Abstract
Colonic inflammation, a hallmark of inflammatory bowel disease, can be influenced by host intrinsic and extrinsic factors. There continues to be a need for models of colonic inflammation that can both provide insights into disease pathogenesis and be used to investigate potential therapies. Herein, we tested the utility of colonoscopic-guided pinch biopsies in mice for studying colonic inflammation and its treatment. Gene expression profiling of colonic wound beds after injury showed marked changes, including increased expression of genes important for the inflammatory response. Interestingly, many of these gene expression changes mimicked those alterations found in inflammatory bowel disease patients. Biopsy-induced inflammation was associated with increases in neutrophils, macrophages, and natural killer cells. Injury also led to elevated levels of sphingosine-1-phosphate (S1P), a bioactive lipid that is an important mediator of inflammation mainly through its receptor, S1P1. Genetic deletion of S1P1 in the endothelium did not alter the inflammatory response but led to increased colonic bleeding. Bacteria invaded into the wound beds, raising the possibility that microbes contributed to the observed changes in mucosal gene expression. In support of this, reducing bacterial abundance markedly attenuated the inflammatory response to wounding. Taken together, this study demonstrates the utility of the pinch biopsy model of colonic injury to elucidate the molecular underpinnings of colonic inflammation and its treatment.
Inflammatory bowel disease (IBD) is a condition in which recurrent bouts of inflammation occur in the intestines of patients, resulting in severe morbidity and increased risk of colorectal cancer.1, 2, 3 IBD-related inflammation is characterized by immune cell infiltration and mucosal destruction, causing diarrhea, rectal bleeding, and abdominal pain. The identification of factors that contribute to inflammation using animal models of disease has led to the development of treatments that reduce disease activity or induce remission. Although therapies are often effective, there is a need for both novel strategies to reduce intestinal inflammation and models to test these interventions.
Sphingosine-1-phosphate (S1P) receptors are G-protein–coupled receptors that function through binding of the bioactive lipid S1P.4, 5 Of the five S1P receptors, S1P1 has been most extensively investigated because of its roles in immune cell trafficking and maintaining blood vessel integrity.6, 7, 8, 9 For example, expression of S1P1 on lymphocytes allows their egress from lymph nodes, resulting in trafficking to other organ sites to exert their effects on target tissues.7, 8 In addition, S1P1 is expressed on blood vessels and is critical for sustaining vascular integrity by maintaining endothelial cell junctions.6, 9 In fact, we previously reported that S1P1 increases in expression and localizes to the colonic vasculature in ulcerative colitis (UC) and maintains vascular integrity in experimental colitis.10 Recently, signaling through S1P1 was found to limit aortic inflammation by decreasing the abundance of proinflammatory adhesion proteins.11 Because of the importance of S1P signaling in inflammation, S1P receptor modulators are being investigated for the treatment of IBD. These agents ameliorate experimental colitis and have shown promise in clinical trials in UC patients.12, 13, 14, 15 However, the effect of targeting vascular S1P signaling on colonic inflammation is unknown.
A critical aspect to maintaining normal gut homeostasis is preventing luminal content from contacting the epithelium. This proinflammatory interaction is prevented by maintaining proper gut barrier function, including an intact mucus layer. Reduced gut barrier function is a common feature of IBD and is believed to be an important mediator of disease pathogenesis.16, 17, 18 Exposure of luminal bacteria to the intestinal mucosa can trigger an immune response, leading to tissue destruction.19 Moreover, the dysbiosis that often occurs in this disease can be an additional contributing factor.20, 21, 22 In fact, several recent studies have demonstrated a direct role for particular bacterial species in triggering gut inflammation.23, 24, 25, 26 Given the importance of host-microbe interactions in colonic inflammation, having an appropriate model to study this interplay is needed.
This study had two main objectives. First, the gene expression changes after wounding in the colonoscopic-guided pinch biopsy model in mice were evaluated, and it was determined whether these changes mimicked human IBD. Second, it was investigated whether targeting host (S1P signaling) and luminal (bacteria) factors altered the biopsy-mediated inflammatory response. Marked inflammatory changes occurred after wounding, many of which mimic changes observed in IBD patients. Although S1P levels increased after biopsy, genetic deletion of vascular S1P1 did not affect the inflammatory response. Bacterial invasion was observed in the wound beds, an effect that contributed to the biopsy-induced inflammatory gene expression changes. Collectively, our findings demonstrate that targeting vascular S1P1 will not adversely affect intestinal inflammation and that microbial invasion after biopsy allows for the study of inflammation induced by host-microbe interactions.
Materials and Methods
Colonoscopy
Mice were anesthetized in an induction chamber using 2% isoflurane with oxygen, then moved to an endoscopic staging platform and maintained under 2% isoflurane (via nose cone) for the remainder of the procedure. Colons were flushed with phosphate-buffered saline to clear fecal material. Colonoscopies were performed using a 1.9-mm rigid bore endoscope coupled to a gas valve for colonic insufflation and a 300-W xenon light source (Karl Storz, Tuttlingen, Germany). The endoscope was inserted 3 cm into the anus of the mouse, and a pinch biopsy was made using miniature biopsy forceps inserted through a working channel. Sham tissues were generated by inserting and removing the endoscope only. To measure wound size, the location of the wound was identified immediately after biopsy, and the closed forceps were extended into the lumen and pressed against the colonic wall above the wound to ensure consistent distance from the lens to the wound. This view was video recorded to generate subsequent still images for quantification of wound size. The perimeter of the wound was measured in a blinded manner (D.C.M.) using ImageJ software version 1.47 (NIH, Bethesda, MD; https://imagej.nih.gov/ij). For tissue harvest after biopsy, mice were euthanized and colons were flushed with ice-cold phosphate-buffered saline and used for multiple end points, as described below. All animal studies were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medicine (New York, NY).
S1pr1ECKO Mice
S1pr1ECKO and S1pr1f/f mice were generated, as previously described.11 Equal numbers of male and female S1pr1ECKO and S1pr1f/f littermates were administered 200 μg tamoxifen at 5 to 8 weeks of age by i.p. injection daily for 5 days to delete S1P1 on blood vessels before biopsy. After tamoxifen administration, mice underwent colonoscopy, as described above. Clot size was measured in wound beds 2 hours after biopsy by measuring the perimeter of the clot under a dissecting microscope ex vivo using ImageJ. Measurements were performed in a blinded manner (D.C.M.).
Tissue Staining
Fluorescence in situ hybridization for bacteria was performed on paraffin sections, as previously described.27 Briefly, sections were deparaffinized, followed by incubation with lysozyme. Slides were then incubated overnight at 46°C with Cy-3–labeled EUB338 universal bacterial probe combined with the irrelevant fluorescein isothiocyanate–labeled non–EUB-338 probe (to control for nonspecific hybridization) (Integrated DNA Technologies, Coralville, IA). Slides were then washed for 30 minutes at 48°C, and Prolong mounting medium (Thermo Fisher Scientific, Inc., Waltham, MA) containing DAPI was added before coverslipping. Probe specificity was confirmed by evaluating sections containing pure bacteria. Images were captured on an Olympus BX51 epifluorescence microscope (Olympus America, Melville, NY).
Coimmunofluorescence of S1P1 and CD31 was performed on frozen sections, as previously described, using anti-S1P1 (H60; Santa Cruz Biotechnology, Dallas, TX) and anti-CD31 (Dianova, Hamburg, Germany) antibodies.10 Primary antibodies were visualized by the addition of anti-rabbit–fluorescein isothiocyanate and anti-rat–Alexa 546 secondary antibodies (Jackson ImmunoResearch, West Grove, PA).
