Abstract
Objective:
The cost-effectiveness of the recently-introduced generic celecoxib in knee OA has not been examined.
Method:
We used the Osteoarthritis Policy (OAPol) Model, a validated computer simulation of knee OA, to evaluate long-term clinical outcomes, costs, and cost-effectiveness of generic celecoxib in persons with knee OA. We examined eight treatment strategies consisting of generic celecoxib, over-the-counter (OTC) naproxen, or prescription naproxen, with or without prescription or OTC proton-pump-inhibitors (PPIs) to reduce gastrointestinal (GI) toxicity. In the base case, we assumed that annual cost was $130 for OTC naproxen, $360 for prescription naproxen, and $880 for generic celecoxib. We considered a willingness-to-pay threshold of $100,000 per quality-adjusted life year (QALY) and discounted costs and benefits at 3% annually. In sensitivity analyses we varied celecoxib toxicity, discontinuation, cost, and pain level.
Results:
In the base case analysis of the high pain cohort (WOMAC 50), celecoxib had an incremental cost-effectiveness ratio (ICER) of $284,630/QALY compared with OTC naproxen. Only under highly favorable cost, toxicity, and discontinuation assumptions (e.g., annual cost below $360, combined with a reduction in the cardiovascular (CV) event rates below baseline values) was celecoxib likely to be cost-effective. Celecoxib might also be cost-effective at an annual cost of $600 if CV toxicity were eliminated completely. In subjects with moderate pain (WOMAC 30), at the base case CV event rate of 0.2%, generic celecoxib was only cost-effective at the lowest plausible cost ($190).
Conclusion:
In knee OA patients with no comorbidities, generic celecoxib is not cost-effective at its current price.
Keywords: osteoarthritis, cost-effectiveness, Celecoxib, NSAIDs
Introduction
Knee osteoarthritis (OA) is a costly, prevalent, and debilitating disease.1,2 Nonsteroidal-anti-inflammatory drugs (NSAIDs) are frequently used for pain relief in patients with knee OA.3–6 However, NSAIDs are associated with non-trivial toxicities, most notably gastrointestinal (GI) complications, including severe bleeding.7–12 Selective NSAIDs that target cyclo-oxegenase-2 (COX-2) have been associated with reduced GI toxicity compared to non-selective NSAIDs.13 Celecoxib, a COX-2 selective NSAID, was first marketed for OA pain under the brand-name Celebrex in the late 1990s. After initial FDA approval, numerous studies found increased rates of cardiovascular (CV) complications associated with the use of Celebrex and other COX-2 selective NSAIDs.13–16 Despite higher CV toxicity, Celebrex was found to be cost-effective in cohorts at high risk of gastrointestinal complications.17,18 These analyses either assumed that the cost of Celebrex was similar to the cost of non-selective NSAIDs in the Veterans’ Administration system or they used Canadian drug pricing.17,18 However, in the general population, due to the combination of expensive brand name pricing (up to $3,700 annually) and elevated CV toxicity, both U.S.-based and international analyses found that Celebrex was associated with lower quality-adjusted life expectancy (QALE) and greater costs compared to conventional NSAIDs.6,18–20
Generic celecoxib was approved by the U.S. FDA in 2014, reducing the annual cost from a brand name average of over $3,500 to a generic price of $880 (assuming a daily dose of 200mg).21 Additionally, the PRECISION (Prospective Randomized Evaluation of Celecoxib Integrated Safety Versus Ibuprofen and Naproxen) trial, mandated by the FDA to assess the cardiovascular safety of celecoxib, found that in a cohort at high risk of cardiovascular complications, celecoxib was non-inferior to naproxen and ibuprofen with regard to CV toxicity; 4.2% of participants experienced a major adverse CV event while on celecoxib compared to 4.3% on naproxen.22 The changes in price and new data on celecoxib’s toxicity raise the question of whether generic celecoxib might now be cost-effective compared to traditional NSAIDs.
