Abstract
Psoriasis is a common complex genetic disease characterized by hyperplasia and inflammation in the skin; however, the relative contributions of epidermal cells and the immune system to disease pathogenesis remain unclear. Linkage studies have defined a psoriasis susceptibility locus (PSORS4) on 1q21, the epidermal differentiation complex, which includes genes for small S100 calcium binding proteins. These proteins are involved in extra- and intracellular signaling during epithelial host defense, linking innate and adaptive immunity. Inflammation-prone psoriatic skin constitutively expresses elevated concentrations of S100A7(psoriasin)/S100A15(koebnerisin) in the epidermis. Here we report that genetically modified mice expressing elevated amounts of doxycycline-regulated mS100a7a15 in skin keratinocytes demonstrated an exaggerated inflammatory response when challenged by exogenous stimuli such as abrasion (Koebner phenomenon). This immune response was characterized by immune cell infiltration and elevated concentrations of Th1 and Th17 proinflammatory cytokines, which have been linked to the pathogenesis of psoriasis and were further amplified upon challenge. Both inflammation priming and amplification required mS100a7a15 binding to the receptor of advanced glycated end products (RAGE). mS100a7a15 potentiated inflammation by acting directly as a chemoattractant for leukocytes, further increasing the number of inflammatory cells infiltrating the skin. Taken together, this study provides a pathogenetic psoriasis model using a psoriasis candidate gene to link the epidermis and innate immune system in inflammation priming, highlighting the S100A7A15-RAGE axis as a potential therapeutic target.
One-sentence summary : Psoriasis candidate genes promote susceptibility to skin inflammation and provide a treatment approach for psoriasis-related inflammation.
Introduction
Psoriasis, one of the most common chronic inflammatory skin diseases, has a strong genetic susceptibility (1, 2). The pathogenesis of psoriasis remains unclear, and it is controversial whether psoriasis results from primary abnormalities in epidermal keratinocytes, from primary dysregulation in the immune system, or from a combination of the two (3, 4). Several lines of evidence suggest the abnormal function of immune cells as a potential cause for psoriasis (5–9). Genetic analysis of psoriasis susceptibility yields association and linkage to loci encoding genes with immune function (10–12). In particular the HLA-C component of the MHC is a strong determinant as are loci that encode IL12B and IL23R (13–15). Coupled to clinical data indicating that drugs targeting effector lymphocytes and antibodies targeting the common p40 subunit of IL-23 and IL-12 are effective anti-psoriatic therapies, the dysregulated immune hypothesis has gained considerable support (16).
Genetic data have also implicated a contribution of epidermal cells to the pathogenesis of psoriasis. The epidermal differentiation locus on 1q21 was an early genetic link to disease susceptibility (PSOR 4), and recent studies have focused on late cornified envelope genes (LCE) in this locus. Among this cluster, deletion of LCE3B and LCE3C is associated with psoriasis susceptibility and the proteins are notably increased in psoriasis lesions (12, 17, 18). Interestingly, deletion of LCE3B and LCE3C is epistatic with the HLA-C genotype, suggesting components of both immune and epidermal function contribute to disease susceptibility.
Transgenic mouse models with epidermal expression of growth and angiogenic factors or signaling molecules shown to be elevated in psoriatic lesions (9, 19–23) develop phenotypes similar to certain aspects of psoriatic skin lesions. A hallmark of psoriatic skin is the increased susceptibility of non-lesional skin to develop inflammatory lesions upon exposure to various environmental triggers, such as inflammatory and mechanical stimuli (Koebner phenomenon). Understanding this phenomenon would help to explain how distinct trigger molecules can lead similarly to full blown disease. Such trigger molecules may interact with genetic components that underlie susceptibility to this disease. However, the functional link to disease susceptibility and disease phenotype is not clear.
S100 proteins are a family of small calcium-binding proteins involved in epithelial host defense through extra- and intracellular signaling linking innate and adaptive immunity (24, 25). Members of the human S100A7 (psoriasin) and S100A15 (koebnerisin) subfamily are upregulated and released during skin inflammation, where the secreted proteins potentiate inflammation by acting as chemokines (26–28). A single mouse ortholog is ancestral to the human S100A7/A15 subfamily (29, 30).
The human S100A7/A15 subfamily is encoded within the psoriasis susceptibility locus (PSORS4, 1q21, Epidermal Differentiation Complex) (18). Here we have investigated a possible functional link between S100A7/S100A15 and skin inflammation susceptibility. Production and release of human S100A7/S100A15 proteins are constitutively enhanced in psoriatic keratinocytes (26, 27). By creating a bitransgenic mouse model, where the single corresponding mouse mS100a7a15 ortholog is conditionally transactivated in mouse epidermis, we recapitulated the situation in psoriatic keratinocytes.
Results
The human S100A7/A15 subfamily is activated in psoriasis
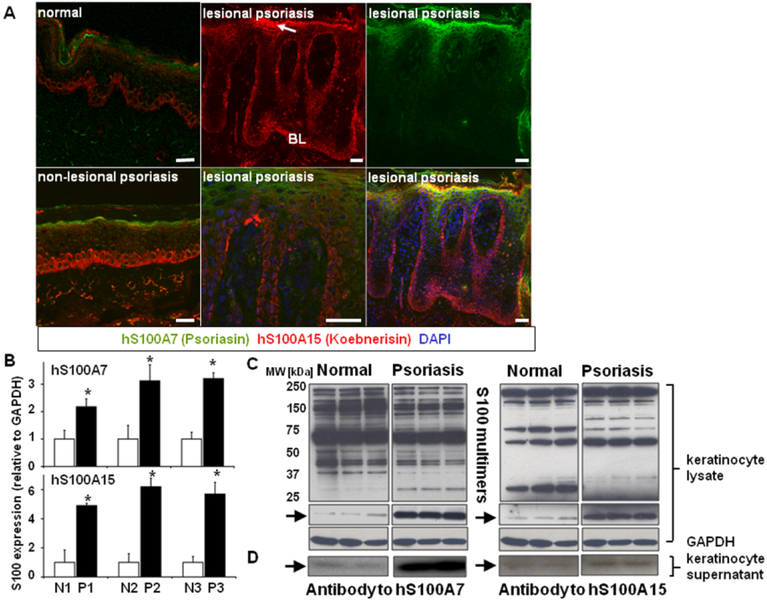
Probing skin sections of biopsies from normal controls and psoriatic patients with specific antibodies to hS100A7 and hS100A15 (26, 27) reveals expanded localization and increased production of both proteins in inflammation-susceptible non-lesional epidermis, which further expands in the lesional thickened psoriatic epidermis (Fig.1A). The cell periphery of subcorneal keratinocytes and the cytoplasm of single basal cells along the dermal-epidermal junction show intense staining (Fig.1A). When deprived of psoriatic environmental/stromal interaction by culture, psoriatic keratinocytes retain intrinsic properties inherently linked to the disease (31–33). Indeed, when cultured, the psoriatic keratinocytes from individual patients also show elevated expression of hS100A7 and hS100A15 transcripts (Fig.1B). Immunoblots of lysates from normal and psoriasis-derived keratinocytes indicate an increased abundance of hS100A7 and hS100A15 protein monomers (arrows, Fig.1C) and specific patterns of higher molecular weight bands characteristic of multimers of these hS100 proteins (34). Furthermore, psoriatic keratinocytes release more proinflammatory hS100A7 and hS100A15 monomers into the culture medium than do normal keratinocytes (Fig.1D, arrow) (24, 28).
Figure 1. Human S100A7/A15 is activated in psoriatic skin.
