Abstract
Background and Aim
The diagnostic and therapeutic modalities of esophageal cancer have recently improved in Asia, and its prognosis is expected to change. This study provides a population‐based report on the epidemiology of esophageal cancer in Korea.
Methods
Cancer incidence data from 1999 to 2013 were obtained from the Korea Central Cancer Registry, covering the entire population. Age‐standardized incidence rates and annual percent changes were calculated according to subsites and histological types. Five‐year relative survival rates were estimated for cases diagnosed between 1993 and 2013. Relative excess rates were compared between patients diagnosed from 2009 to 2013 and 2006 to 2008.
Results
The age‐standardized incidence rates decreased from 8.8 per 100 000 populations in 1999 to 5.9 in 2013 with an annual percent change of −2.6% in men and −2.2% in women. The most common histological type was squamous cell carcinoma, accounting for 90.2% of all esophageal cancers in 2013, followed by adenocarcinomas (3.1%), and their incidences decreased. The proportion of localized and regional cancer tended to increase compared with that of distant cancer. Five‐year relative survival of squamous cell carcinoma improved from 12.1% (1993–1995) to 34.6% (2009–2013). Relative excess rate was 0.72 (95% confidence interval 0.65–0.80) in localized stage and 0.88 (95% confidence interval, 0.82–0.95) in regional stage comparing patients diagnosed from 2009 to 2013 and 2006 to 2008.
Conclusions
The incidence of esophageal cancer has decreased in Korea for the past 15 years, and 5‐year survival rates have improved significantly. These increases may be attributable to more effective detection of early‐stage disease.
Keywords: cancer incidence, cancer registry, esophageal cancer, survival rates
Introduction
Esophageal cancer is the eighth most common form of cancer in incidence and the sixth common cause of death from cancer worldwide, with 456 000 new cases and 400 100 deaths according to the GLOBOCAN 2012.1 Its worldwide incidence is believed to be increasing.2 Age‐standardized incidence and mortality rates of esophageal cancer are the highest in Eastern Asia as well as Southern and Eastern Africa, and around 80% of the cases worldwide occur in less developed regions.1 There are two different histological types, namely, squamous cell carcinoma and adenocarcinoma, although there is only a marginal difference in their treatment strategy. Squamous esophageal cancer is one of most aggressive cancer, and its incidence and mortality have been decreasing in Asian countries including China and Japan.3, 4, 5, 6 However, the incidence of esophageal adenocarcinoma, which is related with gastro‐esophageal reflux disease (GERD), obesity, smoking, and dietary factors, continues to increase, especially in the USA7, 8 and Canada.9
Over the past several decades, correlated with the economic development, the dietary patterns have westernized, increasing the rates of obesity, and the prevalence of Helicobacter pylori infection has decreased during a relatively short period of time in Korea. These changes might be attributable to decrease in the incidence of squamous esophageal carcinoma but increase in the incidence of esophageal adenocarcinoma. On the other hand, gastric adenocarcinoma is the second most common cancer and the third leading cause of cancer deaths in Korea.10 A population‐based screening program for gastric cancer was implemented in 2002 as a part of the National Cancer Screening Program (NCSP) for persons aged 40 years and older by using upper gastrointestinal series or endoscopy biennially.11 This organized screening program may help in the detection of esophageal carcinoma, especially in early stages.
In the present study, we aimed to describe the trends of incidence and survival rates of esophageal cancer during different time periods according to sex, age groups, and histological subtypes from a population‐based cancer registry in Korea.
Methods
Data sources
The Korea Central Cancer Registry (KCCR), a nationwide, hospital‐based cancer registry, was initiated by the Ministry of Health and Welfare, Korea, in 1980.12 Since 1999, the KCCR expanded cancer registration to cover the entire Korean population under the Population‐Based Regional Cancer Registry program.12 In this database, the completeness of cancer incidence was 97.7% in 2012, as determined by the Ajuki method.13 Quality indices for esophageal cancer such as the proportion of death certification, mortality/incidence ratio, and the proportion of microscopic verification are presented in Table S1. Informed consent was not required because of the anonymity of the records in the data registry.
Esophageal cancer incidence
Age‐specific (5‐year intervals) and sex‐specific incidence rates and the number of cases for esophageal cancer patients between 1999 and 2013 were obtained from the Korea National Cancer Incidence Database. Histological subtypes of esophageal cancer were classified as follows: squamous cell carcinoma (International Classification of Diseases for Oncology third edition morphological codes 8050–8078, 8083–8084), adenocarcinoma (8140–8141, 8143–8145, 8190–8231, 8260–8263, 8310, 8401, 8480–8490, 8550–8551, 8570–8574, 8576), other specified (unspecified carcinoma: 8010–8035; sarcoma: 8800–8811, 8830, 8840–8921, 8990–8991, 9040–9044, 9120–9133, 9150, 9540–9581), and unspecified (8000–8005). Anatomical subsites were classified as follows: upper third of the esophagus (International Classification of Diseases 10 code C15.0, C15.3), middle third of the esophagus (C15.4), lower third of the esophagus (C15.5), thoracic (15.1), abdominal (C15.2), and overlapping lesion or not otherwise specified (C15.8–15.9). The stage of diagnosis was defined according to the Surveillance, Epidemiology, and End Results (SEER) summary stage classification. SEER stages were as follows: localized (limited to the organ of origin), regional (tumor extension beyond the limits of the organ of origin), distant (away from the primary tumor), and unknown.
