ABSTRACT
Hepatitis B virus reactivation (HBVr) is emerging as an important clinical entity, with the advent of highly potent immunosuppression licensed for use as the treatment of a widening range of clinical indications. HBVr can lead to severe acute liver failure and death. Risk can be minimised through appropriate screening, monitoring and antiviral prophylaxis. Screening for serological markers at the earliest opportunity is recommended. Risk stratification should then be performed on the basis of characteristics of the underlying disease, markers of viral activity and the potency of proposed immunosuppression. In this review, we summarise the most recent recommendations from the relevant international societies. We also provide suggestions on how a robust multidisciplinary service can be delivered to prevent HBVr in UK clinical practice through optimisation of resources and introduction of checkpoints to prevent the inappropriate administration of immunosuppression to those at significant risk of HBVr.
KEYWORDS: Chronic hepatitis B, reactivation, immunosuppression
Introduction
Hepatitis B virus (HBV) infection remains a major global healthcare challenge with approximately one-third of the worldwide population living with current or past infection. The prevalence of individuals in the UK with serological evidence of present or past infection is increasing, predominantly in urban areas, because of migration patterns. In London, 1% of women undergoing antenatal screening have been identified as being hepatitis B surface antigen (HBsAg) positive, with incidence rates as high as 2.8% in some areas.1
Chronic hepatitis B (CHB) is classically described in distinct disease phases, depending on the host immune response to the virus and the presence or absence of HBsAg.2 Approximately 95% of individuals acutely infected in adulthood will spontaneously seroconvert and lose HBsAg. Conversely, only 5% of infants infected at birth or during early childhood will lose HBsAg, the remainder developing CHB. The correct interpretation of HBV serology is summarised in Table 1.
Table 1.
Definition and interpretation of serological tests for hepatitis B virus
| Markers | Clinical interpretation |
| HBsAg | Hallmark of infection; positive during early phase of acute infection, persistently positive in chronic infection |
| Anti-HBs | Recovery from acute infection (or chronic); immunity following vaccination |
| HBeAg | eAg positivity associated with high replicative state; presence of inflammation and/or fibrosis determines disease phase; eAg negativity reflects a change in disease phase and is usually associated with the emergence of anti-HBe; viral mutations in precore and basal core promoter regions result in eAg-negative hepatitis |
| Anti-HBe | Marker of eAg seroconversion associated with immune control in low viraemic states |
| Anti-HBc (IgM) | Positive in acute infection; may be positive during reactivation of HBV |
| Anti-HBc (IgG) | Exposure to infection and present in association with HBsAg in chronic infection; HBsAg-negative, anti-HBc-positive serology usually indicative of past exposure to virus; anti-HBs may /may not be positive; if anti-HBs negative, a false positive anti-HBc should be considered (eg after IVIG infusion); HBV DNA must be checked to exclude occult infection |
| Tests | Clinical interpretation |
| HBsAg (–) | Indicative of past infection and clinically relevant in the context of immune suppression |
| Total anti-HBc (+) | |
| Anti-HBs (+) | |
| HBsAg (–) | Indicative of prior hepatitis B vaccination |
| Total anti-HBc (–) | |
| Anti-HBs (+) | |
| HBsAg (+) | Indicative of acute HBV infection |
| Total anti-HBc (+) | |
| Anti-HBc IgM (+) | |
| Anti-HBs (–) | |
| HBsAg (+) | HBsAg positivity is the hallmark of chronic HBV infection |
| Total anti-HBc (+) | |
| Anti-HBc IgM (–) | |
| Anti-HBs (–) | |
| HBsAg (–) | Number of potential clinical interpretations: (i) past HBV infection (ii) false-positive anti-HBc (iii) occult chronic hepatitis B if HBV DNA detectable (iv) resolving acute infection |
| Total anti-HBc (+) | |
| Anti-HBs (–) |
Anti-HBs = hepatitis B surface antibody; anti-HBe = hepatitis B ‘e’ antibody; anti-HBc = hepatitis B core antibody; HBeAg = hepatitis B ‘e’ antigen; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; IVIG = intravenous immunoglobulin
Individuals with CHB are likely to be asymptomatic and liver function tests are often normal. Therefore, serological testing is essential to determine the HBV status of an individual. Individuals who are HBsAg negative and hepatitis B core antibody (anti-HBc) positive have evidence of past exposure to HBV. Hepatitis B virus DNA should be checked in these individuals to exclude occult infection. Hepatitis B infection results in the presence of covalently closed circular DNA (cccDNA) in hepatocytes, regardless of whether either HBsAg or HBV DNA are detectable. cccDNA acts as a transcriptional template and can persist indefinitely; it is the formation of this minichromosome that is central to HBV reactivation (HBVr).3,4 As such, the concept of past infection is something of a misnomer; patients who are HBsAg negative, anti-HBc positive and HBV DNA negative will be referred to as ‘anti-HBc positive’ and are considered to have resolved HBV infection. In practice, individuals with prior exposure to infection and undetectable HBV DNA require no specific management or monitoring unless immunosuppressed as a consequence of disease or specific therapies, when HBVr can potentially become a clinically significant entity. Hepatitis B virus reactivation can manifest as asymptomatic viraemia with or without perturbation in serum alanine aminotransferase (ALT); however, a subgroup of patients will develop severe liver injury with jaundice, liver failure or even death.
