Abstract
Background: Multiple Sclerosis (MS) is an autoimmune disease of the central nervous system, mostly affecting young adults. Diamine oxidase is an enzyme essential for histamine production. Histamine which is produced mostly by mast cells can have effects on different aspects of immune system via its different histamine receptors (H1R, H2R, H3R and H4R). The crucial role of diamine oxidase and histamine in immune balance has been documented in different studies and experiments both on MS patients and on experimental allergic encephalomyelitis (EAE). In this regard, we aimed to measure the level of histamine and diamine oxidase in the serum of MS patients. Methods: A total number of 50 relapsing-remitting MS (RRMS) patients and 41 age and sex matched controls were enrolled in this study. Assessments of serum levels of histamine and diamine oxidase enzyme were performed using enzyme-linked immune sorbent assay (ELISA). Results: The serum levels of histamine and diamine oxidase in RRMS patients were lower than healthy controls (P-value = 0.00, for both). Conclusion: Our research team found significant low levels of histamine and diamine oxidase in RRMS patients; however the pathogenesis of this issue was unclear.
Keywords: Multiple sclerosis, histamine, diamine oxidase
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory autoimmune disease of central nervous system (CNS) which is also the leading cause of non-traumatic neurodegenerative disease among young adults that affects almost 2.5 million people worldwide [1]. The precise mechanism and pathogenesis of MS still remains unknown however; immune system and immune cells as well as genetic and environmental factors have been suggested as risk factors for susceptibility to MS [2,3]. Inflammation, demyelination, axonal loss and gliosis are morphological manifestation of MS in which different immune cell types play important roles. Different lines of evidence have revealed important function of T lymphocytes and inflammatory and anti-inflammatory cytokine balance. Different MS models have been studied in order to determine the pathology of MS and factors affecting disease course. At the top of these methods it stands experimental allergic encephalomyelitis (EAE) in which certain myelin peptides are exposed to immune cells [4]. One of the important factors with physiological and pathological roles in our body is histamine [2-4-imidazole ethylamine], a ubiquitous inflammatory mediator with not only neuro-transmitting and vasoactive functions, but also documented pro-inflammatory effects in body. It also should be noted that L-histidine decarboxylase (HDC) is an essential enzyme for histamine bio synthesis from L-histidine. Two major histamine catabolic pathways have been detected for histamine including diamine oxidase and histamine methyl-transferase [5].Along with the established role of histamine in changing the permeability of blood brain barrier (BBB) and its effects on immune cells and cytokine production, a conflicting question has been proposed that whether inhibition of histamine can be considered as a novel and promising therapeutic target [6]. Accumulating lines of evidence have recently suggested that the effects of histamine are mediated through four pharmacologically distinct subtypes of histamine receptors (HR) expressed on different cells including: H1R, H2R, H3R and H4R. It has been also shown that histamine has different functions through each of these G-protein coupled receptors [5]. In this regard, pro-inflammatory effects of histamine are mediated by H1R while its physiological actions are exerted via H2R and H3R. H4R has modulating effects on the immune system [7]. Furthermore, investigations of EAE models have also suggested histamine as a pivotal immune modulating mediator [8]. Evaluation of histamine and diamine oxidase serum levels in MS patients can also add new insights in to the effects of histamine in MS pathogenesis. Therefore, we aimed to measure serum levels of histamine and diamine oxidase in MS patients and healthy controls.
Methods and materials
Our study population was made up of fifty patients (41 females and 9 males) diagnosed with relapsing-remitting form of multiple sclerosis (RRMS) and forty-one (33 females and 8 males). All MS patients with the mean age of 30.82 ± 9.22 years were recruited from MS clinic of Alzahra-hospital in Isfahan, the third large province of Iran. Every one of the MS patients had been diagnosed with definite MS by expert neurologists by brain MRI according to McDonald’s [9] or dissemination in space and dissemination in time and Expanded Disability Status Scale (EDSS) [10] assessments were done also by expert neurologists. Non-inflammatory neurological disease (NIND) individuals serving as our healthy control population, and our control population with the mean age of 31.34 ± 8.19 years volunteered from Iranian Blood Transfusion Organization (IBTO). One of our inclusion criteria described the stable neurological functioning for at least one month prior to study entry for all of our MS patients. Those MS patients having any other autoimmune disease, allergic and hypersensitivity disease and infection diseases were excluded from the study as well as our control subjects. It should also be noted that all the patients and healthy control group signed the written informed consent and also the Ethical Committee on Human Research of Isfahan University of Medical Sciences approved this study (No:293724). For measurements tests, by using routine venipuncture, 3-5 ml of venous blood samples were collected from each one of both MS patients and healthy control subjects. Centrifuging at 2400 g for 10 min, made plasma content to recruit and then it was stored at -80°C. For measuring plasma levels of histamine and diamine oxidase we utilized enzyme-linked immunosorbent assay (ELISA) commercial kits according to the manufacturer’s instructions (Eastbiopharm, Torrance, USA).
