ABSTRACT
Cellular therapy is a key tool to treat haematological malignancies. Over 40,000 allogeneic and autologous haematopoietic stem cell transplants (HSCTs) are performed annually across Europe.1 Since 2017, a new T cell therapy, chimeric antigen receptor-T (CAR-T) cells have been licensed outside clinical trials. CAR-T cells have extremely potent antitumour activity, but also have a profile of toxic side effects not seen before. Cytokine release syndrome (CRS) and CAR-T cell-related encephalopathy syndrome (CRES) are common, predictable and potentially lethal side effects. Patients frequently require admission to intensive care, and management from a number of medical specialties. This exciting and powerful new therapy requires the formation of new multispecialty medical teams for safe delivery and to successfully manage the resultant complications.
KEYWORDS: CAR-T cells, cytokine release syndrome, cancer Immunotherapy, cellular therapy, CAR-T cell related encephalopathy syndrome
Key points
The intensive care team is crucial to enable successful delivery of CAR-T cell therapy within a JACIE-accredited BMT facility
CRS is common and patients can develop multiorgan failure
Tociluzimab, an IL-6 receptor antagonist, is given to patients with severe CRS, and corticosteroids are reserved for patients not responding to tociluzimab
Neurotoxicity/CRES can result in seizures and cerebral oedema
Antiepileptics and steroids may be used to treat neurotoxicity
Introduction
Cellular therapy is well established, with the first published case series of blood transfusion reported in 1824 by the obstetrician James Blundell.2 Although many of his patients died, presumably in part due to ABO incompatibility, he demonstrated the principle that cells transferred from one individual to another could carry out their life-sustaining functions. Over a century later, the first successful haematopoietic stem cell transplant (HSCT) was performed between identical twins. With human leucocyte antigen (HLA) typing this therapy was extended to HLA matched siblings and unrelated donors, but a new condition graft-versus-host disease (GVHD) was observed, whereby donor-derived T-cells attack the recipient.3
Alongside these pivotal moments in transplantation history, it was increasingly recognised that our immune system detects and eliminates cancer cells. Tumour-infiltrating lymphocytes taken from melanoma patients and grown in the laboratory could cause tumour regression when infused back.4 Allogeneic HSCT is now known to not only provide a rescue bone marrow following chemotherapy, but to actually treat leukaemia via the graft versus leukaemia effect (GVL). To enhance GVL, additional lymphocytes can be collected from HSCT donors and given to their recipient patients (donor lymphocyte infusions), but failures occur. This is because cancer cells hide from the immune system by reducing HLA expression (required for T-cells to detect them) and disable the immune system by upregulating inhibitory molecules (immune checkpoints).
T-cells have been genetically engineered to overcome these obstacles resulting in a new therapy, CAR-T cells. However, like the first acute haemolytic transfusion reactions and GVHD, they bring with them a unique set of toxic complications that physicians need to recognise and manage.
Chimeric antigen receptor-T cells
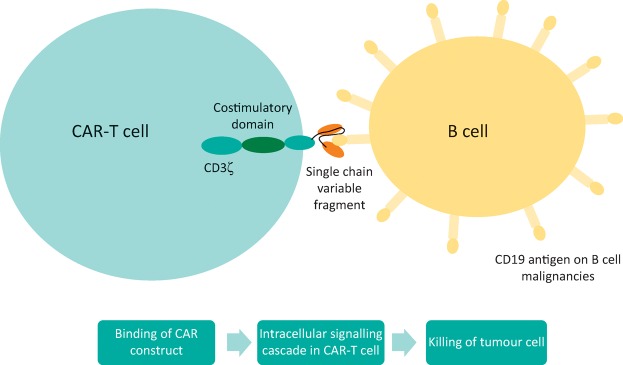
T cells are collected from patients or healthy donors and genetically modified to express an artificial receptor. Patients then receive chemotherapy, prior to CAR-T cell infusion, which depletes immunosuppressive cells thereby aiding CAR-T cell expansion. The extracellular domain of the CAR derives from a monoclonal antibody (usually the single chain variable fragment) which binds to antigens on cancer cells (see Fig 1). Binding initiates signalling in the intracellular domain, CD3ζ (the downstream signalling component of a normal T-cell receptor) and a costimulatory domain (usually 4-1BB or CD28) that allows the T-cells to have sustained antitumour activity. The concept was first developed by Eshhar in the 1980s,5 but is now globally revolutionising how haemato-oncologists treat patients, with the first two CAR-T cell products licensed by the FDA in 2017.6
Fig 1.