There is emerging evidence suggesting that the actual number of individuals presenting with HBVr following commencement of treatment with immunosuppressive agents is increasing. In the UK, this can be attributed to an increase in the prevalence of positive HBV serology in the population in tandem with a rise in the licensed clinical indications for potent immunosuppression, including, but not restricted to, malignancies, inflammatory bowel disease (IBD), autoimmune disorders and rheumatic disease, as well as to the emergence of new agents that appear to account for HBVr.5–7 In view of this, all major medical and scientific societies, including the American Association for the Study of Liver Diseases (AASLD), American College of Rheumatology (ACR), American Gastroenterological Association (AGA), American Society of Clinical Oncology (ASCO), British Society for Rheumatology (BSR), Centers for Disease Control and Prevention (CDC), German Society of Haematology and Medical Oncology (DGHO), European Association for the Study of the Liver (EASL), European Crohn's and Colitis Organisation (ECCO), European Society for Medical Oncology (ESMO), have published guidance on the management of HBVr. However, there is still ambiguity regarding optimal management to prevent HBVr, particularly regarding patients with negative HBsAg but positive anti-HBc. NICE guidelines have highlighted the importance of further research to elucidate the factors driving HBVr and to identify better strategies to screen and manage patients with positive hepatitis B serology receiving immunosuppressive treatments.8
In this review, we summarise the recent international recommendations to prevent HBVr and provide some suggestions based on a multidisciplinary approach for prevention of HBVr in the UK healthcare setting.
Screening
The risk of HBVr can be classified as high (>10%), moderate (1–10%) or low (<1%). Testing for HBV serology before initiating immunosuppressive medication is recommended by international societies across disciplines, yet is poorly performed.9,10 Effective screening should include testing for HBsAg, anti-HBc and hepatitis B surface antibody (anti-HBs). If either HBsAg or anti-HBc is positive, testing for HBV DNA is mandated.8,11 The rate of HBV DNA positivity in patients with isolated positive anti-HBc serology has been estimated to be between 1.7% and 41%.12 Even in individuals with negative HBsAg and positive anti-HBs, cases are reported with detectable HBV DNA. Although this is less common than in those with isolated anti-HBc, it is important that all patients with anti-HBc positivity are screened for occult infection.13 Paul et al recently showed that patients with resolved HBV infection receiving chemotherapy for haematological malignancies without antiviral prophylaxis, are at a decreased risk of HBVr when they have positive anti-HBs.14 More recently, the virological marker Hepatitis B core-related antigen (HBcrAg) was reported to be associated with an increased risk of HBVr in HBsAg-negative and anti-HBc-positive subjects undergoing high-risk immunosuppressive regimens.15 This test remains a research tool and is not routinely used in clinical practice.