Normal distribution of data was assessed using Kolmogorov-Smirnov Z-test, Independent t test and chi square test were used to assess differences between both groups. Also Pearson was used for correlation. Data are shown as number (percent) and mean ± SD and P-value less than 0.05 was deemed as significant threshold.
Results
In this study, Data analyses about gender, age and blood group differences between our MS population and control group showed no significant differences (P-value = 0.85 for gender differences, P-value = 0.36 for age differences and P-value = 0.87 for blood group differences) meaning case and control subjects were matched in ranges of gender, age condition. Further demographic data about our MS patients and control subjects are summarized in Table 1. The mean serum histamine levels for MS patients were 52.00 ± 21.72 ng/ml while on the other hand same measurements for healthy control group indicated serum histamine levels of 86.66 ± 83.06 ng/ml, therefore these decreased histamine levels were significant (P-value: 0.00) (Figure 1). The other factor of our measurements was diamine oxidase serum level which was 99.74 ± 20.23 ng/ml in MS patients and 137.71 ± 75.71 ng/ml in healthy controls (Figure 2). Data analysis demonstrated that this lower amount of diamine oxidase was significant (P-value = 0.00) (Table 1). The Means duration of disease and EDSS for MS patients was 3.52 ± 3.48 years and 1.53 ± 1.26, respectively and mostly of patients treated with Interferon β 1a (38%) (Table 2). In addition, histamine and diamine oxidase serum levels have no significant correlation with EDSS, age and duration of disease (P > 0.05, for all) (Table 3).
Table 1.
Demographic information of subjects
| Characteristics | Patients | Controls | P-value |
|---|---|---|---|
| Number | 50 | 41 | - |
| Mean age (years) (mean ± SD) | 30.82 ± 9.22 | 31.34 ± 8.19 | 0.36 |
| Gender | 0.85 | ||
| Male | 9 (18%) | 8 (19.5%) | |
| Female | 41 (82%) | 33 (19.5%) | |
| Blood group | 0.87 | ||
| A+ | 12 (24%) | 15 (36.6%) | |
| A- | 1 (2%) | 1 (2.4%) | |
| B+ | 3 (6%) | 6 (14.6%) | |
| B- | 1 (2%) | 1 (2.4%) | |
| AB+ | 6 (12%) | 4 (9.8%) | |
| AB | 1 (2%) | 1 (2.4%) | |
| O+ | 17 (34%) | 11 (26.8%) | |
| O- | 3 (6%) | 2 (4.9%) | |
| Histamine blood levels (ng/ml) (mean ± SD) | 52.00 ± 21.72 | 86.66 ± 83.06 | 0.00 |
| Diamine oxidase levels (ng/ml) (mean ± SD) | 99.74 ± 20.23 | 137.71 ± 75.71 | 0.00 |
Figure 1.
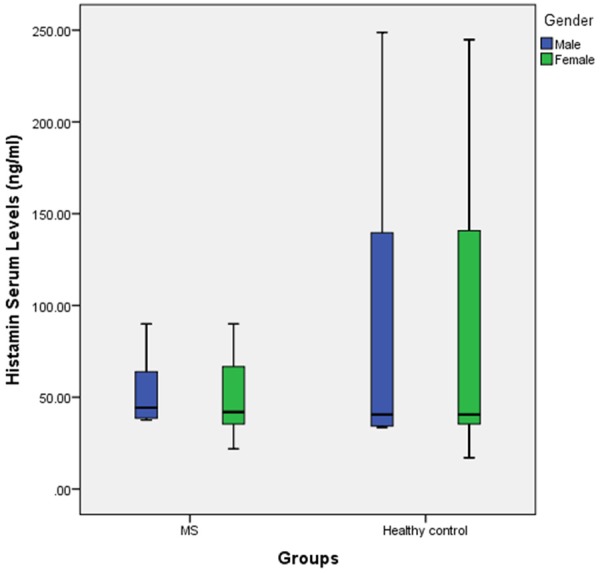
Boxplot of histamine serum level in two groups labeled to gender.
Figure 2.
