Abstract
Background: CHADS2 (congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke/TIA) score has been validated as a risk stratification score to predict stroke in patients with atrial fibrillation (AF). The objective of this analysis was to assess whether patient risk factors, in particular CHADS2 score, identified patients at risk of mortality. Methods: 821 patients with an implantable cardioverter defibrillator were prospectively followed-up in 11 cardiology centers. Patients were grouped in 3 groups according to pre-specified risk classes: low (CHADS2 = 0), moderate (CHADS2 = 1, 2), and high (CHADS2 = 3-6). Information on clinical status and events, were collected during scheduled and unscheduled follow-up visits. Deaths were retrieved from medical records, or through the Regional Office of Vital Statistics. Results: Over a mean follow-up of 44±26 months, 135 deaths occurred in the overall population: 6 (7.7%) in the low-risk population, 69 (13.8%) in moderate-risk patients and 60 (24.6%) in high-risk patients. Kaplan-Meier estimated of patient survival were significantly different in 3 patients groups (93.0%, 90.1%, 78.5% in low, moderate and high risk patients respectively, at 4 years P<0.001). A sub-analysis on patients without history of AF showed similar results. Multivariate regression analysis adjusted for baseline characteristics confirmed the high risk status (HR 1.88, 95% CI 1.27-2.80; P = 0.002) as an independent predictor of mortality adjusted for the baseline characteristics. Conclusions: In our multicenter research, the long-term mortality was higher in patients with high CHADS2 score than in those with lower risk score regardless the presence of history of AF. CHADS2 score could be considered a toll to predict all causes mortality.
Keywords: Implantable cardioverter defibrillator, CHADS2 score, atrial fibrillation, all cause mortality, prediction of mortality
Introduction
CHADS2 score (congestive heart failure, hypertension, age ≥75 years, type 2 diabetes, and previous stroke or transient ischemic attack (TIA) [doubled]) is a well validated tool to stratify the risk for stroke, thromboembolism, and related mortality in patients with Atrial Fibrillation (AF) or Atrial flutter [1-6]. Moreover, CHADS2 score is predictive of first cardiovascular (CV) hospitalization in patients with AF and may be helpful to identify poor prognosis in patients with acute myocardial infarction [5,7]. In heart failure (HF) patients with severe left ventricular systolic dysfunction, who are candidates for cardiac resynchronization therapy together with an implantable defibrillator (CRT-D), pre-implant CHADS2 score is associated with a higher mid-term risk of HF hospitalizations and of the combined endpoint of hospitalizations for HF or mortality [8]. The aim of the present research was to investigate if CHADS2 score could predict long term mortality in the general population with implantable cardiac defibrillator (ICD). We also aimed to evaluate the relative prognostic weight of individual components of CHADS2 score in terms of mortality prediction.
Materials and methods
This is a multicenter prospective observational nationwide analysis involving 11 cardiology Italian centers mainly distributed in northeast regions of the country. The prospective collection of data has been performed in the framework of Medtronic ClinicalService® project, a national cardiovascular data repository and medical care project aiming to describe and improve the use of cardiac implantable electronic device (CIED) in Italian clinical practice. Since 2013, all the participating centers have prospectively collected clinical and device diagnostics data from patients with Medtronic ICD. All consecutive patients who received an ICD or a CRT-D, according to guidelines for CRT or treatment of ventricular arrhythmias, were included in the analysis [8-10]. The project was approved by each site’s Institutional Review Board and Local Ethics Committee and conforms to the principles outlined in the Declaration of Helsinki. Each patient included in the ClinicalService® project provided informed consent for data collection and analysis.
Follow-up and event collection
Patients’ baseline demographic data, clinical characteristics and pharmacological treatment were collected at the time of device implan-tation. The CHADS2 score was calculated at implant; all patients were then re-classified in three pre-specified risk-groups, according to the score: CHADS2 scale 0: low risk, 1-2-: moderate, 3, 4, 5, 6: high risk.
All patients were remotely followed using the Medtronic CareLink® Network [11]. Device data of each patient were automatically transmitted every 3 months and an in-clinic visit was performed annually. Each follow-up, both remote and in-person, included the device interrogation, the analysis of technical and diagnostic data, and the collection of critical adverse events and ICD therapies.
In case of a missed follow up (either a scheduled remote or in-hospital visit), the patient or his/her family was contacted by phone; after two unsuccessful phone contact attempts, information on patients’ life status was collected from the Hospital general Database or from the Regional Office of Vital Statistics. No patient was lost to follow-up. The main objective of this analysis was to evaluate long-term mortality, other clinical outcomes and to correlate these endpoints with the CHADS2 risk score status at baseline.