Early screening for HBV markers enables timely initiation of antiviral prophylaxis or treatment where indicated and reduces the risk of liver failure and death secondary to HBV reactivation in patients receiving chemotherapy.16 Similarly, it also prevents any delay in starting immune suppression while awaiting specialist input and/or additional investigations. Patients with positive serology, particularly those with isolated anti-HBc, can be offered repeat testing.12
Individuals with negative serological markers who are likely to need immune suppression should be immunised against HBV and it is noteworthy that effective immunisation is more challenging in this context. The first dose of anti-HBV vaccine should be administered 1–2 weeks before the administration of treatment and higher doses may be required in patients who are immunocompromised. A minimum of three doses of vaccine administered at monthly intervals are required for effective immunisation in patients who are immunocompetent. ECCO recommends monitoring maintenance of anti-HBs in patients at risk every 1–2 years.17,18 False positive testing for HBsAg can occur for 1–2 weeks following administration of the vaccine, because the assay can also detect surface antigen (sAg) in the vaccine preparation.
Table 2 shows the guidance from international societies on screening for HBV before immune suppression.
Table 2.
Guidelines on screening for hepatitis B virus markers before immunosuppression or chemotherapy
| Society | Who should be screened? | Screening tests |
| AGA | Patients at moderate or high risk of HBVr | HBsAg, anti-HBc + HBV DNA in case of positive results |
| ASCO | Groups at heightened risk for chronic HBV infection or if highly immunosuppressive treatment is planned | HBsAg+- anti-HBc in some populations |
| CDC | All persons receiving cytotoxic or immunosuppressive therapy | HBsAg, anti-HBc, and anti-HBs |
| DGHO | Groups at heightened risk | HBsAg, anti-HBc + HBV DNA in case of positive results |
| ECCO | All IBD patients at diagnosis | HBsAg, anti-HBc, and anti-HBs + HBV DNA in case of positive results |
| EASL | All candidates for chemotherapy and immunosuppression | HBsAg, anti-HBc, and anti-HBs +HBV DNA in case of positive results |
AGA = American Gastroenterological Association; anti-HBc = hepatitis B core antibody; anti-HBs = hepatitis B surface antibody; ASCO = American Society of Clinical Oncology; CDC = Centers for Disease Control and Prevention; DGHO = German Society for Haematology and Medical Oncology; EASL = European Association for the Study of the Liver; ECCO = European Crohn´s and Colitis Organisation; HBsAg = hepatitis B surface antigen; HBV = hepatitis B virus; HBV DNA = hepatitis B virus DNA; HBVr = hepatitis B reactivation; IBD = inflammatory bowel disease
Blood products and intravenous immunoglobulin
Transfusion of blood products or infusion of intravenous immunoglobulin (IVIG) can result in passive transmission of antibodies associated with HBV. This can lead to patients being falsely informed that there is evidence of past HBV or, more importantly, being considered for antiviral prophylaxis in the context of immunosuppression. Baseline anti-HBc should be measured early during the course of disease to avoid this scenario and, if negative, subsequent positive serology can be disregarded in the absence of ongoing risk of acquisition of HBV. Should liver function tests become deranged during the course of immune suppression, HBsAg should be retested, as in any other patient.19
Management of patients who are HBsAg positive
Hepatitis B virus reactivation is more common in individuals who are HBsAg positive, as opposed to HBsAg negative, and is defined as an increase in viral load of at least 100-fold. This may be associated with a transaminitis and, in some cases, will lead to acute liver failure. Patients who are positive for HBsAg should be referred to a clinician with experience in managing CHB, regardless of whether immunosuppressive therapy is planned or the level of HBV DNA.
If immune suppression is planned, patients who are HBsAg positive should start prophylaxis treatment with nucleos(t)ide therapy, namely tenofovir disoproxil fumarate (TDF) or entecavir (ETV), regardless of pretreatment disease activity. The recently licensed tenofovir alafenamide (TAF) may be indicated if there are renal concerns or prior exposure to lamivudine (or virological relapse on entecavir therapy).11,17,20–23
This is the consensus approach from most international societies, with the exception of AGA. AGA exempts from this HBsAg-positive patients considered to be at a low risk of HBVr (<1%), who do not meet standard indications for antiviral treatment. Such groups might include those treated with traditional immunosuppressive agents (eg azathioprine, 6-Mercaptopurine (6MP) or methotrexate), patients treated with intra-articular corticosteroids and those treated with any dose of oral corticosteroids daily for 1 week.20 This may be a reasonable approach in the context of short courses of steroids or intra-articular treatment. However, in the context of long-term immunosuppression with azathioprine, 6MP and/or methotrexate, we support the view of EASL that patients who are HBsAg positive should be offered antiviral prophylaxis, particularly if it is likely that escalation to biologic therapies will be required in the future. Lamivudine should not be used in this group of patients because of the increased risk of viral resistance. NICE guidelines from 2013 recommend the use of lamivudine as prophylaxis in patients with HBV DNA <2,000 IU/mL.8 However, generic formulations of ETV and TDF are now available as cost-effective alternatives and so there is no real barrier to prescribing these drugs as first-line agents in this setting.
