Abstract
Cortical microinfarcts are the most widespread form of brain infarction but frequently remain undetected by standard neuroimaging protocols. Moreover, microinfarcts are only partially detectable in hematoxylin- eosin-stained (H&E) 4-10 μm paraffin sections at routine neuropathological examination. In this short report, we provide two staining protocols for visualizing cortical microinfarcts in 100-300 μm sections. For low-power microscopy, the first protocol combines aldehyde fuchsine staining for detection of lipofuscin granules and macrophages with Darrow red counterstaining for Nissl material. The second protocol combines collagen IV immunohistochemistry with aldehyde fuchsine/Darrow red or with erythrosin-phosphotungstic acid-aniline blue staining for detailed study of the capillary network. In the first protocol, microinfarcts are recognizable as radially- oriented funnel-like accumulations of aldehyde fuchsine-positive macrophages. The second protocol recognizes microinfarcts and alterations of the capillary network, at whose center accumulations of dead neurons and aldehyde fuchsine-positive macrophages cluster. In addition, the second protocol permits visualization of abnormalities within the capillary network associated with more recent microinfarcts. Both protocols can be useful for comparing MRI datasets with cortical microinfarcts in corresponding whole brain sections of 100-300 μm thickness.
Key words: Aldehyde fuchsine, capillary network, CD68, collagen IV immunohistochemistry, cortical microinfarcts, macrophages, post-mortem brain tissue
Introduction
Cortical microinfarcts are probably the single most widespread form of brain infarction. However, they can escape detection in standard in vivo magnetic resonance imaging (MRI) protocols,1-4 and they are only partially detectable in hematoxylineosin (H&E) stained 4-10 μm paraffin sections. The lesions are described as illdefined foci with neuronal loss, gliosis, and cortical pallour,5,6 their reported diameters varying from 50 μm to several millimeters.7 They can be surrounded by glial cells and/or macrophages.1 The burden of cortical microinfarcts is underestimated,3 and the pathomechanisms underlying their development are incompletely understood. 8,9 Rather surprisingly, there have been few prior attempts to develop novel staining or immunolabeling techniques for cortical microinfarcts in humans.1 For instance, one recent protocol used thick (40 μm) paraffin-embedded sections and recommended a combination of heat (microwave, steamer) followed by enzymatic digestion (pepsin) to obtain successful collagen IV immunostaining (immunofluorescence) results of the microvasculature in formaldehyde-fixed human brain tissue; 10 but the authors did not examine/apply this method to cortical microinfarcts. Clearly, both autopsy- and MRI-based studies will be needed, going forward, to determine the exact histopathology of these lesions.1,3
Materials and Methods
All procedures complied with the University of Ulm’s ethics committee guidelines and with German law governing usage of human post-mortem tissue. Two autopsy brains (2 males, 68 and 74 years of age) fixed for 14 days by immersion in a 4% aqueous solution of formaldehyde were used. Tissue blocks embedded in polyethylene glycol (PEG 1000, Merck, Carl Roth Ltd, Karlsruhe, Germany)11,12 were coronally sectioned on a tetrander (Jung, Heidelberg, Germany) at 100-300 μm as previously described.13,14 If paraffin-embedding is preferred, the two staining protocols provided below can be applied to sections of 70-100 μm thickness.15 Histologic sections were viewed and assessed with an Olympus BX61 microscope (Olympus Optical, Tokyo, Japan). Digital micrographs were taken with an Olympus XC50 camera using the Cell DÒ Imaging Software (Olympus, Münster, Germany). The program’s Extended Focal Imaging (EFI) function was used (Figure 1 d,f) to fuse stacks of four differently focused single images into one sharply focused image.
Figure 1.

