Abstract
This is a relative rare case of 0.5% TBSA (total body surface area) burn wound infection caused by Herpes Simplex Virus (HSV). A 1-year-old male infant had deep second degree burn of the left fourth finger with 0.5% TBSA after exposure to a hot object. Blisters and vesicles surrounded by erythema were obvious in the examination of the burned area. The polymerase chain reaction (PCR) and gene sequencing analyses addressed contamination of the burn wound with HSV. Three days after the administration of antibiotics, the wound was relatively healed and finally, the patient was discharged in good general health, and no signs of relapse were observed in the 3-month follow-up. Although HSV infection is rarely reported in non-immunocompromised patients and TBSA burn injuries, due to the high prevalence of HSV infection and its mortality potential in the affected patients, HSV infection should be clinically suspected in the cases with delayed wound healing. In addition, since HSV infection is very contagious, and exposure to patients with HSV infection might be highly problematic for other patients hospitalized in burn wards; hence, proper facilities should be provided for the isolation care of the burn patients with HSV infection.
Keywords: Infection, burn, herpes simplex
Introduction
Herpes Simplex Virus (HSV) is a DNA virus and a member of Herpesviridae family [1], which includes HSV-1 and HSV-2; HSV-1 mostly causes orolabial herpes and HSV-2 genital herpes [2]. The immunodeficiency is the most important factor affecting HSV pathogenicity [2]. Burn is a possible cause of immunodeficiency. In opportunistic infections including HSV, the extent of injury described using the percentages of total body surface area (TBSA) affected by burn should be enough to weaken the T-cell dependent immunity [1-3]. A study by Hayden et al., reported the mean TBSA of 36% in patients with burn wound infection caused by HSV, while the infection was not observed in the cases with slight burns (4% TBSA) [4]. This is a relative rare case of 0.5% TBSA burn that developed to HSV infection.
Care report
A 1-year-old male infant with deep second degree burn of the left fourth finger following the exposure to a hot object had referred to a non-burn physician; his wound was washed and the patient experimentally received a combination regimen of cefotaxime plus silver sulfadiazine and mupirocin. The wound did not heal following the administration of prescribed medicines and 10 days after the first referring, the patient under study referred to the hospital, a burn specialty center. The parents reported good general health for the patient, except unhealed finger burn wound, mild fever started 3 days prior to referring, and slight lethargy. There was no history of special diseases in the patient and his family; he did not take any special medicine, and had timely and complete vaccination. He was conscious at arrival without restlessness, lethargy, and fever and his vital signs were age-appropriately within the normal range. In the physical examinations, blisters and vesicles surrounded by erythema were obvious at the burned area (Figure 1). There were no bleeding or sore discharges in the wound. Also, signs of numbness in extremities, decreased limb movement, and reduced blood pressure were not observed in the patient, except for the burned area and all systematic examinations reported normal results. TBSA was 0.5% for the reported case. First, the hypothesis of misidentification of bacteria or viruses caused wound infection and accordingly, medication errors were introduced and then, the patient received isolation care and underwent washing the wound with sodium hypochlorite as well as dressing with mupirocin ointment twice-a-day. After consultation with burn team including a surgeon and an infectious disease specialist, routine tests as well as polymerase chain reaction (PCR) of burned area swab samples were performed. No abnormality was observed in blood and urine samples tests and infection with Gram-positive cocci was also reported from wound culture test. Based on the results, cloxacillin was intravenously administered to the patient. Due to impaired wound healing, the antibiotic was replaced by cefepime. Based on the results obtained from final PCR products and the fragment sequencing, HSV was identified as a pathogen that caused infection in the burn wound. Three days after the treatment with cefepime, dressing change, and washing the wound properly, a relative improvement was observed in the burned area, and finally the patient was discharged with good general health and HSV infection diagnosis; the parents were informed about warning signs, and after a 3-month follow-up no signs and symptoms of infection relapse were observed.
Figure 1.
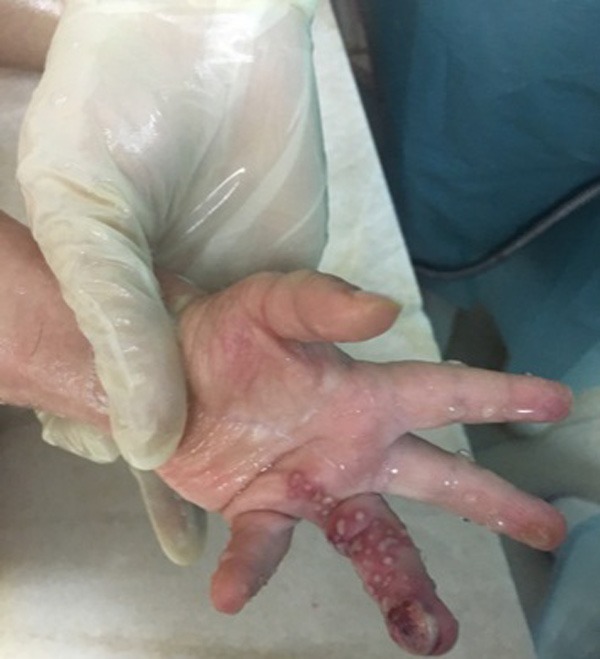
Vesicles surrounded by erythema at the burned area.