Statistical analysis
Continuous data were reported as mean ± standard deviation for normally distributed variables, or median with 25th-75th percentile in the case of skewed distribution. Normality of distribution was assessed by means of the Kolmogorov-Smirnov test. Absolute and relative frequencies were reported for categorical variables. The rate of events (number of deaths, number of episodes treated with shock therapy) was computed per 100 person-years and reported separately for each group, together with their 95% confidence intervals. Rates were compared by means of either a mixed Poisson model or a negative binomial regression model (if overdispersion was present). Incidence Rate Ratio (IRR) were reported as results of the comparison, together with their 95% confidence intervals and p-value.
The analyses of time-to-the-first event were described by means of Kaplan-Meier curves and compared between the groups by means of the log-rank test. Univariate Cox Regression has been used to identify univariate predictors of all-cause death among major baseline characteristics; all of the variables with a univariate P<0.15 have been subsequently tested in a multivariate Cox regression to identify independent predictors of the death. Proportionality of hazards has always been tested and assured. Results of the Cox regression are reported as hazard ratio (HR) together with 95% confidence interval (CI). CHADS2 risk score has been tested both as a unique score and split in all his contributing factors to avoid collinearity and to test, in particular, which of them influenced mortality. A backward selection method has been used to identify significant CHADS2 contributing factors.
All tests were 2-sided and a 2-tailed P value <.05 was considered statistically significant. Stata 12.1 (Stata Corp) was used for computation.
Results
Eight hundred-twenty-one consecutive patients were included in the analysis, and followed up in this analysis. Baseline patient characteristics of patients are described in Table 1. Briefly, mean age was 67±11 years and mean New York Heart Association (NYHA) functional class was 2.2±0.7. Out of 821 patients, 629 (76.6%)% had history of HF, and 406 (49.4%) had an ischemic etiology of cardiomyopathy. The mean ejection fraction (EF) calculated at baseline 2D echocardiogram was 32.3±11.2%. 309 (37.6%) of the whole population had history of atrial tachyarrhythmia. Value of CHADS2 score at baseline for the studied population is shown in Table 2, 78 (9.5%) patients had a CHADS2 score = 0, while 499 (60.8%) had a CHADS2 score equal to 1 or 2. Out of 821 patients, only 319 (38.9%) took oral anticoagulant therapy (OAC). In particular, the patients receiving OAC were 34.7% in the low risk group, 35.4% in the moderate group and 46.7% in the high risk status group. Considering patients with history of AF, 67.8% received OAC, while only 21.4% of patients without AF received OAC (P<0.001).
Table 1.
Baseline characteristics of the population of patients
| Variable | N (%) |
|---|---|
| Male | 660 (80.4%) |
| Age, yrs | 67±11 |
| NYHA class | 2.2±0.7 |
| Heart failure | 629 (76.6%) |
| History of ventricular arrhythmias | 365 (44.5%) |
| History of atrial arrhythmias | 309 (37.6%) |
| Ischemic cardiopathy | 406 (49.4%) |
| Dilatative cardiomyopathy | 523 (63.8%) |
| Hypertension | 506 (61.6%) |
| Diabetes | 187 (22.8%) |
| Chronic kidney disease | 66 (8.2%) |
| Chronic obstructive pulmonary disease | 51 (6.2%) |
| Left Ventricular Ejection Fraction | 32.3±11.2 |
| Left Ventricular end-diastolic diameter | 67.6±15.1 |
| Indication for ICD | |
| Primary | 591 (72.0%) |
| Secondary | 230 (28.0%) |
| Device | |
| Single chamber ICD | 121 (14.7%) |
| Dual chamber ICD | 223 (27.2%) |
| Cardiac Resynchronization ICD | 477 (58.1%) |
| Drugs therapy | |
| Oral anticoagulants | 319 (38.9%) |
| Antiplatelet | 370 (45.1%) |
| Antiarrhythmic | 146 (17.8%) |
| Digitalis | 182 (22.2%) |
| Diuretics | 627 (76.4%) |
| Beta-blockers | 624 (76.1%) |
| ACE inhibitors | 536 (65.4%) |
NYHA: New York Heart Association. ICD: Implantable Cardioverter Defibrillator.
Table 2.
CHADS2 score and the number of patients in the risk status groups according to CHADS2
| CHADS2 score (N = 821) | n (%) | |
|---|---|---|
| Low risk | Score = 0 | 78 (9.5%) |
| Intermediate risk | 499 (60.8%) | |
| Score = 1 | 179 (21.8%) | |
| Score = 2 | 320 (39.0%) | |
| High risk | 244 (29.7%) | |
| Score = 3 | 188 (22.9%) | |
| Score = 4 | 45 (5.5%) | |
| Score = 5 | 11 (1.3%) | |
| Score = 6 | 0 (0 %) |
CHADS2: congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke/TIA.