Age‐standardized rates (ASRs) were calculated using the World Standard Population as the standard population.14 Annual percent changes (APC) in the incidence rates were calculated based on a linear model using the following formula: (exp (b) − 1) × 100, where b is the slope of regression of the natural logarithm of ASR in a calendar year. The 95% confidence intervals (CIs) were obtained with standard error from the fit of the regression and the t‐distribution function.
Esophageal cancer survival
The survival duration for esophageal cancer patients was determined as the interval between the date of initial diagnosis and the date of death, date of loss to follow‐up, or the closing date of follow‐up (December 31, 2013). Survival rates were used from the data available for 1993. Time periods were chosen to allow comparison with those of an earlier study13 and to estimate 5‐year survival rate for more recent data using complete approach.15 Five‐year relative survival rates (RSRs) were calculated using the Ederer II method based on an algorithm written in SAS by Dickman with minor modifications. The 5‐year RSRs of years 1993–2013 by histological subtypes were calculated, and the changes in 5‐year RSRs from 1993–1995 to 2009–2013 were compared. The 1, 3, and 5‐year RSRs of patients diagnosed during 2006–2008 and 2009–2013 were calculated according to the SEER summary stages, which have been available since 2006. In addition, relative excess rates (RER) of patients diagnosed between 2009 and 2013 compared with patients diagnosed between 2006 and 2008 were estimated according to sex and SEER stage. All analyses were stratified by sex. Statistical analysis was performed using Stata/SE 10.0 for Windows (StataCorp LP, College Station, TX, USA) and SAS version 9.3 software (SAS Institute, Inc., Cary, NC, USA).
Results
Time trends of incidence of esophageal cancer
Overall, the number of diagnosed esophageal cancer cases has increased from 1864 in 1999 to 2382 cases in 2013 (Table 1). Although the crude rate of esophageal cancer has increased, the ASRs decreased from 4.06 per 100 000 people in 1999 to 2.91 in 2013 with an APC of −2.2%. The same decreasing trends were observed for both men and women with APCs of −2.6% and −2.2%, respectively (Fig. 1).
Table 1.
Crude incidence rates and age‐standardized incidence rates per 100 000 people† for esophageal cancer and annual percent change by sex and histological subtypes, 1999–2013
| Histological group | Rates | Year | APC | P‐value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | ||||
| Total | ||||||||||||||||||
| Overall | Cases | 1864 | 1769 | 1908 | 1946 | 1932 | 1983 | 2044 | 2046 | 2101 | 2201 | 2166 | 2233 | 2268 | 2354 | 2382 | ||
| CR | 3.95 | 3.72 | 3.99 | 4.04 | 4.00 | 4.09 | 4.20 | 4.19 | 4.28 | 4.46 | 4.36 | 4.48 | 4.53 | 4.68 | 4.71 | |||
| ASR† | 4.06 | 3.69 | 3.88 | 3.77 | 3.62 | 3.56 | 3.54 | 3.39 | 3.32 | 3.34 | 3.14 | 3.13 | 3.03 | 3.02 | 2.91 | −2.2 | < 0.0001 | |
| Squamous cell carcinoma | Cases | 1409 | 1310 | 1491 | 1541 | 1581 | 1625 | 1680 | 1714 | 1770 | 1909 | 1895 | 1985 | 2003 | 2102 | 2149 | ||
| CR | 2.99 | 2.76 | 3.11 | 3.20 | 3.27 | 3.35 | 3.45 | 3.51 | 3.60 | 3.86 | 3.82 | 3.98 | 4.00 | 4.18 | 4.25 | |||
| ASR† | 3.08 | 2.76 | 3.05 | 3.02 | 2.98 | 2.94 | 2.92 | 2.87 | 2.82 | 2.91 | 2.76 | 2.79 | 2.69 | 2.71 | 2.63 | −0.9 | 0.0003 | |
| Adenocarcinoma | Cases | 64 | 59 | 64 | 60 | 64 | 76 | 78 | 76 | 86 | 87 | 78 | 67 | 76 | 69 | 75 | ||
| CR | 0.14 | 0.12 | 0.13 | 0.12 | 0.13 | 0.16 | 0.16 | 0.16 | 0.18 | 0.18 | 0.16 | 0.13 | 0.15 | 0.14 | 0.15 | |||
| ASR† | 0.14 | 0.12 | 0.13 | 0.11 | 0.12 | 0.13 | 0.13 | 0.12 | 0.13 | 0.13 | 0.11 | 0.09 | 0.10 | 0.09 | 0.09 | −2.7 | 0.0006 | |