Discussion
HSV is one of the most common causes of human infection worldwide with 60%-90% frequency [5], which is increasing to such an extent that its prevalence increased from 50% to 85% [6]. HSV annually affects 23 million people worldwide; in 2012 about 11.3% of world population was affected by HSV infection [6,7]. A meta-analysis (2016) in Iran on 7762 patients also reported the prevalence of HSV1, HSV2, and HSV as 42.04%, 6.5%, and 25.7%, respectively [6]. HSV infection is commonly manifested by lesions on lips and face as well as genitalia caused by HSV1 and HSV2, respectively [6], although many cases are asymptomatic and can only be diagnosed with positive antibody. HSV is highly contagious, and is accumulated in nervous ganglia after entering into the body and periodically exhibits symptoms just under particular circumstances [8]. Manifestations can be in the form of opportunistic infection or reactivation of the latent virus. Manifestation depends on the immune system of the host, regardless of some environmental and demographic factors. If the cellular immune system fails to combat HSV infection, the disease is only manifested by some symptoms [2].
Burn is the condition, which weakens the immune system of the host and facilitates opportunistic infections. The most common infection-localizing agents are the bacteria such as Staphylococci. Fungal and viral agents are less prevalent and require weaker immune system for pathogenesis, but if they cause an infection, it is usually associated with high mortality [8,9]. The immune system depends on TBSA in burns. It is evident that as TBSA increases, weaker immune system is expected [10]. The most common viral agents localizing infections on burn wounds are cytomegalovirus (CMV), varicella zoster, herpes zoster, and HSV [8,2]. Herpes burn wound infection was first reported by Scott et al. (1952), in a 2-year-old female with burn injury of the finger that was infected after being kissed by the mother; following the manifestation of vesicular lesions, herpetic whitlow infection was the possible diagnosis [11]. Then after, several studies were conducted worldwide on HSV infection among patients with burn wound and the results indicated the risk of localization of HSV infection in burn wounds [4]. Kegen et al., reported that the risk of high titers of HSV antibodies was 4 times higher in 40% of patients with severe burn injuries [12]. Bourdarias et al., reported that 4% of patients with burn wound also had HSV infection [13]. D’Avignon et al., evaluated the autopsy reports on HSV infection among patients with severe burn wound and showed that 5 out of 97 autopsy reports were attributed to viral infections, of which 4 cases were HSV infection [9].
The percentage of burn in the reported case was very low as 0.5% TBSA. It does not seem that such low percentage of burn could play a significant role in weakening the immune system; on the other hand, the number of white blood cells was at normal limits. Likewise, Hayden et al., showed that more than half of HSV burn wound infections were attributed to children under 5 years old with the mean TBSA of 36%, while no case with <4% TBSA or immunodeficiency was observed among the patients [4]. In addition, Wurzer et al., (2017) showed that infection with Herpesviridae family members usually occurred in larger wounds with 53% TBSA [1]. Similar to the results of the current study, Chen et al., reported a rare case of 21-month infant with HSV1 vesicular lesions developed in burns on arm and chest (4% TBSA) and fever; they showed that although such cases rarely happen, the physicians should pay enough attention to the medical history of the patient as well as clinical examinations and accurate diagnosis [10]. In the current case, the symptoms were less, but more illusory; for example, the patient had no fever and TBSA percentage was really low, however, there were no signs of pathognomonic-vesicular eruptions.
However, wound culture results were another misleading factor. The antibiotic therapy was administered based on the results of wound culture, but no expected healing was observed; therefore, prolonged healing was another factor indicating the HSV infection. Likewise, Sen et al., reported that HSV infection after burn prolongs healing progress and provides the condition for bacterial infection [14]. In the current cases, after being suspicious of viral infection, PCR of wound swab samples was amplified and the diagnosis was confirmed. The clinical diagnosis of HSV infection is difficult due to burn wound lesions and may be neglected; on the other hand, the clinical signs and symptoms of viral infections are not specific. Hence, laboratory methods should be employed in the cases suspected of viral infection; Tzanck smear and culturing are among the methods. Nowadays, PCR is also used as a reference method for HSV infection cases; PCR has 96% sensitivity and 99% specificity [15,16].
Treatment of HSV infection in burn wounds is controversial [1,2]. Some studies believe that in patients with extensive burns developed infection acyclovir should be administered, but some other studies indicated that several patients with moderate burns recovered without any certain treatment [2]; however, acyclovir was not used in the current case report due to patient’s recovery.
