and 57.7% for patients treated with ONU or LNU, respectively, and this difference was statistically significant (p=0.010) (Fig. 1A). The total number of patients showing progression in ONU and LNU groups was 272 (30.0%) and 155 (25.2%), respectively. The 5-year PFS estimates for ONU and LNU were 68.9% and 73.9% respectively, which was not significantly different (p=0.079) (Fig. 1B). Overall, 453 (29.8%) patients died during the study period, including 307 (33.9%) in the ONU group and 146 (23.7%) in the LNU group, and 342 UTUC-related deaths occurred (229 in ONU group and 113 in LNU group). The 5-year CSS estimates and the 5-year OS were 76.4% and 71.4% respectively for patients treated with ONU versus 80.4% and 75.8% for patients treated with LNU. The LNU group showed better 5-year CSS (p=0.032) (Fig. 1C) and OS (p=0.026) (Fig. 1D) than the ONU group.
Table 2.
Survival outcomes after open or laparoscopic nephroureterectomy
| All patients (n=1,521) | ONU (n=906) | LNU (n=615) | p-value | |
|---|---|---|---|---|
| IVRFS | ||||
| No. of events (%) | 631 (41.5) | 396 (43.7) | 235 (38.2) | 0.033 |
| Time to recurrence (mo) | 8.5 (4.8-15.9) | 8.1 (4.7-16.0) | 9.5 (5.1-15.8) | 0.277 |
| Estimated 5-year IVRFS (%) | 53.8 | 51.0 | 57.7 | 0.010 |
| PFS | ||||
| No. of events (%) | 427 (28.1) | 272 (30.0) | 155 (25.2) | 0.040 |
| Time to progression (mo) | 11.1 (5.6-24.2) | 11.1 (4.9-26.2) | 11.0 (6.8-21.2) | 0.780 |
| Estimated 5-year PFS (%) | 70.8 | 68.9 | 73.9 | 0.079 |
| CSS | ||||
| No. of events (%) | 342 (22.5) | 229 (25.3) | 113 (18.4) | 0.002 |
| Time to cancer-specific death (mo) | 24.8 (14.2-40.3) | 24.2 (13.0-41.9) | 25.5 (15.5-38.9) | 0.625 |
| Estimated 5-year CSS (%) | 78 | 76.4 | 80.4 | 0.032 |
| OS | ||||
| No. of events (%) | 453 (29.8) | 307 (33.9) | 146 (23.7) | < 0.001 |
| Time to deaths from any cause (mo) | 27.1 (14.5-45.4) | 27.5 (13.9-53.8) | 26.7 (15.5-40.1) | 0.504 |
| Estimated 5-year OS (%) | 73.1 | 71.4 | 75.8 | 0.026 |
The Shapiro-Wilk normality test was used to investigate the normal distribution of continuous variables. Continuous and non-normally distributed variables are presented as medians with interquartile ranges. ONU, open nephroureterectomy; LNU, laparoscopic nephroureterectomy; IVRFS, intravesical recurrence-free survival; PFS, progression-free survival; CSS, cancer-specific survival; OS, overall survival.
Fig. 1.
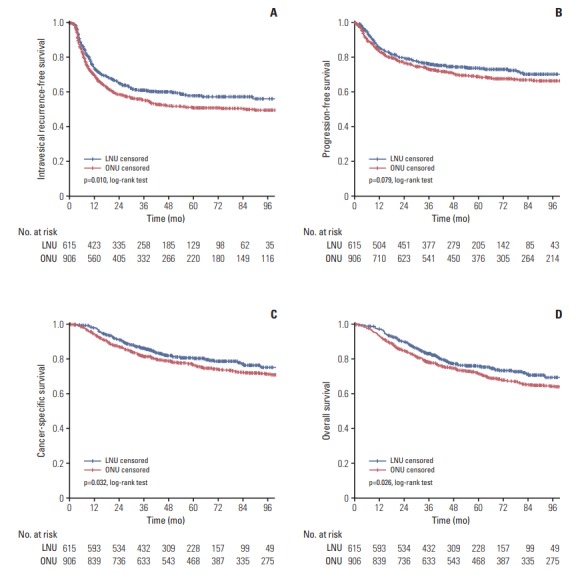
Cumulative survival of 1,521 patients after radical nephroureterectomy for upper tract urothelial carcinoma, stratified by surgical approach. (A) Intravesical recurrence-free survival. (B) Progression-free survival. (C) Cancer-specific survival. (D) Overall survival. LNU, laparoscopic nephroureterectomy; ONU, open nephroureterectomy.