Our goal was to examine the cost-effectiveness of generic celecoxib compared to naproxen with or without the addition of PPIs. We chose naproxen as a comparator because it is one of the most commonly used NSAIDs among patients with knee OA, has been shown to be as effective as celecoxib for improving knee OA pain, and carries a lower risk of GI events compared to other non-selective NSAIDs.21,29,30 This analysis of celecoxib provides a framework for practitioners and payers to understand the toxicity profile, efficacy, and costs that could make generic celecoxib a cost-effective knee OA treatment.
Methods
Analytic Overview
The Osteoarthritis Policy (OAPol) Model is a validated, state-transition, computer simulation model of the natural history and management of knee OA.1,6,23 We used the OAPol Model to evaluate the cost-effectiveness of generic celecoxib in the treatment of knee OA. We focused on knee OA patients without major comorbidities, as this is a population that uses NSAIDs extensively. The primary outcomes were quality-adjusted life years (QALYs), lifetime medical costs, and incremental cost-effectiveness ratios (ICERs). ICERs were calculated as the ratio of change in costs to change in QALYs of two treatment strategies. We discounted costs and QALYs by 3% annually.24 All costs are reported in 2015 USD, and analyses were conducted from a healthcare sector perspective. We conducted sensitivity analyses varying discontinuation, toxicity, and costs to address the impact of data uncertainty on our findings. A strategy was labeled “dominated” if it resulted in both higher costs and lower QALYs compared to any other strategy or combination of other strategies. Strategies were compared incrementally in order of cost to calculate the ICER and whether or not that regimen was dominated.24
The OAPol Model
The OAPol Model generates cohorts of knee OA patients (termed model subjects) based on user-specified demographic and clinical characteristics, including age, sex, body mass index (BMI), and knee OA structural and pain severity. Using Monte Carlo simulation, the model tracks each patient’s progress annually as he or she transitions through different health states that capture changes in knee OA severity and symptoms. The details of the OAPol Model have been published previously.1,6 Each year, patients incur costs associated with OA management as well as non-OA medical costs and quality of life (QoL) decrements or improvements related to changes in pain severity. Background (non-OA related) medical costs are stratified by age and sex.
After subjects are assigned to a knee OA treatment, they continue that treatment regimen until one of the following events occur: major toxicity, discontinuation due to lack of efficacy, voluntary discontinuation due to another reason, or death. Major toxicities include severe side effects that carry a risk of death. Lack of treatment efficacy is defined by failure to relieve pain in the first or subsequent years of treatment. Voluntary discontinuation accounts for subjects who discontinue the treatment due to reasons other than major toxicity or lack of efficacy, such as unpleasant side effects. Once subjects have stopped a particular regimen, they will never return to that regimen. The next OA treatment assigned will be the next treatment depicted in the treatment sequence (Figure 1). The cost of each pharmacologic treatment strategy includes physician office visits, lab tests, and medication, as detailed in previous work.6
Figure 1:

Subjects enter the OAPol Model with knee OA pain and are treated with one of the eight NSAID regimens evaluated (celecoxib, over-the-counter naproxen, or prescription naproxen, with or without PPIs). After failure of the NSAID regimen, subjects may progress to corticosteroid injections, then total knee arthroplasty (TKA), and possibly a revision TKA. Subjects may remain on each treatment for multiple years before progressing to the next state. Death can occur at any year in the model, and between each treatment, subjects can take acetaminophen for pain relief.
Each subject in the model is followed until death, which is governed by mortality rates from US 2011 Life Tables. Results from a large number of individual simulations are aggregated in order to obtain stable estimates of the outcomes of interest and their variance.
Treatment Strategies
We examined eight treatment strategies: 1) OTC naproxen, 2) OTC naproxen and OTC PPIs, 3) prescription naproxen, 4) prescription naproxen and OTC PPIs, 5) prescription naproxen and prescription PPIs, 6) celecoxib, 7) celecoxib and OTC PPIs, and 8) celecoxib and prescription PPIs. For celecoxib, we considered generic formulations and for naproxen we modeled prescription pricing after clinical use. In each of the strategies, upon discontinuing the NSAID-containing regimen, subjects became eligible for corticosteroid injections. After injections, subjects could either undergo total knee arthroplasty (TKA) immediately or take acetaminophen or opioids as needed for pain relief until they became eligible and willing to undergo TKA. TKA recipients could undergo revision TKA upon failure of the primary TKA (Figure 1).