Clinical outcomes
The mean follow-up was 44.3±26.5 months with a total follow-up of 3028 patient-years. During follow-up, 135 patients died with an annual incidence rate of death equal to 4.5 (95% CI, 3.7-5.3) for 100 patient/year. In details, 6 (7.7%) patients died in the low, 69 (13.8%) in the moderate and 60 (24.6%) in the high CHADS2 risk status.
Survival from all-cause mortality is shown in Figure 1.
Figure 1.
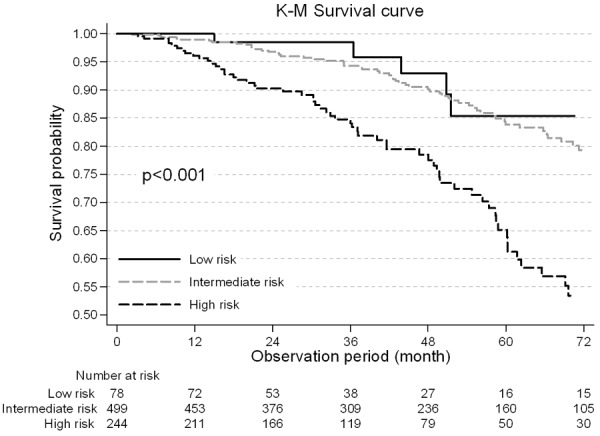
Kaplan-Meier Survival by different CHADS2 risk status (High Risk, Intermediate risk and low risk). In the unadjusted Kaplan-Meier analysis, patients with low and intermediate risk have diminished all-cause survival at 48 months compared with those at high risk (93.0% vs 90.1% vs 78.5%, respectively).
Unadjusted patient survival was significantly lower in the high risk status patients. The cumulative 4-year survival probability was 78.5% in the high risk group as compared with 90.1% and 93.0% in the moderate and low risk group (P<0.001).
Variables associated with increased all-cause mortality on univariate analysis were: male sex, advancing age, NYHA functional class, ischemic cardiomyopathy, chronic kidney disease (CKD), a history of atrial arrhythmias, HF, and CHADS2 ≥3 (Table 3). Multivariate model analysis confirmed ischemic cardiopathy, dilated cardiomyopathy, CKD, and high risk status (CHADS2 ≥3, HR 1.88, 95% CI 1.27-2.80; P = 0.002) as independent predictors of mortality adjusted for baseline characteristics.
Table 3.
Variables associated with increased all-cause mortality on univariate analysis
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| Gender (Male) | 2.08 | 1.19-3.61 | 0.010 | 1.45 | 0.80-2.64 | 0.222 |
| Secondary Prevention | 1.04 | 0.71-1.51 | 0.838 | |||
| NYHA | 1.37 | 1.06-1.76 | 0.015 | 1.23 | 0.91-1.67 | 0.175 |
| VT/VF History (yes) | 0.84 | 0.60-1.19 | 0.325 | |||
| AT/AF History (yes) | 1.83 | 1.30-2.57 | 0.001 | * | ||
| Ischemic Cardiomyopathy (yes) | 1.48 | 1.05-2.08 | 0.026 | 1.69 | 1.10-2.59 | 0.017 |
| Dilated Cardiomyopathy (yes) | 1.42 | 0.97-2.07 | 0.074 | 1.70 | 1.08-2.66 | 0.021 |
| Chronic Kidney Disease (yes) | 4.23 | 2.73-6.53 | <0.001 | 3.26 | 2.03-5.24 | <0.001 |
| COPD (yes) | 1.27 | 0.69-2.38 | 0.442 | |||
| Heart failure (yes) | 2.32 | 1.39-3.86 | 0.001 | |||
| Hypertension (yes) | 1.03 | 0.73-1.46 | 0.860 | |||
| Age ≥75 | 2.80 | 2.00-3.94 | <0.001 | |||
| Diabetes (yes) | 1.35 | 0.92-1.96 | 0.122 | |||
| TIA or Ictus (yes) | 1.24 | 0.55-2.81 | 0.607 | |||
| CHADS2 ≥3 (High risk) | 2.52 | 1.79-3.55 | <0.001 | 1.88 | 1.27-2.80 | 0.002 |
| Device (CRT) | 1.23 | 0.85-1.75 | 0.259 |
NYHA: New York Heart Association. VT: ventricular Tachycardia. VF: ventricular fibrillation. AT: atrial tachycardia. AF: atrial fibrillation. COPD: Chronic obstructive pulmonary disease. TIA: transitory ischemic attack. CHADS2: congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke/TIA. *AT/AF History does not respect the assumption of proportionality of hazards in the multivariate model; therefore it has been decided to drop it out from the model and to repeat the analysis stratifying according to this variable. See “Clinical outcomes” section for the result of the analysis on patients without AT/AF history.