When patients were stratified by pathological T stage, the 5-year IVRFS (p=0.078), PFS (p=0.775), CSS (p=0.994), and OS (p=0.859) of the two groups were similar for patients with organ-confined disease (pTis/pTa/pT1/T2) (Fig. 2). In contrast, 5-year IVRFS (p=0.038) (Fig. 3A), CSS (p=0.022) (Fig. 3C), and OS (p=0.018) (Fig. 3D) were significantly different between the two groups in favor of LNU for patients with locally advanced disease (pT3/pT4). No significant difference in the 5-year PFS (p=0.067) was observed when comparing the two groups for patients with locally advanced disease (pT3/pT4) (Fig. 3B).
Fig. 2.
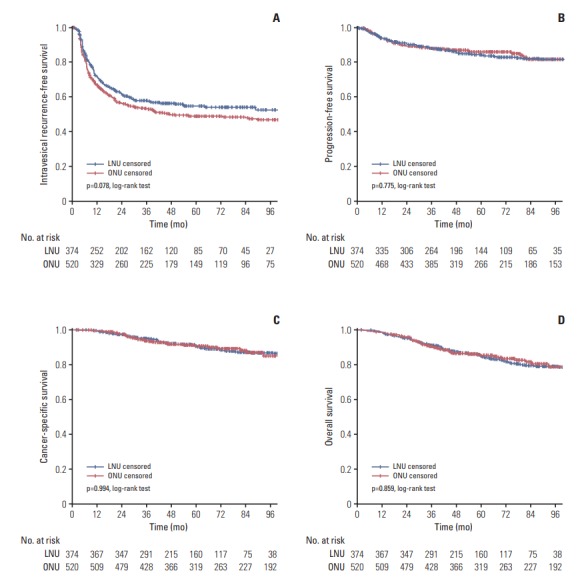
Cumulative survival of 894 patients with organ-confined disease (pTis/pTa/pT1/T2) after radical nephroureterectomy for upper tract urothelial carcinoma, stratified by surgical approach. (A) Intravesical recurrence-free survival. (B) Progression-free survival. (C) Cancer-specific survival. (D) Overall survival. LNU, laparoscopic nephroureterectomy; ONU, open nephroureterectomy.
Fig. 3.
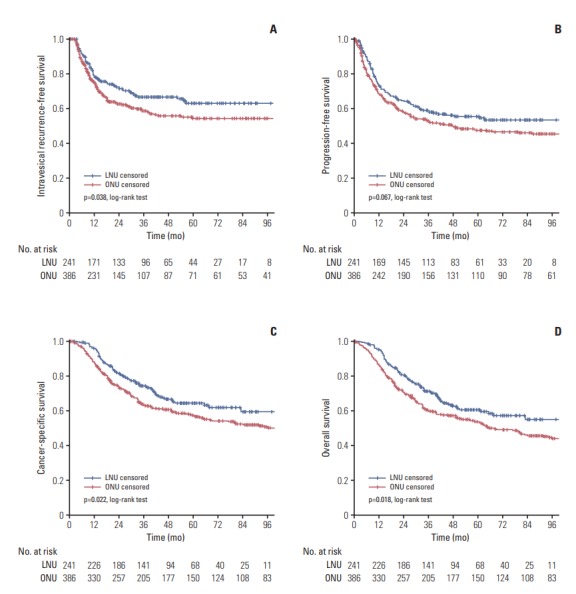
Cumulative survival of 627 patients with locally advanced disease (pT3/pT4) after radical nephroureterectomy for upper tract urothelial carcinoma, stratified by surgical approach. (A) Intravesical recurrence-free survival. (B) Progressionfree survival. (C) Cancer-specific survival. (D) Overall survival. LNU, laparoscopic nephroureterectomy; ONU, open nephroureterectomy
The results of the multivariable Cox regression analysis are shown in Table 3. A history of previous bladder tumor and presence of concomitant bladder tumor were independent predictive factors of intravesical tumor recurrence, progression, death from UTUC, and all-cause death. Age, pathological T stage, tumor grade, presence of concomitant LVI, presence of concomitant CIS, and pathological N stage were significantly associated with progression, death from UTUC, and all-cause death. The surgical approach was revealed as an independent prognostic factor for intravesical tumor recurrence, but was not related to progression, death from UTUC, and all-cause death.