Cortical microinfarcts in PEG-embedded sections of 300 μm and 100 μm thickness. The combination of aldehyde fuchsine and Darrow red in a 300 μm section (framed area in panel a is shown in greater detail in panel b) permits easy recognition of another cortical microinfarct (same individual as in Figure 2, Brodmann area 4). a) Arrow at farleft points to an unstained blood vessel; asterisks indicate gray matter areas that have undergone neuronal loss and glial cell loss; large funnel-like accumulations of aldehyde fuchsine-positive macrophages are oriented radially through multiple cellular cortical layers of the primary motor neocortex. c-f ) The combination of aldehyde fuchsine staining with an immunoreaction against collagen IV (brown chromogen 3,3’-diaminobenzidine tetrahydrochloride, DAB) and a counterstain (eosin-phosphotungstic acid-aniline blue, EPA) permits more detailed study of cortical microinfarctions (100 μm, 74-year-old male); framed areas in panels c and e are shown in greater detail in panels d and f; erythrocytes at upper left in panel d are stained for erythrosine. e,f ) Aldehyde fuchsine staining combined with an immunoreaction for collagen IV and Darrow red displays thickening and puckering of the damaged capillary network in a presumably more recent microinfarction: in contrast to the situation in older microinfarcts (c,d), the central portion of the lesion in panels e and f contains only a few aldehyde fuchsine-positive macrophages. Scale bars: c,e) 200 μm; f ) 100 μm. Objectives used: a) 2:1; b,c,e) 4:1; d,f ) 10:1.
Protocol I - screening for cortical microinfarcts
To provide an overview of cortical microinfarcts for research purposes rather than for routine neuropathology in brain tissue blocks (Figure 2a), we used free-floating sections of 300 μm thickness. Whereas sections of this thickness are not sufficiently penetrated by all commercially available antibodies, they are ideal here for aldehyde fuchsine staining, which selectively visualizes intraneuronal and extraneuronal lipofuscin as well as CD68-positive macro - phages in brain tissue. If desired, counterstaining for Nissl material with Darrow red can be performed (Figure 2b).13
Figure 2.
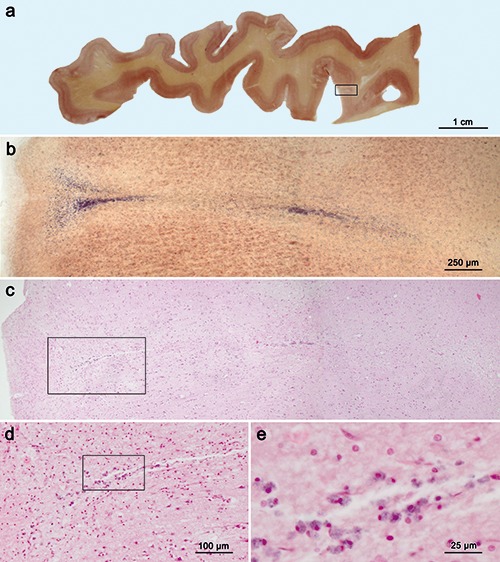
Sections comparing aldehyde fuchsine plus Darrow red staining (300 μm) with H&E staining (5 μm) of a cortical microinfarct in the primary motor neocortex (Brodmann area 4) a). 300 μm PEG-embedded section stained with aldehyde fuchsine and Darrow red for topographical overview (male, 68 years of age). b) Low-power microscopy of such a section permits detection of a cortical microinfarction in the framed area, shown in panel a. c-e) After removing the coverslip, the right-hand one-third of the section that contained the framed area in panel a was re-embedded in paraffin, sectioned at 5 μm, and the immediately adjacent 5 μm section through the microinfarction shown in panel b was stained in panel c with H&E for overview; small infarctions are not easily recognized in standard (5 μm) H&E paraffin sections. d,e) Higher magnification reveals clusters of macrophages; framed area in panel d is shown in greater detail in panel e. Scale bar: c) 250 μm. Objectives used: a) macro 10 mm; b,c) 4:1; d) 10:1; e) 40:1.
Dissolve 1.5 g sodium stearate (Carl Roth, Karlsruhe, Germany) in 100 mL 70% ethanol to remove disruptive cerebrosides from the tissue. Rinse sections in sodium stearate for 1 h at 60°C. Rinse 5 min in 70% ethanol.
Dissolve 1 g sodium percarbonate (Acros Organics, Geel, Belgium) in 100 mL 70% ethanol. Oxidize sections in sodium percarbonate for 1 h at room temperature after heating the sodium percarbonate solution at above 60°C. A white sediment, which is desirable, persists following dissolution. Agitate gently on a shaking table. Rinse sections 5 min in 70% ethanol.
Dissolve 5 mL aldehyde fuchsine solution (Morphisto, Frankfurt am Main, Germany) in 100 mL 70% ethanol and add 20 ml glacial acetic acid. Transfer specimens to aldehyde fuchsine solution for 1 h on a shaking table (gentle setting). Rinse sections twice in 70% ethanol to remove excess dye. When only aldehyde fuchsine staining is desirable, proceed to steps 5 and 6 immediately below.