EASL advise that antiviral treatment should be continued for at least 12 months after the cessation of immune suppression (18 months in the case of rituximab). This protracted course of antiviral prophylaxis is warranted because HBVr has been reported many months after the cessation of rituximab. This represents a shift in practice compared with guidance issued by NICE in 2013 that recommended just 6 months of prophylaxis after cessation of immunosuppression.8 Liver function tests and HBV DNA should be tested every 3–6 months during prophylaxis.11 Patients with baseline HBV DNA levels above the treatment threshold (>2,000 IU/mL) should continue antiviral treatment until endpoints applicable to patients who are immunocompetent are reached.17
We recommend that cases should be discussed in a multidisciplinary setting before starting antiviral treatment. Long-term follow-up and monitoring of HBV markers should be conducted through specialist clinics.
Management of patients with evidence of past exposure to HBV (HBsAg negative and/or anti-HBc positive)
Patients in this group should be tested for HBV DNA and, if detected, should be managed as though they are HBsAg positive (see above).11 Patients who are HBsAg negative, anti-HBc positive and DNA negative are referred to as ‘anti-HBc positive’ and are considered to have resolved HBV infection.
Patients who are anti-HBc positive will be at variable risk of HBVr depending upon host factors, including age and sex, the underlying condition for which immunosuppression is indicated, and the immunosuppressive regimen planned. There is more uncertainty over the management of this group than over those who are HBsAg positive because HBVr rates are variable. Titres of anti-HBs >100 IU/mL have been associated with lower rates of HBVr in patients treated with rituximab for lymphoma,24 as have low titres of anti-HBc.25 At present, none of the main international societies recommend withholding antiviral prophylaxis during rituximab on the basis of thresholds of serological titres for either anti-HBs or anti-HBc.
The underlying disease appears to have an important role in HBVr risk. Non-Hodgkin's lymphomas (NHL) are the haematological malignancies most commonly associated with viral reactivation.26 In a multivariate analysis, acute lymphoid leukaemia (ALL) and multiple myeloma were also found to be independent risk factors for HBVr in patients who were anti-HBc positive.27
Risk can be stratified based upon the drug regimen to be used. In 2013, rituximab received a box warning from the US Food and Drug Administration (FDA) regarding the risk of HBV reactivation.28 Similarly, the European Medicine Agency (EMA) added a special warning for the BCR-ABL tyrosine kinase inhibitors (imatinib, bosutinib, dasatinib, nilotinib and ponatinib); their use in patients with positive HBV serology is also associated with the possibility of HBVr (EMA pharmacovigilance).
Risk assessment based on immune suppression regimens can be categorised as shown in Table 3.
Table 3.
