ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) has a global prevalence of about 25%. Incidence is increasing with rising levels of obesity, type 2 diabetes and the metabolic syndrome, and NAFLD is predicted to become the leading cause of cirrhosis requiring liver transplantation in the next decade. However, the cardiovascular disease is the most common cause of death and only a minority will develop fibrosis and liver-related complications. Therefore, it is imperative to identify patients with advanced disease using non-invasive markers of fibrosis, which include serology-based tests (eg NAFLD Fibrosis Score and ELF test) and imaging (eg transient elastography). This targets appropriate patients for referral to secondary care for additional investigations such as liver biopsy and specialist care. Lifestyle modification and weight loss remains the cornerstone of management, but we are about to enter a new era of promising pharmacotherapies for NASH and fibrosis.
KEYWORDS: non-alcoholic fatty liver disease, fibrosis, diagnosis, management, liver disease
Key points
NAFLD has a global prevalence of 25% and has become one of the leading causes of chronic liver disease
NAFLD is a spectrum of disease including simple fatty liver, NASH, fibrosis and cirrhosis, with a complex ‘multi-hit’ pathophysiology
Diagnosis involves the identification of hepatic steatosis, followed by risk stratification for significant fibrosis (the key prognostic marker)
Cardiovascular risk modification, particularly weight loss, are the mainstay of treatment
Exciting new therapies are emerging that will transform the therapeutic options for patients in the next decade
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined by macrovesicular steatosis in ≥5% hepatocytes, in the absence of a secondary cause such as alcohol or drugs. It encompasses a spectrum of disease from non-alcoholic fatty liver (NAFL) through to non-alcoholic steatohepatitis (NASH), fibrosis and cirrhosis. NAFLD is now a leading cause of chronic liver disease worldwide,1 yet public understanding of the disease remains very limited,2 and the complications of cirrhosis are overlooked in the public discourse on the national obesity epidemic. However, NAFLD is one of the fastest growing areas of liver research and the next decade should witness a transformation in the therapeutic options available for these patients. This review will summarise our current understanding of disease mechanisms, but focus on epidemiology and approaches to diagnosis and management.
Epidemiology and natural history
The worldwide prevalence of NAFLD is about 25%, ranging from 13% in Africa to 23% in Europe and 32% in the Middle East.1 Geographical variation reflects known differences in incidence and severity of disease between different ethnic groups, most notably a protective effect of black ethnicity and conversely higher rates of NASH in Hispanic groups,3 perhaps in part secondary to higher frequency of genetic risk variants (eg rs738409 in PNPLA3) associated with NAFLD.4 There is a close association with type 2 diabetes, central obesity, dyslipidaemia and the metabolic syndrome, each with a respective prevalence of 23%, 51%, 69% and 43% in NAFLD. Consequently, the burden of disease has increased from 15% in 2005 to 25% in 2010 in parallel to rising rates of obesity.1
The most important predictor of adverse outcomes in NAFLD is the presence of fibrosis, rather than histological features of NASH.5,6 There is a small increase in all-cause mortality even at very early fibrosis, which rises on a linear scale with progressive fibrosis stage. Early (F1) fibrosis is not associated with a significant increase in liver-related mortality, but notably this rises exponentially with increasing stage such that mortality rates from liver disease with bridging fibrosis (F3) and cirrhosis (F4) are 7.92 (per 1000 person years follow-up vs stage 0 fibrosis) and 23.3 respectively.7 The rate at which fibrosis develops is typically very slow in NAFLD, although more rapid in patients with NASH (7 years per fibrosis stage) than without NASH (14 years), confirming the importance of NASH in the evolution of fibrosis.8 Risk factors for progressive disease include age, increasing BMI and diabetes.9
The most common cause of death in patients with NAFLD is cardiovascular disease (40%). Although a common set of risk factors contribute to this, recent studies have also shown that NAFLD itself may independently increase risk of heart disease,10 although as yet this has not been incorporated into cardiovascular risk-assessment tools.