Model Inputs
Cohort Characteristics
For each strategy, we simulated two cohorts of patients with symptomatic knee OA, one with high levels of pain and one with moderate pain, each with no prevalent comorbidities. Both cohorts had a mean age of 65 (SD 5), and a starting mean WOMAC Pain (scale 0–100, 100 the worst) of either 50 (high pain) or 30 (moderate pain). The standard deviation of the distributions of pain scores for both pain groups was 15. Neither cohort had any pre-existing comorbid conditions. Race/ethnicity, obesity, and sex distributions were derived from the National Health Interview Survey (NHIS) 2012 cohort with diagnosed knee OA.25 We derived quality of life utilities for knee osteoarthritis based on WOMAC pain, obesity, and age, deriving weights from data from the Osteoarthritis Initiative (OAI) by applying a regression model proposed by Brazier et al. 2004 (Table 1).26,27
Table 1.
Model Inputs
| Cohort Characteristics | ||||||||
| Demographics | ||||||||
| Parameter | Mean (SD) | Data Source | ||||||
| Mean Age (years) | 65 (5) | Assumption | ||||||
| Percent Female | 59% | US Census Bureau 201245 | ||||||
| WOMAC* Pain - High Pain Cohort |
50(15) | NHIS 201225 | ||||||
| WOMAC* Pain- Moderate Pain Cohort |
30(15) | NHIS 201225 | ||||||
| BMI (kg/m2) | 31(7) | NHANES 09–1046 | ||||||
| Quality of Life Weights by Age, Obesity, and WOMAC Pain (rate on a scale of 01) | ||||||||
| WOMAC Pain | Non-obese | Data Source | ||||||
| 45–54 | 55–64 | 65–74 | 75+ | Derived applying regression model proposed by Brazier et al 200424 to OAI26 data |
||||
| 1–16 | 0.82 | 0.82 | 0.85 | 0.83 | ||||
| 16–40 | 0.78 | 0.79 | 0.81 | 0.79 | ||||
| 41–70 | 0.71 | 0.72 | 0.74 | 0.73 | ||||
| 71–100 | 0.66 | 0.66 | 0.69 | 0.67 | ||||
| WOMAC Pain | Obese | |||||||
| 45–54 | 55–64 | 65–74 | 75+ | |||||
| 1–16 | 0.81 | 0.81 | 0.84 | 0.82 | ||||
| 16–40 | 0.77 | 0.78 | 0.8 | 0.78 | ||||
| 41–70 | 0.70 | 0.71 | 0.73 | 0.72 | ||||
| 71–100 | 0.64 | 0.65 | 0.67 | 0.66 | ||||
| Underlying Medical Costs (by Age Group) | ||||||||
| Age Group | Data Source | |||||||
| 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80+ | Pope et al., 200447 MCBS 200948 NHANES 2009–201046 Red Book Online21 CPI35 |
|
| $2,813 | $3,685 | $4,490 | $4,826 | $5,621 | $6,571 | $8,636 | ||
| Treatment Characteristics | ||||||||
| Annual Regimen Cost | ||||||||
|
With or Without PPIs** |
Naproxen OTC | Naproxen Rx | Celecoxib | Data Source | ||||
| Without PPIs | $130 | $360 | $880 | Red Book Online® (February 2016)21 |
||||
| With OTC PPIs | $370 | $600 | $1,120 | |||||
| With Rx PPIs | - | $1,130 | $1,650 | |||||
| Discontinuation in the First 3 Months | ||||||||
| Naproxen OTC | Naproxen Rx | Celecoxib | Data Source | |||||
| 13.3% | 15.1% | 4.1% | Essex et al 201229 | |||||
| WOMAC Pam Decrement in First Year of Treatment (Stratified by Current Pain) | ||||||||
| Current WOMAC Pain | Mean WOMAC Pain Decrement (SD) | Data Source | ||||||
| Naproxen OTC | Naproxen Rx | Celecoxib | Smith et al 201628 | |||||
| 16–40 | 5.6 (17.6) | 6.3 (17.6) | 7.2(17.6) | |||||
| 41–70 | 13.4 (19.0) | 15.2 (19.0) | 17.2 (19.0) | |||||
| 71–100 | 20.9 (19.2) | 23.7 (19.2) | 26.8(19.2) | |||||
| Adverse Events | ||||||||
| Adverse Event Type | Naproxen OTC | Naproxen Rx | Celecoxib | Data Source | ||||
| Minor Toxicity | 55.4% | 63.0% | 63.0% | Bhala et al 201314 | ||||
| Major Toxicity | 0.53% | 0.60% | 0.40% | |||||
|
Gastrointestinal (year 1) |
0.53% | 0.60% | 0.20% | |||||
|
Cardiovascular (year 1) |
0.00% | 0.00% | 0.20% | |||||
|
RR of Gl event on Rx PPIs |