| Other specified | Cases | 50 | 69 | 53 | 58 | 52 | 52 | 70 | 62 | 62 | 69 | 58 | 65 | 82 | 67 | 56 | ||
| CR | 0.11 | 0.15 | 0.11 | 0.12 | 0.11 | 0.11 | 0.14 | 0.13 | 0.13 | 0.14 | 0.12 | 0.13 | 0.16 | 0.13 | 0.11 | |||
| ASR† | 0.11 | 0.14 | 0.11 | 0.11 | 0.10 | 0.09 | 0.12 | 0.10 | 0.10 | 0.10 | 0.09 | 0.09 | 0.11 | 0.08 | 0.07 | −2.6 | 0.0028 | |
| Unspecified | Cases | 341 | 331 | 300 | 287 | 235 | 230 | 216 | 194 | 183 | 136 | 135 | 116 | 107 | 116 | 102 | ||
| CR | 0.72 | 0.70 | 0.63 | 0.60 | 0.49 | 0.47 | 0.44 | 0.40 | 0.37 | 0.28 | 0.27 | 0.23 | 0.21 | 0.23 | 0.20 | |||
| ASR† | 0.72 | 0.67 | 0.59 | 0.53 | 0.42 | 0.40 | 0.36 | 0.30 | 0.27 | 0.20 | 0.18 | 0.15 | 0.13 | 0.13 | 0.12 | −13.0 | < 0.0001 | |
| Men | ||||||||||||||||||
| Overall | Cases | 1708 | 1605 | 1733 | 1785 | 1738 | 1820 | 1885 | 1877 | 1899 | 2003 | 2003 | 2046 | 2073 | 2133 | 2186 | ||
| CR | 7.21 | 6.73 | 7.21 | 7.39 | 7.17 | 7.48 | 7.72 | 7.66 | 7.71 | 8.09 | 8.05 | 8.19 | 8.26 | 8.47 | 8.65 | |||
| ASR† | 8.81 | 7.97 | 8.30 | 8.19 | 7.71 | 7.70 | 7.60 | 7.21 | 6.95 | 6.97 | 6.61 | 6.48 | 6.27 | 6.13 | 5.97 | −2.6 | < 0.0001 | |
| Squamous cell carcinoma | Cases | 1335 | 1218 | 1399 | 1442 | 1468 | 1522 | 1590 | 1604 | 1632 | 1768 | 1781 | 1842 | 1853 | 1930 | 1995 | ||
| CR | 5.64 | 5.10 | 5.82 | 5.97 | 6.06 | 6.26 | 6.51 | 6.55 | 6.63 | 7.14 | 7.16 | 7.37 | 7.39 | 7.66 | 7.89 | |||
| ASR† | 6.81 | 6.00 | 6.64 | 6.55 | 6.45 | 6.38 | 6.37 | 6.14 | 5.96 | 6.13 | 5.86 | 5.83 | 5.58 | 5.54 | 5.44 | −1.4 | < 0.0001 | |
| Adenocarcinoma | Cases | 57 | 49 | 46 | 50 | 51 | 67 | 66 | 63 | 66 | 74 | 69 | 53 | 64 | 55 | 69 | ||
| CR | 0.24 | 0.21 | 0.19 | 0.21 | 0.21 | 0.28 | 0.27 | 0.26 | 0.27 | 0.30 | 0.28 | 0.21 | 0.26 | 0.22 | 0.27 | |||
| ASR† | 0.31 | 0.26 | 0.23 | 0.24 | 0.24 | 0.28 | 0.27 | 0.24 | 0.24 | 0.25 | 0.23 | 0.17 | 0.20 | 0.16 | 0.19 | −3.1 | 0.0008 | |
| Other specified | Cases | 43 | 60 | 46 | 53 | 44 | 47 | 63 | 54 | 51 | 59 | 47 | 57 | 69 | 56 | 42 | ||
| CR | 0.18 | 0.25 | 0.19 | 0.22 | 0.18 | 0.19 | 0.26 | 0.22 | 0.21 | 0.24 | 0.19 | 0.23 | 0.28 | 0.22 | 0.17 | |||
| ASR† | 0.23 | 0.28 | 0.22 | 0.24 | 0.19 | 0.19 | 0.25 | 0.20 | 0.19 | 0.20 | 0.16 | 0.18 | 0.21 | 0.16 | 0.12 | −3.6 | 0.0009 | |
| Unspecified | Cases | 273 | 278 | 242 | 240 | 175 | 184 | 166 | 156 | 150 | 102 | 106 | 94 | 87 | 92 | 80 | ||
| CR | 1.15 | 1.16 | 1.01 | 0.99 | 0.72 | 0.76 | 0.68 | 0.64 | 0.61 | 0.41 | 0.43 | 0.38 | 0.35 | 0.37 | 0.32 | |||
| ASR† | 1.46 | 1.42 | 1.22 | 1.16 | 0.82 | 0.85 | 0.71 | 0.63 | 0.56 | 0.39 | 0.36 | 0.30 | 0.28 | 0.27 | 0.23 | −13.3 | < 0.0001 | |
| Women | ||||||||||||||||||
| Overall | Cases | 156 | 164 | 175 | 161 | 194 | 163 | 159 | 169 | 202 | 198 | 163 | 187 | 195 | 221 | 196 | ||
| CR | 0.66 | 0.69 | 0.73 | 0.67 | 0.81 | 0.67 | 0.66 | 0.69 | 0.82 | 0.80 | 0.66 | 0.75 | 0.78 | 0.88 | 0.78 | |||
| ASR† | 0.56 | 0.55 | 0.58 | 0.51 | 0.59 | 0.47 | 0.43 | 0.47 | 0.52 | 0.50 | 0.38 | 0.44 | 0.43 | 0.48 | 0.41 | −2.2 | 0.0014 | |
| Squamous cell carcinoma | Cases | 74 | 92 | 92 | 99 | 113 | 103 | 90 | 110 | 138 | 141 | 114 | 143 | 150 | 172 | 154 | ` | |
| CR | 0.32 | 0.39 | 0.39 | 0.41 | 0.47 | 0.43 | 0.37 | 0.45 | 0.56 | 0.57 | 0.46 | 0.57 | 0.60 | 0.68 | 0.61 | |||
| ASR† | 0.28 | 0.32 | 0.32 | 0.33 | 0.36 | 0.31 | 0.25 | 0.32 | 0.37 | 0.37 | 0.28 | 0.34 | 0.34 | 0.38 | 0.33 | 0.8 | 0.2396 | |
| Adenocarcinoma | Cases | 7 | 10 | 18 | 10 | 13 | 9 | 12 | 13 | 20 | 13 | 9 | 14 | 12 | 14 | 6 | ||
| CR | 0.03 | 0.04 | 0.08 | 0.04 | 0.05 | 0.04 | 0.05 | 0.05 | 0.08 | 0.05 | 0.04 | 0.06 | 0.05 | 0.06 | 0.02 | |||
| ASR† | 0.02 | 0.03 | 0.06 | 0.03 | 0.04 | 0.03 | 0.04 | 0.04 | 0.05 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.01 | −4.2 | 0.0822 | |