Considering the cohort of patients without history of atrial arrhythmias at implant, 3 (5.1%) patients in the low risk status died, 34 (10.2%) in the moderate and 30 (21.9%) in the high risk status. The annual incidence rate for 100 patient/years was 1.50 (95% CI, 0.31-4.39), 2.56 (95% CI, 1.77-3.58), 6.70 (95% CI 4.52-9.56) respectively in the low, moderate and high risk status group. The incidence rate ratio (IRR) is non-significant for low vs moderate risk group, while IRR = 4.46 (95% CI, 1.39-22.82) for low vs high (P = 0.004) and IRR = 2.62 (95% CI, 1.55-4.40) for moderate vs high (P<0.001). Survival from all-cause mortality is shown in Figure 2.
Figure 2.
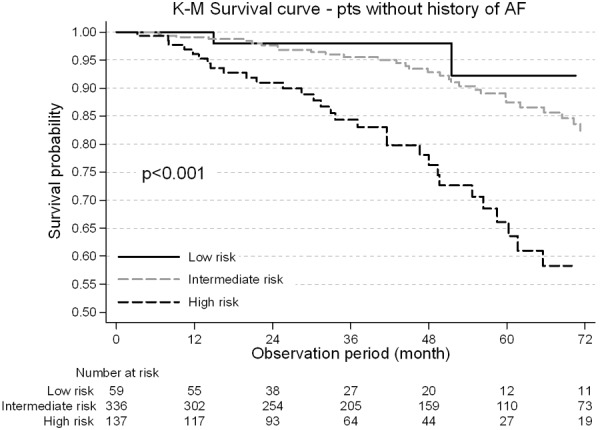
Kaplan-Meier survival curve in the population without atrial arrhythmias by different CHADS2 risk status (High Risk, Intermediate risk and low risk). In the unadjusted Kaplan-Meier analysis, patients with no AF history and with low and intermediate risk have diminished all-cause survival at 48 months compared with those at high risk (98.0% vs 92.8% vs 78.1%, respectively). AF: atrial fibrillation.
Unadjusted patient survival was significantly lower in the high risk status patients. The 4-year survival probability was 78.1% in the high risk group as compared with 92.8% in the moderate and 98.0% in the low risk group (P<0.001). In the overall population, the annual incidence rate of episodes treated with at least one shock was 0.32 (95% CI 0.26-0.39) for 100 patient/years. In particular, the annual incidence rate for 100 patient/years was 0.14 (95% CI, 0.10-0.19), 0.22 (95% CI, 0.20-0.24), 0.35 (95% CI, 0.31-0.40) respectively in the low, moderate and high risk status group. The IRR was equal to 1.60 (95% CI, 1.14-2.30) for low vs moderate risk group (P = 0.004), while IRR was 2.57 (95% CI, 1.82-3.72) for low vs High (P<0.001) and IRR = 1.60 (95% CI, 1.37-1.87) for moderate vs High (P<0.001). Out of 821 patients, 238 (29.0%) had at least an episode of clinically relevant AF during the follow-up.
CHADS2 variables
To investigate which individual components of the CHADS2 score (Congestive heart failure, Hypertension, Age >75, Diabetes mellitus and prior Stroke or TIA) predicted all-cause mortality, a backward sequential multivariate model has been fitted using the variables in Table 3 plus the CHADS2 score. The backward selection started from the full model and sequentially dropped the less significant variable at each step. The procedure stopped when no more variable could be dropped and the final model was reached. The results are shown in Table 4: the variables that were sequentially dropped from the model were Diabetes, TIA/Stroke, NYHA functional class, arterial hypertension, and gender. Consequently, the analysis indicated age >75 years, congestive HF, as well as ischemic cardiopathy, dilated cardiomyopathy, CKD as independent predictors of all-cause mortality. Results of backward selection confirmed what was obtained with the multivariate model showed in Table 3.
Table 4.