RNA-Seq and Data Analysis
Colons were harvested from 8-week–old male C57BL/6J mice after colonoscopy, flushed, and cut open longitudinally, and methylene blue dye was applied to visualize the mucosa. The wound bed and mucosal region immediately surrounding it (0.5 mm) were excised under visualization through a dissecting microscope; mucosa from the same region of the colon was harvested from sham mice. Total RNA was isolated from frozen colon tissue using the RNeasy mini kit (Qiagen, Hilden, Germany). After RNA isolation, total RNA integrity was checked using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA concentrations were measured using the NanoDrop system (Thermo Fisher Scientific, Inc.). Preparation of the RNA sample library and RNA sequencing (RNA-seq) were performed by the Genomics Core Laboratory at Weill Cornell Medicine. mRNA was prepared using TruSeq (Illumina, San Diego, CA), according to the manufacturer's instructions. mRNA was purified using magnetic beads. Samples were then hybridized onto a flow cell and amplified for sequencing. The flow cell was sequenced on a HiSeq2500 sequencer (Illumina) with pair-end 50-bp cycles. Sequencing quality was assessed using FastQC (Babraham Bioinformatics, Cambridge, UK). Raw sequenced reads were aligned to the mouse reference genome version mm10 (University of California, Santa Cruz) using STAR version 2.4.2 aligner (CSC, Espoo, Finland). Aligned reads were quantified against the reference annotation (mm10 from University of California, Santa Cruz) to obtain fragments per kilobase per million and raw counts using CuffLinks version 2.2.1 (Broad Institute, Cambridge, MA) and HTSeq version 0.11.0 (Python Software Foundation; https://www.python.org). For the experiment examining gene expression changes after biopsy, a total of 24,062 genes were measured. Among them, 14,820 had count per million values >1 in at least three samples and were kept for differential expression analysis. For the study involving antibiotics, a total of 24,061 genes were measured. Among them, 14,614 genes had count per million values >1 in at least three samples and were kept for differential expression analysis. The differential gene expression analysis used the limma/voom pipeline with count data as the input, and the trimmed mean of M-values method was used for between-sample normalization.28 In general, genes were considered as differentially expressed if the false discovery rate was <0.05 and the fold change was >2 for comparisons between two independent groups. RNA-seq data were deposited onto Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo; accession number GSE114863). For the antibiotic study, genes that were considered affected by biopsy differently between vehicle- and antibiotic-treated mice were identified by the following criteria: false discovery rate <0.05 for the biopsy by drug treatment interaction; false discovery rate <0.05 and fold change >2 for the biopsy versus sham comparison in either vehicle- or antibiotic-treated mice or both. Significantly changed genes comparing sham with biopsy samples were input into Ingenuity Pathway Analysis (IPA; Qiagen) to determine significantly changed pathways, diseases, and biological functions. Gene Set Enrichment Analysis Software version 6.1 (Broad Institute) was used to test enrichment of gene signatures from other studies.29 Genes were ranked by the t-statistic value obtained from comparisons, and the preranked version of the tool was used to test for enrichment. Pathways enriched with a false discovery rate <0.25 were considered significant.
Immune cell type abundance estimation was performed using CIBERSORT from normalized bulk RNA-seq expression data (fragments per kilobase per million) as input.30 Before inputting data, mouse gene names were converted into their respective human orthologous gene names, using the Mouse Genome Informatics resource (The Jackson Laboratory, Bar Harbor, ME). The relative abundance of cell types quantified was then visualized across all samples.
Quantitative Real-Time PCR
Tissue samples were collected and RNA was extracted, as described above. RNA was reverse transcribed to make cDNA using murine leukemia virus reverse transcriptase and oligo (dT) 16 primer. The resulting cDNA was used for amplification using QuantiTect Primer Assays (Qiagen) for the following genes. Sphk1, Sphk2, Sgpp1, Sgpp2, Sgpl1, S1pr1, S1pr2, S1pr3, S1pr4, S1pr5. Tnf, Il1β, and Il6 were amplified using the following primer sequences: Tnf, 5′-CCAGACCCTCACACTCAGATC-3′ (forward) and 5′-CACTTGGTGGTTTGCTACGAC-3′ (reverse); Il1β, 5′-TGGGCCTCAAAGGAAAGAAT-3′ (forward) and 5′-CAGGCTTGTGCTCTGCTTGT-3′ (reverse); and Il6, 5′-CCAGAGATACAAAGAAATGATGG-3′ (forward) and 5′-ACTCCAGAAGACCAGAGGAAAT-3′ (reverse). Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous normalization control for both commercial and designed primers [5′-AATGTGTCCGTCGTGGATCT-3′ (forward) and 5′-CATCGAAGGTGGAAGAGTGG-3′ (reverse)]. The amplification products of designed primers were verified by sequencing. Quantitative real-time PCR was conducted using 2× SYBR Green PCR master mix on a 7500 real-time PCR system (Applied Biosystems, Foster City, CA). Relative fold induction was determined using the relative quantification analysis protocol.
Immune Cell Profiling
Three biopsies were taken per mouse during colonoscopy, and colons from two mice were combined to generate one sample for analysis. At sacrifice, the distal half of the colons was removed and flushed with ice-cold phosphate-buffered saline, slit open longitudinally, and cut into 1-cm pieces. Cells from the lamina propria were isolated, as previously described.31 Nonspecific binding of isolated cells was blocked by addition of anti-Fc monoclonal antibody, followed by incubation with the following fluorescently conjugated monoclonal antibodies on ice for 30 minutes: F/480–allophycocyanin-cyan7 (1:50), CD11b-allophycocyanin (1:100), Ly6G–fluorescein isothiocyanate (1:50), natural killer 1.1–peridinin chlorophyll protein complex (1:50), CD45–Pacific Blue (1:200), CD3-phycoerythrin (1:100), B220-phycoerythrin-cyan7 (1:400), T-cell receptor-β–phycoerythrin (1:200) (eBioscience, San Diego, CA). Flow cytometry was conducted on an LSRII flow cytometer (BD, Franklin Lakes, NJ), with data analyzed using FlowJo software version 10 (Tree Star, Ashland, OR). Comparisons of inflammatory cell types, on the basis of surface markers using the antibodies described above, were made between sham-treated and biopsied mice or between biopsied S1pr1f/f and S1pr1ECKO to determine the average percentage of inflammatory cells expressing these markers in individual mice.
Antibiotic Treatment and Bacterial PCR
To reduce bacterial abundance, mice were given drinking water containing 0.5-g/L ampicillin, 0.5-g/L metronidazole, 0.25-g/L vancomycin, 0.5-g/L neomycin, 0.5-g/L gentamicin, and 4-g/L artificial sweetener (Splenda; Heartland Consumer Products, Carmel, Indiana) for 3 weeks. The artificial sweetener was included to mask the bad taste of the antibiotics and, therefore, maintain adequate water consumption by mice. This antibiotic cocktail has been successfully used before.32 Control mice were given water containing only artificial sweetener. After treatment, fecal samples were collected and DNA was extracted for quantitative real-time PCR. Briefly, Buffer ASL (Qiagen) was added to fecal samples, followed by vigorous vortex mixing until thoroughly homogenized. DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen), per manufacturer's instructions. For each sample, 8 ng of DNA was used to perform quantitative real-time PCR using Fast SYBR Green PCR master mix and universal bacterial primers (forward: 5′-ACTCCTACGGGAGGCAGCAG-3′ and reverse: 5′-ATTACCGCGGCTGCTGG-3′) on a 7500 Real-Time PCR system (Applied Biosystems). Cycling conditions were the same as described above. After antibiotic or control treatment, mice underwent pinch biopsy and tissues were collected for RNA-seq, as described above.
Determination of Sphingolipids and Ceramides in the Colonic Mucosa
Tissues were extracted and lipids were determined as previously described, with slight modification.33 Briefly, C17-S1P and C16, C18, C20, and C22 ceramide (2 μg/mL; Cayman Chemical Co, Ann Arbor, MI) were added as internal standards. Sphingolipids and ceramides were quantified by liquid chromatography–tandem mass spectrometry using an Agilent 6460 QQQ triple quadrupole mass spectrometer (Agilent Technologies, Palo Alto, CA) equipped with an Agilent HP 1200 binary pump high-performance liquid chromatographic inlet (Agilent Technologies). Sphingolipids and ceramides were separated using a 2 × 100 mm Luna 3-μm C8 analytical column (Phenomenex, Torrance, CA). The mobile phase consisted of 0.2% formic acid and water with 10 mmol/L ammonium formate. The column temperature was maintained at 35°C, and samples were kept at 4°C during the analysis. Individual analytes were detected using electrospray ionization and multiple reaction monitoring; and the m/z transitions of individual lipids were monitored in positive ionization mode, were quantified using authentic standard curves, and were normalized to the amount of protein.