Only a minority of patients with NAFLD will develop complications of chronic liver disease, including 4–8% dying from the complications of cirrhosis and 1–5% from hepatocellular carcinoma (HCC).1 However, the overall number of patients with end-stage liver disease caused by NAFLD is rapidly increasing; there was a 170% increase in cases of NASH on the transplant waiting list in USA from 2004–13,11 and the proportion of liver transplants for NASH increased from 1.2% in 2001 to 9.7% in 2009. It is therefore predicted that NASH will become the leading cause of liver transplantation in the USA in the next decade.12 Moreover, some studies have raised concerns that NAFLD may predispose patients to HCC even in the absence of cirrhosis, but further research is required in the field.13
Pathogenesis
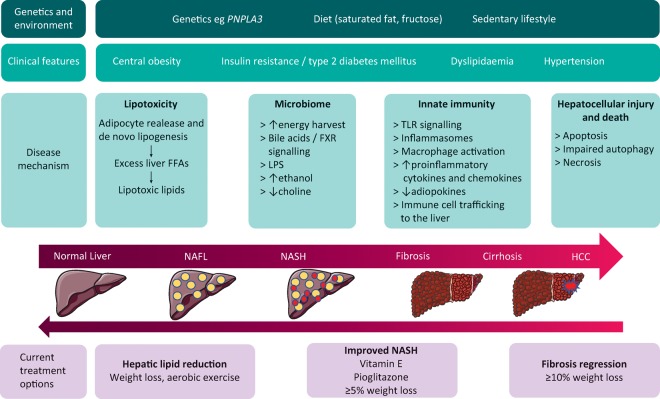
Major advances in our understanding of the pathogenesis have revealed the complexity of the disease. The ‘two-hit’ hypothesis has now been superseded by a ‘multi-hit’ model incorporating multiple interlocking processes, including lipotoxicity, innate immune activation and the microbiome on a background of genetic and environmental factors. A detailed description is beyond the scope of this review but has been well reviewed elsewhere14 and is summarised in Fig 1.
Fig 1.
Pathophysiology and treatment options in NAFLD. DM = diabetes mellitus; FXR = farsinoid X receptor; HCC = hepatocellular carcinoma; LPS = lipopolysaccharide; NAFLD = non-alcoholic fatty liver; NASH = non-alcoholic steatohepatitis; PNPLA3 = patatin-like phospholipase domain-containing protein 3; TLR = toll-like receptor
Diagnosis
The diagnosis of NAFLD consists of identifying steatosis in the absence of a secondary cause (eg alcohol and steatogenic drugs), followed by risk stratification for the presence of NASH and significant fibrosis.10 Investigation is usually initiated in response to elevated liver transaminases, but this approach is limited by many patients with NAFLD having normal liver function tests.15 Therefore, the diagnosis should also be considered in high-risk patients with type 2 diabetes or metabolic syndrome regardless of transaminase levels.16
Steatosis
Traditionally, ultrasound has been the diagnostic modality of choice for identifying steatosis and has the advantage of low cost and high accessibility. However, it has poor sensitivity for detecting <20% steatosis. Alternatively, validated serological markers include the Fatty Liver Index (FLI), NAFLD Fat Score (NAFLD FS), and Hepatic Steatosis Index (HIS), with area under receiver operating characteristic (AUROC) values 0.83, 0.80 and 0.81 respectively.17,18 These tests use simple clinical data which can be entered into free online algorithms and would be particularly useful in settings where imaging is too costly and less accessible.