Backward multivariate analysis
| Full model | Dropped variable from full model | First step | Dropped variable at 1st step | Second step | Dropped variable at 2nd step | Third step | Dropped variable at 3rd step | Fourth step | Dropped variable at 4th step | Final model | HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender (Male) | Gender (Male) | Gender (Male) | Gender (Male) | Gender (Male) | 0.213 | |||||||
| NYHA | NYHA | NYHA | 0.675 | |||||||||
| Ischemic Cardiopathy (yes) | Ischemic Cardiopathy (yes) | Ischemic Cardiopathy (yes) | Ischemic Cardiopathy (yes) | Ischemic Cardiopathy (yes) | Ischemic Cardiopathy (yes) | 1.82 (1.19-2.79) | 0.005 | |||||
| Dilated Cardiomyopathy (yes) | Dilated Cardiomyopathy (yes) | Dilated Cardiomyopathy (yes) | Dilated Cardiomyopathy (yes) | Dilated Cardiomyopathy (yes) | Dilated Cardiomyopathy (yes) | 1.69 (1.07-2.68) | 0.024 | |||||
| Chronic Kidney Disease (yes) | Chronic Kidney Disease (yes) | Chronic Kidney Disease (yes) | Chronic Kidney Disease (yes) | Chronic Kidney Disease (yes) | Chronic Kidney Disease (yes) | 3.88 (2.44-6.17) | <0.001 | |||||
| Heart failure (yes) | Heart failure (yes) | Heart failure (yes) | Heart failure (yes) | Heart failure (yes) | Heart failure (yes) | 1.68 (0.92-3.06) | 0.088 | |||||
| Hypertension (yes) | Hypertension (yes) | Hypertension (yes) | Hypertension (yes) | 0.636 | ||||||||
| Age ≥75 | Age ≥75 | Age ≥75 | Age ≥75 | Age ≥75 | Age ≥75 | 2.51 (1.72-3.68) | <0.001 | |||||
| Diabetes (yes) | 0.965 | |||||||||||
| TIA or Stroke (yes) | TIA or Stroke (yes) | 0.965 |
NYHA: New York Heart Association. TIA: transitory ischemic attack.
Discussion
Main findings
Our research is the first multicenter analysis aimed to establish whether CHADS2 score could be used to predict all-cause mortality in patients implanted with an ICD.
The main result of this research was that higher CHADS2 scores (CHADS2 = 3, 4, 5) were associated with higher risk of long-term all cause mortality. Moreover, similar results were obtained in the ICD population with no history of AF at the time of ICD implantation. Multivariate analysis showed that mortality depended on the risk status of patients.
Long-term clinical outcomes, such as death and arrhythmic profile were found to be worse in patients with a high risks status versus those with low or moderate risk status.
Finally, and in line with current knowledge, CKD confirmed to be a strong predictor of all-cause mortality.
CHADS2 and implantable devices
Many studies have been demonstrated the utility of CHADS2 risk score stratification in assessing the risk for stroke, thromboembolism, and related mortality in patients with AF or Atrial flutter [1-4]. The current evidence suggests that the prevalence of both symptomatic and silent AF is considerable among patients with implanted devices, and that AF is positively correlated with the risk of thromboembolism [12]. For this reason patients implanted with a cardiac device could benefit from the use of CHADS2 score risk.
The TRENDS trial was designed to evaluate the relationship between comprehensive AF burden detected by CIEDs and thromboembolic (TE) risk, and to determine if there was a threshold value of AF burden that increased TE risk. The annualized TE rate in the overall study population of the trial was remarkably low at 1.2%. Nevertheless, the TE rate of 2.4% per year was more than 2 times higher for the high burden group [13]. The mean CHADS2 score in the TRENDS study group was 2.2, very similar compared to our population. In 2009, Botto et al evaluated a combination of the duration of device-detected atrial arrhythmia episodes and CHADS2 score to predict TE events. Five hundred and sixty-eight patients with pacemakers and a history of symptomatic AF were enrolled. In the patients with intermediate CHADS2 score (1 and 2), the combined risk markers (AT/AF duration and CHADS2 score) had a significant value in predicting TE [14].
So far only one study has so far evaluated the mortality risk using CHADS2 status in CRT-D candidates. Perini et al enrolled 559 consecutive HF patients who were candidates to CRTD, and grouped them in three pre-specified risk classes: low (CHADS2/CHA2DS2-VASc 1-2), moderate (CHADS2/CHA2DS2-VASc 3-4), and high (CHADS2 5-6/CHA2DS2-VASc 5-8). At a median follow up of 30 months, event-free survival analysis showed a significant difference according to baseline CHADS2: Kaplan-Meier survival analysis showed a significant higher number of events for patients with higher CHADS2 score (CHADS2 log-rank test P<0.004; P<0.001) [8].