Statistical Analysis
The nonparametric Wilcoxon rank-sum test was used to compare gene expression, lipid levels, percentage of immune cells, and clot and wound size between two experimental groups. For gene expression, data were generated from experiments evaluating effects of biopsy in mice of two different genotypes; multiple linear regression was performed with treatment, genotype, and treatment-by-genotype interaction as covariates. Gene expression data were log transformed to ensure the underlying model assumptions were met. Treatment effects in each genotype and differential treatment effects between mice of different genotypes were evaluated using simultaneous inference for general linear hypotheses. P values were adjusted for multiple comparisons using the Bonferroni-Holm method. All P values were two sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using the R 3.3.1 software package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Marked Tissue Changes Occur after Colonic Pinch Biopsy, Some of Which Mimic IBD
The inflammatory changes that occur after colonoscopic-guided pinch biopsy were first characterized. This model system was chosen because the inflammatory response can be evaluated at precise time points after injury. Gene expression changes after injury were determined by performing RNA-seq on tissue harvested from mice 6 hours after biopsy or sham treatment. Tissue from biopsied mice was collected from the mucosal region approximately 0.5 mm surrounding the wound bed. Normal mucosa was collected from sham-treated mice. RNA-seq revealed many gene expression changes in the wound bed compared with normal mucosa (Table 1). Many genes involved in the inflammatory response were increased, including Il1b, Il6, Trem1, and Trem3, as well as several chemokines (Table 1). The entire data set can be found on the Gene Expression Omnibus website (https://www.ncbi.nlm.nih.gov/geo; accession number GSE114863).
Table 1.
The Top 40 Genes Which Were Either Increased or Decreased in Expression in the Wound Bed 6 Hours after Biopsy
| Gene | LogFC | P value | Adjusted P value |
|---|---|---|---|
| Increased | |||
| Krt6a | 12.6 | 2.9 × 10−14 | 1.5 × 10−12 |
| S100a9 | 11.5 | 7.4 × 10−8 | 4.3 × 10−7 |
| Cxcl3 | 10.9 | 4.4 × 10−7 | 2.1 × 10−6 |
| Cxcl2 | 10.7 | 2.0 × 10−5 | 6.4 × 10−5 |
| Irg1 | 10.5 | 4.6 × 10−6 | 1.7 × 10−5 |
| S100a8 | 10.4 | 5.8 × 10−9 | 4.5 × 10−8 |
| Sprr2h | 10.0 | 1.2 × 10−15 | 1.1 × 10−13 |
| Il1b | 9.9 | 1.9 × 10−7 | 1.0 × 10−6 |
| Trem1 | 9.9 | 2.7 × 10−9 | 2.4 × 10−8 |
| Ccl4 | 9.8 | 7.4 × 10−8 | 4.3 × 10−7 |
| Cxcr2 | 9.5 | 1.8 × 10−11 | 3.3 × 10−10 |
| Prss22 | 9.5 | 5.1 × 10−12 | 1.1 × 10−10 |
| Clec4e | 9.1 | 1.5 × 10−6 | 6.1 × 10−6 |
| 4732456N10Rik | 9.1 | 9.1 × 10−9 | 6.8 × 10−8 |
| Il6 | 8.7 | 5.3 × 10−9 | 4.2 × 10−8 |
| Spp1 | 8.6 | 2.0 × 10−8 | 1.4 × 10−7 |
| Retnlg | 8.5 | 1.1 × 10−13 | 4.4 × 10−12 |
| Gm5483 | 8.5 | 2.2 × 10−18 | 6.6 × 10−16 |
| Ccl3 | 8.3 | 3.9 × 10−5 | 1.2 × 10−4 |
| Sprr1a | 8.2 | 3.7 × 10−23 | 1.4 × 10−19 |
| Il1f9 | 8.1 | 5.4 × 10−9 | 4.3 × 10−8 |
| Pbp2 | 8.1 | 6.5 × 10−16 | 7.0 × 10−14 |
| Osm | 8.1 | 1.6 × 10−9 | 1.5 × 10−8 |
| Il1r2 | 8.0 | 6.4 × 10−10 | 6.9 × 10−9 |
| Arhgap40 | 8.0 | 8.0 × 10−15 | 5.3 × 10−13 |
| Krt16 | 7.9 | 1.2 × 10−12 | 3.5 × 10−11 |
| Hdc | 7.9 | 2.8 × 10−9 | 2.5 × 10−8 |
| Gm10872 | 7.8 | 3.7 × 10−14 | 1.8 × 10−12 |
| Tarm1 | 7.7 | 4.5 × 10−14 | 2.1 × 10−12 |
| Lypd3 | 7.4 | 2.0 × 10−15 | 1.7 × 10−13 |
| Stfa2l1 | 7.4 | 1.5 × 10−13 | 5.5 × 10−12 |
| Krt6b | 7.3 | 2.2 × 10−10 | 2.8 × 10−9 |
| Cxcl1 | 7.2 | 1.1 × 10−8 | 8.0 × 10−8 |
| Chil3 | 7.1 | 1.6 × 10−9 | 1.5 × 10−8 |
| Trem3 | 7.1 | 4.4 × 10−15 | 3.3 × 10−13 |
| Hp | 7.1 | 7.1 × 10−9 | 5.5 × 10−8 |
| Mmp3 | 7.1 | 1.8 × 10−14 | 1.0 × 10−12 |
| Slfn1 | 7.0 | 8.3 × 10−16 | 8.4 × 10−14 |
| Clec4d | 7.0 | 7.2 × 10−14 | 3.1 × 10−12 |
| Cxcl10 | 6.9 | 2.4 × 10−14 | 1.3 × 10−12 |
| Decreased | |||
| Inmt | −4.0 | 5.3 × 10−6 | 1.9 × 10−5 |
| Atp13a4 | −3.9 | 2.8 × 10−9 | 2.4 × 10−8 |
| Col8a2 | −3.9 | 1.7 × 10−8 | 1.2 × 10−7 |
| Pbld1 | −3.8 | 1.2 × 10−11 | 2.3 × 10−10 |
| Fn3k | −3.7 | 1.0 × 10−7 | 5.8 × 10−7 |
| A330033J07Rik | −3.7 | 1.6 × 10−8 | 1.1 × 10−7 |
| Lgi3 | −3.5 | 1.6 × 10−9 | 1.5 × 10−8 |
| 1810064F22Rik | −3.5 | 8.2 × 10−9 | 6.2 × 10−8 |
| Gm13446 | −3.4 | 2.8 × 10−5 | 8.6 × 10−5 |
| Cdh26 | −3.3 | 4.2 × 10−11 | 6.6 × 10−10 |
| Insrr | −3.3 | 5.5 × 10−10 | 6.1 × 10−9 |
| Btnl1 | −3.3 | 3.5 × 10−8 | 2.2 × 10−7 |
| Art5 | −3.2 | 1.6 × 10−7 | 8.4 × 10−7 |
| Gpr34 | −3.2 | 7.8 × 10−11 | 1.1 × 10−9 |
| Adrb3 | −3.1 | 1.6 × 10−7 | 8.7 × 10−7 |
| Rnf32 | −3.1 | 1.2 × 10−12 | 3.5 × 10−11 |
| Igfn1 | −3.1 | 3.6 × 10−8 | 2.3 × 10−7 |
| Slc37a2 | −3.1 | 7.8 × 10−11 | 1.1 × 10−9 |
| Cd300e | −3.0 | 1.6 × 10−9 | 1.5 × 10−8 |
| Ctcfl | −3.0 | 3.1 × 10−10 | 3.6 × 10−9 |
| Gm8979 | −3.0 | 9.1 × 10−12 | 1.8 × 10−10 |
| Abca8a | −3.0 | 1.2 × 10−9 | 1.2 × 10−8 |
| 2900041M22Rik | −3.0 | 7.2 × 10−11 | 1.1 × 10−9 |
| Nxpe2 | −2.9 | 8.3 × 10−12 | 1.7 × 10−10 |
| Gpm6a | −2.9 | 2.7 × 10−11 | 4.6 × 10−10 |
| Slc30a10 | −2.9 | 9.7 × 10−6 | 3.3 × 10−5 |
| A4gnt | −2.9 | 2.3 × 10−10 | 2.9 × 10−9 |
| Mettl7a2Higd1c | −2.8 | 1.3 × 10−7 | 7.3 × 10−7 |
| Hhip | −2.8 | 1.5 × 10−7 | 8.0 × 10−7 |
| Wscd2 | −2.8 | 7.1 × 10−6 | 2.5 × 10−5 |
| Lrrc17 | −2.8 | 5.2 × 10−12 | 1.1 × 10−10 |
| Sprr2b | −2.7 | 2.8 × 10−2 | 4.5 × 10−2 |
| Pck1 | −2.7 | 2.4 × 10−10 | 3.0 × 10−9 |
| Cdkl1 | −2.7 | 2.4 × 10−6 | 9.4 × 10−6 |
| Gdf10 | −2.7 | 7.9 × 10−5 | 2.2 × 10−4 |
| 5033404E19Rik | −2.7 | 5.2 × 10−9 | 4.2 × 10−8 |
| Tnfrsf19 | −2.7 | 4.8 × 10−7 | 2.3 × 10−6 |
| Cyp2f2 | −2.7 | 4.7 × 10−11 | 7.3 × 10−10 |
| Cps1 | −2.7 | 2.3 × 10−10 | 2.8 × 10−9 |
| Kcng1 | −2.6 | 8.9 × 10−4 | 2.0 × 10−3 |
P values were obtained from voom limma analysis and adjusted by the Benjamini-Hochberg method.