(A) Immunofluorescent co-staining of healthy (scale bar, 20 μm), non-lesional (scale bar, 20 μm) and lesional psoriatic skin (scale bar, 50 μm) for hS100A7 (green) and hS100A15 (red). Nuclei were stained with DAPI. The images shown illustrate staining of three sections for each of five individuals. (Arrow indicates subcorneal cells; BL=basal layer) (B) Human S100A7 and human S100A15 mRNA transcripts in extracts of normal (N) and psoriatic (P) keratinocytes detected by RTqPCR. Data are expressed as mean +/−SD. (n=3) from three individuals, * P ≤ 0.05. (C) Immunoblots of lysates and supernatants from cultured normal and psoriatic keratinocytes using specific antibodies for hS100A7 or hS100A15. Arrows indicate the migration of the hS100A15 and hS100A7 monomers. (D) Monomers released into the culture supernatant. Blots were exposed for different time intervals to optimize visibility of the S100 multimers or monomers. Shown is a representative pattern of triplicate determinations from a single patient.
mS100a7a15 mediates inflammation as a chemoattractant through interactions with RAGE
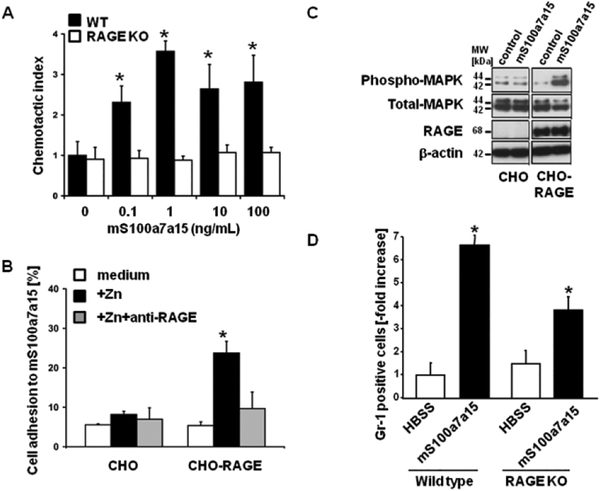
To replicate the biology of S100A7 and S100A15 elevation in human skin, we tested corresponding functions of the single mouse ortholog mS100a7a15 with the goal of using this protein in an animal model. Previously we had shown that both hS100A7 and hS100A15 were chemotactic for leukocytes, and this function of S100A7 was mediated through binding to the receptor of advanced glycated end products (RAGE) (28). Like human S100A7/S100A15, mS100a7a15 attracted leukocytes in a dose-dependent fashion, whereas this chemotaxis was attenuated on leukocytes lacking RAGE (Fig.2A). Further evidence for the interaction of mS100A7/A15 and RAGE comes from studies of CHO cell adhesion. Parental CHO do not express RAGE. When these cells and derivatives reconstituted with RAGE are plated on dishes coated with recombinant mS100a7a15 only RAGE-reconstituted CHO cells adhere (Fig. 2B). This adhesion is disrupted by antibodies against RAGE (Fig. 2B). Further, RAGE-reconstituted CHO cells showed RAGE-dependent activation of MAPK in response to mS100a7a15 (Fig. 2C). mS100a7a15 provoked peritoneal cavity leukocyte infiltration when injected intraperitoneally into C57/BL6 wild type mice (Fig. 2D), and this inflammatory response is substantially attenuated in mice lacking RAGE. These results indicate that mS100a7a15-induced leukocyte chemotaxis is functionally conserved and that it exhibits the same properties as its human S100A7 ortholog (28). Thus, we conclude that the mS100a7a15 could serve as a surrogate for the human proteins to develop a model for S100A7/A15 protein function in skin.
Figure 2. mS100a7a15 exerts its proinflammatory effects through RAGE.
(A) Granulocytes from wild type and RAGE null mice in the upper well of a Boyden chamber were exposed to mS100a7a15 in the lower well. Cells migrating through the filter were stained and counted; *a ratio of migrating cells in treated groups over control (the chemotactic index). Data are expressed as mean +/− SEM (n=3),* P ≤ 0.05. (B) Control or murine RAGE-transfected Chinese hamster ovary (CHO) cells were allowed to adhere to wells coated with mouse S100A7A15 for 60 minutes in the absence and presence of zinc, to enhance S100 RAGE binding. Data are expressed as mean +/− SEM (n=3),* P ≤ 0.05. (C) MAP kinase activation was determined in control or murine RAGE-transfected CHO cells exposed to mS100a7a15 (0.1 ng/mL) for five minutes. Cell lysates were gel-separated and subjected to immunoblotting with the indicated antibodies. (D) Recombinant mS100a7a15 was injected intraperitoneally into C57/BL6 wild-type and RAGE−/− mice and infiltrating leukocytes were aspirated and measured by flow cytometry after 4 hours. Data are expressed as mean +/−SD from five mice, * P ≤ 0.05. Results are representative of three separate experiments.
Epidermal-targeted mS100a7a15 primes skin for inflammation
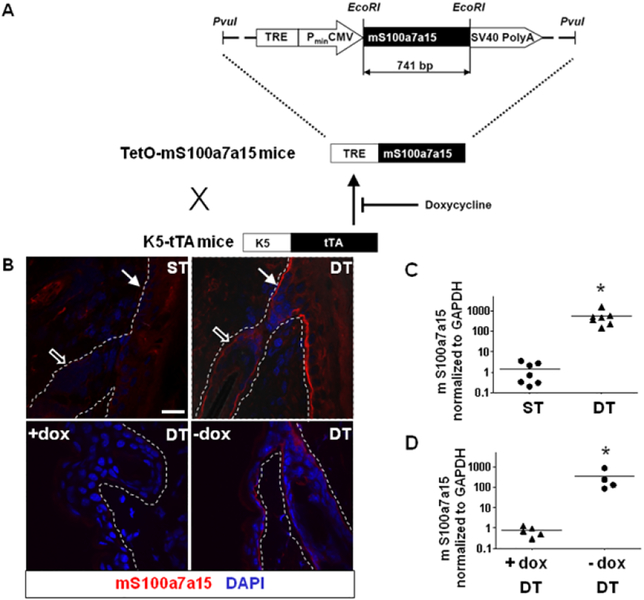
To replicate the activation of human S100A7S100A15 family inherent to psoriatic keratinocytes (Fig.1), the mS100a7a15 ortholog was cloned into the pTRE expression vector to produce TetOmS100a7a15 transgenic mice. These mice were crossed with keratin 5 driven tTA mice (35) to produce K5-tTA/TetOmS100a7a15 double transgenic mice (referred to as tTA/mS100a7a15, DT) (Fig. 3A). Double transgenic mice express abundant mS100a7a15 throughout basal keratinocytes of the epidermis and the outer root sheath of hair follicles (Fig. 3B). Accordingly, elevated expression of transgene and total mS100a7a15 transcripts (Fig. 3C) is detected in skin extracts of tTA/mS100a7a15 mice. mS100a7a15 concentrations were diminished when we switched to doxycycline in the diet of the mice (Fig. 3D), thus establishing a controllable model of mS100a7a15 in skin epithelium resembling the human S100A7/A15 expression in psoriasis.
Figure 3. Mouse S100a7a15 expression is targeted to the skin and controlled by doxycycline.