Alternatively, transfer sections to deionized water and place into Darrow red solution for 1h or more (agitate gently on a shaking table). To do so, dissolve 0.25 g Darrow red (Aldrich/Merck, Darmstadt, Germany) in 1000 mL deionized water, add 15 mL glacial acetic acid, boil for 20 min, filter solution, and add 250 mL of 0.2 Mol (i.e., 6.9 g sodium acetate trihydrate in 250 mL deionized water) sodium acetate solution (Merck, Darmstadt, Germany).
Dehydrate sections through a graded ethanol series. To obtain flatness, place free-floating sections between sheets of filter paper and compress gently between porcelain desiccator plates (140 mm diameter, Inv #119c, Morgan Technical Ceramics Haldenwanger, Germany, or Inv #8104, Lehman Scientific, Wrightsville, PA, USA) in 70%, 96% (30 min each), and 100% ethanol (30 min).
Remove sections from filter paper and dehydrate for another 30 min in 100% ethanol. A thorough dehydration step is crucial when using 300 μm sections. Clear in xylene twice (30 min each), coverslip in a high-refractive medium or synthetic resin (Histomount, National Diagnostics, Atlanta, GA, USA), and mount on slides.
Protocol II - for detailed study of cortical microinfarcts
For additional study purposes, regions of interest from the same PEG blocks as in the prior protocol can be sectioned at 100 μm. In this instance, visualization of the cortical capillary network in and around microinfarcts is best achieved using immunoreactions against collagen IV (Figure 1 c-f).10,16,17 The primary antibody used below displays robust immunoreactivity in archival fixed brain tissue stored in formaldehyde solutions and achieves optimal penetration of free-floating sections at all optical planes during low-power microscopy.16,17 Recently, collagen IV immunohistochemistry was successfully applied to 40 μm paraffin sections by Decker et al.10 Here, we combined the collagen IV immunoreaction with aldehyde fuchsine staining (with or without Darrow red) or in combination with staining of vessel wall components vessel wall in 100 μm PEG sections (Figure 1 c,d).
Perform steps 1-3 as above.
Rinse sections briefly in deionized water, then reduce background immunoreaction by placing them for 30 min in a mixture of 10% methanol (100%) plus 10% hydrogen peroxide (30%) and 80 mL Tris. Rinse 5 min in Tris or PBS buffer. Agitate gently on a shaking table.
Perform blocking with bovine serum albumin for 90 min to prevent non-specific binding, rinse 5 min in Tris or PBS buffer.
Incubate sections with primary polyclonal antibody against collagen IV (Abcam ab6586, Cambridge, UK, 1:5000) at room temperature for 18 h on a shaking table (gentle setting). Rinse 5 min in Tris or PBS buffer.
Transfer specimens to secondary biotinylated antibody (anti-rabbit IgG, BA-1000, Vector Lab, Burlingame, CA, USA, 1:200) at room temperature for 90 min on a shaking table (gentle setting). Rinse 5 min in Tris or PBS buffer.
Visualize immunoreaction with the avidin-biotin complex (ABC Elite, PK- 6100, Vector Lab) at room temperature for 120 min. Rinse 5 min in Tris or PBS buffer.
Transfer sections into 3,3’-diaminobenzidine tetrahydrochloride (DAB, D5637 Sigma, Taufkirchen, Germany) for 30 sec to 5 min, rinse thoroughly in deionized water.
Transfer sections to a solution of 0.1 g erythrosine (Merck, Darmstadt, Germany) in 100 mL deionized water for 10 min. Rinse 2 min in deionized water.
Dissolve 5 g phosphotungstic acid (Merck) in 100 mL 96% in ethanol. Agitate sections gently in phosphotungstic acid solution for 30 min or longer on a shaking table until excess erythrosine is removed except for that in erythrocytes. Rinse 2 min in deionized water.
Prepare aniline blue stock solution by dissolving 1 g phosphotungstic acid and 0.3 g aniline blue (Chroma Waldeck, Münster, Germany) in 100 mL deionized water. Then, add 1 mL glacial acetic acid. Stain specimens in 1 mL stock solution in 50 mL deionized water on a shaking table at gentle setting for 20-30 min. Rinse 1 min in 0.5% acetic acid and dehydrate sections as in protocol I, steps 5 and 6.