FC, fold change.
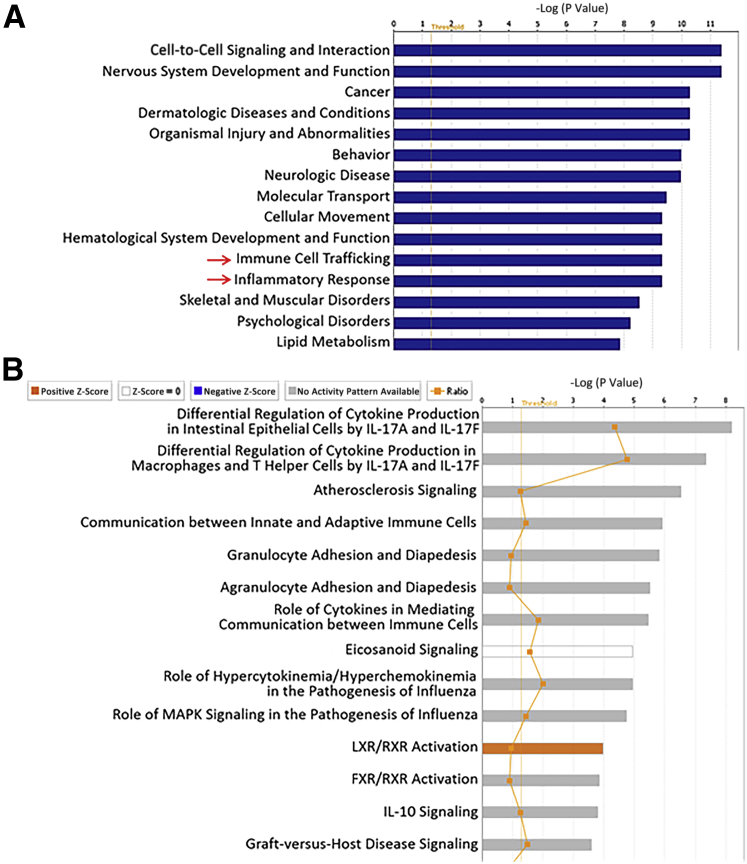
To gain insight into cellular functions, pathways, and diseases associated with these gene expression changes, the RNA-seq data were subjected to pathway analysis using IPA. Diseases and biological functions were first examined, and a significant change in gastrointestinal tract disease was found (Figure 1A). More in-depth probing of this category revealed that the most significantly associated disease in this category was colitis (P = 2 × 10−20), with a total of 82 molecules altered. This prediction suggested that the gene expression changes in the wound bed mimicked those alterations found in IBD. To directly evaluate this, Gene Set Enrichment Analysis was performed to compare the gene expression changes in our study with previously published data analyzing the colonic mucosa of patients with active IBD.29 Impressively, those genes that increased in expression in the wound bed were strongly correlated with gene expression changes observed in UC and Crohn disease (Figure 1B). The selective correlation in increased expression of genes is highlighted by the higher normalized enrichment score, in contrast with those that were decreased (Figure 1B). Most of the changes in gene expression that overlapped between the biopsy model and human IBD related to the inflammatory response, including IL33, NOD2, and IL6, as well as several chemokines (Supplemental Table S1). Many of these genes have been associated with IBD pathogenesis in other studies, indicating the potential utility of the biopsy model to study IBD-related colonic inflammation.
Figure 1.
Mechanical wounding of the colon alters gene expression, consistent with inflammatory bowel disease. A: The top 15 significantly changed categories within diseases and biological functions in wound beds (WBs) 6 hours after biopsy, as determined by Ingenuity Pathway Analysis based on RNA-sequencing (RNA-seq) analysis, are shown. Blue bars represent the −log P value, and the threshold represents a P value of 0.05. Arrows indicate pathways of interest. B: Gene Set Enrichment Analysis was performed on the gene expression data generated by RNA-seq to compare changes in the WB 6 hours after biopsy with previously published gene expression changes in colonic mucosa from patients with ulcerative colitis or Crohn disease compared with healthy controls.29 A higher normalized enrichment score (NES; green line) indicates greater correlation of the gene expression changes comparing the two data sets. The false discovery rate (FDR) is an indicator of significant correlations. The expression of genes that were both increased and decreased in the wound bed was compared, as indicated by the label in the bottom portion of the graph.
Immune Cell Changes Occur in the Wound Bed after Injury
Further examination of the IPA results revealed highly significant changes in other categories, including immune cell trafficking and inflammatory response (Figure 1A). Deeper probing of these categories predicted increased immune cell migration and activation (Supplemental Tables S2 and S3). Given that chemokines can be a major contributor to immune cell trafficking (and would likely cause the pathway analysis results), a panel of genes encoding chemokines was specifically studied and the large majority were found to be strongly increased in the wound bed (Figure 2A). It was next determined whether these gene alterations and predicted biological changes that occur after biopsy are correlated with immune cell influx. Therefore, the RNA-seq data set was first subjected to CIBERSORT, an analytical tool used to estimate immune cell populations.30 This analysis suggested a significant expansion of activated mast cells and a moderate increase in activated natural killer cells in the wound bed compared with sham tissue (Figure 2B). Mast cells are known to be involved in the acute inflammatory response and play a role in IBD.34 To examine immune cell alterations at a later time point, flow cytometry was performed on the immune cells of the lamina propria isolated from colons of mice that were biopsied or sham treated. Increases in CD11b+/Ly6G+ neutrophils, CD11b+/F4/80+ macrophages, and CD3−/T-cell receptor β−/natural killer 1.1+ cells were observed after biopsy (Figure 2C). No meaningful changes occurred in T and B cells (Figure 2C). Taken together, these findings help to characterize the inflammatory changes after biopsy-induced acute colonic injury in mice. Interestingly, many of these alterations are consistent with changes observed in IBD.
Figure 2.
Acute colonic injury induces alterations in immune cells. A: The expression of the genes encoding chemokines, as determined by RNA sequencing (RNA-seq), was compared between normal tissues from sham-treated mice and wound beds (WBs) and shown as a heat map. Green indicates low expression, and red indicates high expression. Each column represents a sample from a single mouse. B: RNA-seq data were subjected to CIBERSORT to infer changes in immune cell populations in the WB compared with tissue from sham-treated mice. Relative percentages of immune cell types are shown for each tissue type. C: Immune cells were isolated from the lamina propria of colons from mice that were biopsied or sham treated 1 day after treatment and identified by flow cytometry on the basis of surface markers within the CD45+ population, as shown. n = 5 samples per group (A); n = 6 samples per group (C). ∗∗P < 0.01. FPKM, fragment per kilobase per million; NK, natural killer; TCR, T-cell receptor.
S1P Production Is Increased in the Colon after Injury
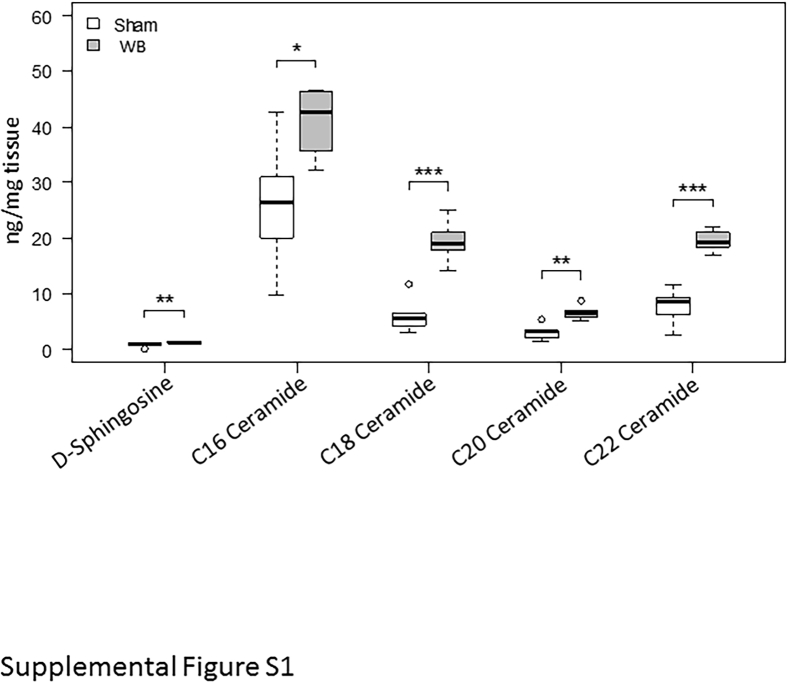
After defining the tissue response to biopsy-induced injury, it was determined whether the S1P signaling axis was affected by biopsy-induced injury. This pathway was chosen given its known importance in controlling inflammation.7, 8, 11, 12, 13, 14, 15 To first determine whether this pathway is relevant for the biopsy model, the expression of genes that control both S1P synthesis (Sphk1 and Sphk2) and degradation (Sgpp1, Sgpp2, and Sgpl1) was measured 6 hours after colonic biopsy by quantitative real-time PCR. Compared with colonic mucosa from sham-treated mice, Sphk1 significantly increased and Sgpp2 decreased, with trends for decreased Sgpp1 and Sgpl1 in the wound bed (Figure 3, A–E). Given that these gene expression changes are consistent with increased S1P production, S1P was directly measured in the wound bed, which revealed elevated levels (Figure 3F). Additional measurements of related sphingolipids showed increased amounts of d-sphingosine and multiple ceramides after wounding (Supplemental Figure S1). Gene expression analysis of the five S1P receptors showed an approximately 40% decrease in S1pr2 in the wound bed, with no significant changes in the levels of the other receptors (Supplemental Figure S2).
