Abstract
Background: Cardiac Infarction/Injury Score (CIIS), an electrocardiographic based scoring system, is a surrogate marker of subclinical myocardial injury (SC-MI) and has shown excellent prognostic value in predicting future cardiovascular mortality. As an association of mild to moderate alcohol consumption with cardiovascular disease (CVD) is conflicting, using an electrocardiographic based scoring system such as CIIS is a simple and cost-effective way to investigate this controversial relationship. Methods: This analysis included 6090 participants (58.42±13.12 years, 54.2% women) free of CVD from the Third National Health and Nutrition Examination Survey (NHANES III). We used multivariable linear regression analysis to examine the cross-sectional association between each alcohol category (non-drinker (reference), 1-6 drinks/week, 7-13 drinks/week, ≥14 drinks/week, and CIIS. SC-MI was defined as CIIS ≥10 units. Results: The prevalence of SC-MI was high among heavy drinkers (≥14 drinks/week) and was lower in participants who were moderate drinkers (7-13 drinks/week). There was a statistically significant and inverse association between moderate alcohol consumption and CIIS (β (95% CI): -0.64 (-1.27, -0.007), P = 0.04) using multivariable linear regression analysis. This inverse association between moderate alcohol consumption and CIIS was more striking among whites compared to non-whites (β (95% CI): -1.06 (-1.93, -0.19) vs. 0.05 (-0.91, 1.00) respectively; interaction p-value = 0.08). Also, the association was stronger among women and older participants, however interaction p-value did not reach statistical significance. Conclusion: There is an inverse association between moderate alcohol consumption and CIIS in participants without manifestations of CVD. As lower CIIS has been associated with low risk of poor outcomes including CVD mortality, these findings further support the existing evidence of the potential benefits of moderate alcohol consumption on cardiovascular health.
Keywords: Alcohol drinking, CIIS, cardiovascular disease, NHANES, subclinical myocardial injury
Introduction
The relationship between alcohol consumption and cardiovascular disease (CVD) has always been intricate. Alcohol consumption is one of the factors that has both protective and detrimental effects on CVD. Most of the studies to date have consistently shown a J-shaped association of alcohol consumption where light to moderate drinkers have lower CVD events than non-drinkers and heavy drinkers [1]. The exact mechanism of the cardioprotective effect of alcohol is unknown. Alcohol consumption is thought to influence CVD risk predominantly by acting on established CVD risk factors, namely, insulin resistance [2], high-density lipoprotein (HDL) [3,4] and inflammation [5].
Results of the studies of the association between alcohol consumption and subclinical CVD have been inconsistent. Previous studies focusing on the association of alcohol intake and subclinical atherosclerosis have shown divergent results. Many previous studies have examined the association between alcohol use and coronary artery calcium (CAC) as a surrogate marker of subclinical atherosclerosis. These studies have shown a U-shaped association [6], a dose-response association [7], or no association [6-11]. Apart from its effect on atherosclerosis, the relationship between alcohol intake and myocardial injury is unclear and not widely studied. The study ex-amining the association of alcohol consumption with subclinical myocardial damage using high sensitivity cardiac troponin-T (hs-cTnT) showed that moderate drinking was associated with lower concentrations of hs-cTnT [12].
The Cardiac Infarction Injury Score (CIIS) is an electrocardiogram classification system that was developed to identify ischemic heart disease [13]. SC-MI as defined by CIIS ≥10 has shown to be associated with an increased CVD mortality. Using CIIS as a continuous variable, increasing CIIS predicted a higher risk of mortality and lower CIIS predicted a low risk of mortality in the previous studies [14,15]. To our knowledge, the association between alcohol consumption and CIIS has not been formally examined in a population-based study. In this study, we comprehensively explored the association between alcohol consumption and CIIS using a sample from the Third National Health and Nutrition Examination Survey (NHANES-III) free of CVD.