| Other specified | Cases | 7 | 9 | 7 | 5 | 8 | 5 | 7 | 8 | 11 | 10 | 11 | 8 | 13 | 11 | 14 | ||
| CR | 0.03 | 0.04 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.03 | 0.05 | 0.04 | 0.06 | |||
| ASR† | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 1.4 | 0.2393 | |
| Unspecified† | Cases | 68 | 53 | 58 | 47 | 60 | 46 | 50 | 38 | 33 | 34 | 29 | 22 | 20 | 24 | 22 | ||
| CR | 0.29 | 0.22 | 0.24 | 0.20 | 0.25 | 0.19 | 0.21 | 0.16 | 0.13 | 0.14 | 0.12 | 0.09 | 0.08 | 0.10 | 0.09 | |||
| ASR† | 0.23 | 0.17 | 0.18 | 0.14 | 0.17 | 0.11 | 0.12 | 0.09 | 0.08 | 0.07 | 0.06 | 0.04 | 0.04 | 0.04 | 0.04 | −12.3 | < 0.0001 | |
The World standard population was used as standard population.
Crude rates and ASRs are expressed per 100 000 people.
APC, annual percent change; ASR, age‐standardized rate; CR, crude rate.
Figure 1.
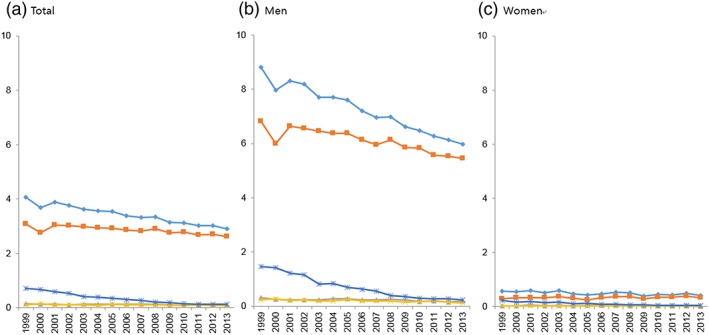
Age‐standardized incidence rates per 100 000 people for esophageal cancer by sex and histological subtypes, 1999–2013. (a) Total, (b) men, and (c) women.  , Overall;
, Overall;  , Squamous cell carcinoma;
, Squamous cell carcinoma;  , Adenocarcinoma;
, Adenocarcinoma;  , Other specified;
, Other specified;  , Unspecified. [Color figure can be viewed at wileyonlinelibrary.com]
, Unspecified. [Color figure can be viewed at wileyonlinelibrary.com]
Trends of esophageal cancer according to histological subtypes
More than 90% of all esophageal cancer cases were men. The most frequent histological subtype was squamous cell carcinoma, constituting 75.5% of all cases in 1999, gradually increasing to 90.2% in 2013 (Table 1). However, after excluding unspecified histology cases, the proportion of squamous cell carcinomas was 92.5% in 1999 and 96% in 2013. The second most common histological subtype was adenocarcinoma, which constituted about 3% of all cases. In men, the ASRs of all histological types significantly decreased, whereas in women, the ASR of unspecified esophageal cancer decreased, but that of other esophageal cancers did not change between 1999 and 2013.
Trends of esophageal cancer according to Surveillance, Epidemiology, and End Results staging
Table 2 shows the SEER summary stage distribution of esophageal cancer patients diagnosed during two periods: 2006–2009 and 2010–2013.
Table 2.
Incidence rates of esophageal cancer per 100 000 people according to age group and SEER stage, 2006–2013
| Year of diagnosis | 2006–2009 | 2010–2013 | |||||
|---|---|---|---|---|---|---|---|
| Cases | (%) | ASR | Cases | (%) | ASR | ||
| Age group | SEER stage | 3.29 | 3.02 | ||||
| Total | Localized | 2559 | (30.1) | 0.99 | 3045 | (33.0) | 1.00 |
| Regional | 2781 | (32.7) | 1.09 | 3487 | (37.8) | 1.15 | |
| Distant | 1514 | (17.8) | 0.59 | 1598 | (17.3) | 0.53 | |
| Unknown | 1660 | (19.5) | 0.62 | 1107 | (12.0) | 0.34 | |
| 0–64 | Localized | 963 | (29.2) | 0.45 | 1226 | (33.0) | 0.48 |
| Regional | 1167 | (35.4) | 0.54 | 1511 | (40.6) | 0.59 | |
| Distant | 669 | (20.3) | 0.31 | 690 | (18.5) | 0.27 | |
| Unknown | 495 | (15.0) | 0.23 | 295 | (7.9) | 0.12 | |
| 65–74 | Localized | 1103 | (31.3) | 8.54 | 1143 | (33.9) | 7.91 |
| Regional | 1150 | (32.6) | 8.93 | 1276 | (37.8) | 8.89 | |
| Distant | 616 | (17.5) | 4.79 | 579 | (17.2) | 4.05 | |
| Unknown | 654 | (18.6) | 5.05 | 377 | (11.2) | 2.57 | |
| 75+ | Localized | 493 | (29.1) | 7.47 | 676 | (31.6) | 7.84 |
| Regional | 464 | (27.3) | 6.96 | 700 | (32.7) | 7.96 | |
| Distant | 229 | (13.5) | 3.44 | 329 | (15.4) | 3.77 | |
| Unknown | 511 | (30.1) | 7.92 | 435 | (20.3) | 5.24 | |
ASR, age‐standardized rate; SEER, Surveillance, Epidemiology, and End Results.