Table 3.
Multivariable Cox proportional hazard regression analyses to predict intravesical tumor recurrence, progression, death from upper tract urothelial carcinoma, and all-cause death in 1,521 patients with upper tract urothelial carcinoma treated with radical nephroureterectomy
| Characteristic | IVRFS |
PFS |
CSS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (continuous) | 1.00 | 0.99-1.01 | 0.749 | 1.02 | 1.00-1.03 | 0.007 | 1.03 | 1.02-1.04 | < 0.001 | 1.04 | 1.03-1.05 | < 0.001 |
| Sex | ||||||||||||
| Male | Reference | Reference | Reference | Reference | ||||||||
| Female | 0.87 | 0.71-1.05 | 0.144 | 1.19 | 0.96-1.48 | 0.116 | 1.09 | 0.85-1.40 | 0.477 | 1.00 | 0.80-1.24 | 0.977 |
| Body mass index (continuous) | 0.98 | 0.95-1.01 | 0.132 | 0.98 | 0.94-1.01 | 0.119 | 0.99 | 0.95-1.02 | 0.435 | 0.97 | 0.94-1.00 | 0.083 |
| ASA | ||||||||||||
| 1 | Reference | Reference | Reference | Reference | ||||||||
| 2 | 1.07 | 0.88-1.30 | 0.518 | 0.82 | 0.65-1.03 | 0.092 | 0.96 | 0.73-1.26 | 0.754 | 0.99 | 0.78-1.27 | 0.951 |
| ≥ 3 | 1.22 | 0.85-1.75 | 0.273 | 0.95 | 0.61-1.46 | 0.800 | 1.15 | 0.72-1.85 | 0.566 | 1.30 | 0.87-1.94 | 0.199 |
| Surgical approach | ||||||||||||
| ONU | Reference | Reference | Reference | Reference | ||||||||
| LNU | 0.82 | 0.69-0.97 | 0.021 | 0.99 | 0.80-1.22 | 0.935 | 0.94 | 0.74-1.19 | 0.612 | 0.95 | 0.77-1.17 | 0.602 |
| Previous bladder cancer | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.81 | 1.44-2.29 | < 0.001 | 1.51 | 1.13-2.02 | 0.005 | 1.74 | 1.26-2.40 | 0.001 | 1.43 | 1.06-1.92 | 0.018 |
| Concomitant bladder cancer | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.94 | 1.46-2.57 | < 0.001 | 1.66 | 1.17-2.36 | 0.005 | 1.78 | 1.22-2.61 | 0.003 | 1.66 | 1.18-2.32 | 0.003 |
| Tumor laterality | ||||||||||||
| Right | Reference | Reference | Reference | Reference | ||||||||
| Left | 0.95 | 0.81-1.11 | 0.485 | 0.93 | 0.76-1.13 | 0.439 | 0.95 | 0.76-1.18 | 0.621 | 0.97 | 0.80-1.18 | 0.778 |
| Tumor location | ||||||||||||
| Renal pelvis | Reference | Reference | Reference | Reference | ||||||||
| Ureter | 0.97 | 0.81-1.17 | 0.770 | 1.15 | 0.91-1.44 | 0.235 | 1.19 | 0.92-1.53 | 0.186 | 1.16 | 0.93-1.45 | 0.178 |
| Both renal pelvis and ureter | 1.21 | 0.96-1.52 | 0.116 | 1.32 | 1.00-1.73 | 0.047 | 1.28 | 0.95-1.73 | 0.111 | 1.22 | 0.93-1.59 | 0.153 |
| Pathological T stage | ||||||||||||
| pTis/pTa/pT1/pT2 | Reference | Reference | Reference | Reference | ||||||||
| pT3/pT4 | 0.90 | 0.73-1.10 | 0.300 | 2.62 | 2.03-3.36 | < 0.001 | 3.15 | 2.36-4.20 | < 0.001 | 2.35 | 1.85-2.99 | < 0.001 |
| Tumor grade | ||||||||||||
| Low | Reference | Reference | Reference | Reference | ||||||||
| High | 1.06 | 0.88-1.27 | 0.576 | 2.25 | 1.64-3.09 | < 0.001 | 1.99 | 1.40-2.83 | < 0.001 | 1.69 | 1.28-2.22 | < 0.001 |
| Concomitant LVI | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.97 | 0.77-1.22 | 0.795 | 1.76 | 1.40-2.20 | < 0.001 | 1.90 | 1.49-2.44 | < 0.001 | 1.85 | 1.48-2.31 | < 0.001 |
| Concomitant CIS | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 1.08 | 0.86-1.37 | 0.510 | 1.01 | 0.78-1.31 | 0.956 | 1.15 | 0.86-1.53 | 0.346 | 1.09 | 0.84-1.41 | 0.528 |
| Pathological N stage | ||||||||||||
| pNx | Reference | Reference | Reference | Reference | ||||||||
| pN0 | 0.96 | 0.81-1.13 | 0.590 | 0.89 | 0.71-1.12 | 0.334 | 0.94 | 0.73-1.21 | 0.630 | 1.19 | 0.96-1.47 | 0.122 |
| ≥ pN1 | 0.68 | 0.45-1.02 | 0.065 | 2.18 | 1.62-2.92 | < 0.001 | 2.01 | 1.44-2.80 | < 0.001 | 2.19 | 1.60-2.98 | < 0.001 |
| Adjuvant chemotherapy | ||||||||||||
| No | Reference | Reference | Reference | Reference | ||||||||
| Yes | 0.79 | 0.62-1.02 | 0.067 | 1.12 | 0.88-1.42 | 0.371 | 1.07 | 0.83-1.39 | 0.595 | 1.09 | 0.86-1.39 | 0.460 |