Addition of cinnamalaldehyde (nD 1.6219 at 20°C) to the mounting medium (8 mL Histomount and 2 m cinnamalaldehyde [Merck-Schuchardt, Hohenbrunn, Germany]) increases the refraction index (nD). When cover-slipping, sections should be mounted in undiluted cinnamalaldehyde after the second clearing in xylene.
Alternatively to steps 8-11, perform counterstaining of Nissl material by transferring sections into Darrow red solution for 1 h or more and agitate gently on a shaking table (follow steps 4-6 in protocol I).
Results and Discussion
In protocol I, microinfarcts are recognizable as funnel-like entities that contain radially-oriented accumulations of aldehyde fuchsine-positive macrophages (Figures 1 a,b and 2b). Protocol II stains not only the microinfarcts but also the affected capillary network, at whose center accumulations of dead neurons and aldehyde fuchsine-positive macrophages cluster (Figure 1c). Very early puckering within the capillary network may indicate recent microinfarcts (Figure 1e) where very little proliferation of aldehyde fuchsine-positive macrophages was observed at the center of such lesions (Figure 1f) in contrast to the dense accumulations of macrophages seen in other infarctions (Figure 1 c,d). Pigment-Nissl staining, which combines aldehyde fuchsine for selective demonstration of neuronal loss – extraneuronal remnants of lipofuscin indicate the positions of dead nerve cells – with a Nissl stain (e.g., Darrow red) for topographical overview in the human brain (Figure 2a), has been in use for many years.13,18,19
However, this stain has not been previously employed to visualize cortical microinfarcts. Here, aldehyde fuchsine effectively stained macrophages in human brain tissue (Figure 2b) and, thus, it can be used instead of CD68 immunoreactions (Figure 3). Here, we also combined pigment- Nissl staining with a sensitive immunoreaction (collagen IV)10 that also renders possible study of cortical microinfarcts with high spatial resolution (Figure 1 c-f). A drawback of both protocols is that they were performed on unconventionally large and thick free-floating tissue sections (≥100 μm), which, although suitable for research purposes, are not amenable to the demands of routine histopathology laboratories that rely on standard embedding cassettes and automated stainers. For this reason, we previously adapted our tissue processing procedures to also make possible the use of 70-100 μm paraffin-embedded free-floating sections,15 a method that has been employed successfully elsewhere.20,21 Current non-invasive in vivo MRI protocols are constantly undergoing improvement to diagnose and treat patients with cerebrovascular changes. While having attained greater importance in the daily clinical routine, such protocols need interpretation. In particular, efforts are in progress to relate in vivo MRI results directly to histopathological results won from post-mortem study of brains with cortical microinfarcts.2 Our staining protocols (Figures 1 and 2 a,b) represent an improvement of and an alternative to current methods in thin H&E as well as CD68 sections (Figures 2 c-e and 3 a-c), and we plan to apply them to compare in vivo MRI datasets with neuropathology of cortical microinfarcts in corresponding 100-300 μm serial whole brain sections.
Figure 3.
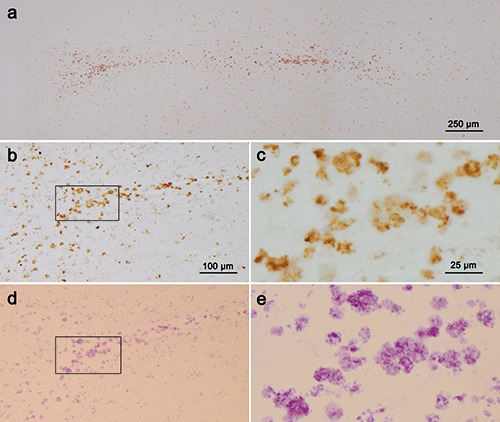
Paraffin sections (5 μm) comparing CD68 immunoreactions (a-c) with aldehyde fuchsine staining of macrophages (d,e) in the cortical microinfarct shown in Figure 2 be. a) The 5 μm section immediately adjacent to the H&E stained section shown in Figure 2c was immunostained for CD68-positive macrophages (here, without nuclear counterstaining); this technique readily recognizes the microinfarction and permits evaluation of the form and extent of the CD68-positive macrophages. b-e) The next adjacent 5 μm section (d,e) only underwent aldehyde fuchsine staining to visualize macrophages (again, without nuclear counterstaining); the framed area in panel d is displayed in greater detail in panel e; after removing the coverslip, a 3% solution of hydrochloric acid-alcohol (3D- 036, Waldeck Gmbh & Co KG, Münster, Germany) was used to rinse out the aldehyde fuchsine stain and CD86 immunostaining was performed (b,c); the framed area in panel b is shown in greater detail in panel c. The macrophages in one and the same 5 μm section are thus compared as they appear in aldehyde fuchsin staining (d, e) and CD68 immunoreaction (b, c), thereby making it possible to use aldehyde fuchsine staining in standard paraffin or thick tissue sections instead of CD68 immunohistochemistry. Scale bars: d) 100 μm; e) 25 μm. Objectives used: a) 4:1; b,d) 10:1; c,e) 40:1.