Methods
Study participants
We analyzed data from the Third National Health and Nutrition Examination Survey (NHANES III), a cross-sectional study conducted between 1988 and 1994 that used a multistage stratified clustered probability design to select a representative sample of the civilian non-institutionalized US population [16]. The NHANES III study was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board (ERB), and documented consent was obtained from participants. Between 1988 and 1994, initial home interviews were conducted to collect baseline information, including demographics (age, sex, race/ethnicity), medication data (e.g., use of antihypertensive and lipid-lowering medications), past medical history (e.g., history of CVD), and behavioral data (e.g., smoking). Subsequently, participants visited mobile examination centers and gave blood samples to record basic laboratory values for each participant (e.g., total cholesterol, plasma glucose).
Alcohol consumption
In NHANES III, alcohol consumption was assessed by the food frequency questionnaire. Participants reported the number of drinks consumed in the past month for three different types of alcohol: 1) beer; 2) wine; and 3) hard liquor. We defined alcohol consumption as 1) currently drinking (≥1 drink of any type per month) or not currently drinking (<1 per month); 2) the number per week of any type of alcoholic drinks (1-6, 7-13, or ≥14); and 3) the number of beverage-specific drinks per month (1-6, 7-13, or ≥14). The not currently drinking category (<1 drink per month) included past drinkers (subjects consuming at least 12 drinks of any type over a lifetime but not currently drinking alcohol) and lifetime abstainers (subjects consuming <12 drinks in their entire lifetime). Also, the frequency of heavy episodic drinking was assessed during the alcohol and drug component of the examination. Participants were classified as binge drinkers if they had at least five alcoholic drinks in a single day during the past 12 months.
Measurement of cardiac infarction/injury score (CIIS)
Resting 12-lead ECG were obtained with a Marquette MAC 12 system (Marquette Medical Systems, Milwaukee, Wisconsin) during the mobile examination visits by trained technicians. Analysis of ECG was achieved through a computerized automated process and visual inspection by a trained technician located in a centralized core laboratory. The calculation of the cardiac infarction/injury score (CIIS) and methodology have been described previously [13]. Briefly, CIIS is based on a weighted scoring system taking several objective electrocardiographic waveform components related to myocardial injury and ischemia, both discrete and continuous, and generating a risk-stratified scoring system. The score is defined by a combination of 11 discrete and 4 continuous features and provides a simple scoring scheme suitable for both visual and computer classification of a standard 12-lead ECG. By design, CIIS values were multiplied by a factor of 10 in NHANES III to avoid using decimal points. We reported CIIS values by dividing by 10. Subclinical myocardial injury (SC-MI) was defined as CIIS values ≥10 points [13,14].
Measurement of other variables
Age (continuous in years), sex (male and female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American and other), income (<$20,000/year, >$20,000/year), smoking status (never, current, and former), leisure time physical activity (number of times engaged in physical activity in past month), were assessed by interview. Height was measured using a wall-mounted stadiometer, and weight was measured using a Toledo digital scale in minimal clothing. BMI was calculated from height and weight measurements. Waist circumference (WC) was measured at the iliac crest after a normal exhalation of breath. Diabetes was defined as a fasting plasma glucose ≥100 mg/dl, or previous use of diabetes-related medications. Blood pressure (mmHg) was measured three times during the in-home interview and three additional times during the participant’s visit to the mobile examination center. Blood samples were collected via venipuncture by a phlebotomist. Samples were analyzed for total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and glucose, using laboratory procedures as reported by NCHS.
For the purpose of this analysis, we only considered NHANES III participants who underwent an ECG recording (n = 8561). We excluded participants with the history of CVD (myocardial infarction, heart failure, or stroke), or with electrocardiographic evidence of myocardial infarction or any major abnormalities on their electrocardiograms according to the Minnesota Code classification. We also excluded participants with cancer on chemotherapy and missing key covariates. After all exclusions (n = 2391), 6107 participants were included in the analysis.
Statistical analysis
Baseline characteristics were compared acro-ss four alcohol categories (Non-drinker, light drinker, moderate drinker, and heavy drinker). Continuous variables were reported as the mean and standard deviation (SD) while categorical variables were reported as frequency and percentage. Analysis of variance (ANOVA) was used to compare the continuous variables while Chi2 was used to compare the categorical variables. We performed multivariable linear regression model with each alcohol categories as independent variable and CIIS as the outcome variable to calculate Beta-Coefficient and 95% CI. Model 1 was adjusted for age, sex, race and socioeconomic status and model 2 adjusted for model 1 plus smoking and physical activity, BMI, insulin resistance, hypertension, CRP, and HDL-cholesterol. Using multiple linear regression model, we also calculated least means square (LSMEANS) and standard error (SE) of CIIS across alcohol categories. Models were adjusted similarly as above.