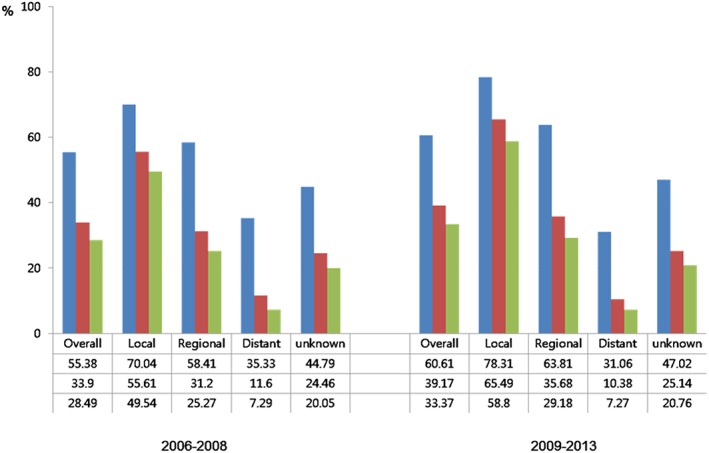
The proportion of patients classified as unknown without staging were 19.5% in 2006–2009 but significantly decreased to 12.5% in 2010–2013. The proportion of patients diagnosed as localized stage increased from 30.1% in 2006–2009 to 33% in 2010–2013 and that of regional stage increased to 37.8% from 32.7%, respectively. The proportion of localized and regional cancer showed a higher tendency to increase than that of distant cancer in all age groups.
Trends of survival rates
Overall RSR markedly improved during the observation period. Overall 5‐RSR was 12.8% between 1993 and 1995 but increased to 33.4% between 2009 and 2013 (Fig. 2). Improvement in the 5‐year RSR was observed in all histological types, except in unspecified histology between 1993 and 2013 (Table 3).
Figure 2.
Relative survival rates (%) of 1, 3, and 5 years of patients with esophageal cancer by period of diagnosis according to Surveillance, Epidemiology, and End Results stage.  , 1‐year;
, 1‐year;  , 3‐year;
, 3‐year;  , 5‐year. [Color figure can be viewed at wileyonlinelibrary.com]
, 5‐year. [Color figure can be viewed at wileyonlinelibrary.com]
Table 3.
Five‐year relative survival (RSR, %) of patients with esophageal cancer by sex and histological subtypes, 1993–2013
| Year of diagnosis | 1993–1995 | 1996–2000 | 2001–2005 | 2006–2010 | 2009–2013 | 1993–2013 | Change‡ (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histological group | No. | RSR† | No. | RSR† | No. | RSR† | No. | RSR† | No. | RSR† | No. | RSR† | |
| Total | |||||||||||||
| Overall | 3377 | 12.8 | 7575 | 15.4 | 8756 | 21.2 | 9290 | 29.6 | 9665 | 33.4 | 34905 | 23.2 | 20.6 |
| Squamous cell carcinoma | 2648 | 12.1 | 6145 | 15.8 | 7419 | 22.0 | 8249 | 30.9 | 8760 | 34.6 | 29833 | 24.3 | 22.5 |
| Adenocarcinoma | 145 | 15.7 | 260 | 18.7 | 291 | 19.3 | 288 | 26.0 | 268 | 29.6 | 1144 | 21.9 | 13.9 |
| Other specified | 118 | 15.9 | 259 | 15.7 | 261 | 21.7 | 268 | 24.7 | 277 | 28.0 | 1081 | 21.1 | 12.1 |
| Unspecified | 466 | 14.7 | 911 | 11.9 | 785 | 14.4 | 485 | 11.8 | 360 | 10.9 | 2847 | 12.9 | −3.8 |
| Men | |||||||||||||
| Overall | 3119 | 11.9 | 6934 | 14.5 | 8036 | 20.5 | 8531 | 29.0 | 8861 | 33.1 | 32007 | 22.5 | 21.2 |
| Squamous cell carcinoma | 2492 | 11.3 | 5714 | 15.0 | 6940 | 21.3 | 7661 | 30.2 | 8107 | 34.4 | 27750 | 23.6 | 23.1 |
| Adenocarcinoma | 120 | 15.4 | 211 | 16.7 | 233 | 19.3 | 236 | 27.3 | 230 | 29.4 | 939 | 21.9 | 14.0 |
| Other specified | 109 | 15.2 | 231 | 14.8 | 231 | 20.9 | 231 | 21.8 | 226 | 23.6 | 942 | 19.3 | 8.4 |
| Unspecified | 398 | 13.4 | 778 | 10.0 | 632 | 11.6 | 403 | 9.8 | 298 | 10.1 | 2376 | 11.0 | −3.3 |
| Women | |||||||||||||
| Overall | 258 | 23.4 | 641 | 24.7 | 720 | 29.6 | 759 | 36.8 | 804 | 36.1 | 2898 | 30.8 | 12.7 |
| Squamous cell carcinoma | 156 | 24.8 | 431 | 25.0 | 479 | 32.1 | 588 | 40.0 | 653 | 37.6 | 2083 | 33.4 | 12.8 |
| Adenocarcinoma | 25 | 17.4 | 49 | 26.6 | 58 | 19.5 | 52 | 20.0 | 38 | 29.5 | 205 | 21.5 | 12.1 |
| Other specified | 9 | 24.6 | 28 | 23.6 | 30 | 28.4 | 37 | 42.4 | 51 | 46.9 | 139 | 33.2 | 22.3 |
| Unspecified | 68 | 22.2 | 133 | 23.2 | 153 | 26.0 | 82 | 21.4 | 62 | 14.4 | 471 | 22.6 | −7.8 |
Five‐year relative survival rate.