Figure 3.
Increased S1P levels are found after acute colonic injury. A–E: Relative expression of genes involved in S1P synthesis or catabolism, as determined by quantitative real-time PCR in the wound bed (WB) 6 hours after biopsy, compared with mucosa from sham-treated mice. F: S1P levels were measured in the wound bed 6 hours after biopsy and in mucosa from sham-treated mice. n = 7 to 9 per group (A–E).
Loss of Vascular S1P1 Enhances Bleeding but Does Not Alter the Colonic Injury Response
The observed increase in S1P levels after colonic wounding suggests that S1P-mediated signaling is relevant in the biopsy-induced injury model. Because the S1P receptor S1P1 is overexpressed in UC and localizes to the vasculature, it was tested whether the response to injury was altered following loss of vascular S1P1.10 To accomplish this, mice with inducible deletion of S1P1 selectively on the endothelium (S1pr1ECKO) and control littermates (S1pr1f/f) were used. Figure 4A shows S1P1 loss on blood vessels after tamoxifen administration in S1P1ECKO mice. Because loss of S1P1 has been shown to reduce blood vessel integrity, it was first determined whether increased bleeding occurs in S1pr1ECKO mice after colonic biopsy. Larger clots formed in the wound beds of S1pr1ECKO mice, indicating increased bleeding (Figure 4, B and C). There was no significant difference in the sizes of the original wounds. It was next determined whether endothelial S1P1 deletion affected the acute inflammatory response. Immune cell populations in the lamina propria and cytokine expression were examined in the wound bed 6 hours after biopsy. These analyses demonstrated that vascular S1P1 loss did not affect either of these biopsy-induced changes (Figure 4, D and E). Taken together, these data demonstrate that loss of vascular S1P1 does not affect the colonic inflammatory response.
Figure 4.
Deletion of S1P1 on the vasculature increases bleeding but does not affect the inflammatory response after wounding in the colon. A: Coimmunofluorescence for CD31 and S1P1 was performed on colon sections from S1pr1f/f and S1pr1ECKO mice after tamoxifen administration. Arrows indicate blood vessels. B: Images of representative clots observed in whole mount colons 2 hours after biopsy in S1pr1f/f and S1pr1ECKO mice. C: Quantification of clot size in S1pr1f/f and S1pr1ECKO mice 2 hours after biopsy. D: The percentage of immune cell subsets was quantified by flow cytometry in the colons from S1pr1f/f and S1pr1ECKO mice 1 day after biopsy. E: The relative expression of genes encoding proinflammatory cytokines was determined by quantitative real-time PCR in sham tissue or wound beds (WBs) from S1pr1f/f and S1pr1ECKO 1 day after treatment. P value represents the magnitude of increase after biopsy comparing genotypes. n = 9 to 11 samples per group (C); n = 6 samples per group (D); n = 5 to 6 samples per group (E). Scale bars = 10 μm (A). Original magnification, ×4 (B). AU, arbitrary units; NK, natural killer; TCR, T-cell receptor.
Colonic Biopsy Results in Bacterial Influx into the Wound Bed, Which Is Partly Responsible for Injury-Induced Gene Expression Changes
To next explore whether biopsy-induced injury is a good model for studying the impact of luminal factors on colonic inflammation, gut luminal bacteria were studied. To begin to determine whether bacteria may be influencing the colonic mucosa of the wound bed, the canonical pathway changes in IPA were mined. In addition to the activation of pathways associated with inflammatory changes (leukocyte extravasation signaling, IL-6 signaling, and TREM1 signaling), consistent with other IPA described in Figure 1A, there was a significant activation of the pathway, the role of pattern recognition receptors in recognition of bacteria and viruses (Figure 5A). This result suggests that the mucosa is responding to microbial exposure. In support of this pathway finding, bacteria were found invading deeply into the wound bed by fluorescence in situ hybridization 2 hours after biopsy, before the observed gene expression changes described in Table 1 (Figure 5B). Normal adjacent tissue with intact mucosa showed no invasion of microbes (Figure 5B). Given the observed exposure of bacteria to the colonic mucosa, it was next determined whether the gene expression changes induced by wounding described above (Table 1 and Figure 1) were, at least in part, because of microbes. To test this idea, RNA-seq was performed on the wound beds of mice 6 hours after biopsy and in normal mucosa from sham-treated mice in the presence or absence of broad-spectrum antibiotics. Before colonoscopy, it was demonstrated that antibiotic treatment reduced luminal bacterial abundance by approximately 1000-fold (Supplemental Figure S3). IPA of the gene expression changes in the wound beds of antibiotic-treated mice showed that the pathways related to inflammation (immune cell trafficking and inflammatory response) that were previously shown to be highly significantly changed in the wound beds of mice (Figure 1A) were now much less significantly altered and were ranked lower compared with other pathways (Figure 6A). Furthermore, examination of canonical pathways showed no activation of leukocyte extravasation signaling, IL-6 signaling, TREM1 signaling, or the role of pattern recognition receptors in recognition of bacteria and viruses (Figure 6B). Closer examination of the effects of antibiotic treatment on the expression of particular genes after wounding shows many that were both attenuated (Table 2) and enhanced (Table 3). Of those genes whose expression was attenuated by antibiotic treatment, Trem2 and Il23a have been shown to be important in modulating inflammation and, therefore, could be contributing to the dampening of the inflammatory response predicted by IPA.35, 36 Taken together, these data support the role of gut microbes in inducing the colonic inflammatory response and for the use of the pinch biopsy model for studying this process.
Figure 5.
Luminal bacteria are exposed to the colonic epithelium after biopsy. A: The top 15 significantly changed canonical pathways in wound beds (WBs) 6 hours after biopsy, as determined by Ingenuity Pathway Analysis based on RNA-sequencing analysis, are shown. The threshold represents a P value of 0.05. The Arrow indicates a pathway of interest. B: Fluorescence in situ hybridization was performed on colon sections from mice 2 hours after biopsy to identify bacteria. Dashed ovals indicate clusters of bacteria visualized by bright orange/reddish staining. Inset: A higher magnification of the wound bed. Original magnifications: ×4 (B, main image); ×200 (B, inset). HMGB1, high mobility group box 1; L, luminal side; LPS, lipopolysaccharide; normal, regions of intact mucosa adjacent to the WB; TREM1, triggering receptor expressed on myeloid cells 1; RXR, retinoid X receptor.
Figure 6.
Reduced bacterial abundance attenuates biopsy-induced gene expression changes. A: The top 15 significantly changed categories within diseases and biological functions in wound beds 6 hours after biopsy in mice given antibiotics, as determined by Ingenuity Pathway Analysis (IPA), based on RNA-sequencing (RNA-seq) analysis, are shown. Blue bars represent the −log P value, and the threshold represents a P value of 0.05. Arrows indicate pathways of interest. B: The top 15 significantly changed canonical pathways in wound beds 6 hours after biopsy in mice given antibiotics, as determined by IPA based on RNA-seq analysis, are shown. The threshold represents a P value of 0.05. FXR, farnesoid X receptor; LXR, liver X receptor; MAPK, mitogen-activated protein kinase; RXR, retinoid X receptor.
Table 2.