IVRFS, intravesical recurrence-free survival; PFS, progression-free survival; CSS, cancer-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ASA, American Society of Anesthesiologists; ONU, open nephroureterectomy; LNU, laparoscopic nephroureterectomy; LVI, lymphovascular invasion; CIS, carcinoma in situ.
Discussion
The current gold standard treatment for UTUC is RNU with bladder cuff excision [3]. ONU, the conventional surgical approach that supports favorable cancer control, has long been accepted as the standard surgical approach for UTUC, especially for large or locally advanced UTUC. Recently, minimally invasive approaches, such as LNU or robotic nephroureterectomy, have gained wide acceptance as viable alternatives to traditional ONU due to their faster recovery time and a decreased likelihood of perioperative complications. Actually, after the first successful LNU procedure in 1991 by Clayman et al. [19], widespread use of LNU was initially limited by concerns over tumor cell dissemination in a pneumoperitoneal environment and the significant operator learning curve. With increased operative skills and the demonstration of comparable oncologic outcomes, utilization of LNU has gradually increased. In the United States, utilization of LNU or robotic nephroureterectomy increased from 57.7% to 71.5% from 2010 to 2013, whereas use of ONU decreased from 42.3% to 28.6% [20].
Numerous studies have evaluated the oncologic outcomes of ONU versus LNU and demonstrated comparable oncologic results between the two different surgical techniques [5-13]. A randomized trial by Simone et al. [5] compared 40 ONU patients with 40 LNU patients. They found that IVRFS, metastasis-free survival, and CSS were not significantly different between the two groups at a median follow-up of 44 months. The 5-year CSS was 89.9% and 79.8% for the ONU and LNU patients, respectively (p=0.2). Similarly, several multicenter retrospective studies showed oncologic equivalence between ONU and LNU with regard to RFS and CSS [8,9]. Walton et al. [8] retrospectively evaluated 703 ONU patients and 70 LNU patients at nine centers worldwide with median follow-up of 34 months. They reported 5-year RFS of 73.7% and 63.4% (p=0.124) and 5-year CSS of 75.4% and 75.2% (p=0.897) for the ONU and LNU groups, respectively. Likewise, Ariane et al. [9] also demonstrated that LNU could provide equivalent oncologic outcomes compared to ONU with median follow-up of 27 months in a large French multicenter collaborative study. In their study, no significant difference was observed in the 5-year RFS between the ONU (50.7%) and LNU (52.2%) patients (p=0.7). The 5-year CSS was 78% for the ONU patients and 90.7% for the LNU patients, but this difference was not statistically significant (p=0.06). Of note, in subgroup analysis by tumor stage, there were no significant differences between the two procedures in the 5-year RFS or the 5-year CSS for any of the pathological tumor stages. A meta-analysis published by Ni et al. [14] including 21 publications also reported no significant differences in the 5-year RFS and 5-year OS between LNU and ONU. However, a few studies have reported that 5-year CSS and OS rates were lower in LNU patients than in ONU patients with locally advanced disease. Simone et al. [5] reported that CSS was significantly different between the two surgical techniques in favor of ONU for pT3 tumors (p=0.039). A retrospective comparative analysis by Kim et al. [13] demonstrated that LNU patients showed inferior 5-year CSS (p=0.015) and OS (p=0.027) compared with ONU patients. Moreover, the 5-year CSS and OS for pT3/pT4 tumors were significantly lower in the LNU patients than in the ONU patients (p=0.005 and p=0.007, respectively).