Change (%) in the 5‐year relative survival rates from 1993–1995 to 2009–2013.
RSR, relative survival rate.
Between 1993 and 1995, the 5‐RSRs were 12.1% for squamous cell carcinoma and 15.7% for adenocarcinoma, but between 2009 and 2013, the former was 34.6% and the latter was 29.6%. Therefore, the survival rate of squamous cell carcinoma increased more than that of adenocarcinoma. The overall survival rates of squamous cell carcinoma and adenocarcinoma were similar. When comparing the RSRs by gender and histological subtypes, there was a remarkable improvement in RSR of men with squamous cell carcinoma, which account for the majority of esophageal carcinoma patients.
Improvements in RSR were observed in localized and regional cancer patients diagnosed in 2009–2013 compared with patients diagnosed in 2006–2008. Particularly, the 5‐year survival rate of localized cancer was 49.5% during 2006–2008, and it improved significantly to 58.5% between 2009 and 2013 (Fig. 2). RER was 0.72 (95% CI 0.65–0.80) in localized cancer and 0.88 (95% CI, 0.82–0.95) in regional cancer among patients diagnosed in 2009–2013, compared with those in 2006–2008. RER was most reduced in men with localized stage (Table 4).
Table 4.
Estimated relative excess risks of esophageal cancer in Korea
| SEER stage | Both | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RER | 95% CI | LR test | RER | 95% CI | LR test | RER | 95% CI | LR test | |
| Local | |||||||||
| 2006–2008 | 1.00 | Reference | < 0.0001 | 1.00 | Reference | < 0.0001 | 1.00 | Reference | 0.6238 |
| 2009–2013 | 0.72 | 0.65–0.80 | 0.69 | 0.62–0.77 | 1.08 | 0.79–1.49 | |||
| Regional | |||||||||
| 2006–2008 | 1.00 | Reference | 0.0005 | 1.00 | Reference | 0.0007 | 1.00 | Reference | 0.4473 |
| 2009–2013 | 0.88 | 0.82–0.95 | 0.88 | 0.82–0.95 | 0.90 | 0.70–1.17 | |||
| Distant | |||||||||
| 2006–2008 | 1.00 | Reference | 0.2396 | 1.00 | Reference | 0.1800 | 1.00 | Reference | 0.6722 |
| 2009–2013 | 1.05 | 0.97–1.15 | 1.06 | 0.97–1.16 | 0.93 | 0.67–1.29 | |||
| Unknown | |||||||||
| 2006–2008 | 1.00 | Reference | 0.4089 | 1.00 | Reference | 0.3342 | 1.00 | Reference | 0.7582 |
| 2009–2013 | 0.96 | 0.86–1.06 | 0.95 | 0.85–1.06 | 1.06 | 0.75–1.49 | |||
CI, confidence interval; LR, likelihood risk; RER, relative excess risks; SEER, Surveillance, Epidemiology, and End Results.
Discussion
Esophageal cancer incidence has decreased in both men and women. More than 90% of esophageal cancers are squamous cell carcinomas, and the incidences of both squamous cell carcinoma and adenocarcinoma have decreased significantly. Similar to our results, squamous cell carcinoma is the most common type of esophageal cancer in Japan and China5; however, in the USA and British Columbia in Canada, adenocarcinoma is the most common type in men, and squamous cell carcinoma is the most common type in women.8, 9 A decrease in incidence as well as mortality have been observed in both developing and developed countries.16 In contrast, the prevalence of adenocarcinoma continuously increased between 1975 and 2009 in the USA, although the increasing trend has slowed since 1998.7
Risk factors for esophageal cancer differ by histological subtype. As established risk factor for esophageal adenocarcinoma is bodyfat and that for squamous cell carcinoma is alcohol consumption according to a systematic review by World Cancer Research Fund/American Institute for Cancer Research.17 In addition, cigarette smoking and insufficient intake of vegetable and fruits are more strongly related with the risk of squamous cell carcinomas.18, 19 Prevalence of cigarette smoking, especially among men, has dramatically decreased during the last few decades in Korea.20 However, the prevalence of high‐risk alcohol consumers has slightly increased in both men (19.9% to 20.8%) and women (3.4% to 5.8%) between 2005 and 2015,21 and prevalence of insufficient consumption of non‐salted vegetables and fruits did not change significantly between 1998 and 2012.22 In addition, the prevalence of obesity gradually increased from 25.7% in 1998 to 35.1% in 2005 and 37.9% in 2013–2014 in men, when obesity was defined as having a body mass index of 25 kg/m2 or higher; however, the prevalence of obesity in women decreased during the same period.23 The prevalence of GERD tends to increase,24 whereas Barrett's esophagus, which is a precancerous lesion of esophageal adenocarcinoma, is still very rare in Korea.25 In addition to dynamic changes in risk factors in both elevating the risk (GERDs, high‐risk alcohol consumption, and obesity in men) and lowering the risk (decrease in smoking prevalence), it is possible that gastric cancer occurred in cardia was diagnosed as adenocarcinoma occurred in the lower esophagus until AJCC 8 edition clearly defined esophago‐gastric junction cancer.26 Stomach cancer in Korea including cardia, body, and antrum is decreasing10, therefore, we cannot completely rule out the possibility of potential misclassification of the cancer site.