Using multivariable linear regression analysis, we also conducted subgroup analysis stratified by age (using 65 years as a cut point), sex and race (whites vs. non-whites). Models were adjusted for age, sex, race, socioeconomic status, smoking, physical activity, BMI, insulin resistance, hypertension, CRP, and HDL cholesterol.
As an additional analysis, we used multivariable logistic regression analysis to compute odds ratios (OR) and 95% confidence interval (CI) for the cross-sectional association between each alcohol category (light drinkers, moderate drinkers, heavy drinkers, and non-drinkers (reference) and SC-MI. The models were adjusted for confounder as mentioned above.
All statistical analyses were performed using with SAS version 9.4 (SAS Institute Inc, Cary, NC) and p-values were considered significant if <0.05.
Results
Our analysis included 6090 participants (58.42±13.12 years, 54.2% women and 50.3% Non-Hispanic Whites). Table 1 shows the characteristics of participants stratified by alcohol categories. Moderate and heavy drinkers were more likely to be young, male, white, current, and former smokers, high socioeconomic status, elevated SBP, elevated DBP and insulin resistance. Prevalence of SC-MI was 23.2%, 19.4%, 20.1%, and 22% for heavy drinkers, moderate drinkers, light drinkers, and non-drinkers respectively (Figure 1).
Table 1.
Baseline characteristics of study participants
| Characteristics Mean ± SD or n (%) | Non-Drinker N = 3417 | Mild alcohol use (0-6 drinks/week) N = 1973 | Moderate alcohol use (7-13 drinks/week) N = 545 | High alcohol use (≥14 drinks/week) N = 155 | P-Value† |
|---|---|---|---|---|---|
| Age (years) | 60.4±13.4 | 55.1±12.0 | 57.7±12.6 | 58.1±12.0 | <.0001 |
| Male (%) | 1216 (35.5%) | 1081 (54.7%) | 357 (65.5%) | 131 (84.5%) | <.0001 |
| Race | <.0001 | ||||
| Non-Hispanic White | 1651 (48.3%) | 1003 (50.8%) | 327 (60%) | 88 (56.7%) | |
| Non-Hispanic Black | 765 (22.3%) | 433 (21.9%) | 103 (18.9%) | 30 (19.3%) | |
| Mexican American | 835 (24.4%) | 465 (23.7%) | 104 (19.0%) | 32 (20.6%) | |
| Other | 166 (4.8%) | 72 (3.6%) | 11 (2.0%) | 5 (3.2%) | |
| Total Annual Family Income <20,000 | 1748 (52.1%) | 690 (35.5%) | 183 (34.0%) | 54 (35.2%) | <.0001 |
| Systolic Blood Pressure (mmHg) | 132.5±19.9 | 128.5±17.6 | 131.2±17.0 | 133.6±16.5 | <.0001 |
| Diastolic Blood Pressure (mmHg) | 75.6±10.0 | 77.0±9.8 | 77.0±9.9 | 80.0±9.8 | <.0001 |
| Insulin resistance (%) | 1418 (41.6%) | 704 (35.7%) | 207 (38.1%) | 69 (44.8%) | 0.0002 |
| HDL Cholesterol | 50.0±15.1 | 51.8±16.9 | 57.9±18.8 | 59.5±20.8 | <.0001 |
| C-reactive protein | 0.52±0.81 | 0.44±0.64 | 0.42±0.64 | 0.49±0.75 | 0.0001 |
| Body mass index (Kg/m2) | 27.9±5.4 | 27.5±5.2 | 26.0±4.5 | 26.5±4.7 | <.0001 |
| Smoking (%) | |||||
| Current Smoker | 607 (17.7%) | 530 (26.8%) | 195 (35.7%) | 61 (39.3%) | <.0001 |
| Former Smoker | 984 (28.8%) | 679 (34.4%) | 210 (38.5%) | 66 (42.5%) | <.0001 |
| Never Smoker | 1826 (53.4%) | 764 (38.7%) | 140 (25.6%) | 28 (18.0%) | <.0001 |
| Physical Activity (METs per week)* | 7.32 (0-27.4) | 12.79 (3.2-34.6) | 15.11 (3.2-36.2) | 13.6 (3.2-34.8) | <.0001 |
| SC-MI (%) | 754 (22.0%) | 397 (20.1%) | 106 (19.4%) | 36 (23.2%) | 0.23 |
| Cardiac Injury Score | 5.40±6.92 | 4.90±6.60 | 4.92±6.23 | 5.44±6.96 | 0.04 |
p-value for calculated by ANOVA for continuous and chi2 for categorical variables.