(A) TetOmS100a7a15 mice were generated using full-length mS100a7a15 cDNA inserted after the TRE-responsive CMV promoter through EcoRI restriction sites (vector pTRE tight). Crossing with mice containing the TRE driven by the keratin 5 promoter (K5-tTA) conditionally targets mS100a7a15 to the basal epidermis (K5-tTA/TetOmS100a7a15) regulated by doxycycline. (B) Representative immunostaining of frozen skin sections from single-transgenic (ST) or double-transgenic (DT) mice for mS100a7a15 in the presence or absence of doxycycline (dox). Follicular (hollow arrows) and interfollicular (solid arrows) epithelium is shown (scale bar: 20 μm); three to four mice per group, four independent experiments. The dotted line indicates the basement membrane. (C, D) RTqPCR analysis performed in duplicate of cDNA from skin of single-transgenic (ST) or double-transgenic (DT) mice in the presence or absence of doxycycline (dox) from the experiments described in (B). Each dot represents an individual mouse and line represents the mean value, * P ≤ 0.05
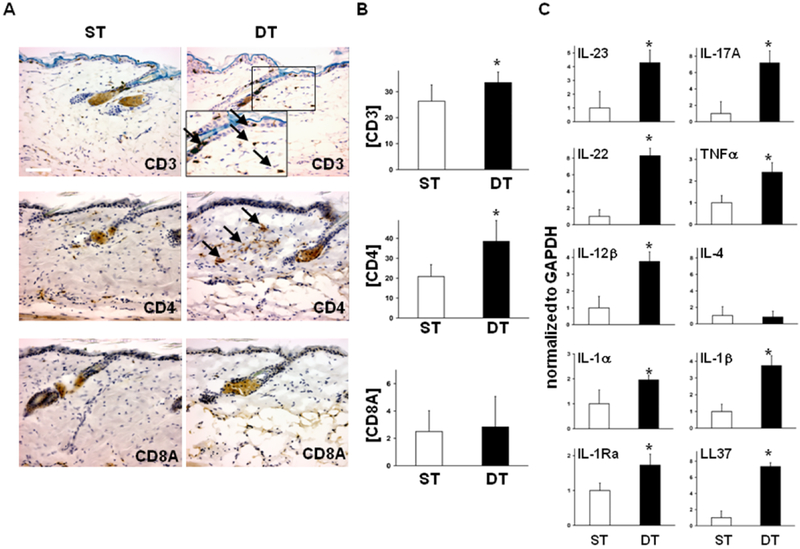
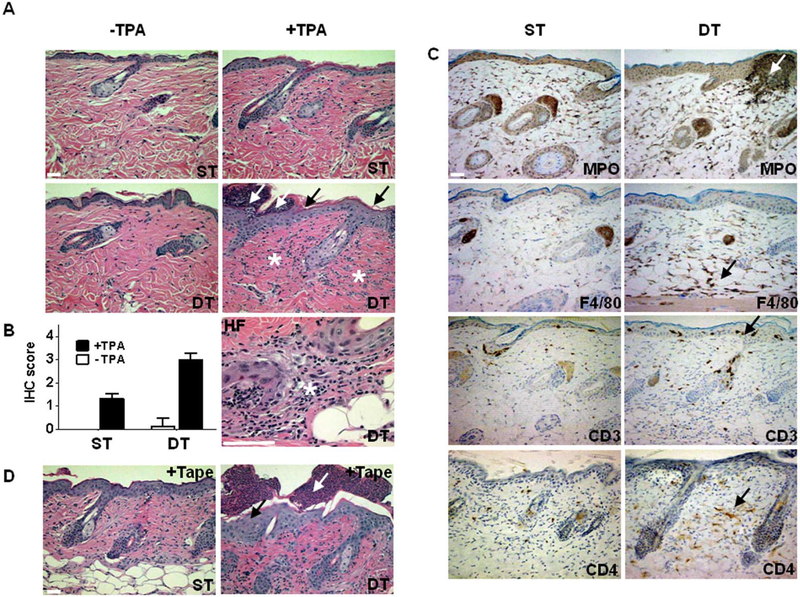
In the absence of doxycycline, tTA/mS100a7a15 mice are viable, and their skin appears normal at birth with no substantial gross alterations found with ageing. Histological evaluation of resting skin from single transgenic (ST) and double transgenic (DT) mice revealed no substantial morphological differences similar to healthy and non-lesional psoriatic skin (Fig. 4A). However, using specific immunohistochemical stains we observed an increased infiltration of CD3+ and CD4+ T lymphocytes in DT non-lesional skin (Fig. 4A, B) and increased transcripts for psoriasis-relevant Th1- and Th17-cytokines as well as cathalecidin/ LL-37 in whole skin lysates relative to GAPDH; IL-4 was barely detectable (Fig. 4C).
Figure 4. Epidermal mS100a7a15 primes the skin for a Th1/Th17 inflammatory response.
(A) Representative immunostaining of sections from resting, uninvolved skin from single-transgenic (ST) and double-transgenic (DT) adult mice for the immunocyte markers CD3, CD4, and CD8A; arrows, positive cells in the epidermis and dermis in DT mice (scale bar, 50μm) (B) Quantification of the immunocyte markers CD3, CD4, and CD8A from resting single-transgenic (ST) or double-transgenic mouse skin (DT). Positive cells were counted in a total of three high powered fields. Data are expressed as mean+/−SD, * P ≤ 0.05. (C) Expression of transcripts for indicated inflammatory markers relative to GAPDH in resting skin from ST and DT mice by RTqPCR analysis. Data are expressed as mean +/−SD (n=6),* P ≤ 0.05.
Because the skin priming pattern is similar to that of inflammation-susceptible non-lesional skin in human psoriasis (36, 37), we challenged tTA/mS100a7a15 mice with pro-inflammatory stimuli. The phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) is a known skin irritant. TPA-treated sites showed a marked epidermal thickening with collections of neutrophils (Kogoj and Munro’s abscesses) in the epidermis and an extensive inflammatory infiltrate in the dermis compared to ST-controls (Fig. 5A, B). TPA-induced skin lesions in the tTA/mS100a7a15 mice shared other histological features of psoriatic inflammation: vasodilatation, perivascular and perifollicular leukocyte infiltrates. CD3/CD4 positive lymphocytes, F4/80 positive macrophages, and MPO positive granulocytes in the epidermis and dermis of tTA/mS100a7a15 were substantially increased (Fig. 5C). To confirm that the changes seen upon TPA challenge were not unique to this stimulus, mouse skin was exfoliated with tape stripping. This stimulus causes perturbation of the skin permeability barrier. Exfoliation is associated with early clinical manifestations of psoriasis as exemplified by the Koebner phenomenon (38). Tape-stripping induces lesions that are exaggerated in tTA/mS100a7a15 mice and similar to those seen with TPA, including enhanced hyperplasia, vasodilatation, invasion of inflammatory cells, and accumulation of neutrophils in the subcorneal epidermis (Munro abscesses) (Fig. 5D).
Figure 5. Epidermal mS100a7a15 increases skin inflammation.
(A) Representative hematoxylin-eosin stained skin sections from single-transgenic (ST) and double-transgenic (DT) adult mice 18h after topical treatment with acetone or TPA. HF, hair follicle; black arrows, acanthosis; white arrows, Munro microabscesses; asterisks, dermal inflammatory infiltrates in DT skin, (scale bar, 50μm) The study involved three to four mice per group and five independent experiments. (B) Quantification of inflammation of single-transgenic (ST) or double-transgenic (DT) mice 18h after acetone or TPA treatment. The IHC score was determined from serial sections of skin from experiments described in (A), stained for specific inflammatory cell subsets and graded 1–4. (C) Representative immunostaining of skin sections from single-transgenic (ST) and double-transgenic (DT) adult mice from experiments described in (A) for leukocyte markers MPO, F4/80, CD3, and CD4 18h after topical treatment with TPA; arrows, accumulation of inflammatory cells, (scale bar, 50μm). (D) Representative hematoxylin-eosin stained skin sections of skin from single-transgenic (ST) and double-transgenic (DT) adult mice 2 days after exfoliation by tape stripping; black arrow, acanthosis; white arrow, Munro microabscesses; (scale bar, 50μm). Exfoliation experiments were independently performed three times with three mice per group.