Schematic diagram of CAR-T cells.
The success of this therapy in B-cell malignancies is unprecedented. Adult B-acute lymphoblastic leukaemia (B-ALL) patients relapsing post allograft have a median survival of ∼7 months.7 Following CAR-T cell therapy >80% of relapsed and refractory patients achieved a complete remission (CR),8–12 resulting in 50–86% of patients being alive 1 year later.11,12 Similarly, B-cell lymphoma patients with chemorefractory disease have a median survival of just 6.3 months.13 More than 50% were in CR following CAR-T cells,14,15 with more than half alive 18 months post infusion.14 A proportion of patients are expected to achieve a long-term remission, although relapses do occur. Reduced expression of the target antigen on tumour cells and rejection of CAR-T cells are known mechanisms of resistance. Numerous other CAR-T cells are being evaluated in clinical trials for haematological and solid organ malignancies with some promising early data for myeloma.16 The responses in some key trials are summarised in Table 1.
Table 1.
Summary data of key clinical trials of CAR-T cells
| Clinical trial | Patient group | Objective response rate | Complete remission rate | Overall survival | Reference |
|---|---|---|---|---|---|
| ELIANA (Novartis) | Children and young adults with relapsed and refractory B-ALL | 81% | 81% | Median survival 19.1 months | 11 |
| MSKCC | Adults with relapsed B-ALL | - | 83% | Median survival 12.9 months | 12 |
| ZUMA-1 (Kite Pharma) | Adults with refractory large B-cell lymphoma | 82% | 54% | 52% at 18 months | 14 |
| JULIET (Novartis) | Adults with relapsed DLBCL or follicular lymphoma | 64% | 43% DLBCL 71% follicular lymphoma | DLBLC median survival 22.2 months, follicular lymphoma not reached | 15 |
| CRB-401 (Bluebird bio / Celgene) | Relapsed and refractory multiple myeloma | 89% | 22% | Not available | 16 |
B-ALL = B-acute lymphoblastic leukaemia; DLBCL = diffuse large B-cell lymphoma
Both licensed products target CD19, an antigen expressed on B-cells. Tisagenlecleucel, brought to market by Novartis, and Axicabtagene Ciloleucel, developed by Kite Pharma / Gilead, are indicated for B-ALL and B-cell lymphoma respectively. The European Medicines Agency (EMA) are reviewing both products, and a decision is expected imminently.
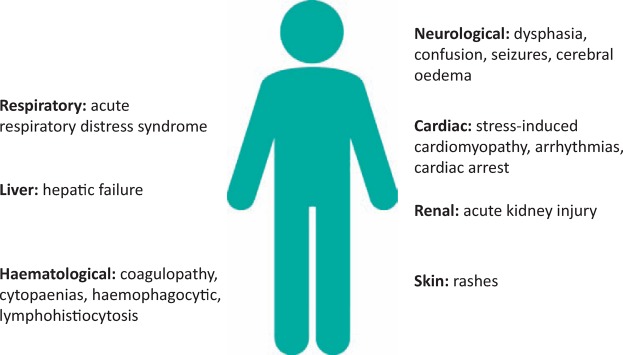
However, these new treatments have potentially lethal side effects (see Fig 2). CAR-T cells have created a novel list of complications in patients receiving them. The most significant, cytokine release syndrome (CRS) and CAR-T cell-related encephalopathy syndrome (CRES), require expertise from a range of medical specialties. Currently, it is known that some patients will succumb to these complications. There is a pressing need for improved understanding and management of these iatrogenic conditions.
Fig 2.
Organ dysfunction following CAR-T cell therapy.
