Abstract
To improve access to high-quality HIV care in underserved regions of Western Washington (WA) State, we collaborated with the WA State Department of Health (DOH) and community partners to launch four satellite HIV clinics. Here, we describe this innovative clinical care model, present an estimate of costs, and evaluate patient care outcomes, including virologic suppression rates. To accomplish this, we assessed virologic suppression rates 12 months before and 12 months after the satellite clinics opened, comparing people living with HIV (PLWH) who enrolled in the satellite clinics versus all PLWH in the same regions who did not. We also determined virologic suppression rates in 2015 comparing satellite clinic versus non-satellite clinic patients and compared care quality indicators between the satellite clinics and the parent academic clinic. Results demonstrate that the change in virologic suppression rate 12 months before to 12 months after the satellite clinics opened was higher for patients who enrolled in the satellite clinics compared to all those in the same region who did not (18% versus 6%, p < 0.001). Virologic suppression in 2015 was significantly higher for satellite clinic than non-satellite clinic patients at three of four sites. Care quality indicators were met at a high level at the satellite clinics, comparable to the parent academic clinic. Overall, through community partnerships and WA DOH support, the satellite clinic program increased access to best practice HIV care and improved virologic suppression rates in difficult-to-reach areas. This model could be expanded to other regions with inadequate access to HIV practitioners, though financial support is necessary.
Keywords: HIV, AIDS, delivery of healthcare, continuity of care, rural
Introduction
The epidemic of HIV in the United States (US) is shifting more to non-metropolitan areas, where people living with HIV (PLWH) access healthcare less and have lower rates of retention in care and viral suppression (Nelson et al., 2016; Schafer et al., 2017; Uphold & Mkanta, 2005). Many factors lead PLWH in less populated communities to seek care at long distances from home, including geographic isolation, provider discrimination, stigma, confidentiality concerns, financial constraints, and lack of confidence in local medical systems (Heckman et al., 1998; Mainous & Matheny, 1996; McKinney, 2002; Pellowski, 2013; Schur et al., 2002). Insufficient access to mental health and addiction treatment services is common in these areas (Wood, 2008). These barriers may lead to delayed receipt of new HIV treatments, fewer provider visits, higher likelihood of receiving care from a lowvolume HIV provider, and higher probability of AIDS diagnosis at baseline or progression to AIDS (Cohn et al., 2001; Lahey et al., 2007; Lopes, Eron, Mugavero, Miller, & Napravnik, 2017; Ohl et al., 2010; Ohl et al., 2013; Weissman et al., 2015; Wilson et al., 2011). This, along with the predicted attrition of the HIV provider workforce and growing size of the population of PLWH, underscores the widening gap between HIV care capacity and patient demands and the need for innovative ways to improve access to high-quality HIV care, especially outside of major cities (Weiser et al., 2016).
To help address HIV care disparities in Western Washington (WA) State and to improve HIV treatment outcomes in under-resourced areas, we launched a satellite clinic program in 2007. To establish the satellite clinics, we targeted areas of the state in which patients lacked local access to providers with HIV training, including rural and urban underserved regions. We developed partnerships with local community-based clinics or health districts, which provide the facility, support staff, supplies, and basic equipment for the clinic sessions. Clinical HIV specialists from our academic medical center (Harborview Medical Center, a component of University of Washington Hospitals) travel to the community sites weekly or biweekly to deliver direct patient care. The Washington State Department of Health (WA DOH) provides funding to both Harbor-view Medical Center and three of the four local community partners to support the program.
Here, we describe this innovative clinical care model and present patient care outcomes, including virologic suppression rates and other quality of care indicators, and illustrate challenges we encountered plus solutions we devised in order to sustain the program. The goal is to inform other regions and institutions that struggle to deliver care to PLWH outside major cities.
Methods
For the purpose of describing the satellite clinics, we use the term “rural” to describe a city with population below 50,000, “urban” as population 50,000 to 499,999, and “metropolitan” as above 500,000 (Nelson et al., 2016). Population figures are based on 2014 US Census data (United States Census Bureau, 2014). Descriptions of the satellite clinic patient population, such as the number who were previously out of care or who previously drove long distances to care, come from a one-time survey administered to all PLWH who enroll at one of the satellite clinics at their first visit to that clinic.