Our results confirm and complete the findings by Perini et al: not only ICD patients in high risk status based on CHADS2 classification had a lower survival respect to those in low or moderate risk status, but they have a great annual incidence rate of episodes treated with at least a shock. However, it is, important to underline some methodological differences between the two analysis: a) the data evaluated by Perini et al were gathered from patients implanted only with CRT ICD, whereas our project involved patients implanted also with single/dual or CRT defibrillators, b) in our research high risk status according to CHADS2 is (HR = 1.88, 95% CI 1.27-2.80; P = 0.002) an independent predictors of mortality; c) we evaluated the increased risk of mortality for high risk patients also in patients with no history of AF. Recently, Zhou at al conducted a systematic meta-analysis to evaluate the utility of the CHADS2 score for the prediction of ischemic stroke or TIA and death in patients with coronary artery disease (CAD) with and without AF [15]. Including more than 29000 patients, the meta-analysis showed that the CHADS2 score is strongly predictive of death in patients with CAD, regardless of the presence or absence of AF. In patients of CHADS2 score ≥2, the risk of death is more than doubled, and it is greater in patients without than with AF [15]. In our population, the half of patients had CAD history, confirming Zhou et al results. In our analysis among the CHADS2 score risk factors, like HF and age were found to be independent predictors of all-cause mortality. Particularly, HF may contribute to both left atrial remodeling [16,17], potentially resulting in blood stasis and an increased risk of thromboembolism independent of AF [18] Additionally HF may also be associated with a worse prognosis especially for patients with reduced left EF [19].
CHADS2 score and patients with no history of AF
Kondo et al retrospectively studied 127 consecutive patients with stable in Chronic heart failure (CHF), left ventricular ejection fraction (LVEF) <40% and no history of AF and showed that CHADS2 score was predictive of ischemic stroke [20]. Moreover, recently Biffi et al have demonstrated the diagnostic capability of CHADS2 ≥2 in predicting AF recurrence in the follow-up in a population indicated to single chamber defibrillators. Interestingly, CHADS2 score independently predicted new-onset AT/AF occurrence in patients without atrial arrhythmias history or pacing/resynchronization therapy indications [21]. In the Heart and Soul Study CHADS2, in the absence of a history of AT/AF, was associated to an increased risk of ischemic stroke [22]. Chan et al found that CHA2DS2-VASc and CHADS2 predict cardiovascular death among 579 high-risk cardiovascular outpatients without AF [23].
CHADS2 seems to have prognostic value also in ischemic cardiopathy irrespective of the presence of AF. Higher CHADS2 score has been found to be correlated with incidence of major adverse cardiovascular events (MACE) after acute myocardial infarction [7]. Dy et al found that CHADS2 score provided an efficient risk model to predict of MACE and overall mortality in patients with coronary artery disease undergone coronary bypass surgery [24]. In the CARISMA sub-study, the authors found that in the post-myocardial infarction setting CHADS2 score could identify population at high risk of cardiac arrhythmias, including ventricular tachycardia [25]. In patients with acute coronary syndrome CHADS2 score seems to be a simple and reliable tool to identify those at high risk of stroke and death even without AF. Finally, a recent meta-analysis suggests that CHADS2 score may predict mortality in a population with coronary artery disease irrespective of presence of AF [26]. Overall these studies taken together seem to suggest a possible role of CHADS2 score to predict cardiovascular events and death in a population without AF [15]. In our research a high CHADS2 score increased risk of total mortality of 4 times.
Clinical implications
At the best of our knowledge our data provide new insights on the association between CHADS2 score and all-cause mortality in ICD/CRT-D recipients. The complex management of ICD/CRT-D patients could benefit from remote monitoring, periodic in clinic visits and assessment of clinical history however, to improve efficiency, the frequency of the visits may be adjusted based on the risk status of each patient. The complex management of ICD/CRT-D patients could benefit from remote monitoring, periodic in clinic visits and assessment of clinical history however, to improve efficiency, the frequency of the visits may be adjusted based on the risk status of each patient.
We can argue why a score traditionally used for stratifying stroke risk in AF seems to be a reliable and simple tool for prediction of mortality in patients suffering from different heart disease without AF. The most powerful predictors among individual components of CHADS 2 seem to be age >75 years and congestive HF: advance age intrinsically implicates a reduction of residual survival, and congestive HF, including mostly patients with reduced EF, reasonably is a clinical condition intrinsically determining an adverse prognosis per se.
Assuming these simple statements and the unproved exclusion of any other confounder or unmeasured variable, a reasonable interpretation of the role of CHADS2 score in determining cumulative survival in different clinical contexts, could be that the variable included in this score have a prognostic implication independently of the presence of AF.
Finally, in a pure hypothesis generating perspective and in the light of continuous research of the best cost effective therapy, we could consider CHADS2 score as a potential tool to improve prognostic stratification of CRT candidates patients. A high CHADS2 score, that seems to be associated with an increased risk of overall mortality, may justify the additional value of defibrillation therapy to resynchronization therapy.