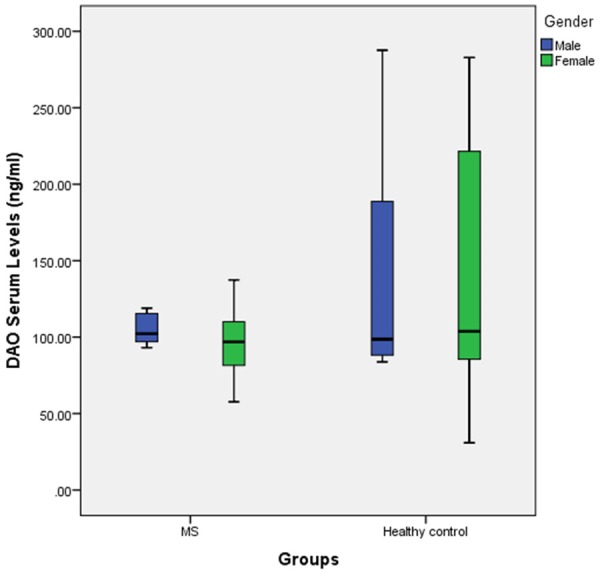
Boxplot of Diamine oxidase serum level in two groups labeled to gender.
Table 2.
Clinical and para clinical information of patients
| Variables | RRMS Patients | |
|---|---|---|
| EDSS (mean ± SD) | 1.53 ± 1.22 | |
| Duration of disease (years) (mean ± SD) | 3.52 ± 3.48 | |
| Onset | Sensory | 11 (22%) |
| Weakness | 1 (2%) | |
| Visual | 23 (46%) | |
| Cerebellar | 8 (16%) | |
| Drug | Interferon β 1a | 19 (38%) |
| Non Interferon β 1a | 7 (14%) | |
EDSS: Expanded Disability Status Scale.
Table 3.
The correlation between Histamine and Diamine oxidase with variables
| Correlation | Age | Duration of disease | EDSS | |
|---|---|---|---|---|
| Histamine | P-Value | 0.30 | 0.22 | 0.49 |
| Pearson correlation | -0.11 | -0.17 | -0.09 | |
| Diamine oxidase | P-Value | 0.81 | 0.88 | 0.58 |
| Pearson correlation | -0.02 | 0.02 | 0.09 | |
Discussion
Until now many intrinsic and extrinsic factors including histamine have been studied with the aim of investigation of their roles in the MS pathogenesis. This study was performed in order to investigate the differences in serum levels of histamine and diamine oxidase in MS patients and healthy individuals. In this paper we have reported, histamine and diamine oxidase levels in the serum of RRMS patients were lower than control subjects. As described before, histamine is an important product of diamine oxidase and its functions in body are mediated via different receptors (H1R, H2R, H3R and H4R). These functions include, but are not limited to, changing permeability of vessels, mediating allergic reactions, antibody switching, changing Th1/Th2 balance and also bronchospasm. As Jadidi-Niaragh and colleagues report, histamine performs its proinflammatory actions such as increasing BBB permeability and increasing immune cells infiltration into CNS via H1R [7]. These actions result in worsening the disease condition and as Lock and colleagues report [11], there is an overexpression of H1R gene in chronic plaques of MS patients. Histamine is also considered to promote Th1 and cell mediated immune which will in turn lead to more inflammatory cytokine (i.e. INF-g, TNF and IL-6) production and exacerbating autoimmune condition. On the other hand H2R is reported to mediate physiological actions of histamine including gastric acid secretion [12]. Histamine actions via H2R also decrease both Th1 and Th2 responses of immune system and are believed to ameliorate MS condition. As Emerson and colleagues [13] report, activation of histamine H2R receptor resulted in suppressed inflammatory agents such as production of O2-, TNF-a and IL-12 [12]. It was suggested by Musio and colleagues [14] that EAE severity was promoted in HDC deficient mice which were unable to produce histamine. Splenocytes of HDC-/- mice were also reported unable of producing histamine in response to myelin antigen resulting in higher production of INF-g, TNF and leptin. These results are in line with the findings of Dunford which report elevated production of Th1 inflammatory cytokine and chemokine H4R blockage [15]. Effects of exogenous histamine on provoking re-myelination were considered affecting other important factors such as T3 and vitamin B12 [7,16]. Most experiments had been investigated to measure histamine levels in CSF of patients and less attention has been given to diamine oxidase levels. In the study by Rozinecki and colleagues [17] reported no significant differences between CSF histamine levels in MS patients compared to healthy controls. But it’s also been shown by Molnár and Moldován that elevated histamine level exist in CSF of MS patients compared with controls [18]. Also recently, Kallweit and colleagues investigated histamine level in CSF of 36 MS patients and they found significant elevation in CSF histamine levels of MS patients than in control group [19]. It must also be considered that increased histamine levels in MS patients can be due to increased diamine oxidase levels but all these increased histamine levels were observed in CSF of MS patients. Another article which found higher level of histamine in CSF of MS patients was performed by Tuomisto and colleagues that reported 60% higher histamine level in their 26 MS patients while compared to their control group because histamine-N-methyltransferase (HMT) activity decreased significantly in MS [20].