Risk of hepatitis B virus reactivation stratified by immunosuppressive regimen
| HBsAg positive | HBsAg negative – anti-HBc positive | |
| High risk >10% |
|
|
| Moderate risk 1-10% |
|
|
| Low risk <1% |
|
|
Anti-HBc = hepatitis B core antibody; HBsAg = hepatitis B surface antigen; HBVr = hepatitis B virus reactivation; HCC = hepatocellular carcinoma; HDIs = histone deacetylase inhibitors; TACE = transarterial chemoembolization; TNF = tumour necrosis factor
High risk (>10%)
Rituximab and other B-cell depleting therapies pose a particular risk for HBVr and all patients with active or past exposure to hepatitis B infection should receive antiviral prophylaxis. We recommend that ETV/TDF is used first line for antiviral prophylaxis in patients who are anti-HBc positive. Prophylaxis should continue throughout rituximab treatment and for at least 18 months afterwards (and indefinitely in those with ongoing immunosuppression as a result of active haematological malignancy).29 Monitoring for reactivation should continue for a further 12 months after the cessation of antiviral prophylaxis.11 Recently, the PREBLIN study, a randomised prospective study, demonstrated a trend suggesting a prophylactic effect of TDF in preventing HBVr in patients receiving rituximab-based regimens for haematological malignancies.30
Haematopoietic stem cell transplantation (HSCT), in particular allogeneic HSCT, is associated with a significantly increased risk of HBVr, with the highest rate (>50%) reported in patients who are HBsAg positive.31 Patients who are anti-HBc positive are also at risk of HBVr, reported to be as high as 20%.32 Rituximab, the number of chemotherapy cycles, as well as low anti-HBs levels were all reported to be risk factors for HBVr.33 Of note, in such patients, the risk of seroreversion can persist for years.34
Moderate risk (1–10%)
Table 3 shows drugs deemed to be of medium risk of HBVr. EASL advises that patients who are anti-HBc positive can receive medium risk immunosuppression with careful monitoring and pre-emptive therapy. Their recommendation is that HBsAg and/or HBV DNA should be monitored every 1–3 months during and after immunosuppressive therapy; antiviral treatment should be initiated if either is positive.11
A recent comprehensive review suggested that patients who are anti-HBc positive can be appropriately managed during immunosuppression with either monitoring or antiviral prophylaxis.3 The guidance from international societies varies on this issue, with some in favour of initiating antiviral treatment in patients on immunosuppressive treatment only in the event of detectable HBV DNA or elevated aminotransferases. AASLD considers that, in patients who are anti-HBc positive, reactivation is infrequent and, hence, they should be monitored, and antiviral therapy only initiated upon HBVr occurrence.22,23 ECCO also recommends against routine prophylaxis and suggests monitoring of HBV DNA and ALT/aspartate aminotransferase (AST), taking into consideration the fact that HBVr of patients who are anti-HBc positive rarely occurs during the treatment of IBD.17
The inhibition of tumour necrosis factor (TNF)-α signalling could lead to increased HBV replication and, hence, reactivation of the virus. Anti-TNF-α agents (eg infliximab, adalimumab, certolizumab, golimumab and etanercept) are widely used to treat patients with rheumatic disease, IBD and psoriasis, among other conditions, and have been implicated in HBVr; the estimated risk of viral reactivation with anti-TNF-α monotherapy varied in different studies from 1% to 10% for individuals who were HBsAg positive and was significantly lower in patients who were anti-HBc positive. It is still unclear whether the risk of HBVr varies between the anti-TNF-α agents.7,35–37 In a meta-analysis of patients treated with anti-TNF-α for rheumatic diseases, patients who were anti-HBc positive had a HBVr rate of 1.7%, while a study of patients who were HBsAg positive reported HBVr in 12.3% of patients.38,39 The risk of HBVr with novel agents, such as ustekinumab, natalizumab, alemtuzumab and vedolizumab, remains unclear.5,37
Low risk (1%)
Patients on immune suppressive regimens with low risk of HBVr generally only require monitoring as outlined above. If there is likely to be treatment escalation, a prolonged duration of immunosuppression, or the underlying disease predisposes to immune suppression, then antiviral prophylaxis may be appropriate.
Steroids
Prednisolone constitutes an independent risk factor for HBVr when used as a monotherapy or, more importantly, when combined with other immunosuppressive medications.35 All professional international societies suggest that, when a patient is due to be immunosuppressed with corticosteroids, an appropriate level of risk stratification should be undertaken, because it has been shown that the immunosuppressive effect of steroids is dose and duration dependent.40 Guidelines from both DGHO and AGA in 2015 highlight the high risk of HBVr (10%) in patients who are HBsAg positive treated with moderate- (prednisolone 10–20 mg or equivalent) or high-dose (prednisolone >20 mg or equivalent) corticosteroids for ≥4 weeks.