Limitations
The present results were obtained from a retrospective analysis of prospectively collected data. In our analysis there is a limited number of patients with CHADS2 score = 0 and 6. Moreover, this is an observational research and therefore all intrinsic limitations associated with this type of research should be considered, especially the lack of a control population without ICD or CRT. Nevertheless, the use of remote monitoring strengthens the follow up. The ventricular episodes detected by ICD/CRTD were not classified as appropriate or not appropriate. Therefore, the number of episodes with at least a shock could be affected by a certain percentage of inappropriate shocks. Finally, the heterogeneity of the population, including patients with single/dual chamber ICD as well as with a CRT-D may be a potential limitation.
Conclusions
In a population of ICD and CRT-D patients CHADS2 score could be considered a risk stratification tool for all causes mortality. The long-term mortality of patients with high risk CHADS2 score differed from those of patients with lower risk factor regardless the presence of the history of AF.
Acknowledgements
Data collection and analysis was performed in the framework of the ClinicalService®, a project supported by Medtronic Italia, an affiliate of Medtronic Inc.
Disclosure of conflict of interest
None.
Abbreviations
- AF
atrial fibrillation
- AT
atrial tachycardia
- CAD
coronary artery disease
- CHADS2
congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, previous stroke or transient ischemic attack (doubled)
- CHF
chronic heart failure
- CI
confidence interval
- CIED
cardiac implantable electronic device
- CKD
chronic kidney disease
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization therapy defibrillator
- CV
cardiovascular
- EF
ejection fraction
- HF
heart failure
- HR
hazard ratio
- ICD
implantable cardioverter defibrillator
- IRR
incidence rate ratio
- MACE
major adverse cardiovascular event
- NYHA
New York Heart Association
- OAC
oral anticoagulant drug
- TE
thromboembolic
- TIA
transient ischemic attack
References
- 1.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national registry of atria fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American college of cardiology foundation/American heart association task force on practice guidelines developed in partnership with the European society of cardiology and in collaboration with the European heart rhythm association and the heart rhythm society. J Am Coll Cardiol. 2011;57:101–e198. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Crandall MA, Horne BD, Day JD, Anderson JL, Muhlestein JB, Crandall BG, Weiss JP, Osborne JS, Lappé DL, Bunch TJ. Atrial fibrillation significantly increases total mortality and stroke risk be yond that conveyed by the CHADS2 risk factors. PACE. 2009;32:981–986. doi: 10.1111/j.1540-8159.2009.02427.x. [DOI] [PubMed] [Google Scholar]
- 4.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 5.Naccarelli GV, Panaccio MP, Cummins G, Tu N. CHADS2 and CHA2DS2-VASc risk factors to predict first cardiovascular hospitalization among atrial fibrillation/atrial flutter patients. Am J Cardiol. 2012;109:1526–1533. doi: 10.1016/j.amjcard.2012.01.371. [DOI] [PubMed] [Google Scholar]
- 6.Henriksson KM, Farahmand B, Johansson S, Asberg S, Terént A, Edvardsson N. Survival after stroke--the impact of CHADS2 score and atrial fibrillation. Int J Cardiol. 2010;141:18–23. doi: 10.1016/j.ijcard.2008.11.122. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Chen YH, Chan WL, Huang PH, Chen JW, Lin SJ. Usefulness of the CHADS2 score for prognostic stratification of patients with acute myocardial infarction. Am J Cardiol. 2014;114:1309–14. doi: 10.1016/j.amjcard.2014.07.063. [DOI] [PubMed] [Google Scholar]
- 8.Perini AP, Bartolini S, Pieragnoli P, Ricciardi G, Perrotta L, Valleggi A, Vergaro G, Michelotti F, Boggian G, Sassone B, Mascioli G, Emdin M, Padeletti L. CHADS2 and CHA2DS2-VASc scores to predict morbidity and mortality in heart failure patients candidates to cardiac resynchronization therapy. Europace. 2014;16:71–80. doi: 10.1093/europace/eut190. [DOI] [PubMed] [Google Scholar]
- 9.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, Ponikowski P, Priori SG, Sutton R, van Veldhuisen DJ Committee for Practice Guidelines of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG) 2010 focused update of ESC guidelines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the heart failure association and the European heart rhythm association. Eur J Heart Fail. 2010;12:1143–1153. doi: 10.1093/eurjhf/hfq192. [DOI] [PubMed] [Google Scholar]