For the analysis, we assessed the patient and community-level impact of the satellite clinic system through three methods: (1) an assessment of HIV RNA (viral load, VL) suppression rates one year before and one year after each clinic opened, comparing PLWH who attended each satellite clinic to all PLWH living in the same region who did not attend the satellite clinics, (2) a snapshot of the proportion of PLWH with VL suppression in 2015 comparing those in each region who attended the satellite clinics versus those who did not, and (3) a comparison of quality of care indicators, including performance on various HIV primary care-related clinical measures at the satellite clinics versus at the academic clinic in Seattle (Harborview Medical Center’s HIV Clinic, also known as Madison Clinic and hereafter referred to as Madison Clinic).
For the first objective, an assessment of VL suppression rates before and after each satellite clinic opened, we identified satellite clinic patients from our electronic medical record with documented medical visits within 12 months of the date when each satellite clinic opened. Using the state HIV surveillance registry, the Enhanced HIV/AIDS Reporting System (eHARS), we evaluated the proportion of these patients with virologic suppression (at or below 200 copies/mL), using the last VL within the 12 months before and 12 months after the clinics opened. As a comparison group, we used the eHARS registry to identify all PLWH residing in the same regions who did not attend the Madison satellite clinics and evaluated VL suppression using measurements from the same timeframes. We defined the region as the same county for three of the satellite clinics and as the same city for the Federal Way clinic (because the Federal Way clinic lies in King County, which also includes the city of Seattle, where Madison Clinic is located). Both satellite and non-satellite clinic patient groups were limited to individuals who were diagnosed at least 12 months before the clinics opened in order to exclude newly-diagnosed patients who may not yet be taking ART. We calculated the difference in these proportions of VL suppression (12 months before and 12 months after clinic opening) for the two groups using the 2-sample Chi-squared test. Surveillance data for this analysis and subsequent objectives were based on cases and laboratory results reported to WA DOH as of 31 August 2016.
For the second objective, a snapshot of VL suppression rates, we identified satellite clinic patients who had at least one documented clinic visit in 2015. Using eHARS, we evaluated the proportion of these patients with VL suppression (HIV RNA below 200 copies/mL) compared to all PLWH in the same region who were not satellite clinic patients. Both groups were limited to individuals diagnosed before 1 January 2015. We compared the proportion of VL suppression between these groups, as well as baseline characteristics, using the Chi-squared test.
For the third objective, an evaluation of clinical quality of care indicators, we compared satellite clinic patients to those followed at the parent Madison Clinic in Seattle. Because we did not have access to full clinical data for non-Madison patients in the satellite clinic regions, we did not include that group in this comparison. For Madison satellite patients and Madison patients in Seattle, we assessed the proportion who received or achieved the following healthcare indicators: prescription of antiretroviral therapy (ART), VL suppression (HIV RNA below 200 copies/mL), lipid screening, hepatitis B virus (HBV) and hepatitis C virus (HCV) serologic testing, HBV vaccination (for anti-HBV surface antibody-negative persons), tetanus-diptheria-pertussis (TDaP) vaccination, and pneumococcal vaccination. We evaluated these outcomes among those patients who attended two or more medical visits within an 18-month time period (1 January 2015 to 30 June 2016) at one of the satellite clinics or at Madison Clinic. To examine retention in care, we identified individuals with at least one provider visit in the first six-month period (1 January 2015 to 30 June 2015) and considered them to be retained in care if they had a subsequent visit within the next 12 months (1 July 2015 to 30 June 2016).
Finally, to provide a description of program costs, we reviewed expenses and revenue for one of the satellite clinics (Bremerton, Kitsap County) for one fiscal year (2015 to 2016) and summarized these budget figures.
This study was considered minimal risk and thus exempt from IRB review.