An increase in the survival of esophageal cancer patients may be explained by introduction of the NCSP. According to the Korean National Cancer Screening Survey, the lifetime usage of upper endoscopy for stomach cancer screening has gradually increased from 32.4% in 2004 to 64.4% in 2013,27 and it may have helped in the early detection and treatment of precancerous lesions as well as related conditions such as GERD.
The relative survival of esophageal cancer has improved recently in localized and regional stages, but not in the distant stage. The overall survival in Korea is higher compared with reports from the USA (20.1% for 2005–2011) and Canada (15% for 2006–2008) and lower than that of Japan (33.7% for 2003–2005). When compared with US SEER data for 1998–2009, our results showed a higher 5‐year relative survival in all SEER stages. RER showed an improvement of 31% in male patients with localized stage and 18% in male patients with regional stage, indicating that the highest increase in RSR was in men with early‐stage esophageal cancer. Aggressive surgical treatment and endoscopic submucosal dissection (ESD) have improved the survival rate of early‐stage esophageal cancer. ESD has the advantage of permitting en bloc and complete histological resection of early esophageal cancer. Recent studies have shown that ESD of superficial esophageal cancer yielded a comparable survival rate with that of surgery and improved the patients' quality of life without complications accompanying the surgery.28, 29
The five‐year RSRs were higher among women than men in all histological types. Based on a previous study from the KCCR, women showed a higher survival rate than men even after adjustments for years of follow‐up, age, SEER summary stage, and casemix, and the differences in survival rates were statistically significant among the age group of 50–74 years old.30 Consistent with our results, female esophageal cancer patients showed better survival than male patients in US SEER data,31 the British Columbia Cancer Registry of Canada,9 Europe,32 and China.33 In previous analyses, women were more likely to be presented with localized stage than men.30 Although the stage and casemix were considered in the analysis, we still cannot exclude the possibility of differential casemix between men and women even within the same SEER stage groups.
The strength of the current study is it being the first population‐based approach by using cancer registry data with high quality and completeness in Korea. The limitation, however, includes relatively high proportions of unspecified subsites and unknown SEER stages especially in the early period of the registry, which makes interpretation by time difficult. The proportion of unspecified histology has decreased from 18% in 1999 to 4% in 2013; therefore, an increase in the proportion of squamous cell carcinoma can be partly explained by improvements in reporting of morphology information. When esophageal cancers were classified by anatomical subsites, around half of all patients were coded as “overlapping or not otherwise specified (NOS)” during 1999 and 2003, and the proportion of “overlapping or NOS” cancers gradually decreased until 2013 (Table S2). Esophageal cancer occurred most frequently in the mid‐esophagus, and the upper and lower esophagus showed a similar incidence. Although significant trends in incidence of subtypes were observed, it is difficult to interpret whether it was a true change because of the substantially high proportion of NOS tumors. Second, because of limited treatment information, we could not analyze the influence of treatment approaches on changes in the survival rates, which is an important prognostic factor. Lastly, the effect of lead‐time bias on improvement of esophageal cancer patients, which could be introduced after the application of NCSP, could not be evaluated.
In conclusion, this study indicates a continuous decrease in the incidence of esophageal cancer and an increase in its survival rate. Improvement in survival may be affected by the relative increase in early detection of localized cancer. Esophageal cancer is difficult to detect early, and surgery is enforced restrictively despite definitive treatment options. Further studies on improvements in the survival rate of esophageal carcinoma in relation to early detection and subsequent shifts in the treatment modality by time should be pursued.
Supporting information
Table S1. Data quality indices for esophageal cancer, the Korea Central Cancer Registry, 1999–2013.
Table S2. Age‐standardized esophageal cancer incidence rates per 100 000 and annual percent changes (APC) by subsites, 1999–2013.
Shin, A. , Won, Y.‐J. , Jung, H.‐K. , Kong, H.‐J. , Jung, K.‐W. , Oh, C.‐M. , Choe, S. , and Lee, J. (2018) Trends in incidence and survival of esophageal cancer in Korea: Analysis of the Korea Central Cancer Registry Database. Journal of Gastroenterology and Hepatology, 33: 1961–1968. 10.1111/jgh.14289.
Declaration of conflict of interest: The funding organization has not had any role in the design and in conducting the study.