Controlled attenuation parameter (CAP) is a relatively recent function of transient elastography machines (FibroscanTM, Echosens, Paris) and uses ultrasound waves to quantify liver fat. A recent systematic review including 2735 patients identified 248, 268 and 280 dB/m as optimal cut-offs for diagnosing mild, moderate and severe steatosis respectively.19 However, there is significant overlap and CAP is generally poor at discriminating between stages of steatosis, although the clinical significance of increasing grades of steatosis is questionable. Overall a score of 250 dB/m is an acceptable cut-off to confirm the diagnosis of NAFLD.20
Fibrosis
It is crucial to risk stratify all patients with NAFLD based on the presence or absence of significant fibrosis, the key prognostic feature in NAFLD.5,6 Multiple serological tests for staging fibrosis have been developed, using simple biochemical and clinical parameters which incur no additional cost to routine haematology and biochemistry investigations and can be easily calculated using online tools (eg NALFD Fibrosis Score [NFS], BARD, FIB-4), as well as more expensive commercial tests (eg Enhanced Liver Fibrosis Score [ELF test], Fibrotest, FibroMeter NAFLD). A common feature of the tests are split cut-offs to optimise the sensitivity or specificity, with a greater capacity to identify and rule out advanced rather than mild fibrosis.20
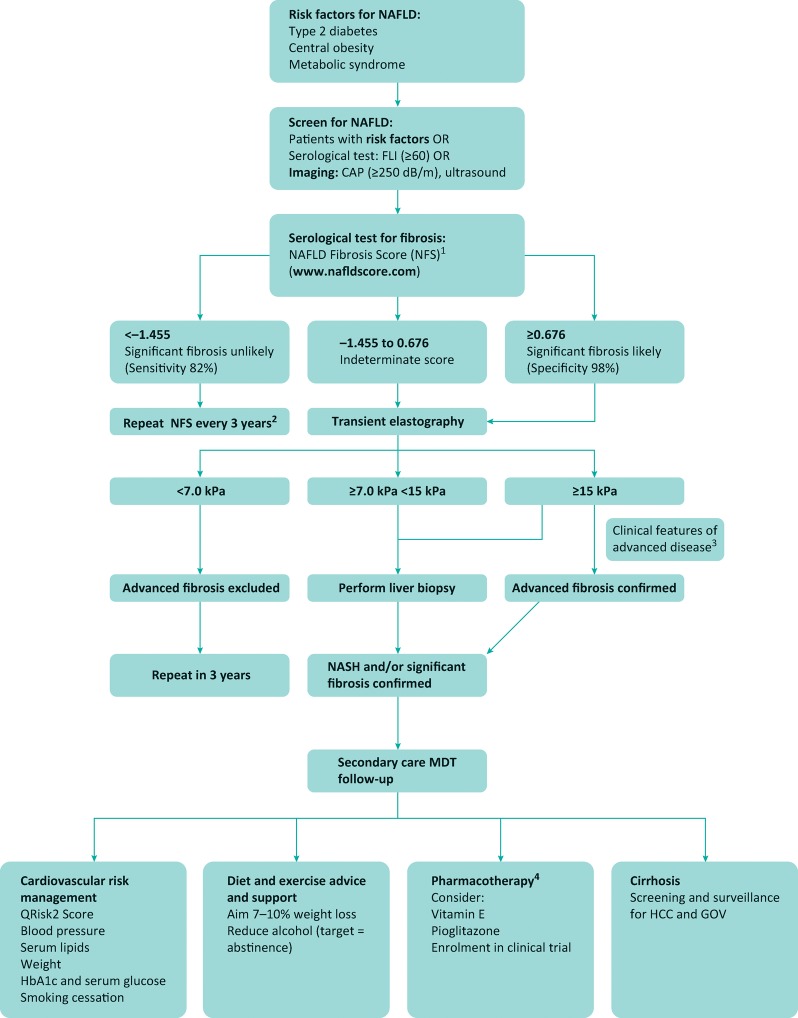
Therefore, a common strategy is to use simple serological markers to ‘rule out’ significant fibrosis, which could be an effective pathway to filter referrals from primary to secondary care,21 at which point more detailed evaluation can be performed using additional risk-stratification tools such as transient elastogrpahy (TE) (Fig 2). Variable cut-offs have been used in studies evaluating TE, but a meta-analysis of TE performance in NAFLD has demonstrated a liver stiffness of 7.5–10.4 kPa has a sensitivity and specificity of 0.82 (0.74–0.88) and 0.84 (0.78–0.89) respectively for the diagnosis of F3 fibrosis.22 Additional bedside imaging modalities are emerging as alternative options, such as acoustic wave force impulse (ARFI) and supersonic shear imaging (SSI), which have demonstrated comparable results.23
Fig 2.