The following groups are deemed to be at moderate risk (1–10%) of HBVr during treatment with steroids: patients who are HBsAg positive treated with low-dose (prednisolone <10 mg daily or equivalent) corticosteroids for ≥ 4 weeks, and patients who are anti-HBc positive treated with moderate- (prednisolone 10–20 mg daily or equivalent) or high-dose (prednisolone >20 mg daily or equivalent) corticosteroids daily for ≥ 4 weeks.
Patients at low risk (<1%) of HBVr include those treated with intra-articular corticosteroids or a course of steroids lasting less than a week at any dose.20,21
Practical considerations
Data from UK practice demonstrate that the introduction of local policies and raising awareness of the risk of HBVr can significantly improve the uptake of screening before starting rituximab.9 Audits of local practice will help inform whether guidance is being adhered to and to ascertain the local prevalence of at-risk patients to inform resource allocation. For high-risk immunosuppression, such as rituximab, it would be prudent to introduce checkpoints within pharmacies and on infusion units to ensure that HBV serology has been checked before drug dispensing and/or administration.
More evidence is needed to facilitate decisions of whether to initiate monitoring or prophylaxis in the medium-risk group. Factors that may favour prophylaxis over monitoring alone are: patient age (or generally physiologically frail), male sex, haematological malignancy, treatment with more than one low-to-medium-risk immunosuppressive agent, or a reasonable likelihood of treatment escalation. If monitoring is chosen, then consideration needs to be given to how this will be delivered in clinical practice and who is the responsible physician.
In situations where monitoring cannot be offered reliably in the moderate-risk group, then antiviral prophylaxis might be a more appropriate and pragmatic approach. ETV and TDF are considered safe and well tolerated and the emergence of generic formulations in the UK make this a cost-effective option. The wishes of the patient should also be considered in this situation.
In our experience, antiviral treatment and monitoring during immune suppression can be safely delivered by specialist nurses with experience in managing HBV. However, we recommend that all patients with positive HBV serology should be discussed in a multidisciplinary setting under the guidance of a physician with an interest in HBV before the initiation of immunosuppression and management in a nurse-led clinic.
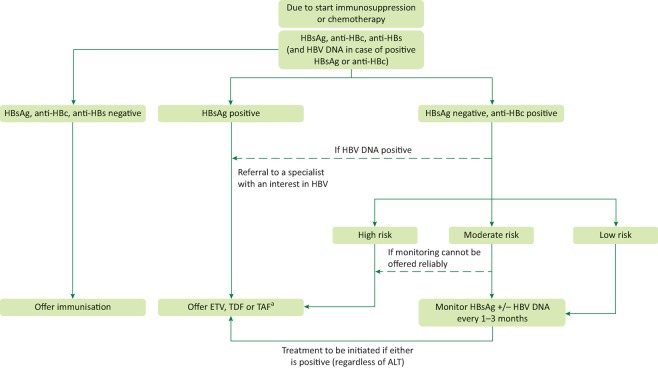
A multidisciplinary model could be developed as shown in Fig 1 to help identify areas where increased resources might be required and referral pathways streamlined.
Fig 1.
Guidance on the management of hepatitis B virus reactivation in patients who are due to be initiated on immunosuppression or chemotherapy. aThe risk of HBVr is low in the context of intra-articular steroids or a course of oral steroids for ≤ 7 days. As such, antiviral prophylaxis is not usually indicated in this setting. anti-HBc = hepatitis B core antibody; anti-HBs = hepatitis B surface antibody; ETV = entecavir; HBsAg = hepatitis B surface antigen; HBVr = hepatitis B virus reactivation; TAF = tenofovir alafenamide; TDF = tenofovir disoproxil fumarate
Conclusion
HBVr is likely to be of increasing clinical significance as potent immunosuppressive regimens are used more widely across all medical specialties and, as such, should be managed using a multidisciplinary approach. HBVr will be of particular importance in clinical practice where the population served includes migrants from endemic areas, and treating physicians should be aware of the potential risk for patients with resolved HBV infection. In an era of safe and inexpensive potent antivirals, there is now a paradigm shift to offer antiviral prophylaxis to more patients who are at risk of HBVr and to extend the duration of both prophylaxis and subsequent monitoring. Further research to improve risk stratification is required, including the evaluation of novel virological markers, such as HBcrAg, as well as anti-HBc and anti-HBs titres and their potential utility in clinical practice.