- 10.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, Smith SC Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association and the Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death-executive summary: a report of the American college of cardiology/American heart association task force and the European society of cardiology committee for practice guidelines developed in collaboration with the ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death-executive summary: a report of the American college of cardiology/American heart association task force and the European society of cardiology committee for practice guidelines developed in collaboration with the European heart rhythm association and the heart rhythm society. Eur Heart J. 2006;27:2099–2140. doi: 10.1093/eurheartj/ehl199. [DOI] [PubMed] [Google Scholar]
- 11.Slotwiner D, Varma N, Akar JG, Annas G, Beardsall M, Fogel RI, Galizio NO, Glotzer TV, Leahy RA, Love CJ, McLean RC, Mittal S, Morichelli L, Patton KK, Raitt MH, Ricci RP, Rickard J, Schoenfeld MH, Serwer GA, Shea J, Varosy P, Verma A, Yu CM. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular electronic implantable devices. Heart Rhythm. 2015;12:69–100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Glotzer TV, Ziegler PD. Silent atrial fibrillation as a stroke risk factor and anticoagulation indication. Can J Cardiol. 2013;29:S14–23. doi: 10.1016/j.cjca.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 14.Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, Favale S, Molon G, Ricci R, Biffi M, Russo G, Vimercati M, Corbucci G, Boriani G. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241–248. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Cao K, Kou S, Qu S, Li H, Yu Y, Wang C, Liu Y, Li P, Li D. Usefulness of CHADS2 score for prognostic stratification of patients with coronary artery disease: a systematic review and meta-analysis of cohort studies. Int J Cardiol. 2017;228:906–911. doi: 10.1016/j.ijcard.2016.11.114. [DOI] [PubMed] [Google Scholar]
- 16.Owan TE, Hodge TO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. NEJM. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 17.Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA. 2012;308:2108–2117. doi: 10.1001/jama.2012.14785. [DOI] [PubMed] [Google Scholar]
- 18.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J PEP-CHF Investigators. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;19:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 19.Kontogeorgos S, Thunström E, Johansson MC, Fu M. Heart failure with preserved ejection fraction has a better long-term prognosis than heart failure with reduced ejection fraction in old patients in a 5-year follow-up retrospective study. Int J Cardiol. 2017;232:86–92. doi: 10.1016/j.ijcard.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Kondo T, Yamada T, Morita T, Furukawa Y, Tamaki S, Iwasaki Y, Kawasaki M, Kikuchi A, Kawai T, Takahashi S, Ishimi M, Hakui H, Ozaki T, Sato Y, Seo M, Sakata Y, Fukunami M. The CHADS2 score predicts ischemic stroke in chronic heart failure patients without atrial fibrillation: comparison to other stroke risk scores. Heart Vessels. 2017;32:193–200. doi: 10.1007/s00380-016-0861-7. [DOI] [PubMed] [Google Scholar]
- 21.Biffi M, Ziacchi M, Ricci RP, Facchin D, Morani G, Landolina M, Lunati M, Iacopino S, Capucci A, Bianchi S, Infusino T, Botto GL, Padeletti L, Boriani G. Can we predict new AF occurrence in single-chamber ICD patients? Insights from an observational investigation. Int J Cardiol. 2017;230:275–280. doi: 10.1016/j.ijcard.2016.12.126. [DOI] [PubMed] [Google Scholar]
- 22.Welles CC, Whooley MA, Na B, Ganz P, Schiller NB, Turakhia MP. The CHADS2 score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: data from the heart and soul study. Am Heart J. 2011;162:555–561. doi: 10.1016/j.ahj.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YH, Yiu KH, Lau KK, Yiu YF, Li SW, Lam TH, Lau CP, Siu CW, Tse HF. The CHADS2 and CHA2DS2-VASc scores predict adverse vascular function, ischemic stroke and cardiovascular death in high-risk patients without atrial fibrillation: role of incorporating PR prolongation. Atherosclerosis. 2014;237:504–13. doi: 10.1016/j.atherosclerosis.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Lu DY, Huang CC, Huang PH, Chen JW, Chen TJ, Lin SJ, Chan WL, Lee CY, Leu HB. Usefulness of the CHADS2 score for prognostic stratification in patients with coronary artery disease having coronary artery bypass grafting. Am J Cardiol. 2017;119:839–844. doi: 10.1016/j.amjcard.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 25.Ruwald AC, Gang U, Thomsen PE, Jørgensen RM, Ruwald MH, Huikuri HV, Jons C. The predictive value of CHADS2 risk score in post myocardial infarction arrhythmias-a Cardiac Arrhythmias and Risk Stratification after Myocardial infarction (CARISMA) substudy. Int J Cardiol. 2014;173:441–6. doi: 10.1016/j.ijcard.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Poçi D, Hartford M, Karlsson T, Herlitz J, Edvardsson N, Caidahl K. Role of the CHADS2 score in acute coronary syndromes: risk of subsequent death or stroke in patients with and without atrial fibrillation. Chest. 2012;141:1431–40. doi: 10.1378/chest.11-0435. [DOI] [PubMed] [Google Scholar]