METs reported as median and IQR.
MET, metabolic equivalent; HDL, high-density cholesterol; SC-MI, subclinical myocardial injury. Insulin resistance defined as fasting blood sugar ≥100 mg/dl, or self-reported history of diabetes or taking medications.
Figure 1.
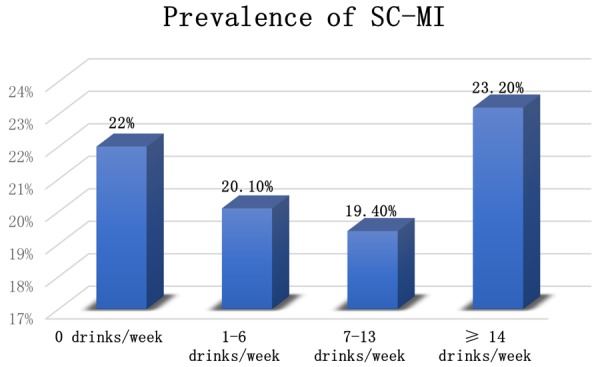
Prevalence of Subclinical Myocardial Injury Across Alcohol categories.
Using CIIS as continuous variable, in a linear regression model adjusted for demographics, moderate alcohol consumption was associated with lower values of CIIS compared with non-drinker (β (95% CI): -0.56 (-1.18, 0.05), P = 0.07) and this negative association of moderate alcohol consumption with CIIS did not change after adjustment for CVD risk factors and potential mediators (β (95% CI): 0.64 (-1.27, -0.007), P = 0.04). Light and heavy drinking were also associated with lower values of CIIS, but results were not statistically significant in models adjusted for demographics as well as in fully adjusted models (Table 2).
Table 2.
Multivariable Beta-coefficient and 95% CI of association between alcohol categories and CIIS
| Alcohol categories | Model 1 | Model 2 | ||
|---|---|---|---|---|
|
| ||||
| Beta-Coefficient (95% CI) | p-value | Beta-Coefficient (95% CI) | p-value | |
| 0 drinks/week | Reference | Reference | ||
| 1-6 drinks/week | -0.20 (-0.58, 0.18) | 0.30 | -0.22 (-0.61, 0.17) | 0.26 |
| 7-13 drinks/week | -0.56 (-1.18, 0.05) | 0.07 | 0.64 (-1.27, -0.007) | 0.04 |
| ≥14 drinks/week | -0.19 (-1.27, 0.89) | 0.72 | -0.37 (-1.47, 0.72) | 0.50 |
Beta coefficient and 95% confidence interval calculated from a multivariable linear regression analysis. Model 1 adjusted for age, sex, race, and socioeconomic status. Model 2 adjusted for model 1 plus physical activity, smoking, BMI, HTN, insulin resistance, CRP and HDL. CIIS, cardiac injury score; CRP, C-reactive protein; HDL, high-density lipoprotein; BMI, body mass index. Hypertension defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 or taking antihypertensive medications. Insulin resistance defined as fasting blood sugar ≥100 mg/dl, or self-reported history of diabetes or taking medications.
Using multiple linear regression analysis, we also calculated least mean square and standard error (SE) of association alcohol categories with CIIS. We found that never drinkers had the higher mean values of CIIS, while moderate drinkers had the lowest mean values of CIIS (Table 3 and Figure 2).