0.35 | Rostom et al 200249 | ||||||
|
RR of Gl event on OTC PPIs |
0.40 | |||||||
| Sensitivity Analyses† | ||||||||
| Variable | Values‡ | |||||||
| Annual Cost of generic celecoxib |
$1,320 | $880 | $600 | $360 | $190 | |||
| Discontinuation Rate (first 3 months) |
4% | 8% | 12% | 15% | ||||
| Gl Toxicity Rate (first year) |
0.2% | 0.3% | 0.4% | 0.5% | 0.6% | |||
| CV Toxicity Rate (first year) |
0.2% | 0.1% | 0.05% | 0% | ||||
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index, an osteoarthritis pain scale used in the OAPol model
PPI: proton-pump-inhibitor
Values for sensitivity analyses were chosen to assist with the comparison between naproxen and celecoxib. For cost, the highest value reflects the cost of a 300 mg daily dose of celecoxib (200 mg in base case) and the lowest cost was the price of the cheapest generic celecoxib formulation available per Red Book. Finally, $360 was selected as that is the current cost of naproxen and $600 was chosen as an arbitrary value in between our base case and the cost of naproxen. Values for discontinuation rate and toxicity rates were varied between our base case scenario and the values for naproxen with at least two intermediate values selected to aid comparison.
Underlined values are the base case celecoxib values and bold values are equivalent to prescription naproxen
Treatment Characteristics
Efficacy
The efficacy of naproxen and celecoxib was determined using data from a meta-analytical review of NSAID efficacy.6,28 The mean pain decrement for the NSAID regimen was stratified by pain severity upon starting the regimen (Table 1). For example, subjects with a WOMAC Pain score between 41 and 70 received a mean pain decrement of 15.2 (SD = 19.0) when taking naproxen and 17.2 (SD = 19.0) when taking celecoxib (Table 1). As OTC formulations contain a lower dose than their prescription analogs (880 mg in OTC formulations versus 1000 mg in prescription), we assumed a 12% efficacy reduction for OTC naproxen, as described previously.6
For the base case analysis, we used a 15.1% discontinuation rate for naproxen and a 4.1% discontinuation rate for celecoxib in the first three months of treatment, according to published literature.29 We used these discontinuation rates to derive pain decrements by multiplying the average WOMAC Pain reduction for persons on the regimen for an entire year by the proportion of the cohort remaining on the regimen at the end of the year.
Toxicity
The model distinguishes between major and minor toxicities. While all toxicities have associated costs and QoL decrements, major toxicities carry a risk of death and result in discontinuation of treatment. Major toxicities included CV events (e.g. myocardial infarction, stroke) or GI events (e.g. GI bleed). Prescription naproxen was associated with a 0.60% probability of a major GI toxicity in the first year of treatment.14 In the base case analysis, celecoxib was associated with a 0.20% risk of a major GI toxicity in the first year on treatment.14 Mortality from major GI events was 11.6% for both treatment strategies.6,14,30 Because PPIs lower GI event risk, the relative risk of having a major GI event while on prescription or OTC PPIs was 0.35 and 0.40, respectively.31 In the base case, celecoxib carried a 0.20% risk of a major CV event with a 24.9% probability of death from the event, while naproxen did not carry CV risks.7,12 Due to a lack of long-term data, we assumed that the GI toxicity rate in subsequent years would be half the first year rate, but CV toxicity rate would remain the same.