Results
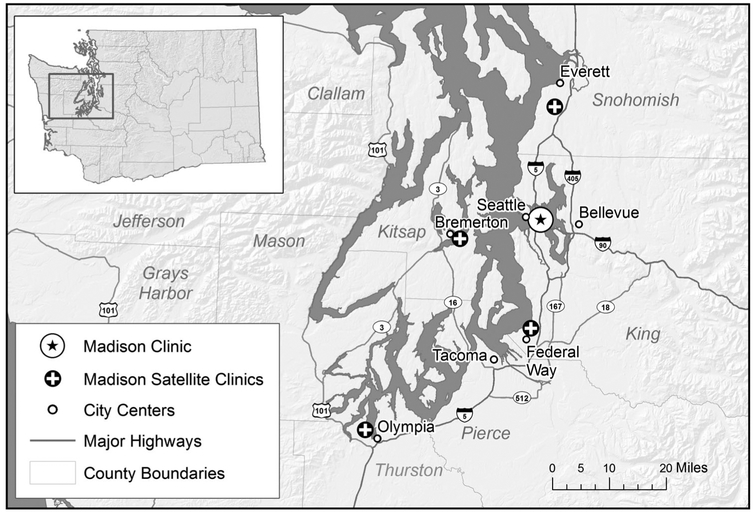
To date, we have established four satellite clinics by partnering with distinct partners (Figure 1) in four different communities surrounding metropolitan Seattle. Two of the clinics are located in rural regions and two in urban cities with limited access to HIV care. The first clinic launched in 2007 in Bremerton, WA (population 38,572) through a partnership with the Kitsap Public Health District. To reach this clinic, an HIV specialist crosses a large body of water (the Puget Sound) by a one-hour ferry ride. The second clinic launched in 2008 in Everett, WA (population 106,736) in partnership with Community Health Centers of Snohomish County. Subsequently, in 2014, we established a third clinic in Olympia, WA (population 49,218) in partnership with SeaMar Community Health Centers and a fourth clinic in Federal Way, WA (population 93,425) in collaboration with the University of Washington Neighborhood Clinic Network. The satellite clinics are located a mean 35.4 miles from Seattle (range 23.2 to 60.9 miles). We hold weekly, day-long clinic sessions at three of the clinics and biweekly sessions at the Olympia clinic.
Figure 1.
Map of WA state satellite clinics.
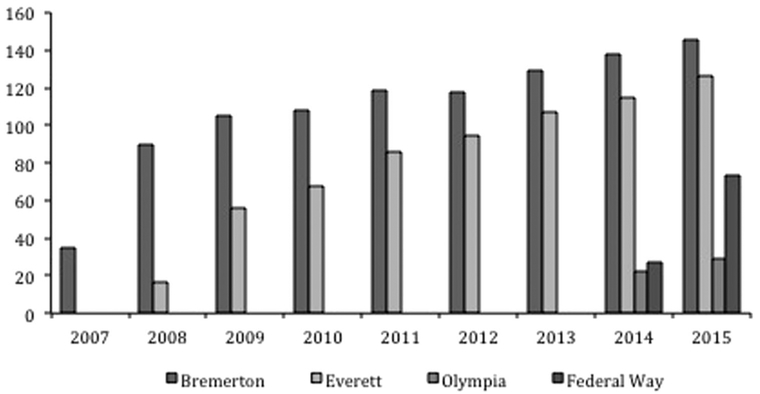
Since the satellite clinics started, 694 patients have enrolled and completed 5162 visits (as of 12/31/2015). Enrollment increased steadily in the years since each clinic was established (Figure 2). Based on patient survey data (completed by 477 patients; response rate 69%), as of 1 July 2016 the satellite clinic attendees included 28 patients who had never previously sought care for HIV infection and 58 patients who had been out of care for more than 12 months. Thirty-nine newly diagnosed patients were linked to care at one of the satellite clinics. Travel distance was reduced for 125 patients to less than 25 miles, including for 64 patients who had previously traveled greater than 50 miles to receive care.
Figure 2.
Satellite clinic patient enrollment over time.
Demographic data for satellite clinic patients as compared to non-satellite clinic patients living in the same regions, pooled for the four counties in 2015, are presented in Table 1. Satellite and non-satellite patients were comparable in gender, age, race/ethnicity, and CD4 counts (the difference in race/ethnicity met criteria for statistical significance, but the absolute difference between the categories of race/ethnicity were small). A higher prevalence of satellite clinic patients reported a history of injection drug use compared with non-satellite clinic patients (25% versus 15%).
Table 1.