Table 3.
Least Mean Square and SE of CIIS across alcohol categories
| Alcohol categories | Model 1 | Model 2 |
|---|---|---|
|
| ||
| Mean ± SE | Mean ± SE | |
| 0 drinks/week | 5.32±0.11 | 5.34±0.11 |
| 1-6 drinks/week | 5.12±0.15 | 5.12±0.15 |
| 7-13 drinks/week | 4.75±0.28 | 4.71±0.29 |
| ≥14 drinks/week | 5.13±0.53 | 4.97±0.54 |
Least mean square and standard error calculated from multiple linear regression analysis. Model 1 adjusted for age, sex, race, and socioeconomic status. Model 2 adjusted for model 1 plus physical activity, smoking, BMI, HTN, insulin resistance, CRP and HDL. CIIS, cardiac injury score; CRP, C-reactive protein; HDL, high-density lipoprotein; BMI, body mass index; SE, standard error.
Figure 2.
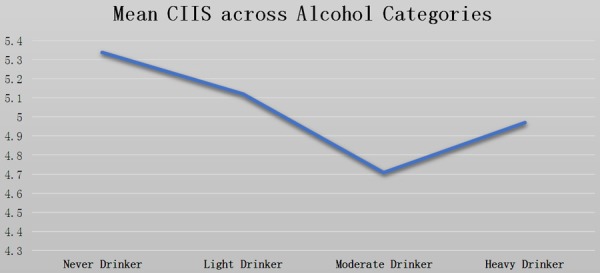
Least Mean Square (LSMEANS) of Cardiac Infarction/Injury Score (CIIS) Across Alcohol Categories after adjustment of all potential confounders.
In the subgroup analysis by age, sex, and race, we found heterogeneities in the association between alcohol categories and CIIS. Using linear regression analysis, in a fully adjusted model, females and older participants had lower values of CIIS compared to males, and younger participants in all alcohol categories. However, there was no significant interaction by sex or age. Whites tend to have lower values of CIIS compared to non-whites, especially with moderate alcohol consumption (β (95% CI): -1.06 (-1.93, -0.19) vs. 0.05 (-0.91, 1.00) respectively. The interaction p-value was close to the level of significance (P = 0.08) (Table 4).
Table 4.
Multivariable beta coefficient and 95% CI for the association between Alcohol categories and CIIS in Subgroups
| Alcohol Categories | Beta-coefficient (95% CI) | Interaction P-value | |
|---|---|---|---|
| Male | 1-6 drinks/week | -0.04 (-0.62, 0.54) | 0.68 |
| 7-13 drinks/week | -1.47 (-1.00, 0.70) | ||
| ≥14 drinks/week | -0.02 (-1.29, 1.24) | ||
| Female | 1-6 drinks/week | -0.45 (-0.98, 0.07) | |
| 7-13 drinks/week | -1.35 (-2.35, -0.36) | ||
| ≥14 drinks/week | -1.51 (-4.16, 1.14) | ||
| Whites | 1-6 drinks/week | -0.45 (-1.02, 0.13) | 0.08 |
| 7-13 drinks/week | -1.06 (-1.93, -0.19) | ||
| ≥14 drinks/week | -1.05 (-2.58, 0.48) | ||
| Non-Whites | 1-6 drinks/week | 0.07 (-0.46, 0.59) | |
| 7-13 drinks/week | 0.05 (-0.91, 1.00) | ||
| ≥14 drinks/week | 0.51 (-1.08, 2.10) | ||
| Age >65 years | 1-6 drinks/week | -0.77 (-1.65, 0.11) | 0.45 |
| 7-13 drinks/week | -1.47 (-2.76, -0.17) | ||
| ≥14 drinks/week | 0.47 (-1.93, 2.86) | ||
| Age ≤65 years | 1-6 drinks/week | -0.15 (-0.57, 0.26) | |
| 7-13 drinks/week | -0.42 (-1.14, 0.30) | ||
| ≥14 drinks/week | -0.74 (-1.95, 0.47) |
Beta coefficient and 95% confidence interval calculated from a multivariable linear regression analysis. Reference group = 0 drinks/week. Model adjusted for Age, Sex, Race, Socioeconomic status, Smoking, Physical Activity, BMI, insulin resistance, Hypertension, high-density cholesterol, CRP. CIIS, cardiac injury score; CRP, C-reactive protein; HDL, high-density lipoprotein; BMI, body mass index. Hypertension defined as systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 or taking antihypertensive medications. Insulin resistance defined as fasting blood sugar ≥100 mg/dl, or self-reported history of diabetes or taking medications.