Acknowledgments
The authors wish to thank Mr. David Ewert, University of Ulm, for skillful technical assistance (figure layout). In memoriam: Rev. Gerard Herman Ettlinger, S.J. (September 30, 1935 – July 15, 2018).
Funding Statement
Funding: This work was supported by the Foundation for Research on Alzheimer Disease (FRA), Paris.
References
- 1.Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 2011;82:126e135. [DOI] [PubMed] [Google Scholar]
- 2.van Veluw SJ, Zwanenburg JJ, Engelen- Lee J, Spliet WG, Hendrikse J, Luijten PR, et al. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J Cereb Blood Flow Metab 2013;33:322-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Veluw SJ, Zwanenburg JJM, Rozemuller AJM, Luijten PR, Spliet WGM, Biessels GJ. The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. J Cereb Blood Flow Metab 2015;35:676-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dalen JW, Scuric EE, van Veluw SJ, Cann MW, Nederveen AJ, Biessels BJ, et al. Cortical microinfarcts detected in vivo on 3 Tesla MRI, clinical and radiological correlates. Stroke 2015;46: 255-57. [DOI] [PubMed] [Google Scholar]
- 5.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012;11:272-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvanitakis Z, Capuano JW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The relationship of cerebral vessel pathology to brain microinfarcts. Brain Pathol 2016;27:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ. Cerebral microinfarcts: a systematic review of neuropathological studies. J Cereb Blood Flow Metab 2012;32:425-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westover MB, Bianchi MT, Yang C, Schneider J, Greenberg SM. Estimating cerebral microinfarct burden from autopsy samples. Neurology 2013;80:1365-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 2017;16:730-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decker Y, Müller A, Németh E, Schulz-Schaeffer WJ, Fatar M, Menger MD, et al. Analysis of the vasculature by immunohistochemistry in paraffinembedded brains. Brain Struct Funct 2018;223:1001-15. [DOI] [PubMed] [Google Scholar]
- 11.Smithson KG, MacVicar BA, Hatton G. Polyethylene glycol embedding: a technique compatible with immunocytochemistry, enzyme histochemistry, histofluorescence and intracellular staining. J Neurosci Methods 1983;7: 27-41. [DOI] [PubMed] [Google Scholar]
- 12.Klosen P, Maessen X, van den Bosch de Aquilar P. PEG embedding for immunocytochemistry: application to the analysis of immunoreactivity loss during histological processing. J Histochem Cytochem 1993;41:455-63. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol 1991;1:213-16. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer’s disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006;112:389-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldengut S, Del Tredici K, Braak H. Paraffin sections of 70-100 μm: a novel technique and its benefits for studying the nervous system. J Neurosci Methods 2013;15:241-44. [DOI] [PubMed] [Google Scholar]
- 16.Challa VR, Thore CR, Moody DM, Brown WR, Anstrom JA. A threedimensional study of brain string vessels using celloidin sections stained with anti-collagen antibodies. J Neurol Sci 2002; 203-204:165-67. [DOI] [PubMed] [Google Scholar]
- 17.Brown WR, Thore CR. Review: Cerebral microvascular pathology in ageing and neurodegeneration. J Neuropathol Appl Neurobiol 2011;17: 56-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braak H, Rüb U, Del Tredici K. Involvement of precerebellar nuclei in multiple system atrophy. J Neuropathol Appl Neurobiol 2003;29:60-76. [DOI] [PubMed] [Google Scholar]
- 19.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol 2012;124:643-66. [DOI] [PubMed] [Google Scholar]
- 20.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brettschneider J, Suh E, Robinson JL, Fang L, Lee EB, Irwin DJ, et al. Converging patterns of α-synuclein pathology in multiple system atrophy. J Neuropathol Exp Neurol 2018;77:1005-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