Demographic characteristics of people living with HIV (PLWH) attending Madison satellite clinics versus non-Madison satellite clinic patients in same regions in 2015.
| Madison Satellite | Non-Satellitea | ||||
|---|---|---|---|---|---|
| Number | Percent | Number | Percent | P value | |
| Total | 362 | 100% | 1498 | 100% | |
| Sex at birth | |||||
| Male | 287 | 79% | 1220 | 81% | |
| Female | 75 | 21% | 278 | 19% | 0.35 |
| Current age | |||||
| <20 | 1 | 0% | 20 | 1% | |
| 20–29 | 31 | 9% | 83 | 6% | |
| 30–39 | 64 | 18% | 234 | 16% | |
| 40–49 | 104 | 29% | 421 | 28% | |
| 50–59 | 114 | 31% | 510 | 34% | |
| ≥60 | 48 | 13% | 230 | 15% | 0.09 |
| Race and Hispanic origin | |||||
| White | 219 | 60% | 981 | 65% | |
| Black | 58 | 16% | 270 | 18% | |
| Hispanic (all races) | 46 | 13% | 151 | 10% | |
| Otherb | 39 | 11% | 96 | 6% | 0.01 |
| HIV transmission category | |||||
| Men who have sex with men (MSM) | 185 | 51% | 842 | 56% | |
| Injection drug use (IDU) | 40 | 11% | 87 | 6% | |
| MSM and IDU | 51 | 14% | 128 | 9% | |
| Heterosexual | 57 | 16% | 230 | 15% | |
| Otherc | 29 | 8% | 211 | 14% | <0.001 |
| CD4 count (cells/mm3)d | |||||
| <200 | 120 | 33% | 454 | 30% | |
| 200–349 | 52 | 14% | 235 | 16% | |
| 350–499 | 59 | 16% | 245 | 16% | |
| ≥500 | 130 | 36% | 511 | 34% | 0.79 |
This includes Kitsap County, Snohomish County, Thurston County, and Federal Way (City). Based on HIV surveillance data reported to WA DOH through August 31, 2016.
Asian, Native Hawaiian/Pacific Islander, American Indian/Alaska Native.
Mother-to-child transmission, blood transfusion, no identified risk.
First available CD4 in 2015, among 361 satellite patients and 1445 non-satellite patients.
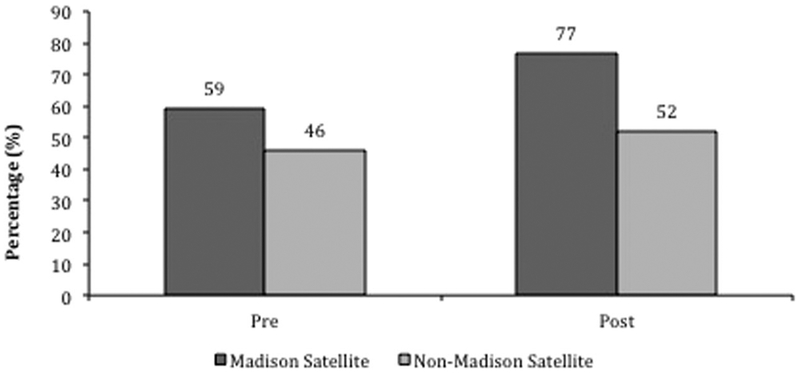
Collectively, at 12 months prior to each of the satellite clinics opening, the proportion of PLWH with VL at or below 200 copies/mL was 59% for those who enrolled in the satellite clinics versus 46% for those in the same regions who did not enroll; 12 months after the launch of the satellite clinics, the proportion with VL suppression increased to 77% versus 52%, respectively (net change in rates of VL suppression 18% for PLWH who enrolled in the satellite clinics versus 6% for those who did not; p < 0.001 by Chi-Square test; Figure 3).
Figure 3.
Viral load suppression 12 months pre and 12 months post satellite clinic launch, comparing PLWH who enrolled at the satellite clinics to all those PLWH in the same regions who did not (net change in proportion with VL suppression 18% for patients who enrolled in the satellite clinics versus 6% for those who did not; p < 0.001 by Chi-Square test).