Gene Expression Changes 6 Hours after Colonic Biopsy That Were Attenuated by Antibiotics Treatment
| Gene | logFC |
Adjusted P value | |
|---|---|---|---|
| Control | Antibiotics | ||
| Hamp | 5.64 | 2.65 | 0.03 |
| Rbm4 | 2.91 | 1.19 | 0.00 |
| Il23a | 2.82 | −0.11 | 0.05 |
| Homer2 | 2.44 | 1.29 | 0.02 |
| Trib1 | 1.96 | 0.85 | 0.02 |
| Fermt1 | 1.87 | 1.07 | 0.01 |
| Ippk | 1.84 | 1.29 | 0.04 |
| Cd3eap | 1.81 | 1.27 | 0.05 |
| Snord52 | 1.76 | 0.72 | 0.04 |
| Wdr43 | 1.66 | 1.22 | 0.05 |
| Tagln2 | 1.55 | 0.99 | 0.05 |
| Rbm14 | 1.47 | 0.33 | 0.00 |
| 4931428F04Rik | 1.47 | 0.64 | 0.01 |
| Slc29a2 | 1.39 | 0.77 | 0.04 |
| Dapk2 | 1.38 | 0.75 | 0.01 |
| Ctsf | 1.38 | 0.01 | 0.01 |
| Chst4 | 1.35 | 0.56 | 0.01 |
| Gtpbp4 | 1.31 | 0.89 | 0.02 |
| Qtrtd1 | 1.28 | 0.78 | 0.01 |
| Ube2f | 1.25 | 0.80 | 0.03 |
| Spata5 | 1.23 | 0.59 | 0.02 |
| Timm9 | 1.22 | 0.68 | 0.02 |
| Timm8a1 | 1.21 | 0.22 | 0.01 |
| Heatr3 | 1.18 | 0.73 | 0.03 |
| Rsl1d1 | 1.16 | 0.78 | 0.04 |
| Tprn | 1.13 | 0.58 | 0.04 |
| Ppid | 1.12 | 0.54 | 0.01 |
| Wsb1 | 1.03 | 0.44 | 0.01 |
| Nol9 | 1.03 | 0.68 | 0.02 |
| Gm11545 | 1.03 | 0.40 | 0.03 |
| 1810032O08Rik | 1.01 | 0.37 | 0.02 |
| Col6a1 | −1.01 | −0.09 | 0.04 |
| Ulk1 | −1.02 | −0.54 | 0.05 |
| Tbx2 | −1.03 | −0.27 | 0.02 |
| Rarg | −1.04 | −0.04 | 0.01 |
| Prr12 | −1.04 | −0.41 | 0.03 |
| Kifc3 | −1.05 | −0.41 | 0.04 |
| Bcas1 | −1.06 | −0.33 | 0.02 |
| Tcp11l2 | −1.08 | −0.45 | 0.03 |
| Wnt4 | −1.09 | 0.03 | 0.02 |
| Erbb3 | −1.10 | −0.09 | 0.04 |
| Vash1 | −1.14 | −0.18 | 0.05 |
| Cplx2 | −1.16 | −0.32 | 0.04 |
| Slitrk6 | −1.16 | −0.18 | 0.03 |
| Atoh8 | −1.18 | 0.10 | 0.00 |
| Zer1 | −1.18 | −0.65 | 0.02 |
| Scube1 | −1.19 | −0.23 | 0.01 |
| Dchs1 | −1.20 | −0.22 | 0.04 |
| Smg6 | −1.20 | −0.76 | 0.05 |
| Vstm4 | −1.24 | −0.39 | 0.01 |
| Maf | −1.26 | −0.12 | 0.00 |
| Ctdspl | −1.26 | −0.73 | 0.02 |
| Prrx2 | −1.26 | −0.12 | 0.04 |
| Aatk | −1.28 | 0.08 | 0.05 |
| Thra | −1.28 | −0.59 | 0.03 |
| F13a1 | −1.29 | −0.07 | 0.02 |
| Rgs10 | −1.29 | −0.69 | 0.04 |
| Ano5 | −1.34 | 0.14 | 0.04 |
| Tcf21 | −1.35 | −0.60 | 0.02 |
| Smad6 | −1.35 | −0.14 | 0.00 |
| Efs | −1.37 | −0.39 | 0.04 |
| Agbl5 | −1.37 | −0.78 | 0.03 |
| Sgsm2 | −1.39 | −0.91 | 0.03 |
| Tox | −1.41 | −0.76 | 0.03 |
| Ttc12 | −1.43 | −0.44 | 0.04 |
| Prrt2 | −1.47 | −0.47 | 0.04 |
| Dyrk1b | −1.48 | −0.84 | 0.02 |
| Rab3il1 | −1.49 | −0.69 | 0.03 |
| Zfp395 | −1.55 | −0.55 | 0.01 |
| Angptl2 | −1.57 | −0.49 | 0.02 |
| Lef1 | −1.57 | 0.03 | 0.00 |
| Dnm1 | −1.66 | −0.41 | 0.01 |
| Trem2 | −1.67 | −0.45 | 0.01 |
| Lamc3 | −1.74 | −0.36 | 0.05 |
| Wipi1 | −1.75 | −0.97 | 0.01 |
| Cyp2s1 | −1.77 | −0.80 | 0.00 |
| Scrn1 | −1.79 | −0.13 | 0.01 |
| Fbxl20 | −1.79 | −0.86 | 0.01 |
| Zfyve28 | −1.81 | −0.71 | 0.05 |
| Trerf1 | −1.84 | −0.84 | 0.04 |
| Notch3 | −1.85 | −0.84 | 0.01 |
| Gpihbp1 | −1.85 | −0.62 | 0.05 |
| Cygb | −1.88 | −1.02 | 0.02 |
| Galnt15 | −1.88 | −0.28 | 0.02 |
| Tlr7 | −1.95 | −0.21 | 0.01 |
| Ano2 | −1.97 | −0.23 | 0.01 |
| Pid1 | −1.99 | −0.88 | 0.00 |
| Stab1 | −2.02 | −1.04 | 0.02 |
| Fam13c | −2.04 | −0.26 | 0.05 |
| P2rx7 | −2.09 | −1.11 | 0.02 |
| Ccdc85a | −2.11 | −0.72 | 0.03 |
| Ccdc8 | −2.20 | −1.11 | 0.03 |
| Pcdh20 | −2.69 | −0.92 | 0.05 |
| Wscd1 | −2.92 | −1.47 | 0.03 |
Adjusted P values were obtained by voom limma analysis, followed by adjustment using the Benjamini-Hochberg method. They indicate the difference in gene expression changes comparing the control with the antibiotic group after biopsy.
FC, fold change.
Table 3.
Gene Expression Changes 6 Hours after Colonic Biopsy That Were Enhanced by Antibiotics Treatment
| Gene | logFC |
Adjusted P value | |
|---|---|---|---|
| Control | Antibiotics | ||
| Ttc9 | 2.38 | 3.70 | 0.03 |
| Rufy4 | 2.34 | 4.21 | 0.01 |
| Vill | 2.05 | 3.13 | 0.00 |
| Lman1l | 1.96 | 3.74 | 0.01 |
| Snai1 | 1.28 | 2.39 | 0.03 |
| Apln | 1.22 | 2.09 | 0.03 |
| Dio3 | 1.03 | 3.26 | 0.04 |
| Car9 | 0.91 | 1.50 | 0.01 |
| Igfbp3 | 0.74 | 1.81 | 0.02 |
| Adam12 | 0.73 | 2.29 | 0.04 |
| Ptk7 | 0.62 | 1.16 | 0.01 |
| Slc12a4 | 0.60 | 1.40 | 0.04 |
| Por | 0.58 | 1.31 | 0.00 |
| Tbc1d1 | 0.55 | 1.16 | 0.01 |
| Smad7 | 0.47 | 1.09 | 0.04 |
| Gpr158 | 0.40 | 2.46 | 0.05 |
| Frmd4a | 0.36 | 1.32 | 0.01 |
| Lox | 0.36 | 1.62 | 0.01 |
| Cep170 | 0.33 | 1.12 | 0.05 |
| Bcl6b | 0.26 | 1.29 | 0.03 |
| Pdgfb | 0.26 | 1.13 | 0.02 |
| C3ar1 | 0.19 | 1.52 | 0.02 |
| Tbx3 | 0.15 | 1.26 | 0.01 |
| Jag2 | 0.15 | 1.28 | 0.00 |
| Plxna4 | 0.10 | 1.39 | 0.05 |
| Acsl6 | 0.06 | 1.42 | 0.04 |
| Olfml3 | −0.01 | 1.09 | 0.03 |
| Nmnat2 | −0.10 | 1.20 | 0.01 |
| Mtfp1 | −0.42 | −1.11 | 0.04 |
| Crabp1 | −0.48 | 1.61 | 0.04 |
| Nccrp1 | −0.48 | −1.72 | 0.05 |
| Gca | −0.55 | −1.16 | 0.03 |
| Npy | −0.64 | 1.54 | 0.04 |
| Dpp7 | −0.64 | −1.21 | 0.04 |
| Nipsnap1 | −0.70 | −1.18 | 0.05 |
| Sult1a1 | −0.87 | −1.89 | 0.04 |
| Gsdmc3 | −0.95 | −1.98 | 0.01 |
| Gstm3 | −1.03 | −2.13 | 0.02 |
| Hao2 | −1.64 | −3.32 | 0.05 |
| Fmo1 | −1.70 | −2.52 | 0.04 |
Adjusted P values were obtained by voom limma analysis, followed by adjustment using the Benjamini-Hochberg method. They indicate the difference in gene expression changes comparing the control with the antibiotic group after biopsy.