We also calculated OR and 95% CI for SC-MI entered in the models as binary outcome variable defined as CIIS ≥10. There was no significant association between alcohol consumption and SC-MI using logistic regression analysis. However, among alcohol categories, odds of SC-MI was lowest with moderate alcohol consumption (OR (95% CI): 0.79 (0.60-1.04), P = 0.29) (Table S1).
Discussion
In this nationally representative sample of 6090 participants free of CVD at baseline, we found statistically significant cross-sectional associations between moderate alcohol consumption (7-13 drinks/week) and low CIIS. This inverse association between moderate alcohol consumption and CIIS score persisted despite rigorous adjustment for confounders. Although light and heavy drinking were also associated with decreased CIIS score, this association was not statistically significant. Moderate alcohol consumption relative to non-drinkers was associated with lower CIIS score among whites and not among the non-white population. Moreover, the inverse association was much stronger among females and older population (>65 years) compared to men and young (<65 years) respectively.
Most of the previous studies have focused on examining the relationship between alcohol consumption and subclinical atherosclerosis using CAC or carotid intima-media thickness (c-IMT). Vliegenthart et al. [6] found a U-shaped association between alcohol consumption and coronary calcification while Pletcher et al. [7] discovered dose-response relation. A more recent study by Yun et al. [17] found that higher levels of alcohol consumption were associated with an increased risk of coronary calcification in Korean men. But most of the other epidemiological studies have found no association between alcohol use and CAC [8,9,11,18]. The few studies evaluating the effect of alcohol consumption and c-IMT have also provided conflicting results [18-21]. Furthermore, its duplicated an analysis from the Atherosclerosis Risk in Communities (ARIC) study showed that compared to never drinkers, persons who consumed 2-7 drinks per week were less likely to have increased levels of high sensitivity troponin T assays, another measure of SC-MI [12]. To the best of our knowledge, there has not been a prior study linking alcohol consumption levels to SC-MI as measured by ECG scoring system such as CIIS in a relatively healthy cohort.
The mechanisms by which alcohol consumption may influence subclinical myocardial injury are not entirely clear. Several proposed mechanisms of al-cohol that may explain its cardioprotective effect include increasing high-density lipoprotein (HDL) [3,4], decreasing c-reactive protein [5], increasing NO release [22], decreasing platelet aggregation [23] and increasing insulin sensitivity [2,24]. Our finding of moderate alcohol consumption having a protective association with SC-MI is consistent with existing evidence which suggests that moderate alcohol consumption is associated with decreased risk of CVD [1]. Our study showed the stronger inverse relationship between alcohol consumption and CIIS in the older population. As we can see from the potential mechanisms of the cardioprotective effects of alcohol, some effects are mediated through mechanisms that are not reflected by CIIS score. Hence, the protective effect of alcohol may differ in older individuals. Also, the finding of a strong association of alcohol consumption with lower CIIS score among female may suggest a more favorable dietary pattern that might have accentuated the protective effect of alcohol.
A large number of patients with SC-MI will be missed if abnormalities are examined separately. The application of CIIS has been shown to predict CVD and all-cause mortalities among a large group of persons without apparent CVD [14]. The application of CIIS in this study was to find individuals without clinical CVD but who were at an increased risk of CVD and all-cause mortalities. Other electrocardiographic markers which are also associated with increased mortality include QRS duration and minor Q waves, but they only rely on 1 or 2 data points [25,26]. In contrast, the application of a scoring system, such as CIIS may be a more sensitive indicator of myocardial injury. It has also been shown that the CIIS improves the accuracy of the standard 12-lead electrocardiogram to identify patients with SC-MI [15].