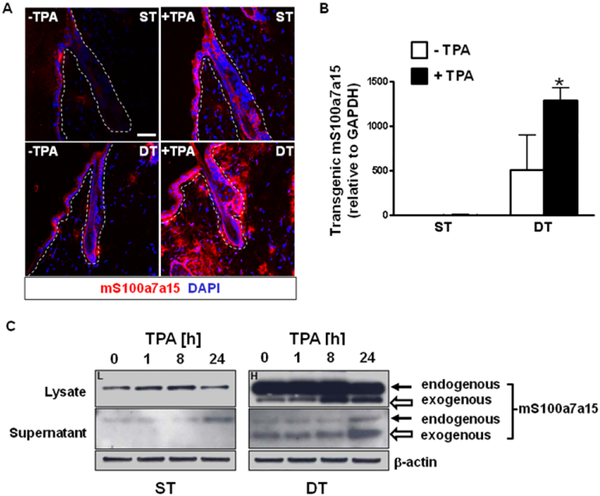
Immunofluorescence staining of TPA-treated skin sections revealed that production of mS100a7a15 protein was enhanced in response to inflammation and particularly pronounced in double transgenic mouse skin with intense epidermal-dermal and perifollicular staining (Fig. 6A). Substantial amounts of the protein appeared to be extracellular in TPA-provoked skin of DT mice. TPA caused a significant increase in the amount of transcripts for the transgene in DT mice (Fig. 6B). Immunoblot analysis of lysates from cultured keratinocytes of both genotypes showed a higher production and release of endogenous mS100a7a15 in response to TPA treatment (Fig. 6C). Transgenic mS100a7a15 protein was also increased in keratinocyte lysates in response to TPA, and substantial amounts of the transgenic protein were released into the culture supernatant, consistent with the extracellular location of the transgenic protein in TPA treated skin (Fig. 6C). Collectively, the data indicate that constitutive elevation of mS100a7a15 in mice increased the sensitivity of the epidermis to inflammation triggered by exposure to external stimuli, similar to the classical clinical feature of psoriasis known as the Koebner phenomenon (39).
Figure 6. An inflammatory stimulus increases mS100a7a15 activation in the transgenic epidermis.
(A) Representative skin sections from single-transgenic (ST) and double-transgenic (DT) adult mice 18h after topical treatment with TPA and stained for mS100a7a15 (red). Nuclei were stained with DAPI (blue), scale bar: 50 μm, three to four mice per group, five independent experiments. (B) RTqPCR analysis using specific primers that amplify transcripts for the transgene. Results represent mean +/− SD (n=5) * P ≤ 0.05. (C) Immunoblots of keratinocyte lysates and supernatants from TPA-treated cultured keratinocytes from single-transgenic (ST) and double-transgenic (DT) mice over a 24 hour timecourse; solid arrow-endogenous mS100a7a15, hollow arrow- transgenic mS100a7a15. Blots were exposed for different time intervals [ST (L=low exposure) and DT (H=high exposure)] to optimize visibility of the endogenous or transgenic mS100a7a15. Results are representative of three independent experiments.
Psoriasis-associated cytokines are induced in the skin of mS100a7a15-expressing mice
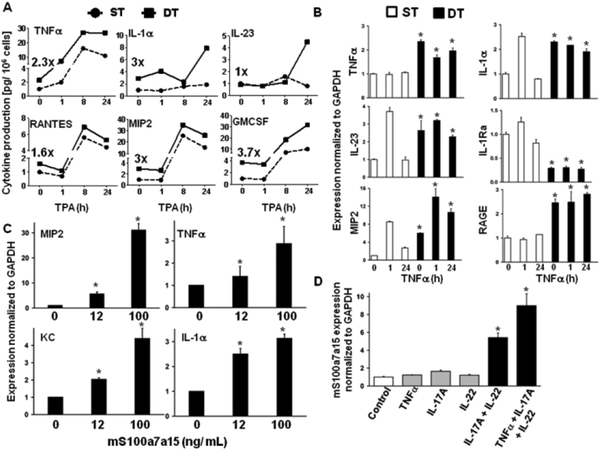
Th1 and Th17 associated cytokines are considered to be critical for the development and maintenance of psoriatic skin lesions (40–42). We tested the contribution of elevated epidermal mS100a7a15 to this environment using cultured keratinocytes. The release of pathogenesis-relevant proinflammatory cytokines involved in Th1 response, Th17- polarization, lymphocyte and granulocyte proliferation and migration was enhanced in mS100a7a15-transgenic keratinocytes and further amplified and sustained upon TPA treatment (Fig. 7A). Furthermore, mouse keratinocytes also produced IL-23, a factor essential to promote and expand the Th17- subtype of T lymphocytes. The production of RANTES and GM-CSF was also amplified by TPA in mS100a7a15 keratinocytes. TNFα is a key inflammatory cytokine in psoriasis (43). Transcripts for key proinflammatory mediators including TNFα itself and IL-1α, IL-23 and MIP2 were elevated by TNFα to a greater extent in double transgenic keratinocytes compared to single transgenic keratinocytes, a response that is similar to the TPA effect (Fig. 7B). Concordantly, transcripts for the anti-inflammatory IL-1 receptor antagonist (IL-1Ra) were suppressed (Fig. 7B). Of particular interest are the elevated amounts of RAGE in transgenic keratinocytes, implicating a positive feedback-loop in the primed keratinocytes. Furthermore, other key mediators of inflammation (MIP2, KC, TNFα, IL-1α) were elevated in wild-type keratinocytes exposed to recombinant mS100a7a15 (Fig. 7C) indicating that extracellularly released mS100a7a15 could mediate production of proinflammatory factors.
Figure 7. mS100a7a15 transgenic keratinocytes are sensitized for expression and release of proinflammatory cytokines.
(A) ELISA for the indicated inflammatory cytokines was performed with supernatant of cultured keratinocytes from single-transgenic (ST) and double-transgenic (DT) mice stimulated with TPA over the indicated time intervals. The numbers indicate the relative difference detected at base line. Data are expressed as mean+/−SD (n = 4) (B) RTqPCR was performed with cDNA from cultured keratinocytes from single-transgenic (ST) and double-transgenic (DT) mice stimulated with TNFα over indicated time. The Y axis documents the expression normalized to GAPDH over ST at baseline. (C) RTqPCR was performed with cDNA from cultured wild-type keratinocytes stimulated with increasing amounts of recombinant mS100a7a15.. (D) RTqPCR for mS100a7a15 performed with cDNA from cultured wild-type keratinocytes stimulated with indicated T cell-derived recombinant proinflammatory cytokines. Data are expressed as mean+/−SEM (n=3–5), * P ≤ 0.05.
Several lines of evidence indicate that lymphocyte-derived Th1 and Th17 cytokines drive the pathogenesis of psoriatic lesions (40, 41). The combination of IL-17A and IL-22 elevated the production of mS100a7a15 in wild-type keratinocytes, and this was further increased by TNFα (Fig. 7D). Together, these data indicate that elevated mS100a7a15 in keratinocytes primed the cells for enhanced production and release of immunotropic cytokines and in return represented a downstream transcriptional target for key Th1 and Th17 cytokines from immunocytes involved in the pathogenesis of cutaneous inflammation. These data suggest an amplification loop between epidermal and immune systems in the pathogenesis of mS100a7a15-mediated inflammation.