FC, fold change.
Discussion
Intestinal inflammation in IBD is accompanied by tissue destruction and severe morbidity in patients. Although current therapies target inflammatory mediators, better treatment options and models of inflammation are needed. Both tissue and luminal factors affect the inflammatory response.37, 38 This study comprehensively defined the inflammatory response after colonoscopic-guided pinch biopsies in mice and evaluated the effects of disrupting S1P signaling as well as the role of luminal microbes on inflammation. Colonic injury induced a marked inflammatory response that mimicked IBD. Deletion of endothelial S1P1 did not affect biopsy-induced inflammation, but reducing bacterial abundance strongly attenuated inflammatory gene expression changes. These findings suggest that the pinch biopsy model is useful for studying colonic inflammation and potential therapies.
The use of colonoscopic-guided pinch biopsies in mice was first reported by Stappenbeck and colleagues39 in 2009. This work carefully examined the histologic changes and select inflammatory responses that occur after biopsy, in addition to developing the method to measure wound closure.39 These findings laid the groundwork for later studies interrogating how inflammatory mediators, stem cells, and oxidation-reduction molecules affect the wound healing process.39, 40, 41, 42, 43, 44, 45, 46 Although mainly focused on wound healing, a previous study showed increased neutrophils and macrophages in and around the wound bed by immunostaining 6 hours after biopsy, as well as gene expression changes of type 1 and type 2 helper T-cell–related cytokines at both early and late stages of the injury response.39 In the current study, comprehensive RNA-seq analysis of the wound bed was performed after colonic biopsy. This analysis revealed many gene expression changes, including many that are related to immune cell recruitment and activation, as suggested by pathway analysis. In fact, immune cell profiling by flow cytometry 1 day after biopsy revealed strong increases in myeloid cells, including neutrophils and macrophages in the colons. Given the small size of wound beds generated by this method of injury, there is a limited amount of tissue to perform meaningful studies of single cells other than by histology. To overcome this limitation, the approach was modified for assessing single-cell populations by taking multiple biopsies per colon, thus generating ample tissue containing immune cells in both the wound bed and surrounding regions. Because this approach proved useful for analyzing immune cell populations by flow cytometry, this method could be used for studying complex immune cell populations that may be relevant for the inflammatory response. Additionally, our gene expression analysis suggested that biopsy-induced changes were similar to those observed in UC and Crohn disease, many of which were inflammation related. These findings highlight the potential utility of this model for studying the pathogenesis of intestinal inflammation.
Given the robust inflammatory response after biopsy-induced injury, it was determined which mediators may play a role in this tissue response. Disrupting S1P signaling is a recognized strategy for suppressing colitis.12, 13, 14, 15 Although the anti-inflammatory effect of agents that interfere with S1P signaling is attributable to reduced lymphocyte trafficking, whether S1P signaling in the vasculature contributes to colonic inflammation is unknown. S1P1, one of the five S1P receptors, is known to be critical for maintaining blood vessel integrity and controlling inflammation.10, 11 S1P1 is expressed on the colonic endothelium, and its levels increase in UC as a result of enhanced blood vessel density.10 In this study, colonic levels of S1P increased in response to tissue injury. The associated gene expression changes suggest that this increase in S1P levels may reflect a combination of increased synthesis and reduced degradation. Interestingly, selective deletion of S1P1 on blood vessels increased bleeding, whereas the inflammatory response was unaffected. The increased bleeding can be explained by the key role that S1P1 plays in maintaining endothelial cell junctions and, thus, vascular barrier function.5, 9, 10 Although S1P1 expression in the colon is limited to the endothelium, the possibility that S1P signaling via other receptors in the epithelium may also be relevant in the pathogenesis of inflammation cannot be ruled out.47, 48 Taken together, these data suggest that disruption of vascular S1P signaling does not affect the acute colonic inflammatory response. The fact that bleeding is not a common adverse effect of agents targeting S1P signaling likely reflects the fact that pharmacologic targeting of a receptor does not disrupt signaling as strongly as genetic deletion.
In addition to the role of host tissue components in intestinal inflammation, gut luminal factors are also important. Recent work has demonstrated that luminal bacteria quickly enter the wound bed after biopsy, followed by a reestablishment of the colonic barrier several days after injury.40 This bacterial influx contributed to the proliferation and migration of epithelial cells to heal the injured region.40 In line with these findings, this study showed an influx of bacteria into the wound bed shortly after biopsy. More in-depth analysis determined which biopsy-induced gene expression changes were dependent on gut microbes. Interestingly, reducing bacterial abundance attenuated the normally strong inflammatory response induced by wounding. This was associated with both the attenuation and enhancement of biopsy-induced expression of select genes. For example, antibiotic treatment attenuated the increase in Trem2, a gene expressed in myeloid cells known to contribute to tissue inflammation.35 In addition, the increase in Il23a expression was lessened by antibiotic treatment. This gene is known to be a key mediator of the inflammatory response to bacteria, and its genetic deletion ameliorates experimental colitis.36 Given the known importance of gut bacteria in IBD, these data suggest that the colonoscopic-guided pinch biopsy model may be useful for further elucidating the importance of host-microbe interactions in intestinal inflammation and mucosal homeostasis.
Acknowledgments
We thank Dr. Robert Benezra for numerous helpful discussions; and Francis Davis for technical assistance with fluorescence in situ hybridization.
Contributor Information
David C. Montrose, Email: dam2040@med.cornell.edu.
Andrew J. Dannenberg, Email: ajdannen@med.cornell.edu.
Supplemental Data
Supplemental Figure S1.
Lipids increase in colonic wound beds (WBs). d-Sphingosine and ceramide levels were measured by liquid chromatography–tandem mass spectrometry in WBs and normal mucosa from sham-treated mice 6 hours after colonoscopy. n = 6 to 7 samples per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Supplemental Figure S2.
S1P receptor expression changes after acute injury. Expression levels of the five S1P receptors were measured by quantitative real-time RT-PCR in wound beds (WBs) and normal mucosa from sham-treated mice 6 hours after colonoscopy. n = 5 to 6 samples per group (A–E).
Supplemental Figure S3.
Broad-spectrum antibiotic treatment reduces fecal bacterial abundance by approximately 1000-fold. Bacterial-specific PCR was performed on DNA extracted from feces of mice given plain drinking water containing artificial sweetener (Splenda; vehicle) or broad-spectrum antibiotics (antibiotics and artificial sweetener). Results are shown at the Ct value for each sample (diamonds) as well as the average value for each group (line). P = 3 × 10−7, as determined by unpaired t-test comparing the vehicle with the antibiotic group.