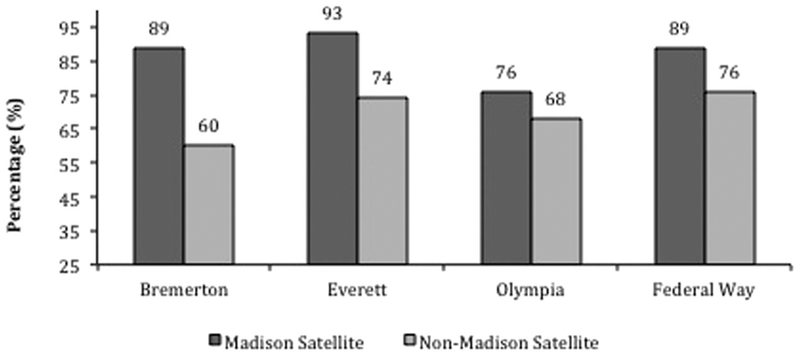
A snapshot of VL suppression rates in 2015 comparing PLWH in each region who attended the satellite clinics versus those who did not showed the following rates of VL suppression: 89% versus 60% for Bremerton (p < 0.001), 93% versus 74% for Everett (p < 0.001), 76% versus 68% for Olympia (p = 0.44), and 89% versus 79% for Federal Way (p = 0.017; Figure 4).
Figure 4.
Viral load suppression as of 2015 comparing PLWH attending satellite clinics versus all PLWH in the same regions not attending satellite clinics (p < 0.001 for Bremerton, p < 0.001 for Everett, p = 0.44 for Olympia, and p = 0.017 for Federal Way p = 0.017).
Among PLWH who attended two or more medical visits within an 18-month time period (1 January 2015 to 30 June 2016) at one of the satellite clinics or at Madison Clinic, the proportion who met a variety of quality of care indicators including immunization and screening was similar for patients attending the satellite clinics and those attending the parent academic clinic in Seattle (Table 2).
Table 2.
Quality of care measures comparing satellite clinics to parent academic HIV clinic (Madison Clinic), 2015–2016.
| Quality Metric | ||
|---|---|---|
| Satellite Clinics (n = 364) | Madison Clinic (n = 2649) | |
| ART (ever prescribed) | 92% | 98% |
| Viral load suppression (past 18 months) | 93% | 88% |
| Lipid screening (past 18 months) | 77% | 68% |
| Hepatitis C screening (ever) | 87% | 95% |
| Hepatitis B screening (ever) | 87% | 93% |
| TDaP vaccine (ever) | 68% | 75% |
| Pneumococcal vaccine (PCV-13 or PPSV-23 ever) | 81% | 88% |
| Satellite Clinics (n = 89) | Seattle Clinic (n = 676) | |
| Hepatitis B vaccine (for those with surface antibody titer < 10 IU) | 62% | 52% |
| Satellite Clinic (n = 319) | Seattle Clinic (n = 2265) | |
| Retention in care (>2 visits, follow-up within 12 months) | 91% | 92% |
Abbreviations: ART = antiretroviral therapy; TDaP = tetanus-diptheria-pertussis; PCV-13 = 13-valent pneumococcal conjugate vaccine; PPSV-23 = 23-valent pneumococcal polysaccharide vaccine; IU = international units.
The primary costs incurred by the University of Washington and Harborview Medical Center for operating the satellite clinics are the costs of salaries and benefits for providers and support staff. The principal source of revenue is financial support from WA DOH. A financial summary of one clinic demonstrates that costs would not be covered without this financial support (Table 3).
Table 3.
Financial analysis of one HIV satellite clinic (expenses and revenue for the Bremerton clinic for the 2015–2016 fiscal year).
| $/Year | |
|---|---|
| Costs | |
| Salaries and benefits (0.2 MD, 0.3 RN, 0.5 MA, 0.275 admin support) | 134,636 |
| Meds supplies, intranet, transportation, space rental | 3270 |
| Overhead | 23,186 |
| Total costs | 161,092 |
| Revenue | |
| Financial support from DOH | 164,468 |
| Profees | 27,891 |
| Total revenue | 192,359 |
Discussion
One of the US National HIV/AIDS Strategy goals is to increase the percentage of PLWH with virologic suppression to over 80% in all regions (National HIV/AIDS Strategy for the United States: Updated to 2020 2015). To accomplish this, access to comprehensive care and well-trained providers must be a priority, including for PLWH living outside major cities. Here, we present a description and evaluation of a program that extends HIV care to rural and urban-underserved regions in Western WA via academic satellite clinics, one of the first programs of its kind documented in a developed country. Our principal findings are that: (1) partnership between an academic medical center and multiple community entities, with fiscal support from a state department of health, reduced geographic barriers to care, (2) implementation of a decentralized clinic system led to improved virologic suppression rates in under-resourced regions of the state, and (3) quality of care at the remote clinics is high and comparable to the metropolitan academic HIV clinic.