TNFα and mS100a7a15-RAGE regulate skin inflammation
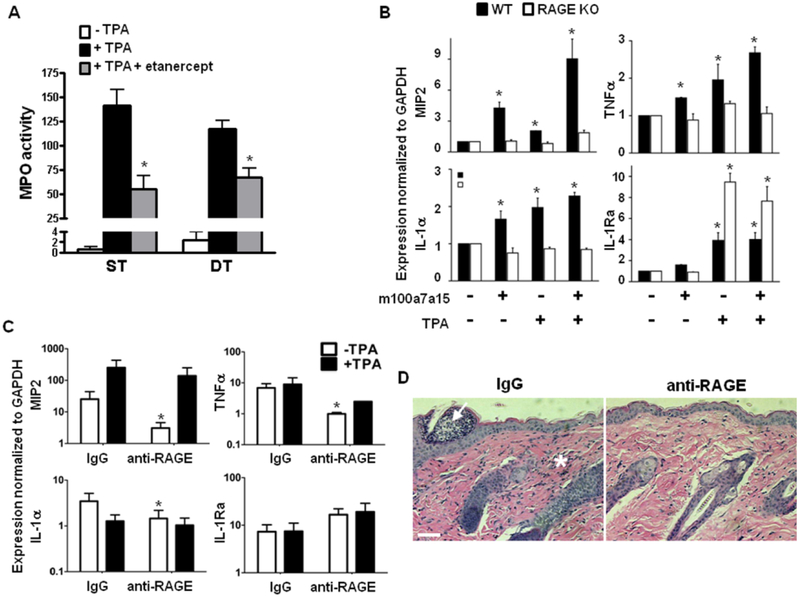
TNFα is a key cytokine mediating the inflammatory milieu of psoriatic skin (16). Our DT data indicated that TNFα and mS100a7a15 crosstalk in skin to regulate other pro-inflammatory factors as well as each other. The relevance of the pro-inflammatory contribution of TNFα in human psoriasis was emphasized by the effectiveness of anti-TNFα therapy (etanercept, Enbrel) to reduce inflammation during disease exacerbations. We tested intraperitoneal etanercept in our ST and DT model as an anti-inflammatory agent to reduce cutaneous myeloperoxidase (MPO) activity after topical TPA. DT mice had constitutively increased MPO activity (Fig. 8A). Eighteen hours after TPA, cutaneous MPO activity was markedly increased in both groups, and pretreatment with etanercept was effective in reducing this response. However, the effectiveness of etanercept was greater in ST mice (61% reduction) than in DT mice (43% reduction) suggesting a more complex inflammatory cascade controls the cutaneous infiltrate when excess S100 proteins contribute to the response. We also tested whether mS100a7a15-mediated keratinocyte priming and amplification of cytokine production was mediated through RAGE (Fig. 8B). The induction of proinflammatory cytokines MIP2, IL-1α and TNFα was amplified by TPA in keratinocytes (Fig. 8B). Both induction and amplification were diminished in the absence of RAGE (44). In contrast, the anti-inflammatory molecule IL-1Ra was induced by TPA and further enhanced in RAGE null keratinocytes, suggesting that RAGE activation was blocking the expression of this anti-inflammatory molecule. Similarly, in vivo systemic treatment with a RAGE neutralizing antibody reduced baseline expression of the same pro- inflammatory cytokines in double-transgenic skin suggesting RAGE-dependent inflammation priming (Fig. 8C). However, anti-RAGE did not significantly alter the amount of IL-1Ra in vivo. Anti-RAGE antibody also suppressed TPA-mediated skin inflammation in mS100a7a15 overexpressing mice consistent with the reduction of several proinflammatory cytokines (Fig. 8C, D). Together these results confirm that mS100a7a15/RAGE activation is an important component of skin inflammation.
Figure 8. TNFα and mS100a7a15-RAGE mediate susceptibility to inflammation.
(A) ST and DT mice (n=5 per group) were either untreated or injected intraperitoneally with 25mg/kg etanercept for 24 hours and again 1 hour before topical application of TPA (5μg). After 18 hours, 8 mm punch biopsies of dorsal skin were lysed and assayed for myeloperoxidase (MPO) activity. Bars= mean +/−SD,* P ≤ 0.05. (B) RTqPCR for inflammatory cytokines with cDNA from cultured wild type (WT) and RAGE null (KO) keratinocytes stimulated with mS100a7a15 and TPA. Data are expressed as mean+/−SD (n=4),* P ≤ 0.05. (C) RTqPCR for inflammatory cytokines from cDNA of total skin from double-transgenic (DT) mice topically treated with TPA for 18h and injected intraperitoneally with antibody to RAGE or control IgG. The data were normalized to mGAPDH expression. Each bar represents mean +/− SD (n=4), * P ≤ 0.05. Data are representative of two independent experiments. (D) Hematoxylin-eosin stained skin sections from double-transgenic (DT) adult mice topically treated with TPA for 18h and injected with antibody to RAGE or control IgG as described in (C) (scale bar, 50 μm). Arrow indicates microabscess; * indicates inflammatory infiltrate.
Discussion
Psoriasis is a prevalent chronic inflammatory skin disease that involves epidermal thickening, hypervascularization and skin inflammation. When exposed to environmental factors, non-lesional psoriatic skin has an increased susceptibility to develop an inflammatory response (Koebner phenomenon). Genetic linkage studies suggest several psoriasis susceptibility loci (45, 46). Members of the human S100A7/S100A15 subfamily are encoded within the psoriasis susceptibility region (PSORS4) on chromosome 1q21 and are enhanced in inflammation-prone psoriatic skin (30, 47). This locus also encodes members of the late cornified envelope family, two of which, LCE3B and LCE3C, are also genetically linked to the disease. In common, the LCE proteins and the S100 proteins are incorporated into cornified envelopes and are integral components of the skin barrier (17, 29). This raises the possibility that one way that these proteins contribute to the disease is through barrier dysfunction, but this awaits additional studies.
We report here that both hS100A7 and hS100A15 are overexpressed and released in psoriatic patients by keratinocytes, which retain intrinsic properties inherently linked to the disease. When active extracellularly, S100A7 potentiates the inflammatory response as a leukocyte chemoattractant through RAGE, which is important in sustaining inflammation (28). Analysis of inducible tTA/mS100a7a15 mice allowed us to conclude that skin targeted overexpression of the corresponding S100a7a15 mouse ortholog primes skin for inflammation resembling a pattern similar to nonlesional psoriatic skin. This inflammation susceptible skin was prone to develop lesions that histologically and molecularly recapitulate key features of psoriatic inflammation. Clinically, skin lesions on tTA/mS100a7a15 mice could be induced with several forms of chemical and mechanical wounding reminiscent of Koebner phenomenon in human psoriasis (39). Thus our present data suggest that elevated expression of mS100a7a15 in keratinocytes, subsequent induction of mS100a7a15-immunotropic cytokines, and enhanced presence of CD3/CD4 positive immunocytes are sufficient for priming and subsequent development of inflammatory psoriatic lesions in transgenic mice.
In turn, mS100a7a15 is a downstream target for key cytokines from Th1 and Th17 immunocytes. This parallels the pathophysiological activation of the human S100A7/S100A15 subfamily by disease-relevant mediators in human psoriasis and models the proposed interaction between epidermal and immune compartment for disease pathogenesis (48)(Fig. 9). The genetic linkage and the activation of the human S100A7A15 homologs in psoriatic keratinocytes support their importance for susceptibility, development, and sustainment of psoriasis.
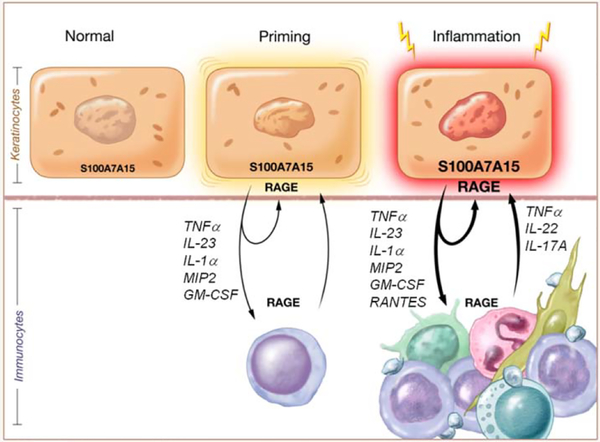
Figure 9.