A detailed description and derivation of minor toxicity rates has been published elsewhere.1,6 Prescription naproxen and celecoxib both carried a 63.0% probability of minor toxicity (e.g. skin rash) in each treatment year.32 We assumed a 12% reduction in all toxicity rates for OTC naproxen because of the lower dosage.6
Costs
Costs in the base case analysis were derived to reflect the current average price of naproxen, generic celecoxib, and proton pump inhibitors from a healthcare sector perspective. Costs were based on the following doses: prescription naproxen 500mg twice a day, OTC naproxen 220mg four times a day, and celecoxib 200mg once a day. PPIs included once daily lansoprazole or omeprazole at 15 and 20mg, respectively. To derive these prices, all available average wholesale prices (AWP) for each drug were downloaded from Red Book Online, averaged, and converted to average sale price (ASP) by reducing brand name and generic costs by 26% and 68% respectively. 19,22,34 The annual cost of physician office visits and lab tests were added to the drug costs to capture annual OA-related medical costs.33–35 Drug prices were based on available data from February 2016.21 Our derived annual costs used in the base case analysis were $360 for prescription naproxen, $130 for OTC naproxen, and $880 for celecoxib. Prescription PPIs cost an additional $770, and OTC PPIs cost an additional $240 each year.33,36
Sensitivity Analyses
To understand the impact of data uncertainty on the model’s output, we varied the discontinuation rate, GI and CV toxicity rates, and annual cost of celecoxib in sensitivity analyses by increasing them to the levels consistent with those reported in the literature on discontinuation rates of naproxen (Table 1). Similarly, we reduced the discontinuation rates of naproxen to the levels of discontinuation reported in the literature for celecoxib. This led to the creation of ‘best’ and ‘worst’ case scenarios for each drug with respect to discontinuation rates. Discontinuation rates ranged from 4–15%, GI toxicity rates from 0.20–0.60%, and CV toxicity rates from 0–0.20%.14,29 The annual cost for celecoxib ranged from $190 to $1,320 per year. The highest price represents a 50% increase in our daily dose, and the lowest price is based on an AWP set by a distributor that sells predominantly to the Veteran’s Administration, which reflects the lowest celecoxib price offered in the U.S. as of early 2016. A probabilistic sensitivity analysis was also performed on the base case parameters of CV and GI toxicity rates and discontinuation rates for naproxen and celecoxib. 100 iterations of each possible treatment regimen in question (Naproxen OTC or Rx with or without PPIs, generic celecoxib with or without PPIs) were generated and analyzed (see technical appendix for input details).
Results
Base case
Table 2 presents costs, quality-adjusted life expectancies (QALE), and ICERs for the eight strategies. OTC naproxen was the least expensive regimen. Subjects treated with OTC naproxen had a QALE of 10.892 QALYs and average lifetime medical costs of $100,300. Adding OTC PPIs to OTC naproxen resulted in an ICER of $59,000/QALY. Celecoxib-based strategies (with and without PPI) cost more and offered fewer benefit than naproxen-based strategies. By convention, we label these strategies “dominated.”24 The cohort with moderate pain had similar results; celecoxib was dominated by naproxen-based strategies. 0.261% and 0.347% of patients on celecoxib experienced a GI and CV toxicity respectively while 0.786% of patients on naproxen experienced a GI toxicity.
Table 2.