These findings have implications for systems of HIV care on a large scale. Nationally, linkage to and retention in care are two principal challenges in HIV medicine. Improving these measures would lead to higher treatment and VL suppression rates and could dramatically decrease HIV transmissions, deaths, and costs of care (Sabin et al., 2017; Shah, Risher, Berry, & Dowdy, 2016). A recent US-based study reported that retention in care and VL suppression rates are significantly lower in rural and urban areas compared to metropolitan areas (Nelson et al., 2016). Furthermore, rising numbers of PLWH are living in non-metropolitan areas (Schafer et al., 2017). Our findings suggest that a sustainable satellite clinic system that brings HIV expertise to non-metropolitan communities significantly improves VL suppression rates. We found improvement in the proportion with VL suppression by 12 months after the satellite clinics opened and a significantly higher proportion with VL suppression in a 2015 snapshot for those PLWH who were engaged at the satellite clinics as compared with PLWH in the same regions who were not. Viral suppression rates were above US National HIV/AIDS Strategy and CDC 2015 goals (80% or higher) at three of four satellite clinics, whereas rates in the same counties for PLWH not engaged with the satellite clinics fell below this goal in every region. The proportion of PLWH retained in care at the satellite clinics also exceeded national targets (Centers for Disease Control and Prevention National HIV Prevention Progress Report, 2015; National HIV/AIDS Strategy for the United States: Updated to 2020, 2015).
We attribute the success of the satellite clinic program to the delivery of care to the communities where patients reside, thus overcoming geographic isolation and transportation barriers. For example, prior to the Bremerton clinic opening, patients in that region had to travel to Bremerton, catch a ferry to Seattle, make their way to the county hospital, and then complete the return trip – a costly journey that took many hours. We have also learned that the success of our program relies heavily on close relationships with community partners and establishing a secure funding mechanism. Our analysis demonstrates that clinical revenues from the satellite clinic system are not self-sustaining and the program would not be viable without close collaboration and financial support from the state DOH.
Our satellite HIV clinics have faced a number of challenges since their inception. Some of the most significant hurdles involved integrating into the local communities and developing an efficient means for obtaining local referrals and procedures, including radiologic imaging (which is unavailable at three of four satellite clinics), coordinating local hospitalizations, and addressing urgent care needs on days when the clinics were not in session. Access to local mental health resources in these regions is difficult. Furthermore, the rapid growth of the satellite clinics led to extended wait times for provider visits, and the need for preventive services for partners and other HIV-uninfected at-risk individuals quickly became evident. Additionally, the clinics utilize a variety of electronic health record (EHR) systems and some have their own credentialing processes.
We addressed these challenges in a number of ways. We collaborated with local primary care providers (PCP’s) who helped care for patients on days when the satellite clinics were not in session. To handle increased demand, we added a second HIV specialist provider two days per month and a medical assistant weekly at two of the satellite clinics. We opened all of the clinics to partner and preventive services, including HIV testing and pre-exposure prophylaxis (PrEP), and we worked closely with local case managers to coordinate referrals. We also developed a plan to institute tele-psychiatry consultations for one of the satellite clinics where access to mental health resources is especially problematic. Finally, we collaborated with local partners at each of the non-University of Washington network clinics to make a plan that would allow for patient documentation to occur in the Harborview EHR and also remain visible in the local EHR. An added benefit of the satellite clinic system has been enhanced educational opportunities for students and trainees. Medical students and residents now accompany faculty to the satellite clinics regularly and experience the inherent differences of providing HIV primary care in community-based settings versus at a large academic medical center.