Cytokine release syndrome
CRS is the most commonly seen and expected life-threatening complication following CAR-T cell infusion. Indeed, the absence of CRS raises concern that CAR-T cells are failing to expand sufficiently to eradicate the malignant cells. In clinical trials of the licensed products, it occurred in 57–97% of patients,11,14,15 leading to 47% of patients in one study requiring admission to intensive therapy unit (ITU).11 Each agent has its own specific toxicity profile.
CRS is a state of immune over-activation. CAR-T cells receive an exaggerated signal through the artificial receptor stimulating them to secrete cytokines. This activates other cells of the immune system, including macrophages and drives expansion of infused CAR-T cells. IL-6 at very high levels is thought to directly cause some organ toxicity seen in CRS although the exact pathophysiology is still being elucidated.
Fever is an early sign. Patients can rapidly develop multisystem complications including: acute respiratory distress syndrome (ARDS), acute kidney injury (AKI), liver failure, disseminated intravascular coagulation (DIC), cardiomyopathy and arrhythmias.17 There have been cases of patients with severe CRS developing fulminant haemophagocytic lymphohistiocytosis (HLH), a serious condition with a similar pattern of immune over-activation.17
Serum ferritin and C-reactive protein (CRP) are typically elevated supporting a diagnosis of CRS. Cytokine levels, if available, provide additional diagnostic information. The current management strategy follows a step-wise approach. Mild CRS with fever is treated with paracetamol and fluid hydration. Intravenous (IV) antibiotics are given to cover the differential diagnosis of neutropaenic sepsis. Hypotension is treated with an IV fluid bolus. If patients require repeated fluid boluses or inotropes to maintain their blood pressure then specific IL-6 blocking therapy should be considered. Tociluzimab, an IL-6 receptor antagonist, is approved by the FDA for severe CRS;6 it has shown good efficacy, with 69% of patients having recovered at 2 weeks.6 In patients not responding to tociluzimab, or in whom organ dysfunction has developed, a short course of corticosteroids should be given.17 Patients with severe CRS should be managed within the ITU with appropriate organ support.
CAR-T cell-related encephalopathy syndrome
CRES ranges from mild confusion to fatal cerebral oedema. Typically, patients develop an encephalopathy with dysphasia and disorientation. Seizures can occur, as can focal motor symptoms.18 In some but not all patients experiencing these symptoms, CAR-T cells have been detected in the cerebrospinal fluid (CSF).18 The pathophysiology is not fully understood.
Fundoscopic examination for papilloedema, and neuroimaging with computed tomography (CT) / magnetic resonance imaging (MRI) should be carried out.18 If it can be performed safely, a lumbar puncture measuring CSF pressure should be done. The incidence of seizures is high and an electroencephalogram (EEG) is indicated in those with CRES. One group found non-convulsive status epilepticus in 10% of patients who received CAR-T cells.17 However, the severity and frequency of CRES varies between different CAR-T cell products.
In those with raised intracranial pressure, high-dose corticosteroids should be initiated. Other therapies aimed at lowering intracranial pressure should be trialled, including acetazolamide, hyperosmolar therapy, and hyperventilation in liaison with neurosurgical and neuro-ITU teams.17 If seizure-like activity is seen on EEG, antiepileptics are indicated.18 If status epilepticus is present benzodiazepines are given with further specialist input from neurology. In those receiving CAR-T cell products associated with CRES prophylactic antiepileptics should be considered.17
Challenges of delivering CAR-T cell therapy
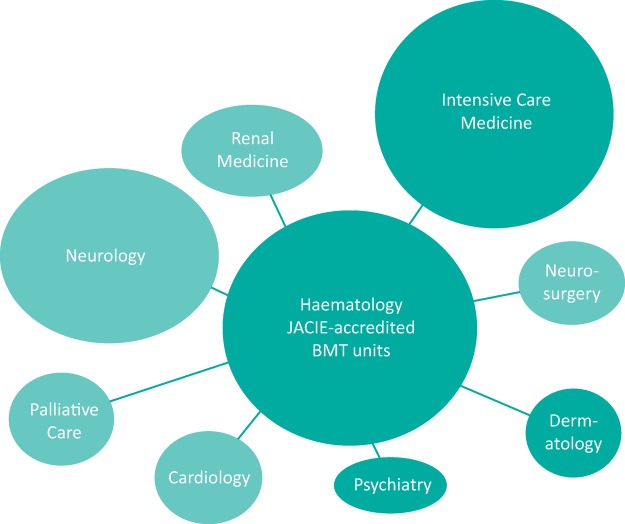
Hospitals delivering CAR-T cell therapy need to establish a multidisciplinary team, with an education programme and expertise in managing the complications of this therapy. NHS England are currently developing service specifications for both products and centres will be assessed against these. Centres will need to be JACIE (Joint Accreditation Committee-ISCT & EBMT) accredited bone marrow transplant (BMT) units. Alongside haematologists, intensivists are vital. Neurologists, cardiologists and renal physicians should also be part of these specialist teams (see Fig 3).