Generally, although HSV infection rarely happens in the cases without immunodeficiency and lower TBSA, and it is almost asymptomatic, due to high prevalence of such infections and their potential for high mortality, in the cases with prolonged recovery, viral infection should be considered; however, since HSV is highly contagious, exposure to other patients with such infections is associated with high risk of HSV infection in patients with severe immunodeficiency admitted to burn centers. Therefore, proper facilities should be provided for the isolation care of the burn patients. On the other hand, if HSV infection is not diagnosed properly in burn patients, the infection is exacerbated in case of undergoing debridement and skin graft surgery; the current case was managed properly by early and accurate diagnosis and such measures were postponed until achieving stable conditions in the patient.
Disclosure of conflict of interest
None.
References
- 1.Wurzer P, Cole MR, Clayton RP, Hundeshagen G, Nunez Lopez O, Cambiaso-Daniel J, Winter R, Branski LK, Hawkins HK, Finnerty CC, Herndon DN, Lee JO. Herpesviradae infections in severely burned children. Burns. 2017;43:987–992. doi: 10.1016/j.burns.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wurzer P, Guillory A, Parvizi D, Clayton RP, Branski LK, Kamolz LP, Finnerty CC, Herndon DN, Lee JO. Human herpes viruses in burn patients: a systematic review. Burns. 2017;43:25–33. doi: 10.1016/j.burns.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, Suman OE, Mlcak RP, Herndon DN. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayden FG, Himel HN, Heggers JP. Herpesvirus infections in burn patients. Chest. 1994;106:15S–21S. doi: 10.1378/chest.106.1_supplement.15s. discussion 34S-35S. [DOI] [PubMed] [Google Scholar]
- 5.Marchi S, Trombetta CM, Gasparini R, Temperton N, Montomoli E. Epidemiology of herpes simplex virus type 1 and 2 in Italy: a seroprevalence study from 2000 to 2014. J Prev Med Hyg. 2017;58:E27–E33. [PMC free article] [PubMed] [Google Scholar]
- 6.Malary M, Abedi G, Hamzehgardeshi Z, Afshari M, Moosazadeh M. The prevalence of herpes simplex virus type 1 and 2 infection in Iran: a meta-analysis. Int J Reprod Biomed (Yazd) 2016;14:615–624. [PMC free article] [PubMed] [Google Scholar]
- 7.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGill SN, Cartotto RC. Herpes simplex virus infection in a paediatric burn patient: case report and review. Burns. 2000;26:194–9. doi: 10.1016/s0305-4179(99)00057-1. [DOI] [PubMed] [Google Scholar]
- 9.D’Avignon LC, Hogan BK, Murray CK, Loo FL, Hospenthal DR, Cancio LC, Kim SH, Renz EM, Barillo D, Holcomb JB, Wade CE, Wolf SE. Contribution of bacterial and viral infections to attributable mortality in patients with severe burns: an autopsy series. Burns. 2010;36:773–9. doi: 10.1016/j.burns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Chen CC, Chen CL, Chiang CH, Pan SC. Herpes simplex infection in a minor burn wound: a case report. J Burn Care Rehabil. 2005;26:453–5. doi: 10.1097/01.bcr.0000176889.39355.41. [DOI] [PubMed] [Google Scholar]
- 11.Scott TF, Coriell L, Blank H, Burgoon CF. Some comments on herpetic infection in children with special emphasis on unusual clinical manifestations. J Pediatr. 1952;41:835–43. doi: 10.1016/s0022-3476(52)80303-8. [DOI] [PubMed] [Google Scholar]
- 12.Kagan RJ, Naraqi S, Matsuda T, Jonasson OM. Herpes simplex virus and cytomegalovirus infections in burned patients. J Trauma. 1985;25:40–5. doi: 10.1097/00005373-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bourdarias B, Perro G, Cutillas M, Castede JC, Lafon ME, Sanchez R. Herpes simplex virus infection in burned patients: epidemiology of 11 cases. Burns. 1996;22:287–90. doi: 10.1016/0305-4179(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 14.Sen S, Szoka N, Phan H, Palmieri T, Greenhalgh D. Herpes simplex activation prolongs recovery from severe burn injury and increases bacterial infection risk. J Burn Care Res. 2012;33:393–7. doi: 10.1097/BCR.0b013e3182331e28. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan RL, Schulz JT, Weber JM, Ryan CM, Pasternack MS, Tompkins RG. Cutaneous herpetic infections complicating burns. J Burns. 2000;26:621–4. doi: 10.1016/s0305-4179(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 16.Bordes J, Kenane N, Meaudre E, Asencio Y, Montcriol A, Prunet B, Palmier B. A case of atypical and fatal herpes simplex encephalitis in a severe burn patient. Burns. 2009;35:590–3. doi: 10.1016/j.burns.2008.05.006. [DOI] [PubMed] [Google Scholar]