The satellite clinic model has limitations. There is a geographic limitation based on feasible travel for providers. We feel this satellite clinic model could be effectively combined with provider-to-patient telemedicine visits to extend the reach and benefits and we are exploring this option. Another ongoing need is improving the capacity of local providers to deliver quality HIV preventive and treatment services. We have given trainings related to HIV care and prevention to staff at the satellite clinic sites, distributed resources related to PrEP, and supported PrEP initiation by PCP’s. In this way, connections made at the satellite clinics have helped to increase capacity of local providers. We also believe the satellite clinic model is complemented well by programs that support care capacity through longitudinal distance mentoring of community practitioners, such as Project ECHO (a telehealth program that creates virtual communities of practice for providers across broad regions). Through collaborations at the satellite clinic sites, we have helped multiple PCP’s and other staff members enroll in our HIV and hepatitis C Project ECHO programs (Scott et al., 2012; Wood et al., 2016).
Limitations of this study include the cross-sectional nature and reliance on surveillance data, which may have missing information and may misrepresent where PLWH resided within a given time period. The analysis may not fully account for unmeasured confounders. For the quality of care metrics, some values may be underestimates, especially for satellite clinic patients, who may have received vaccines or other measures from outside providers. Additionally, while we were able to summarize cost and revenue, we were not able to assess value for money or cost effectiveness (Drummond, 2005).
In summary, while specialized care is often localized to metropolitan hubs, we found that a satellite clinic system can reach PLWH in underserved areas and affect clinically important outcomes. While much has been studied about decentralized care in resource-limited countries, data for programs to address disparities in care in better-resourced nations are scant. Given recent data showing increased proportions of PLWH residing in less-populated areas, worse outcomes along the continuum of care in rural regions, plus recent HIV outbreaks in rural parts of the US, these findings are especially noteworthy. This model could be replicated and expanded in other regions that struggle to provide HIV care to under-resourced communities.
Acknowledgments
The authors would like to thank the Washington State Department of Health for ongoing support and collaboration, as well as our multiple community partners: Kitsap Public Health District, Community Health Centers of Snohomish County, SeaMar Community Health Centers, and the University of Washington Neighborhood Clinic Network. We are grateful for the outstanding nurses, case managers, and other staff at all of the sites who help make this program possible.
Funding
The satellite HIV clinic system described here is supported by a contract with the Washington State Department of Health (contract #N21329).
Footnotes
Data in this manuscript were presented at the ID Week 2016 Conference in New Orleans, LA, USA (Oct. 26–30, 2016). Title: “Washington State Satellite HIV Clinic Program: Delivering Highly Effective Decentralized Care for Patients in Underserved Communities.” Abstract #499.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Centers for Disease Control and Prevention (CDC) National HIV Prevention Progress Report. (2015). https://www.cdc.gov/hiv/pdf/policies/progressreports/cdc-hiv-nationalprogressreport.pdf
- Cohn SE, Berk ML, Berry SH, Duan N, Frenkel MR, Klein JD, & Bozzette SA (2001). The care of HIV-infected adults in rural areas of the United States. JAIDS Journal of Acquired Immune Deficiency Syndromes, 28(4), 385–392. [DOI] [PubMed] [Google Scholar]
- Drummond M (2005). Methods for the economic evaluation of health care programmes (3rd ed.). Oxford: Oxford University Press. [Google Scholar]
- Heckman TG, Somlai AM, Peters J, Walker J, Otto-Salaj L, Galdabini CA, & Kelly JA (1998). Barriers to care among persons living with HIV/AIDS in urban and rural areas. AIDS Care, 10(3), 365–375. [DOI] [PubMed] [Google Scholar]
- Lahey T, Lin M, Marsh B, Curtin J, Wood K, Eccles B, & von Reyn C (2007). Increased mortality in rural patients with HIV in New England. AIDS Research and Human Retroviruses, 23(5), 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes BLW, Eron JJ Jr., Mugavero MJ, Miller WC, & Napravnik S (2017). HIV care initiation delay among rural residents in the Southeastern United States, 1996 to 2012. JAIDS Journal of Acquired Immune Deficiency Syndromes, 76(2), 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous AG 3rd, & Matheny SC (1996). Rural human immunodeficiency virus health service provision. Indications of rural-urban travel for care. Archives of Family Medicine, 5(8), 469–473. [DOI] [PubMed] [Google Scholar]