Fig 3.
Key teams involved in the delivery of CAR-T cell therapy.
Delivery of therapy should be coordinated with intensive care who are aware of all CAR-T cell patients and alerted at the time of cell infusion. Complications tend to follow a predictable timeline (although this varies between CAR-T cell products), helping to inform bed-planning decisions. Once patients develop early CRS, ITU should be updated regularly, with outreach team review, so that patients are transferred at the appropriate time. With the planned establishment of cell therapy units which include intensive care beds it is hoped patient care and survival of complications will improve.
Conclusions
Cellular therapy and cancer immunotherapy are now firmly positioned at the forefront of anticancer treatment. Patients with chemorefractory disease are accessing new, genetically-engineered T cell therapies, particularly CAR-T cells. As these treatments move into the mainstream, physicians will encounter and be expected to manage the resultant complications. New teams, consisting of haematologists, intensivists and other medical specialists, need to be established to allow successful delivery of these powerful new treatments.
Conflicts of interest
Charlotte Graham receives research funding from Servier and has received funding to attend educational meetings from Gilead and Pfizer. Antonio Pagliuca has received advisory board fees for Novartis and Bluebird BIO, clinical input into the KCH Cellectis trial and is the National Clinical Lead for Regenerative Medicine NHSE. Reuben Benjamin receives research funding from Servier and Pfizer, participated in advisory board meetings for Servier, Pfizer, Novartis and Gilead, and is principal investigator of a Servier sponsored CAR-T cell trial.
References
- 1.Passweg JR. Baldomero H. Bader P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. Bone Marrow Transplant. 2016;51:786–92. doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blundell J. Researches Physiological and Pathological. London: E. Cox Co. Sons; 1824. [Google Scholar]
- 3.Thomas ED. A history of haemopoietic cell transplantation. Br J Haematol. 1999;105:330–9. doi: 10.1111/j.1365-2141.1999.01337.x. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME. Wunderlich JR. Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross G. Waks T. Eshhar Z. Expression of immunoglobulin T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food & Drug Administration . FDA approves tisagenlecleucel for B-cell ALL and tociluzimab for cytokine release syndrome. FDA; 2017. www.fda.gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. [Accessed 26 February 2018] [Google Scholar]
- 7.Kantarjian H. Stein A. Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukaemia. N Engl J Med. 2017;376:836–47. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brentjens RJ. Rivière I. Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp SA. Kalos M. Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude SL. Frey N. Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukaemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maude SL. Laetsch TW. Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukaemia. N Engl J Med. 2018;378:439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH. Riviere I. Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukaemia. N Engl J Med. 2018;378:449–59. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump M. Neelapu SS. Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neelapu SS. Locke FL. Bartlett N, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large b-cell lymphoma. N Engl J Med. 2017;377:2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuster SJ. Svoboda J. Chong E, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berdeja JG. Lin Y. Raje N, et al. Durable clinical responses in heavily pretreated patients with relapsed/refractory multiple myeloma: updated results from a multicentre study of bb2121 anti-Bcma CAR T cell therapy. Blood. 2017;130(Suppl 1):740. [Google Scholar]
- 17.Neelapu SS. Tummala S. Kebriaei P, et al. Chimeric antigen receptor T-cell therapy assessment and management of toxicities. Nat Rev Clin Onc. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davila ML. Riviere I. Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]