Proposed investigation and management algorithm for NAFLD.1 NICE guidelines recommend ELF test. Where this is available, cut-off values are: –1.068 (moderate ≥F2 fibrosis) and 0.3576 (advanced fibrosis). FIB-4 is also well validated, with cut-off values ≤1.30 (low risk of significant fibrosis) and ≥2.67 (high risk of significant fibrosis).2 Further studies are required on the optimal frequency of follow-up;3 eg clinical stigmata of chronic liver disease, type 2 diabetes and/or obesity (BMI >30), platelets <150 cells/mm3, radiological evidence of cirrhosis.4 Always consider comorbidities and potential side effects, which include weight gain and congestive cardiac failure (pioglitazone), and possible increased risk of haemorrhagic stroke and prostate cancer (vitamin E). BMI = body mass index; CAP = controlled attenuation parameter; ELF = enhanced liver fibrosis; FLI = fatty liver index; GOV = gastro-oesophageal varices; HCC = hepatocellular carcinoma; MDT = multidisciplinary team; NICE = National Institute for Health and Care Excellence
There are some limitations with the non-invasive markers of fibrosis. Most of the tests perform more poorly at discriminating between earlier stages of fibrosis, with a range of indeterminate values where significant disease cannot be accurately ruled out or in. Fibroscan also has a higher failure rate in obesity, which has improved with the development of the XL probe. Finally, there is no validated non-invasive test for NASH. Therefore, liver biopsy remains a valuable tool in diagnosing NASH and fibrosis in patients identified through non-invasive markers to be at risk of significant disease (Fig 2). The NASH Clinical Research Network (CRN) score is the most commonly used histological reporting system, although in the future there may be a move away from semi-quantitative scores in favour of automated analytical software to objectively quantify features such as fat and fibrosis to reduce intra- and inter-observer variability.
Management
Cardiovascular risk factor modification
The assessment and management of blood pressure, lipids, weight, smoking status and diabetes control are the cornerstone of managing NAFLD. This will mainly be done in primary care, but can be incorporated into a multidisciplinary specialist clinic model (Fig 2).
Diet and exercise
There is good evidence that weight loss not only reduces liver fat content, but histological resolution of NASH can occur with as little as 5% weight loss, and almost half of patients with 10% weight loss will have fibrosis regression.24 However, lifestyle modification is difficult for most patients, where even in a trial setting only 10% patients achieved ≥10% weight loss.24 Patients should be offered a review by a dietician and information on local weight loss support groups. Novel interventions such as smartphone apps to monitor dietary intake and exercise and set goals are increasingly popular and should be a focus of ongoing research.
Pharmacotherapies
Lifestyle modification alone will be insufficient for a subset of patients, either due to an inability to achieve the required weight loss or the advanced nature of disease. A 96-week course of the antioxidant vitamin E has demonstrated benefit in NASH resolution in an RCT of non-diabetic patients (43% vs 19%, p=0.001).25 Post-hoc analysis of combined trial data has shown this is also an option for diabetics, although widespread use has been limited by concerns over potential risks of long-term treatment such as haemorrhagic stroke and prostate cancer.26 The same trial also studied pioglitazone, a peroxisome proliferator-activator receptor (PPAR)-γ agonist, and found some histological improvement in patients with NASH (34% vs 19% placebo, p=0.04) but this did not meet prespecified cut-offs for efficacy.25 There is muted guidance to ‘consider’ these therapies in the current The National Institute for Health and Care Excellence (NICE) guidance,16 but due to ongoing safety concerns with vitamin E and problematic weight gain with pioglitazone, neither treatment has been widely adopted in clinical practice.
However, drug therapies for NASH is a rapidly advancing field. Promising future treatments currently undergoing phase 3 trials include obeticholic acid (farnesoid X receptor agonist),27 cenicriviroc (cysteine-cysteine motif chemokine ligand receptor 2 and 5 [CCR2/5] antagonist), elafibranor (PPAR α/δ agonist) and selonsertib (apoptosis signal-regulating kinase [ASK] inhibitor) (Table 1; for a full review of pharmacotherapies in current development, see Rotman et al28). It is therefore anticipated that there will be major advances in our treatment options for NASH in the next 5 years.
Table 1.