In contrast to these results, two comprehensive metaanalyses in the literature reported that LNU was superior to ONU in terms of CSS. Zhang et al. [12] conducted a systemic review and meta-analysis to evaluate the oncologic outcome associated with two different surgical approaches (ONU and LNU) across 21 retrospective studies. They demonstrated that LNU showed better CSS compared with ONU (hazard ratio, 0.79; 95% confidence interval, 0.68 to 0.91). Likewise, Ni et al. reported that the 5-year CSS rate was notably higher for patients who underwent LNU (9%) than for those who underwent ONU (p=0.03) [14]. In this study, IVRFS was significantly lower in LNU patients, at 17% (p=0.02).
The present study from the Urothelial Cancer-Advanced Research and Treatment study group extended previous studies to evaluate the associations between surgical approach and oncologic outcomes in a larger cohort of patients with UTUC. The 5-year CSS of this study was 76.4% and 80.4% for ONU and LNU, respectively. These data were similar to those of previous studies, which reported 5-year CSS of 73-90% and 75-91% for ONU and LNU patients, respectively [5,6,8,9]. A summary of our results shows significant differences in IVRFS, CSS, and OS between ONU and LNU. The 5-year IVRFS, CSS, and OS rates were lower in the ONU group than in the LNU group and the benefit of LNU was especially apparent in the subgroup with locally advanced disease (pT3/pT4). The type of surgical approach for RNU was not a significant predictor of oncologic results; significance was achieved for IVRFS, but not for PFS, CSS, and OS. However, these results should be interpreted with caution. The reason for superiority of oncologic outcomes in the LNU group is uncertain and might be affected by several factors, including patient’s clinical and pathological characteristics, surgical experience, and extent of regional lymph node dissection [21]. Above all, it could be mainly affected by selection bias. The choice of surgical approach was usually determined by the surgeon’s preference in addition to the patient’s baseline characteristics. Although absolute indications for each surgical approach are not clearly defined, it is important to select appropriate patients to ensure optimal oncologic outcomes and safety. Many surgeons performing LNU tend to select patients who generally have a good comorbidity profile and typically offer the ONU procedure to patients with more aggressive and bulky UTUC. Therefore, although there were no differences in pathological T stage and tumor grade between two surgical approaches it is possible that ONU patients had more aggressive tumors, which may have affected the oncologic outcome. Indeed, the LNU group was less likely to have lymph node metastases relative to the ONU group in the present study (Table 1).
The present study has some other limitations. Foremost, due to the retrospective and nonrandomized study design, unidentified confounding variables may have been present. Second, multiple surgeons in five different centers performed ONU and LNU. Thus, the individual learning curve of each surgeon could have been a source of bias. Furthermore, the patients analyzed in ONU and LNU groups are not contemporary; the outcomes from the ONU group are based on less contemporary patient cohorts. Because we did not consider the effect of surgeon or time period, it remains unclear whether these factors influenced the results. Third, although all participating centers in this study usually followed recommendations and institutional protocols [3], there was a lack of standardization of the selection criteria and surgical approaches. In addition, we excluded patients with largely incomplete information from our analysis, which could possibly create selection bias. As previously mentioned, this selection bias might affect evaluation of the real impact of surgical approach on oncologic outcomes.