Our study has several limitations. Alcohol consumption was self-reported, and participants may have underreported heavy consumption. This would most likely have attenuated the associations. Also, there were relatively few heavy drinkers, which limited our ability to explore alcohol associations among this subset. Finally, we adjusted for several confounders, but residual confounding remains a possibility. Our study has many strengths. The strength of our study includes its large sample size, community-based and multiracial population and better generalizability of the US population. Also, we were able to adjust for many potential confounders and mediators including lifestyle variables and CVD risk factors.
In conclusion, moderate alcohol consumption was associated with lower CIIS. As lower CIIS is associated with decreased risk of CVD mortality, it is, therefore plausible that moderate alcohol consumption may be associated with low risk of poor future outcomes. We also observed heterogeneity in association of alcohol consumption with CIIS by age and sex, so these subgroup analyses should be considered exploratory and hypothesis generating. If these associations are causal, further research is needed to understand the mechanisms by which moderate alcohol consumption confers protection in these subgroups.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppes LL, Dekker JM, Hendriks HF, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes-a meta-analysis of prospective observational studies. Diabetes Care. 2005;28:719–725. doi: 10.2337/diacare.28.3.719. [DOI] [PubMed] [Google Scholar]
- 3.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JM, Mukamal KJ. An update on alcohol and atherosclerosis. Curr Opin Lipidol. 2004;15:673–680. doi: 10.1097/00041433-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 6.Vliegenthart R, Oei HH, van den Elzen AP, van Rooij FJ, Hofman A, Oudkerk M, Witteman JC. Alcohol consumption and coronary calcification in a general population. Arch Intern Med. 2004;164:2355–2360. doi: 10.1001/archinte.164.21.2355. [DOI] [PubMed] [Google Scholar]
- 7.Pletcher MJ, Varosy P, Kiefe CI, Lewis CE, Sidney S, Hulley SB. Alcohol consumption, binge drinking, and early coronary calcification: findings from the coronary artery risk development in young adults (CARDIA) study. Am J Epidemiol. 2005;161:423–433. doi: 10.1093/aje/kwi062. [DOI] [PubMed] [Google Scholar]
- 8.McClelland RL, Bild DE, Burke GL, Mukamal KJ, Lima JA, Kronmal RA. Alcohol andcoronary artery calcium prevalence, incidence, and progression: results from the multi-ethnic study of atherosclerosis (MESA) Am J Clin Nutr. 2008;88:1593–1601. doi: 10.3945/ajcn.2008.26420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tofferi JK, Taylor AJ, Feuerstein IM, O’Malley PG. Alcohol intake is not associated with subclinical coronary atherosclerosis. Am Heart J. 2004;148:803–809. doi: 10.1016/j.ahj.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Yang T, Doherty TM, Wang ND, Detrano RC. Alcohol consumption, coronary calcium, and coronary heart disease events. Am J Cardiol. 1999;84:802–806. doi: 10.1016/s0002-9149(99)00440-3. [DOI] [PubMed] [Google Scholar]
- 11.Ellison RC, Zhang YQ, Hopkins PN, Knox S, Djousse L, Carr JJ. Is alcohol consumption associated with calcified atherosclerotic plaque in the coronary arteries and aorta? Am Heart J. 2006;152:177–182. doi: 10.1016/j.ahj.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Lazo M, Chen Y, McEvoy JW, Ndumele C, Konety S, Ballantyne CM, Sharrett AR, Selvin E. Alcohol consumption and cardiac biomarkers: the atherosclerosis risk in communities (ARIC) study. Clin Chem. 2016;62:1202–1210. doi: 10.1373/clinchem.2016.255778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rautaharju PM, Warren JW, Jain U, Wolf HK, Nielsen CL. Cardiac infarction injury score: an electrocardiographic coding scheme for ischemic heart disease. Circulation. 1981;64:249–256. doi: 10.1161/01.cir.64.2.249. [DOI] [PubMed] [Google Scholar]