Cost-effectiveness of celecoxib using base case parameter estimates
| Regimen | QALE* | Cost | ICER† |
|---|---|---|---|
| High Pain Cohort | |||
| OTC** Naproxen | 10.892 | $100,300 | |
| Rx†† Naproxen | 10.894 | $100,700 | Dominated‡ |
| OTC Naproxen + OTC PPIs‡‡ | 10.899 | $100,700 | $59,000/QALY§ |
| Rx Naproxen + OTC PPIs | 10.900 | $101,100 | $314,700/QALY |
| Celecoxib | 10.897 | $101,700 | Dominated |
| Rx Naproxen + Rx PPIs | 10.901 | $102,000 | $1,644,900/QALY |
| Celecoxib + OTC PPIs | 10.899 | $102,200 | Dominated |
| Celecoxib + Rx PPIs | 10.900 | $103,300 | Dominated |
| Moderate Pain Cohort | |||
| OTC Naproxen | 11.310 | $97,700 | |
| OTC Naproxen + OTC PPIs | 11.317 | $98,100 | $61,500 |
| Rx Naproxen | 11.311 | $98,100 | Dominated |
| Rx Naproxen + OTC PPIs | 11.317 | $98,600 | $1,126,600 |
| Celecoxib | 11.311 | $99,300 | Dominated |
| Rx Naproxen + Rx PPIs | 11.317 | $99,600 | Dominated |
| Celecoxib + OTC PPIs | 11.314 | $99,800 | Dominated |
| Celecoxib + Rx PPIs | 11.314 | $101,000 | Dominated |
QALE stands for Quality-Adjusted Life Expectancy
ICER stands for Incremental Cost-Effectiveness Ratio
Dominated indicates that the regimen cost more than the previous regimen in the table and resulted in a lower quality adjusted life expectancy
QALY stands for Quality-Adjusted Life Years
OTC stands for over-the-counter
Rx stands for prescription
PPI stands for proton-pump-inhibitor
Sensitivity Analyses
Toxicity
In the high pain cohort, the cost-effectiveness of celecoxib was sensitive to the interplay between CV and GI toxicity, with generic celecoxib likely to be cost-effective only under the most favorable conditions (Figure 2a). With higher CV toxicity, generic celecoxib had ICERs below $100,000/QALY only at the lowest cost considered (with lower rates of GI toxicity and/or discontinuation rates) or at a cost below $360/year with the lowest discontinuation and GI toxicity rates (4% and 0.2% respectively). Lower CV toxicity led to more combinations of GI toxicity and discontinuation under which celecoxib was cost-effective. When the CV event rate was lowered to 25% of the base case (0.05%), the scenarios under which celecoxib was cost-effective increased substantially, especially for costs of $190-$360. Additionally, at a cost of $600/year and a CV event rate of 0.05%, celecoxib was cost-effective with an ICER of $78,300/QALY, assuming a base case discontinuation rate (4%) and GI toxicity rate (0.2%).
Figure 2a:

Sensitivity analyses consisted of varied annual drug costs, discontinuation rates, gastrointestinal (GI) toxicity, and cardiovascular (CV) toxicity for generic celecoxib. For each of these parameters, the values were varied between the best estimated value from the literature on celecoxib and the value for prescription naproxen. Annual drug costs were varied between $190 and $1,320, discontinuation rates between 4% and 15%, GI toxicity between 0.2% and 0.6%, and CV toxicity between 0% and 0.2%. Base case values for generic celecoxib are indicated with an asterisk and values equivalent to prescription naproxen with bolded text. Incremental cost-effectiveness ratios (ICERs) were calculated for each set of parameters, comparing generic celecoxib without PPIs to all other naproxen-containing regimens and to celecoxib with PPIs in a high pain cohort. The shading in the figure denotes the resultant ICER ranging from <$25,000/QALY to values exceeding $150,000/QALY as well as instances where the strategy is dominated (costs more and confers fewer QALYs) as cost, GI and CV toxicity, and discontinuation are varied. Grey hash marks on the figure indicate an area where the ICER for generic celecoxib was calculated using a different comparator regimen than adjacent cells. For example, at an annual cost of $360, the most frequent comparator to generic celecoxib was prescription naproxen without PPIs. However, when discontinuation was at 4%, CV toxicity at 0.1%, and GI toxicity at 0.3% or 0.4%, the comparator was OTC naproxen with OTC PPIs.