References
- 1.Public Health England Hepatitis B epidemiology in London: 2012 data. London: PHE; 2014. [Google Scholar]
- 2.Gill US. Kennedy PT. New insights in the management of chronic hepatitis B. Clin Med. 2015;15:191–6. doi: 10.7861/clinmedicine.15-2-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loomba R. Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang TJ. Block TM. McMahon BJ, et al. Present and future therapies of hepatitis B: From discovery to cure. Hepatology. 2015;62:1893–908. doi: 10.1002/hep.28025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessone F. Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol. 2016;8:385–94. doi: 10.4254/wjh.v8.i8.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin ST. Cardwell SM. Nailor MD. Gabardi S. Hepatitis B reactivation and rituximab: a new boxed warning and considerations for solid organ transplantation. Am J Transplant. 2014;14:788–96. doi: 10.1111/ajt.12649. [DOI] [PubMed] [Google Scholar]
- 7.Perrillo RP. Gish R. Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221–44. doi: 10.1053/j.gastro.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Sarri G. Westby M. Bermingham S, et al. Diagnosis and management of chronic hepatitis B in children, young people, and adults: summary of NICE guidance. BMJ. 2013;346:f3893. doi: 10.1136/bmj.f3893. [DOI] [PubMed] [Google Scholar]
- 9.Dyson JK. Jopson L. Ng S, et al. Improving testing for hepatitis B before treatment with rituximab. Eur J Gastroenterol Hepatol. 2016;28:1172–8. doi: 10.1097/MEG.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung C. Tsoi E. Burns G. Sievert W. An argument for the universal prophylaxis of hepatitis B infection in patients receiving rituximab: a 7-year institutional experience of hepatitis screening. Oncologist. 2011;16:579–84. doi: 10.1634/theoncologist.2010-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Wu T. Kwok RM. Tran TT. Isolated anti-HBc: The Relevance of Hepatitis B Core Antibody-A Review of New Issues. Am J Gastroenterol. 2017;112:1780–8. doi: 10.1038/ajg.2017.397. [DOI] [PubMed] [Google Scholar]
- 13.Anastasiou OE. Widera M. Korth J, et al. Clinical patterns associated with the concurrent detection of anti-HBs and HBV DNA. J Med Virol. 2018;90:282–90. doi: 10.1002/jmv.24942. [DOI] [PubMed] [Google Scholar]
- 14.Paul S. Dickstein A. Saxena A, et al. Role of surface antibody in hepatitis B reactivation in patients with resolved infection and hematologic malignancy: a meta-analysis. Hepatology. 2017;66:379–88. doi: 10.1002/hep.29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seto WK. Wong DH. Chan TY, et al. Association of hepatitis B core-related antigen with hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol. 2016;111:1788–95. doi: 10.1038/ajg.2016.436. [DOI] [PubMed] [Google Scholar]
- 16.Hwang JP. Suarez-Almazor ME. Cantor SB, et al. Impact of the timing of hepatitis B virus identification and anti-hepatitis B virus therapy initiation on the risk of adverse liver outcomes for patients receiving cancer therapy. Cancer. 2017;123:3367–76. doi: 10.1002/cncr.30729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahier JF. Magro F. Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–68. doi: 10.1016/j.crohns.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Rubin LG. Levin MJ. Ljungman P, et al. IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay I. Gorton RL. Patel M, et al. Transmission of hepatitis B core antibody and galactomannan enzyme immunoassay positivity via immunoglobulin products: a comprehensive analysis. Clin Infect Dis. 2016;63:57–63. doi: 10.1093/cid/ciw222. [DOI] [PubMed] [Google Scholar]
- 20.Reddy KR. Beavers KL. Hammond SP. Lim JK. Falck-Ytter YT. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215–9. doi: 10.1053/j.gastro.2014.10.039. quiz e16–7. [DOI] [PubMed] [Google Scholar]