Emerging drugs for non-alcoholic steatohepatitis in phase III trials
| Mechanism of action | Drug | Primary endpoint(s) | Clinical trial | Expected completion date |
|---|---|---|---|---|
| PPAR α/δ agonist | Elafibranor | • Resolution of NASH without worsening of fibrosis | RESOLVE-IT NCT02704403 | December 2021 |
| • Composite endpoint: all-cause mortality, cirrhosis and liver-related clinical outcomes | ||||
| ASK1 inhibitor | Selonsertib | • ≥1 stage improvement in fibrosis | STELLAR 4 | January 2020 |
| • Event-free survival at 240 weeks | NCT03053063 | |||
| FXR agonist | Obeticholic acid | • Composite endpoint: all-cause mortality, MELD score ≥15, liver transplant, histological progression to cirrhosis, hepatic decompensation (HCC, ascites, variceal bleed, hepatic encephalopathy, SPB) | REGENERATE NCT02548351 | October 2021 |
| • Fibrosis improvement composite endpoint: improvement in ≥1 stage of fibrosis; NASH resolution with no worsening of fibrosis | ||||
| CCR2/5 antagonist | Cenicriviroc | • Improvement in ≥1 stage of fibrosis and no worsening of NASH | AURORA NCT03028740 | July 2019 |
| • Clinical outcomes composite endpoint: histological progression to cirrhosis, liver-related clinical outcomes, all-cause mortality |
ASK = apoptosis signal-regulating kinase; CCR = cysteine-cysteine motif chemokine receptor; FXR = farnesoid X receptor; MELD = model for end stage liver disease; NASH = non-alcoholic steatohepatitis; PPAR = peroxisome proliferator-activator receptors
Cirrhosis
Patients with NASH cirrhosis require appropriate screening for HCC, oesophageal varices and other complications of cirrhosis. Decompensation with ascites, variceal bleeding or hepatic encephalopathy should prompt early assessment by a transplant centre.
Conclusion
NAFLD is a rapidly growing cause of chronic liver disease, mirroring the rising incidence of obesity and the metabolic syndrome. Management requires a multidisciplinary approach with clear risk stratification. The therapeutic options for advanced disease stages will significantly improve in the next decade.
References
- 1.Younossi ZM. Koenig AB. Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease – Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Ghevariya V. Sandar N. Patel K, et al. Knowing what's out there: awareness of non-alcoholic fatty liver disease. Front Med. 2014;1:4. doi: 10.3389/fmed.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bambha K. Belt P. Abraham M, et al. Ethnicity and nonalcoholic fatty liver disease. Hepatology. 2012;55:769–80. doi: 10.1002/hep.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo S. Kozlitina J. Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekstedt M. Hagström H. Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P. Kleiner DE. Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–97.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulai PS. Singh S. Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S. Allen AM. Wang Z. Prokop LJ. Murad MH. Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. doi: 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angulo P. Hui JM. Marchesini G, et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–54. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 10.Sinn DH. Kang D. Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017. pp. 323–9. [DOI] [PubMed]
- 11.Wong RJ. Aguilar M. Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 12.Charlton MR. Burns JM. Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Mittal S. El-Serag HB. Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14:124–31.e1. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wree A. Broderick L. Canbay A. Hoffman HM. Feldstein AE. From NAFLD to NASH to cirrhosis – new insights into disease mechanisms. Nat Rev Gastroenterol Hepatol. 2013;10:627–36. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]
- 15.Portillo-Sanchez P. Bril F. Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100:2231–8. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence Non-alcoholic fatty liver disease (NAFLD): assessment and management. NICE guideline [NG49]. NICE; 2016. [PubMed] [Google Scholar]
- 17.Fedchuk L. Nascimbeni F. Pais R, et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–22. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 18.Byrne CD. Targher G. EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1141–4. doi: 10.1007/s00125-016-3910-y. [DOI] [PubMed] [Google Scholar]
- 19. Karlas T. Petroff D. Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Castera L. Noninvasive evaluation of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:291–303. doi: 10.1055/s-0035-1562948. [DOI] [PubMed] [Google Scholar]
- 21.Majumdar A. Crossan C. Thorburn D, et al. Referral pathways for patients with non-alcoholic fatty liver disease based on non-invasive fibrosis tests: diagnostic accuracy and cost analysis of a two-tier approach (PS-089) J Hepatol. 2017;66:51. [Google Scholar]
- 22.Kwok R. Tse Y. Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease – the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2013;39:254–69. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 23.Cassinotto C. Boursier J. de Lédinghen V, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–27. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 24.Vilar-Gomez E. Martinez-Perez Y. Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–78.e5. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 25. Sanyal AJ. Chalasani N. Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowdley KV. Wilson LA. Van Natta ML. Pai RK. Sanyal AJ. Efficacy and safety of vitamin E in nonalcoholic steatohepatitis patients with and without diabetes: pooled analysis from the PIVENS and FLINT NIDDK NASH CRN trials. Hepatology. 2015;62:261–5. [Google Scholar]
- 27. Neuschwander-Tetri BA. Loomba R. Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotman Y. Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2016;66:180–90. doi: 10.1136/gutjnl-2016-312431. [DOI] [PubMed] [Google Scholar]