Similar trends were seen for the moderate pain cohort (Figure 2b). In subjects with moderate pain, celecoxib was only cost-effective at the base case CV event rate of 0.2% at a $190 annual cost assuming base GI and discontinuation rates.
Figure 2b:

Sensitivity analyses consisted of varying the annual drug cost, discontinuation rates, gastrointestinal (GI) toxicity, and cardiovascular (CV) toxicity for generic celecoxib were conducted utilizing the same conditions as Figure 2a. Base case values for generic celecoxib are indicated with an asterisk and values equivalent to prescription naproxen with bolded text. ICERs were calculated for each set of parameters, comparing generic celecoxib without PPIs to all other naproxen-containing regimens and to celecoxib with PPIs in a moderate pain cohort. The shading in the figure denotes the resultant ICER ranging from <$25,000/QALY to values exceeding $150,000/QALY as well as instances where the strategy is dominated (costs more and confers fewer QALYs) as cost, GI and CV toxicity, and discontinuation are varied. Grey hash marks on the figure indicate an area where the ICER for generic celecoxib was calculated using a different comparator regimen than adjacent cells.
Cost
In both the high and moderate OA pain cohorts, celecoxib was never cost-effective at the base case cost of $880/year. Its cost-effectiveness at lower prices was sensitive to discontinuation and toxicity rates.
Discontinuation
For the high pain cohort, the cost-effectiveness of celecoxib was sensitive to the discontinuation rate. Generic celecoxib was cost-effective under more toxicity and cost scenarios when a lower discontinuation rate was assumed (Figure 2a). At the lowest discontinuation rate (base case; 4%), generic celecoxib was cost-effective at $190/year (lowest cost generic celecoxib reported in Red Book; sold to the VA21) and $360/year under most scenarios except when the highest toxicity rates for both CV and GI were used (0.2% CV (base case); 0.6% GI (250% of base case)). At an annual cost of $600, celecoxib was only cost-effective if the CV toxicity was 0.05% (25% of base case) or less.
As the discontinuation rate was increased, the number of scenarios in which generic celecoxib was cost-effective decreased. When a discontinuation rate similar to that of naproxen (15%) was modeled, generic celecoxib was no longer cost-effective at an annual cost of $600 or $880 regardless of GI and CV toxicity rate. Celecoxib was cost-effective at an annual cost of $360 if its toxicity was markedly lower than the base case values. At an annual cost of $190, celecoxib was cost-effective at CV toxicity rates lower than 0.2%.
For the moderate pain cohort (Figure 2b), celecoxib was only cost-effective at a discontinuation rate of 4% in six scenarios at cost greater than $190 annually: five at a cost of $360, and only one at a cost of $600 (Figure 2b). For a discontinuation rate of 15%, the highest cost at which celecoxib was cost-effective was $360 and only if CV and GI toxicity rates were 0% and 0.2% respectively.
Probabilistic Sensitivity Analyses
In the high pain cohort, celecoxib without PPIs was cost-effective in 1% of cases at a willingness-to-pay (WTP) threshold of $100,000 per QALY (Figure 3a). Celecoxib was never the cost-effective option of the eight regimens evaluated at lower WTP thresholds. Celecoxib was the cost-effective treatment option in 10% and 13% of scenarios when the WTP threshold was raised to $150,000 and $200,000 per QALY respectively. Celecoxib with PPIs (either OTC or prescription) was never cost-effective at a WTP threshold of $100,000 per QALY. At a WTP threshold of $100,000 per QALY, OTC naproxen with OTC PPIs was the cost-effective treatment in 49% of cases, the most of the eight regimens (Figure 3a).