The present study shows that LNU provided better oncologic control of IVRFS, CSS, and OS compared to ONU for the management of patients with UTUC. The clinical benefit was more pronounced among patients with locally advanced disease. Further multicenter randomized trials are necessary to definitively prove that LNU is a safe alternative surgical approach to ONU in patients with UTUC, especially those with more advanced disease.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2014. Cancer Res Treat. 2017;49:292–305. doi: 10.4143/crt.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roupret M, Babjuk M, Comperat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology guidelines on upper urinary tract urothelial cell carcinoma: 2015 update. Eur Urol. 2015;68:868–79. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 4.Tsujihata M, Nonomura N, Tsujimura A, Yoshimura K, Miyagawa Y, Okuyama A. Laparoscopic nephroureterectomy for upper tract transitional cell carcinoma: comparison of laparoscopic and open surgery. Eur Urol. 2006;49:332–6. doi: 10.1016/j.eururo.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Simone G, Papalia R, Guaglianone S, Ferriero M, Leonardo C, Forastiere E, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56:520–6. doi: 10.1016/j.eururo.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Capitanio U, Shariat SF, Isbarn H, Weizer A, Remzi M, Roscigno M, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol. 2009;56:1–9. doi: 10.1016/j.eururo.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 7.Favaretto RL, Shariat SF, Chade DC, Godoy G, Kaag M, Cronin AM, et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur Urol. 2010;58:645–51. doi: 10.1016/j.eururo.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walton TJ, Novara G, Matsumoto K, Kassouf W, Fritsche HM, Artibani W, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int. 2011;108:406–12. doi: 10.1111/j.1464-410X.2010.09826.x. [DOI] [PubMed] [Google Scholar]
- 9.Ariane MM, Colin P, Ouzzane A, Pignot G, Audouin M, Cornu JN, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): results from a large French multicenter collaborative study. Ann Surg Oncol. 2012;19:301–8. doi: 10.1245/s10434-011-1841-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Dai YB, Zhou FJ, Long Z, Li YH, Xie D, et al. Laparoscopic versus open nephroureterectomy to treat localized and/or locally advanced upper tract urothelial carcinoma: oncological outcomes from a multicenter study. BMC Surg. 2017;17:8. doi: 10.1186/s12893-016-0202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rieken M, Xylinas E, Kluth L, Trinh QD, Lee RK, Fajkovic H, et al. Diabetes mellitus without metformin intake is associated with worse oncologic outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol. 2014;40:113–20. doi: 10.1016/j.ejso.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Luo Y, Wang C, Fu SJ, Yang L. Long-term oncologic outcomes of laparoscopic nephroureterectomy versus open nephroureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis. PeerJ. 2016;4:e2063. doi: 10.7717/peerj.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HS, Ku JH, Jeong CW, Kwak C, Kim HH. Laparoscopic radical nephroureterectomy is associated with worse survival outcomes than open radical nephroureterectomy in patients with locally advanced upper tract urothelial carcinoma. World J Urol. 2016;34:859–69. doi: 10.1007/s00345-015-1712-3. [DOI] [PubMed] [Google Scholar]
- 14.Ni S, Tao W, Chen Q, Liu L, Jiang H, Hu H, et al. Laparoscopic versus open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2012;61:1142–53. doi: 10.1016/j.eururo.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–48. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612–8. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung HH, Jeon HG, Han DH, Jeong BC, Seo SI, Lee HM, et al. Diagnostic ureterorenoscopy is associated with increased intravesical tecurrence following tadical nephroureterectomy in upper tract urothelial carcinoma. PLoS One. 2015;10:e0139976. doi: 10.1371/journal.pone.0139976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayman RV, Kavoussi LR, Figenshau RS, Chandhoke PS, Albala DM. Laparoscopic nephroureterectomy: initial clinical case report. J Laparoendosc Surg. 1991;1:343–9. doi: 10.1089/lps.1991.1.343. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez JF, Packiam VT, Boysen WR, Johnson SC, Smith ZL, Smith ND, et al. Utilization and outcomes of nephroureterectomy for upper tract urothelial carcinoma by surgical approach. J Endourol. 2017;31:661–5. doi: 10.1089/end.2017.0086. [DOI] [PubMed] [Google Scholar]
- 21.Fairey AS, Kassouf W, Estey E, Tanguay S, Rendon R, Bell D, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: results from the Canadian Upper Tract Collaboration. BJU Int. 2013;112:791–7. doi: 10.1111/j.1464-410X.2012.11474.x. [DOI] [PubMed] [Google Scholar]