- 14.O’Neal WT, Shah AJ, Efird JT, Rautaharju PM, Soliman EZ. Subclinical myocardial injury identified by cardiac infarction/injury score and the risk of mortality in men and women free of cardiovascular disease. Am J Cardiol. 2014;114:1018–1023. doi: 10.1016/j.amjcard.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson K, Engel G, Yamazaki T, Chun S, Froelicher VF. Electrocardiographic damage scores and cardiovascular mortality. Am Heart J. 2005;149:458–463. doi: 10.1016/j.ahj.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Plan and operation of the third national health and nutrition examination survey, 1988-94. series 1: programs and collection procedures. Vital Health Stat 1. 1994:1–407. [PubMed] [Google Scholar]
- 17.Yun KE, Chang Y, Yun SC, Smith GD, Ryu S, Cho SI, Chung EC, Shin H, Khang YH. Alcohol and coronary artery calcification: an investigation using alcohol flushing as an instrumental variable. Int J Epidemiol. 2017;46:950–962. doi: 10.1093/ije/dyw237. [DOI] [PubMed] [Google Scholar]
- 18.Mukamal KJ, Kronmal RA, Mittleman MA, O’Leary DH, Polak JF, Cushman M, Siscovick DS. Alcohol consumption and carotid atherosclerosis in older adults-the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2003;23:2252–2259. doi: 10.1161/01.ATV.0000101183.58453.39. [DOI] [PubMed] [Google Scholar]
- 19.Zyriax BC, Lau K, Klahn T, Boeing H, Volzke H, Windler E. Association between alcohol consumption and carotid intima-media thickness in a healthy population: data of the strategy study (stress, atherosclerosis and ECG study) Eur J Clin Nutr. 2010;64:1199–1206. doi: 10.1038/ejcn.2010.144. [DOI] [PubMed] [Google Scholar]
- 20.Demirovic J, Nabulsi A, Folsom AR, Carpenter MA, Szklo M, Sorlie PD, Barnes RW. Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The atherosclerosis risk in communities (ARIC) study investigators. Circulation. 1993;88:2787–2793. doi: 10.1161/01.cir.88.6.2787. [DOI] [PubMed] [Google Scholar]
- 21.Zureik M, Gariepy J, Courbon D, Dartigues JF, Ritchie K, Tzourio C, Alperovitch A, Simon A, Ducimetiere P. Alcohol consumption and carotid artery structure in older French adults: the three-city study. Stroke. 2004;35:2770–2775. doi: 10.1161/01.STR.0000147968.48379.c3. [DOI] [PubMed] [Google Scholar]
- 22.Toda N, Ayajiki K. Vascular actions of nitric oxide as affected by exposure to alcohol. Alcohol Alcohol. 2010;45:347–355. doi: 10.1093/alcalc/agq028. [DOI] [PubMed] [Google Scholar]
- 23.Mukamal KJ, Massaro JM, Ault KA, Mittleman MA, Sutherland PA, Lipinska I, Levy D, D’Agostino RB, Tofler GH. Alcohol consumption and platelet activation and aggregation among women and men: the framingham offspring study. Alcohol Clin Exp Res. 2005;29:1906–1912. doi: 10.1097/01.alc.0000183011.86768.61. [DOI] [PubMed] [Google Scholar]
- 24.Danziger J, Young RL, Shea MK, Tracy RP, Ix JH, Jenny NS, Mukamal KJ. Vitamin K-dependent protein activity and incident ischemic cardiovascular disease: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36:1037–1042. doi: 10.1161/ATVBAHA.116.307273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badheka AO, Singh V, Patel NJ, Deshmukh A, Shah N, Chothani A, Mehta K, Grover P, Savani GT, Gupta S, Rathod A, Marzouka GR, Mitrani RD, Moscucci M, Cohen MG. QRS duration on electrocardiography and cardiovascular mortality (from the national health and nutrition examination survey-III) Am J Cardiol. 2013;112:671–677. doi: 10.1016/j.amjcard.2013.04.040. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Dawood FZ, Chen H, Jain A, Walsh JA 3rd, Alonso A, Lloyd-Jones DM, Soliman EZ. Minor isolated Q waves and cardiovascular events in the MESA study. Am J Med. 2013;126:450.e9–450.e16. doi: 10.1016/j.amjmed.2012.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