S100A7A15 primes keratinocytes for skin inflammation Psoriasis is genetically linked to the human S100A7/S100A15subfamily encoded within the psoriasis susceptibility locus at chromosome 1q21. Inflammation-prone psoriatic keratinocytes are characterized by constitutively elevated amounts of S100A7/S100A15. In this psoriasis model, inflammation susceptibility of keratinocytes is mediated by increased production and release of S100A7A15. When extracellular, it drives production of several proinflammatory cytokines (autocrine loop), co-attracts immunocytes (paracrine effect) and subsequently establishes a subtle Th1/Th17 inflammation-prone environment (inflammation priming). Upon Koebnerization through an external stimulus, this proinflammatory cascade is amplified by further increased extracellular S100A7A15 leading to an enhanced inflammatory response through further cytokine production and potentiation of S100A7A15 chemotactic activity (inflammation amplification). Both inflammation priming and amplification are RAGE dependent. Thus, targeting S100-RAGE may provide a therapeutic approach for treatment of susceptibility and inflammation in psoriasis.
The contribution of disturbed epidermis versus altered immune function in the pathogenesis of psoriasis has been elusive in terms of disease etiology (49–51). In the current study, we showed that induction of mS100a7a15 in keratinocytes activates an inflammatory cascade consisting of multiple cytokines of the innate and adaptive immune pathways. These include key cytokines, such as TNFα and IL-23a, which reflect and sustain the Th1 and Th17 polarization important in the pathogenesis of psoriasis (41, 52). In psoriasis, members of the human S100A7A15 family are induced by mediators released from disease-relevant Th1 and Th17 cells infiltrating psoriatic skin (48). It can be hypothesized that the constitutively increased numbers of myeloid and CD3/CD4 positive cells in non-lesional mS100a7a15 overexpressing skin resembles an important susceptibility factor to initiate the psoriatic inflammation (53). This is consistent with the observation that psoriatic conversion takes place preferentially in the graft skin derived from psoriatic patients (7).
Extracellular mS100a7a15 ligand and its receptor RAGE on keratinocytes were required to induce proinflammatory cytokines in an autocrine loop. Exogenous proinflammatory stimuli further amplified their inflammatory response. Thus, our data indicated that the enhanced release of mS100a7a15 in unstimulated skin sensitizes the skin to inflammation (Fig. 3). In addition to the autocrine amplification of inflammation of keratinocytes, our data suggested that the mS100a7a15 further potentiates an inflammatory response as a chemoattractant mainly through a RAGE-dependent paracrine mechanism on leukocytes. Thus, mS100a7a15 mediated inflammation priming and amplification both require RAGE. The evolutionary and functional homology of genomic organization, protein structure and processing, and regulation shown in previous studies predict that this mechanism is conserved with the proposed role of human S100A7 in inflammation (28, 29).
Recently, transgenic psoriasis mouse models have been created that reflect single or combined epidermal, vascular, or inflammatory changes resembling psoriatic lesions (19, 23, 54). Most of these models were constructed using growth stimulatory factors or activated signaling molecules that regulate growth enhancing pathways targeted to the epidermis. They provide a constitutive phenotype. The tTA/mS100a7a15 transgenic model uses a different approach by emphasizing a relevant molecule associated with genetic susceptibility. This model expresses a more florid phenotype after an inflammatory stimulus. In this way the host is primed for disease exacerbation and the transgenic protein functions through a link between the epidermal and immune compartments. Our model suggests a pathogenetic mechanism linking genetic susceptibility and inflammation priming in psoriasis. In this regard, the S100A7/A15 subfamily is an important regulator of factors that are involved in the development of the psoriatic phenotype. The S100A7A15–RAGE axis may represent a model to test drugs that may prevent exacerbations or treat active psoriasis, particularly targeted to RAGE mediated pathways.
Material and Methods
Skin samples
Punch biopsies (5mm) were obtained from patients with psoriasis and healthy volunteers at the Dermatology Department, Dusseldorf University with informed consent.
Skin-targeted mS100a7a15-inducible mice
The full-length mS100a7a15 transcript (AY465109) (24) was subcloned into the pTRE expression vector (Clontech). Founders of transgenic mice were generated in FVB/N background using pronuclear injection (55) and were sequenced to ensure correct genomic introduction. Compared to the native S100A7A15 monomer protein that is mainly expressed in suprabasal cells of the epidermis, the transgene targeted to the basal cells is expressed at a slightly lower molecular weight (29). However, coding sequences of the transgene and wild-type mS100a7a15 among the gene donor and host strains were identical. Saponification of keratinocyte lysate eliminated the molecular weight difference of both native and transgene mS100a7a15, suggesting posttranslation lipidification of mS100A715 in the suprabasal layers (native) compared to basal (transgene).
Exfoliation and TPA treatment
Mice were subjected to tape stripping by using twenty strokes with transparent tape on dorsal skin following hair removal with Nair or were shaved on the dorsal side, and treated for 18h with 5μg/200 uL12-O-tetradecanoylphorbol-13-acetate (TPA) (Alexis). Full thickness skin excisions on the backs of 6–9 week old mice were made after euthanasia by CO2.
Myeloperoxidase assay
Back whole skin samples were used for myeloperoxidase assay as previously described (33). In brief, samples were homogenized in potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide (HTAB), sonicated, and freeze-thawed three times, after which sonication was repeated. The suspension was centrifuged at 40,000 g for 15 min, and 10 μl of supernatant was added to 290 μl of potassium phosphate buffer (pH 6.0) containing 0.167 mg/ml o-dianisidine dihydrochloride (Sigma) and 0.0005% hydrogen peroxide. Changes in OD were monitored at 460 nm at 25°C, over a 4 minute period.
Regulation of mS100a7a15 ligand transactivaton and receptor activity
To suppress the mS100a7a15 transgene, mice were fed with doxycycline chow (1000ppm) five days prior to treatment. In some experiments mice were injected intraperitoneally with 100μg/mL anti-RAGE antibodies (R&D Systems) or IgG isotype control antibodies. To neutralize TNFα pharmacologically in mice, a first dose of etanercept (25mg/kg) was administrated intraperitoneally (i.p) and 24h later a second identical treatment was performed 1h prior to TPA painting. Etanercept was reconstituted at a concentration of 25mg/ml as suggested by the manufacturer (Amgen).
Protein expression and purification
mS100a7a15 cDNA was placed into a baculovirus expression system (Invitrogen). Purified recombinant mS100a7a15 (Mr ~ 12 kDa) was purged of endotoxin by chromatography onto Detoxigel columns (Pierce) with endotoxin amounts < 0.05 EU/ μg protein (29).
Cell culture
Normal and psoriatic keratinocytes were cultured in standard keratinocyte growth medium (Cascade Biologics). Keratinocytes isolated from newborn tTA/mS100a7a15, single-transgenic, RAGE null or wildtype control mice were cultured as described and experiments were performed under pre-confluent conditions (56). CHO cells were cultured in HAM’s F12 (Invitrogen) with Zeocin (200 μg/ mL) for selection of RAGE transfectants (57).
Cytokine stimulation assays
Keratinocytes starved overnight were treated with recombinant mS100a7a15 alone or in combination with TPA (10ng/mL), TNFα (20ng/mL), IL-17A (20 ng/mL), or IL-22 (200 ng/mL) (Preprotech) for the indicated time. Cell free supernatants of cultures of mouse keratinocytes were subjected to Muliplex-ELISA assays (Searchlight) to determine amounts of secreted proinflammatory cytokines (Pierce).