Financial support: Y.‐J. Won was supported by a National Cancer Center grant (NCC‐1610200).
Contributor Information
Young‐Joo Won, Email: astra67@ncc.re.kr.
Hye‐Kyung Jung, Email: junghk@ewha.ac.kr.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 2. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet 2013; 381: 400–412. [DOI] [PubMed] [Google Scholar]
- 3. He Y, Wu Y, Song G et al Incidence and mortality rate of esophageal cancer has decreased during past 40 years in Hebei Province, China. Chin. J. Cancer Res. 2015; 27: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He YT, Hou J, Qiao CY et al An analysis of esophageal cancer incidence in Cixian county from 1974 to 1996. World J. Gastroenterol. 2003; 9: 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin Y, Totsuka Y, He Y et al Epidemiology of esophageal cancer in Japan and China. J. Epidemiol. 2013; 23: 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Dhakal IB, Zhao Z, Li L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur. J. Cancer Prev. 2012; 21: 480–489. [DOI] [PubMed] [Google Scholar]
- 7. Hur C, Miller M, Kong CY et al Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013; 119: 1149–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998‐2003. Int. J. Cancer 2008; 123: 1422–1428. [DOI] [PubMed] [Google Scholar]
- 9. Bashash M, Shah A, Hislop G, Brooks‐Wilson A, Le N, Bajdik C. Incidence and survival for gastric and esophageal cancer diagnosed in British Columbia, 1990 to 1999. Can. J. Gastroenterol. 2008; 22: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung KW, Won YJ, Oh CM et al Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2014. Cancer Res. Treat. 2017; 49: 292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee S, Jun JK, Suh M et al Gastric cancer screening uptake trends in Korea: results for the National Cancer Screening Program from 2002 to 2011: a prospective cross‐sectional study. Medicine (Baltimore). 2015; 94: e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shin HR, Won YJ, Jung KW et al Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res. Treat. 2005; 37: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung KW, Won YJ, Kong HJ et al Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res. Treat. 2015; 47: 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Segi M. Cancer Mortality for Selected Sites in 24 Countries (1950–1957). 1960.
- 15. Brenner H, Gefeller O. Deriving more up‐to‐date estimates of long‐term patient survival. J. Clin. Epidemiol. 1997; 50: 211–216. [DOI] [PubMed] [Google Scholar]
- 16. Collaboration GBoDC . The global burden of cancer 2013. JAMA Oncol. 2015; 1: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Research WCRFIAIfC . Contunuous update project report: diet, nutrition, physical activity and oesophageal cancer. 2016.
- 18. Engel LS, Chow WH, Vaughan TL et al Population attributable risks of esophageal and gastric cancers. J. Natl. Cancer Inst. 2003; 95: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 19. Freedman ND, Abnet CC, Leitzmann MF et al A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am. J. Epidemiol. 2007; 165: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 20. Jee YH, Shin A, Lee JK, Oh CM. Decreases in smoking‐related cancer mortality rates are associated with birth cohort effects in Korean men. Int. J. Environ. Res. Public Health 2016; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prevention KCFDCa . Health behavior & Chronic disease facts, 2015.
- 22. Kim EK, Ha AW, Choi EO, Ju SY. Analysis of Kimchi, vegetable and fruit consumption trends among Korean adults: data from the Korea National Health and Nutrition Examination Survey (1998‐2012). Nutr. Res. Pract. 2016; 10: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin HY, Kang HT. Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: The Korean National Health and Nutrition Examination Survey from 1998 to 2014. J. Epidemiol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J. Neurogastroenterol. Motil. 2011; 17: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee IS, Choi SC, Shim KN et al Prevalence of Barrett's esophagus remains low in the Korean population: nationwide cross‐sectional prospective multicenter study. Dig. Dis. Sci. 2010; 55: 1932–1939. [DOI] [PubMed] [Google Scholar]
- 26. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann. Cardiothorac. Surg. 2017; 6: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suh M, Choi KS, Park B et al Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004‐2013. Cancer Res. Treat. 2016; 48: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Joo DC, Kim GH, Park DY, Jhi JH, Song GA. Long‐term outcome after endoscopic submucosal dissection in patients with superficial esophageal squamous cell carcinoma: a single‐center study. Gut Liver. 2014; 8: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park HC, Kim DH, Gong EJ et al Ten‐year experience of esophageal endoscopic submucosal dissection of superficial esophageal neoplasms in a single center. Korean J. Intern. Med. 2016; 31: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jung KW, Park S, Shin A et al Do female cancer patients display better survival rates compared with males? Analysis of the Korean National Registry data, 2005‐2009. PLoS One 2012; 7: e52457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: a SEER database analysis. J. Gastroenterol. Hepatol. 2016; 31: 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Micheli A, Mariotto A, Giorgi Rossi A, Gatta G, Muti P, EUROCARE Working Group . The prognostic role of gender in survival of adult cancer patients. Eur. J. Cancer 1998; 34: 2271–2278. [DOI] [PubMed] [Google Scholar]
- 33. Zeng H, Zheng R, Guo Y et al Cancer survival in China, 2003‐2005: a population‐based study. Int. J. Cancer 2015; 136: 1921–1930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Data quality indices for esophageal cancer, the Korea Central Cancer Registry, 1999–2013.
Table S2. Age‐standardized esophageal cancer incidence rates per 100 000 and annual percent changes (APC) by subsites, 1999–2013.