Furthermore, another study by Iarosh and colleagues [21] indicated low level of blood histamine in those MS patients with disease length over 5 years while in the same paper, they report high levels of blood histamine in those MS patients with disease length lower than 5 years. These results conflict with what was observed in other studies that histamine levels were increased in MS patients who might be due to their small study group and their site of measurement (CSF).
Conclusion
In conclusion, our data analyses indicated significant changes in histamine and diamine oxidase levels in the serum of MS patients compared to control group and serum levels of histamine and diamine oxidase in RRMS patients were lower than healthy controls. These results were in line with some studies; on the other hand some others reported elevated levels of histamine in CSF of MS patients than compared to healthy individuals. Hence, further studies will have to clarify the potential role of both histamine and diamine oxidase enzyme in the pathogenesis of MS.
Disclosure of conflict of interest
None.
References
- 1.Steinman L. Immunology of relapse and remission in multiple sclerosis. Annu Rev Immunol. 2014;32:257–281. doi: 10.1146/annurev-immunol-032713-120227. [DOI] [PubMed] [Google Scholar]
- 2.Hoglund RA, Maghazachi AA. Multiple sclerosis and the role of immune cells. World J Exp Med. 2014;4:27–37. doi: 10.5493/wjem.v4.i3.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broux B, Stinissen P, Hellings N. Which immune cells matter? The immunopathogenesis of multiple sclerosis. Crit Rev Immunol. 2013;33:283–306. doi: 10.1615/critrevimmunol.2013007453. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;15:393–410. doi: 10.1016/j.cytogfr.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Jutel M, Watanabe T, Akdis M, Blaser K, Akdis CA. Immune regulation by histamine. Curr Opin Immunol. 2002;14:735–740. doi: 10.1016/s0952-7915(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 7.Jadidi-Niaragh F, Mirshafiey A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology. 2010;59:180–189. doi: 10.1016/j.neuropharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, Sobel RA, Steinman L. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc Natl Acad Sci U S A. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 12.Huang JF, Thurmond RL. The new biology of histamine receptors. Curr Allergy Asthma Rep. 2008;8:21–27. doi: 10.1007/s11882-008-0005-y. [DOI] [PubMed] [Google Scholar]
- 13.Emerson MR, Orentas DM, Lynch SG, LeVine SM. Activation of histamine H2 receptors ameliorates experimental allergic encephalomyelitis. Neuroreport. 2002;13:1407–1410. doi: 10.1097/00001756-200208070-00012. [DOI] [PubMed] [Google Scholar]
- 14.Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, Ohtsu H, Galli SJ, Mantegazza R, Steinman L, Pedotti R. A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol. 2006;176:17–26. doi: 10.4049/jimmunol.176.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Dunford PJ, O’Donnell N, Riley JP, Williams KN, Karlsson L, Thurmond RL. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176:7062–7070. doi: 10.4049/jimmunol.176.11.7062. [DOI] [PubMed] [Google Scholar]
- 16.Gillson G, Wright JV, DeLack E, Ballasiotes G. Transdermal histamine in multiple sclerosis, part two: a proposed theoretical basis for its use. Altern Med Rev. 2000;5:224–248. [PubMed] [Google Scholar]
- 17.Rozniecki JJ, Hauser SL, Stein M, Lincoln R, Theoharides TC. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 18.Molnár G, Moldován J. Histamine content of the cerebrospinal fluid in multiple sclerosis. A preliminary communication. Acta Med Acad Sci Hung. 1966;22:271–274. [PubMed] [Google Scholar]
- 19.Kallweit U, Aritake K, Bassetti CL, Blumenthal S, Hayaishi O, Linnebank M, Baumann CR, Urade Y. Elevated CSF histamine levels in multiple sclerosis patients. Fluids Barriers CNS. 2013;10:19. doi: 10.1186/2045-8118-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuomisto L, Kilpelainen H, Riekkinen P. Histamine and histamine-N-methyltransferase in the CSF of patients with multiple sclerosis. Agents Actions. 1983;13:255–257. doi: 10.1007/BF01967346. [DOI] [PubMed] [Google Scholar]
- 21.Iarosh OO, Kanevs’ka SA. [The characteristics of the blood histamine indices and of the pathomorphological changes in the gastric mucosa of patients with multiple sclerosis] Lik Sprava. 1992:75–76. [PubMed] [Google Scholar]