RTqPCR
cDNA was analyzed by the SYBR green fluorescein RQPCR detection system (Biorad). Resulting CT values using primers for total mS100a7a15 (29), mS100A715 transgene (forward: 5’GCTCGTTTAGTGAACCGTCAG3’, reverse: 5’ GGAGTCCTCCACTGGTGTGT3’, tTA (35), GMCSF (58), MIP2, KC, TNFα, IL-23, IL-22, IL-17A, IL-12a, IL-1α, IL-1β, IL-1Ra, IL-4, LL-37/CAMP2 (Qiagen), RAGE (Super Array), hS100A7, hS100A15 were normalized to GAPDH using the deltaCT method (34). Some experiments were also normalized to L32 to confirm GAPDH results. Each cDNA was analyzed in duplicate or triplicates, and error bars represent SEM.
Immunoassays, immunostaining, inflammation assessment
Total cell lysates of cultured cells were gel-separated, transferred and incubated with primary antibody (anti-mS100a7a15, 1ug/mL (24); anti-hS100A15, 1μg/mL (custom); anti-hS100A7 antibody (abcam), 1μg/mL; anti-phospho-ERK1/2, anti-total ERK1/2, 1:1000, (Cell Signaling); anti-RAGE, 1:250, (Santa Cruz); anti-β-actin, 1:20,000 and anti-GAPDH (Chemicon) overnight, followed by secondary antibody for 1 hour.
Acetone-fixed frozen sections were blocked and incubated overnight with anti-mS100a7a15, anti-hS100A15 or anti-hS100A7 (5 μg/ml each) followed by Donkey anti-rabbit cy3 (1:250) or donkey anti-mouse FITC (1:250) (Jackson Laboratory), mounted and analyzed by confocal microscopy (Zeiss). Immunohistochemistry for leukocyte subsets was performed on paraffin sections using primary antibody (anti-CD3, anti-CD4, anti-CD8A, anti-MPO, and anti-F4/80). Intensity of inflammation was quantified by a grading score (0= none, 1 = minimal, 2 = mild, 3 = moderate, 4 = severe; IHC score)
Chemotaxis assays
Suspended percoll-purified (95%) granulocytes from wild-type and RAGE null (KO) mice (59) were exposed to mS100a7a15 and MIP-2 (PreproTech) in a Boyden chamber separated by a polycarbonate filter (pore size: 5 μm). Migrated cells were counted and the averages were expressed as ratio (chemotactic index) of the numbers of cells in the treated sample versus control (spontaneous migration).
Cell adhesion assays
Control vector or RAGE transfected CHO cells were allowed to adhere to immobilized mS100a7a15 (10 μg/ mL each) and to bovine serum albumin (as control) in the absence or presence of neutralizing anti-RAGE (5 μg/ mL, R&D).
In vivo peritonitis model
C57/Bl6 RAGE null mice were characterized previously (60). HBSS (Invitrogen) or recombinant mS100a7a15 (20 μg in 1 mL HBSS / mouse) was injected intraperitoneally into C57/Bl6 wild-type and RAGE null mice. Aspirated peritoneal fluid was stained and analyzed as previously described (28).
Statistical analysis
Data were analyzed for statistical significance by Student’s T-test and ANOVA with post-hoc Bonferroni analysis using SPSS (version 15.0) with P ≤ 0.05 being considered significant.
Acknowledgements:
We are grateful to S. Wincovich and S. Garfield for skillful assistance with confocal microscopy and B. Taylor for advice and help with the flow cytometry. We thank W. Gillette and J. Hartley for recombinant proteins, P. Goldsmith and M. Gunsior for generation of S100 antibodies, R. Smith for quantitative cell analysis, and H. Rager for endotoxin analysis. We particularly thank A. Ryscavage for maintaining the mouse colonies and M. Anver and S. Lawrence for support in evaluation of the skin sections. We thank C. Voscopoulous for skillful help with the immunostainings and E.Y. Choi for help with granulocyte isolations. We further thank P. Nawroth and A. Bierhaus for providing RAGE−/− mice. We thank J. Anders for excellent editorial assistance.
Funding: This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Ronald Wolf is funded by the German Research Foundation (DFG, Wo843/3–1).
Footnotes
Competing Interests: The authors declare that they have no competing interests.
References and Notes
- 1.Gottlieb AB, Bos JD, Recombinantly engineered human proteins: transforming the treatment of psoriasis. Clin.Immunol. 105, 105 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, Barker JN, Pathogenesis and clinical features of psoriasis. Lancet 370, 263 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Nickoloff BJ, Keratinocytes regain momentum as instigators of cutaneous inflammation. Trends Mol.Med. 12, 102 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Lowes MA, Bowcock AM, Krueger JG, Pathogenesis and therapy of psoriasis. Nature 445, 866 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Gudjonsson JE, Johnston A, Sigmundsdottir H, Valdimarsson H, Immunopathogenic mechanisms in psoriasis. Clin.Exp.Immunol. 135, 1 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eedy DJ, Burrows D, Bridges JM, Jones FG, Clearance of severe psoriasis after allogenic bone marrow transplantation. BMJ 300, 908 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrone-Smith T, Nickoloff BJ, Dermal injection of immunocytes induces psoriasis. J.Clin.Invest 98, 1878 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilhar A, David M, Ullmann Y, Berkutski T, Kalish RS, T-lymphocyte dependence of psoriatic pathology in human psoriatic skin grafted to SCID mice. J.Invest Dermatol. 109, 283 (1997). [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M, Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J.Exp.Med. 202, 135 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowcock AM, Krueger JG, Getting under the skin: the immunogenetics of psoriasis. Nat.Rev.Immunol. 5, 699 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, Wise C, Miner A, Malloy MJ, Pullinger CR, Kane JP, Saccone S, Worthington J, Bruce I, Kwok PY, Menter A, Krueger J, Barton A, Saccone NL, Bowcock AM, A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS.Genet. 4, e1000041 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang XJ, Huang W, Yang S, Sun LD, Zhang FY, Zhu QX, Zhang FR, Zhang C, Du WH, Pu XM, Li H, Xiao FL, Wang ZX, Cui Y, Hao F, Zheng J, Yang XQ, Cheng H, He CD, Liu XM, Xu LM, Zheng HF, Zhang SM, Zhang JZ, Wang HY, Cheng YL, Ji BH, Fang QY, Li YZ, Zhou FS, Han JW, Quan C, Chen B, Liu JL, Lin D, Fan L, Zhang AP, Liu SX, Yang CJ, Wang PG, Zhou WM, Lin GS, Wu WD, Fan X, Gao M, Yang BQ, Lu WS, Zhang Z, Zhu KJ, Shen SK, Li M, Zhang XY, Cao TT, Ren W, Zhang X, He J, Tang XF, Lu S, Yang JQ, Zhang L, Wang DN, Yuan F, Yin XY, Huang HJ, Wang HF, Lin XY, Liu JJ, Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat.Genet. 41, 205 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Matthews D, Fry L, Powles A, Weber J, McCarthy M, Fisher E, Davies K, Williamson R, Evidence that a locus for familial psoriasis maps to chromosome 4q. Nat Genet 14, 231 (1996); published online EpubOct (10.1038/ng1096–231). [DOI] [PubMed] [Google Scholar]
- 14.Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, Browne J, Barber R, Terwilliger J, Lathrop GM, Barker JN, Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome-wide search in psoriasis. Hum Mol Genet 6, 813 (1997); published online EpubMay (dda094 [pii]). [DOI] [PubMed] [Google Scholar]
- 15.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, Gudjonsson JE, Li Y, Tejasvi T, Feng BJ, Ruether A, Schreiber S, Weichenthal M, Gladman D, Rahman P, Schrodi SJ, Prahalad S, Guthery SL, Fischer J, Liao W, Kwok PY, Menter A, Lathrop GM, Wise CA, Begovich AB, Voorhees JJ, Elder JT, Krueger GG, Bowcock AM, Abecasis GR, Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat.Genet 41, 199 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR, Nair RP, Molecular dissection of psoriasis: integrating genetics and biology. J Invest Dermatol 130, 1213 (2010). [DOI] [PubMed] [Google Scholar]