- McKinney MM (2002). Variations in rural AIDS epidemiology and service delivery models in the United States. The Journal of Rural Health, 18(3), 455–466. [DOI] [PubMed] [Google Scholar]
- National HIV/AIDS Strategy for the United States: Updated to 2020. (2015). https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf
- Nelson JA, Kinder A, Johnson AS, Hall HI, Hu X, Sweet D, … Harris J (2016). Differences in selected HIV care continuum outcomes among people residing in rural, urban, and metropolitan areas- 28 US jurisdictions. The Journal of Rural Health. [Epub ahead of print]. doi: 10.111/jrh.12208 [DOI] [PubMed] [Google Scholar]
- Ohl M, Lund B, Belperio PS, Goetz MB, Rimland D, Richardson K, & Vaughan-Sarrazin M (2013). Rural residence and adoption of a novel HIV therapy in a national, equal-access healthcare system. AIDS and Behavior, 17(1), 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl M, Tate J, Duggal M, Skanderson M, Scotch M, Kaboli P, & Justice A (2010). Rural residence is associated with delayed care entry and increased mortality among veterans with human immunodeficiency virus infection. Medical Care, 48(12), 1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellowski JA (2013). Barriers to care for rural people living with HIV: A review of domestic research and health care models. Journal of the Association of Nurses in AIDS Care, 24(5), 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin CA, Howarth A, Jose S, Hill T, Apea V, Morris S, & Burns F (2017). Association between engagement in care and mortality in HIV-positive persons: A cohort study. AIDS, 31(5), 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer KR, Albrecht H, Dillingham R, Hogg RS, Jaworsky D, Kasper K, & Ohl ME (2017). The continuum of HIV care in rural communities in the United States and Canada: What is known and future directions. JAIDS Journal of Acquired Immune Deficiency Syndromes, 75(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur CL, Berk ML, Dunbar JR, Shapiro MF, Cohn SE, & Bozzette SA (2002). Where to seek care: An examination of people in rural areas with HIV/AIDS. The Journal of Rural Health, 18(2), 337–347. [DOI] [PubMed] [Google Scholar]
- Scott JD, Unruh KT, Catlin MC, Merrill JO, Tauben DJ, Rosenblatt R, & Spach DH (2012). Project ECHO: A model for complex, chronic care in the Pacific Northwest region of the United States. Journal of Telemedicine and Telecare, 18(8), 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Risher K, Berry SA, & Dowdy DW (2016). The epidemiologic and economic impact of improving HIV testing, linkage, and retention in care in the United States. Clinical Infectious Diseases, 62(2), 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Census Bureau. (2014). https://www.census.gov/programs-surveys/acs/news/data-releases/2014.html
- Uphold CR, & Mkanta WN (2005). Review: Use of health care services among persons living with HIV infection: State of the science and future directions. AIDS Patient Care and STDs, 19(8), 473–485. [DOI] [PubMed] [Google Scholar]
- Weiser J, Beer L, West BT, Duke CC, Gremel GW, & Skarbinski J (2016). Qualifications, demographics, satisfaction, and future capacity of the HIV care provider workforce in the United States, 2013–2014. Clinical Infectious Diseases, 63(7), 966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman S, Duffus WA, Iyer M, Chakraborty H, Samantapudi AV, & Albrecht H (2015). Rural-urban differences in HIV viral loads and progression to AIDS among new HIV cases. Southern Medical Journal, 108(3), 180–188. [DOI] [PubMed] [Google Scholar]
- Wilson LE, Korthuis T, Fleishman JA, Conviser R, Lawrence PB, Moore RD, & Gebo KA (2011). HIV-related medical service use by rural/urban residents: A multistate perspective. AIDS Care, 23(8), 971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SA (2008). Health care services for HIV-positive substance abusers in a rural setting: An innovative program. Social Work in Health Care, 47(2), 108–121. [DOI] [PubMed] [Google Scholar]
- Wood BR, Unruh KT, Martinez-Paz N, Annese M, Ramers CB, Harrington RD, & Spach DH (2016). Impact of a telehealth program that delivers remote consultation and longitudinal mentorship to community HIV providers. Open Forum Infectious Diseases, 3(3), ofw123 (eCollection). [DOI] [PMC free article] [PubMed] [Google Scholar]