- 21.Sandherr M. Hentrich M. von Lilienfeld-Toal M, et al. Antiviral prophylaxis in patients with solid tumours and haematological malignancies–update of the Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society for Hematology and Medical Oncology (DGHO) Ann Hematol. 2015;94:1441–50. doi: 10.1007/s00277-015-2447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lok AS. McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 23.Lok AS. McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 24.Cho Y. Yu SJ. Cho EJ, et al. High titers of anti-HBs prevent rituximab-related viral reactivation in resolved hepatitis B patient with non-Hodgkin's lymphoma. J Med Virol. 2016;88:1010–7. doi: 10.1002/jmv.24423. [DOI] [PubMed] [Google Scholar]
- 25.Matsubara T. Nishida T. Shimoda A, et al. The combination of anti-HBc and anti-HBs levels is a useful predictor of the development of chemotherapy-induced reactivation in lymphoma patients with resolved HBV infection. Oncol Lett. 2017;14:6543–52. doi: 10.3892/ol.2017.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcucci F. Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood. 2011;117:1792–8. doi: 10.1182/blood-2010-06-275818. [DOI] [PubMed] [Google Scholar]
- 27.Han JW. Yang H. Lee HL, et al. Risk factors and outcomes of hepatitis B virus reactivation in hepatitis B surface antigen negative patients with hematological malignancies. Hepatol Res. 2016;46:657–68. doi: 10.1111/hepr.12603. [DOI] [PubMed] [Google Scholar]
- 28.Mitka M. FDA: Increased HBV reactivation risk with ofatumumab or rituximab. JAMA. 2013;310:1664. doi: 10.1001/jama.2013.281115. [DOI] [PubMed] [Google Scholar]
- 29.Marrone A. Capoluongo N. D’Amore C, et al. Eighteen-month-lamivudine-prophylaxis on preventing occult hepatitis b virus (hbv) infection reactivation in patients with hematologic malignancies receiving immunosuppression therapy. J Viral Hepat. 2018;25:198–204. doi: 10.1111/jvh.12802. [DOI] [PubMed] [Google Scholar]
- 30.Buti M. Manzano ML. Morillas RM, et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: the Preblin study. PLoS ONE. 2017;12(9):e0184550. doi: 10.1371/journal.pone.0184550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Potential risk of hepatitis B reactivation after treatment with BCR-ABL Tyrosine Kinase Inhibitors. 2016. www.esmo.org/Oncology-News/Potential-Risk-of-Hepatitis-B-Reactivation-After-Treatment-With-BCR-ABL-Tyrosine-Kinase-Inhibitors. [Accessed 7 February 2017]
- 32.Hammond SP. Borchelt AM. Ukomadu C, et al. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049–59. doi: 10.1016/j.bbmt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Lalazar G. Rund D. Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699–712. doi: 10.1111/j.1365-2141.2006.06465.x. [DOI] [PubMed] [Google Scholar]
- 34.Palmore TN. Shah NL. Loomba R, et al. Reactivation of hepatitis B with reappearance of hepatitis B surface antigen after chemotherapy and immunosuppression. Clin Gastroenterol Hepatol. 2009;7:1130–7. doi: 10.1016/j.cgh.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seetharam A. Perrillo R. Gish R. Immunosuppression in patients with chronic hepatitis B. Curr Hepatol Rep. 2014;13:235–44. doi: 10.1007/s11901-014-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzano-Alonso ML. Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011;17:1531–7. doi: 10.3748/wjg.v17.i12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reynolds JA. Manch RA. Gish RG. Medical interventions associated with HBV reactivation: common and less common. Clin Liver Dis. 2015;5:32–4. doi: 10.1002/cld.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YH. Bae SC. Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol. 2013;31:118–21. [PubMed] [Google Scholar]
- 39.Lee YH. Bae SC. Song GG. Hepatitis B virus reactivation in HBsAg-positive patients with rheumatic diseases undergoing anti-tumor necrosis factor therapy or DMARDs. Int J Rheum Dis. 2013;16:527–31. doi: 10.1111/1756-185X.12154. [DOI] [PubMed] [Google Scholar]
- 40.Gu HR. Shin DY. Choi HS, et al. HBV reactivation in a HBsAg-negative patient with multiple myeloma treated with prednisolone maintenance therapy after autologous HSCT. Blood Res. 2015;50:51–3. doi: 10.5045/br.2015.50.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]