References
- 1.Danese S., Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Doulberis M., Panagopoulos P., Scherz S., Dellaporta E., Kouklakis G. Update on ischemic colitis: from etiopathology to treatment including patients of intensive care unit. Scand J Gastroenterol. 2016;51:893–902. doi: 10.3109/00365521.2016.1162325. [DOI] [PubMed] [Google Scholar]
- 3.El Feghaly R.E., Bangar H., Haslam D.B. The molecular basis of Clostridium difficile disease and host response. Curr Opin Gastroenterol. 2015;31:24–29. doi: 10.1097/MOG.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 4.Blaho V.A., Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem Rev. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez T., Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 6.McVerry B.J., Garcia J.G. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Pappu R., Schwab S.R., Cornelissen I., Pereira J.P., Regard J.B., Xu Y., Camerer E., Zheng Y.W., Huang Y., Cyster J.G., Coughlin S.R. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 8.Rivera J., Proia R.L., Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Dudek S.M. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montrose D.C., Scherl E.J., Bosworth B.P., Zhou X.K., Jung B., Dannenberg A.J., Hla T. S1P(1) localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J Lipid Res. 2013;54:843–851. doi: 10.1194/jlr.M034108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvani S., Sanson M., Blaho V.A., Swendeman S.L., Obinata H., Conger H., Dahlback B., Kono M., Proia R.L., Smith J.D., Hla T. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8:ra79. doi: 10.1126/scisignal.aaa2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deguchi Y., Andoh A., Yagi Y., Bamba S., Inatomi O., Tsujikawa T., Fujiyama Y. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep. 2006;16:699–703. [PubMed] [Google Scholar]
- 13.Sanada Y., Mizushima T., Kai Y., Nishimura J., Hagiya H., Kurata H., Mizuno H., Uejima E., Ito T. Therapeutic effects of novel sphingosine-1-phosphate receptor agonist W-061 in murine DSS colitis. PLoS One. 2011;6:e23933. doi: 10.1371/journal.pone.0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandborn W.J., Feagan B.G., Wolf D.C., D'Haens G., Vermeire S., Hanauer S.B., Ghosh S., Smith H., Cravets M., Frohna P.A., Aranda R., Gujrathi S., Olson A. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–1762. doi: 10.1056/NEJMoa1513248. [DOI] [PubMed] [Google Scholar]
- 15.Scott F.L., Clemons B., Brooks J., Brahmachary E., Powell R., Dedman H., Desale H.G., Timony G.A., Martinborough E., Rosen H., Roberts E., Boehm M.F., Peach R.J. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–1792. doi: 10.1111/bph.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson M.E., Gustafsson J.K., Holmen-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjovall H., Hansson G.C. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleessen B., Kroesen A.J., Buhr H.J., Blaut M. Mucosal and invading bacteria in patients with inflammatory bowel disease compared with controls. Scand J Gastroenterol. 2002;37:1034–1041. doi: 10.1080/003655202320378220. [DOI] [PubMed] [Google Scholar]
- 18.Swidsinski A., Loening-Baucke V., Theissig F., Engelhardt H., Bengmark S., Koch S., Lochs H., Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M.L., Sundrud M.S. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:1157–1167. doi: 10.1097/MIB.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halfvarson J., Brislawn C.J., Lamendella R., Vazquez-Baeza Y., Walters W.A., Bramer L.M., D'Amato M., Bonfiglio F., McDonald D., Gonzalez A., McClure E.E., Dunklebarger M.F., Knight R., Jansson J.K. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2:17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascal V., Pozuelo M., Borruel N., Casellas F., Campos D., Santiago A., Martinez X., Varela E., Sarrabayrouse G., Machiels K., Vermeire S., Sokol H., Guarner F., Manichanh C. A microbial signature for Crohn's disease. Gut. 2017;66:813–822. doi: 10.1136/gutjnl-2016-313235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw K.A., Bertha M., Hofmekler T., Chopra P., Vatanen T., Srivatsa A., Prince J., Kumar A., Sauer C., Zwick M.E., Satten G.A., Kostic A.D., Mulle J.G., Xavier R.J., Kugathasan S. Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016;8:75. doi: 10.1186/s13073-016-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassett S.A., Young W., Barnett M.P., Cookson A.L., McNabb W.C., Roy N.C. Changes in composition of caecal microbiota associated with increased colon inflammation in interleukin-10 gene-deficient mice inoculated with Enterococcus species. Nutrients. 2015;7:1798–1816. doi: 10.3390/nu7031798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eun C.S., Mishima Y., Wohlgemuth S., Liu B., Bower M., Carroll I.M., Sartor R.B. Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10-/- mice. Infect Immun. 2014;82:2239–2246. doi: 10.1128/IAI.01513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison P.J., Bending D., Fouser L.A., Wright J.F., Stockinger B., Cooke A., Kullberg M.C. Th17-cell plasticity in Helicobacter hepaticus-induced intestinal inflammation. Mucosal Immunol. 2013;6:1143–1156. doi: 10.1038/mi.2013.11. [DOI] [PubMed] [Google Scholar]
- 26.Wick E.C., Rabizadeh S., Albesiano E., Wu X., Wu S., Chan J., Rhee K.J., Ortega G., Huso D.L., Pardoll D., Housseau F., Sears C.L. Stat3 activation in murine colitis induced by enterotoxigenic Bacteroides fragilis. Inflamm Bowel Dis. 2014;20:821–834. doi: 10.1097/MIB.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janeczko S., Atwater D., Bogel E., Greiter-Wilke A., Gerold A., Baumgart M., Bender H., McDonough P.L., McDonough S.P., Goldstein R.E., Simpson K.W. The relationship of mucosal bacteria to duodenal histopathology, cytokine mRNA, and clinical disease activity in cats with inflammatory bowel disease. Vet Microbiol. 2008;128:178–193. doi: 10.1016/j.vetmic.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Law C.W., Chen Y., Shi W., Smyth G.K. voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014;15:R29. doi: 10.1186/gb-2014-15-2-r29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holgersen K., Kutlu B., Fox B., Serikawa K., Lord J., Hansen A.K., Holm T.L. High-resolution gene expression profiling using RNA sequencing in patients with inflammatory bowel disease and in mouse models of colitis. J Crohns Colitis. 2015;9:492–506. doi: 10.1093/ecco-jcc/jjv050. [DOI] [PubMed] [Google Scholar]
- 30.Newman A.M., Liu C.L., Green M.R., Gentles A.J., Feng W., Xu Y., Hoang C.D., Diehn M., Alizadeh A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montrose D.C., Nakanishi M., Murphy R.C., Zarini S., McAleer J.P., Vella A.T., Rosenberg D.W. The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat. 2015;116-117:26–36. doi: 10.1016/j.prostaglandins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., Paley M.A., Antenus M., Williams K.L., Erikson J., Wherry E.J., Artis D. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaner R.L., Allegood J.C., Park H., Wang E., Kelly S., Haynes C.A., Sullards M.C., Merrill A.H., Jr. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50:1692–1707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton M.J., Frei S.M., Stevens R.L. The multifaceted mast cell in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:2364–2378. doi: 10.1097/MIB.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klesney-Tait J., Turnbull I.R., Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 36.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., Murphy E., Sathe M., Cua D.J., Kastelein R.A., Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman O.I., Haller D. Bacterial signaling at the intestinal epithelial interface in inflammation and cancer. Front Immunol. 2017;8:1927. doi: 10.3389/fimmu.2017.01927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishida A., Inoue R., Inatomi O., Bamba S., Naito Y., Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 39.Seno H., Miyoshi H., Brown S.L., Geske M.J., Colonna M., Stappenbeck T.S. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam A., Leoni G., Quiros M., Wu H., Desai C., Nishio H., Jones R.M., Nusrat A., Neish A.S. The microenvironment of injured murine gut elicits a local pro-restitutive microbiota. Nat Microbiol. 2016;1:15021. doi: 10.1038/nmicrobiol.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alam A., Leoni G., Wentworth C.C., Kwal J.M., Wu H., Ardita C.S., Swanson P.A., Lambeth J.D., Jones R.M., Nusrat A., Neish A.S. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuhn K.A., Manieri N.A., Liu T.C., Stappenbeck T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS One. 2014;9:e114195. doi: 10.1371/journal.pone.0114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leoni G., Alam A., Neumann P.A., Lambeth J.D., Cheng G., McCoy J., Hilgarth R.S., Kundu K., Murthy N., Kusters D., Reutelingsperger C., Perretti M., Parkos C.A., Neish A.S., Nusrat A. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manieri N.A., Mack M.R., Himmelrich M.D., Worthley D.L., Hanson E.M., Eckmann L., Wang T.C., Stappenbeck T.S. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. J Clin Invest. 2015;125:3606–3618. doi: 10.1172/JCI81423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miyoshi H., Ajima R., Luo C.T., Yamaguchi T.P., Stappenbeck T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neurath M.F., Wittkopf N., Wlodarski A., Waldner M., Neufert C., Wirtz S., Gunther C., Becker C. Assessment of tumor development and wound healing using endoscopic techniques in mice. Gastroenterology. 2010;139:1837–1843.e1. doi: 10.1053/j.gastro.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Eisenhoffer G.T., Loftus P.D., Yoshigi M., Otsuna H., Chien C.B., Morcos P.A., Rosenblatt J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–549. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Y., Forostyan T., Sabbadini R., Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J Cell Biol. 2011;193:667–676. doi: 10.1083/jcb.201010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.