- 17.de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, Escaramis G, Ballana E, Martin-Ezquerra G, den Heijer M, Kamsteeg M, Joosten I, Eichler EE, Lazaro C, Pujol RM, Armengol L, Abecasis G, Elder JT, Novelli G, Armour JA, Kwok PY, Bowcock A, Schalkwijk J, Estivill X, Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 41, 211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Semprini S, Capon F, Tacconelli A, Giardina E, Orecchia A, Mingarelli R, Gobello T, Zambruno G, Botta A, Fabrizi G, Novelli G, Evidence for differential S100 gene over-expression in psoriatic patients from genetically heterogeneous pedigrees. Hum.Genet. 111, 310 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Schon MP, Animal models of psoriasis - what can we learn from them? J.Invest Dermatol. 112, 405 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Li AG, Wang D, Feng XH, Wang XJ, Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 23, 1770 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS, Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102, 161 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF, Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437, 369 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J, Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nature Medicine 11, 43 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Donato R, Intracellular and extracellular roles of S100 proteins. Microsc.Res.Tech. 60, 540 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Eckert RL, Broome AM, Ruse M, Robinson N, Ryan D, Lee K, S100 proteins in the epidermis. J.Invest Dermatol. 123, 23 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, Kiil J, Walbum E, Andersen AH, Basse B, Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J.Invest Dermatol 97, 701 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Wolf R, Mirmohammadsadegh A, Walz M, Lysa B, Tartler U, Remus R, Hengge U, Michel G, Ruzicka T, Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 17, 1969 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Wolf R, Howard OMZ, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH, Chemotactic activity of S100A7 (psoriasin) is mediated by RAGE and potentiates inflammation with highly homologous but functionally distinct S100A15. J.Immunol 181, 1499 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf R, Voscopoulos CJ, FitzGerald PC, Goldsmith P, Cataisson C, Gunsior M, Walz M, Ruzicka T, Yuspa SH, The mouse S100A15 ortholog parallels genomic organization, structure, gene expression, and protein-processing pattern of the human S100A7/A15 subfamily during epidermal maturation. J.Invest Dermatol. 126, 1600 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Wolf R, Ruzicka T, Yuspa SH, Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function. Amino Acids, (2010); published online EpubJul 2 (10.1007/s00726-010-0666-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickoloff BJ, Mitra RS, Varani J, Dixit VM, Polverini PJ, Aberrant production of interleukin-8 and thrombospondin-1 by psoriatic keratinocytes mediates angiogenesis. American Journal of Pathology 144, 820 (1994). [PMC free article] [PubMed] [Google Scholar]
- 32.Giustizieri ML, Mascia F, Frezzolini A, De Pita O, Chinni LM, Giannetti A, Girolomoni G, Pastore S, Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J.Allergy Clin.Immunol. 107, 871 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Cataisson C, Pearson AJ, Tsien MZ, Mascia F, Gao JL, Pastore S, Yuspa SH, CXCR2 ligands and G-CSF mediate PKCalpha-induced intraepidermal inflammation. J.Clin.Invest 116, 2757 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf R, Voscopoulos C, Winston J, Dharamsi A, Goldsmith P, Gunsior M, Vonderhaar BK, Olson M, Watson PH, Yuspa SH, Highly homologous hS100A15 and hS100A7 proteins are distinctly expressed in normal breast tissue and breast cancer. Cancer Letters 277, 101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond I, Owolabi T, Marco M, Lam C, Glick A, Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J.Invest Dermatol. 115, 788 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Uyemura K, Yamamura M, Fivenson DF, Modlin RL, Nickoloff BJ, The cytokine network in lesional and lesion-free psoriatic skin is characterized by a T-helper type 1 cell-mediated response. J.Invest Dermatol. 101, 701 (1993). [DOI] [PubMed] [Google Scholar]
- 37.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, Gorman DM, Smith K, de Waal MR, Kastelein RA, McClanahan TK, Bowman EP, IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J.Exp.Med. 203, 2577 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghadially R, Reed JT, Elias PM, Stratum corneum structure and function correlates with phenotype in psoriasis. J.Invest Dermatol. 107, 558 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Miller RA, The Koebner phenomenon. Int.J.Dermatol. 21, 192 (1982). [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W, Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Sabat R, Philipp S, Hoflich C, Kreutzer S, Wallace E, Asadullah K, Volk HD, Sterry W, Wolk K, Immunopathogenesis of psoriasis. Exp.Dermatol 16, 779 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal MR, Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat.Immunol. 8, 950 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Fantuzzi F, Del GM, Gisondi P, Girolomoni G, Targeting tumor necrosis factor alpha in psoriasis and psoriatic arthritis. Expert.Opin.Ther.Targets 12, 1085 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P, RAGE signaling sustains inflammation and promotes tumor development. J.Exp.Med. 205, 275 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhalerao J, Bowcock AM, The genetics of psoriasis: a complex disorder of the skin and immune system. Hum.Mol.Genet. 7, 1537 (1998). [DOI] [PubMed] [Google Scholar]
- 46.Elder JT, Nair RP, Voorhees JJ, Epidemiology and the genetics of psoriasis. J.Invest Dermatol. 102, 24S (1994). [DOI] [PubMed] [Google Scholar]
- 47.Marenholz I, Volz A, Ziegler A, Davies A, Ragoussis I, Korge BP, Mischke D, Genetic analysis of the epidermal differentiation complex (EDC) on human chromosome 1q21: chromosomal orientation, new markers, and a 6-Mb YAC contig. Genomics 37, 295 (1996). [DOI] [PubMed] [Google Scholar]
- 48.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA, Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J.Exp.Med 203, 2271 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos JD, de Rie MA, Teunissen MB, Piskin G, Psoriasis: dysregulation of innate immunity. Br.J.Dermatol. 152, 1098 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L, Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens? Immunol.Today 16, 145 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Christophers E, The immunopathology of psoriasis. Int.Arch.Allergy Immunol. 110, 199 (1996). [DOI] [PubMed] [Google Scholar]
- 52.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, Khatcherian A, Gonzalez J, Pierson KC, White TR, Pensabene C, Coats I, Novitskaya I, Lowes MA, Krueger JG, Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br.J.Dermatol. 159, 1092 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nickoloff BJ, Wrone-Smith T, Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. American Journal of Pathology 155, 145 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gudjonsson JE, Johnston A, Dyson M, Valdimarsson H, Elder JT, Mouse models of psoriasis. J.Invest Dermatol. 127, 1292 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Labosky PA, Barlow DP, Hogan BL, Mouse embryonic germ (EG) cell lines: transmission through the germline and differences in the methylation imprint of insulin-like growth factor 2 receptor (Igf2r) gene compared with embryonic stem (ES) cell lines. Development 120, 3197 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Lichti U, Anders J, Yuspa SH, Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat.Protoc 3, 799 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, Chavakis T, A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 26, 1129 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cataisson C, Pearson AJ, Torgerson S, Nedospasov SA, Yuspa SH, Protein kinase C alpha -mediated chemotaxis of neutrophils requires NF- kappa B activity but is independent of TNF alpha signaling in mouse skin In vivo. J.Immunol. 174, 1686 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T, Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 322, 1101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chavakis T, Bierhaus A, Al Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP, The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J.Exp.Med 198, 1507 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]