Figure 3a:
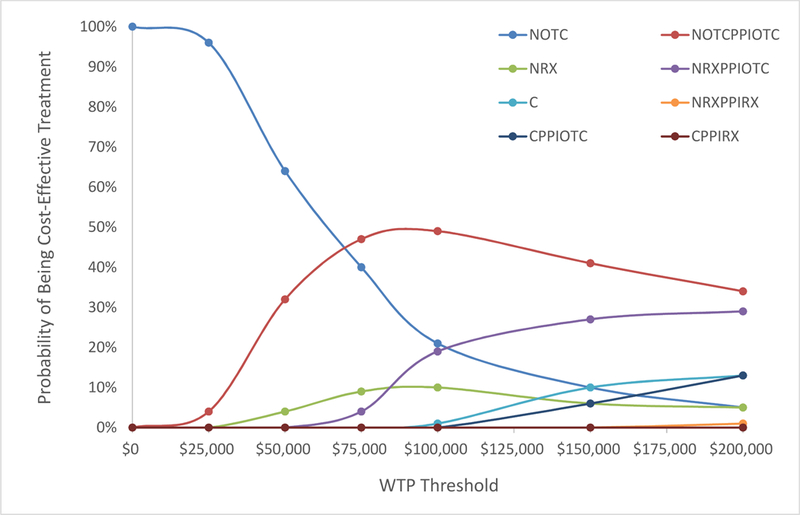
Probabilistic sensitivity analyses were performed in the high pain cohort varying the base case discontinuation rate (4% celecoxib, 15% naproxen) and cardiovascular (0.2% celecoxib, 0% naproxen) and gastrointestinal (0.2% celecoxib, 0.6% naproxen) toxicity rates of each celecoxib and naproxen regimen. 100 iterations for each regimen (celecoxib with or without prescription or over-the-counter (OTC) proton-pump-inhibitors (PPIs); prescription or OTC naproxen with or without prescription or OTC PPIs) were generated. ICERs were calculated for each set of parameters, comparing generic celecoxib without PPIs to all other naproxen-containing regimens and to celecoxib with PPIs in a high pain cohort. The resulting cost-effectiveness acceptability curves (CEAC) were generated based on the number of times each regimen was the cost-effective option at varying willingness-to-pay (WTP) thresholds ranging from $0 to $200,000 per quality-adjusted-life-year.
In the moderate pain cohort, celecoxib with or without PPIs was never the cost-effective option at a WTP threshold of $100,000 per QALY and was cost-effective in 1% and 4% of cases at WTP thresholds of $150,000 per QALY and $200,000 per QALY respectively (Figure 3b). OTC naproxen with OTC PPIs was most frequently the cost-effective option, being cost-effective in 59% of cases at a WTP threshold of $100,000 per QALY.
Figure 3b:
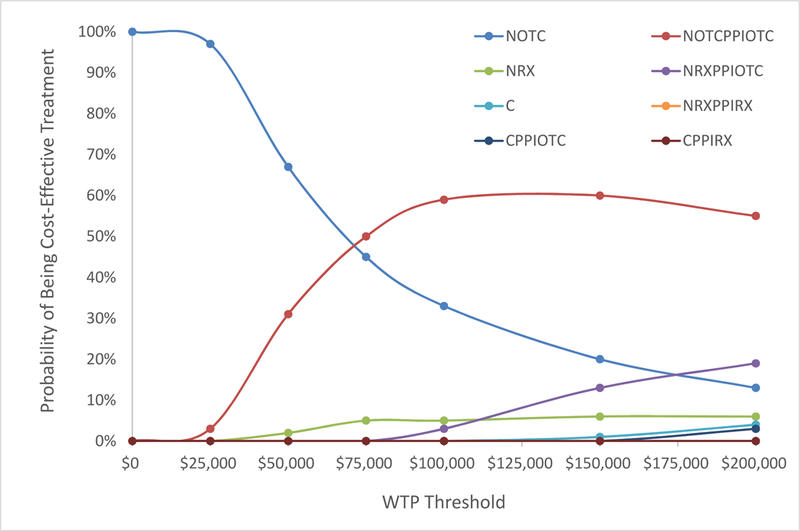
Probabilistic sensitivity analyses were performed in the moderate pain cohort varying the base case discontinuation rate (4% celecoxib, 15% naproxen) and cardiovascular (0.2% celecoxib, 0% naproxen) and gastrointestinal (0.2% celecoxib, 0.6% naproxen) toxicity rates of each celecoxib and naproxen regimen. 100 iterations for each regimen (celecoxib with or without prescription or over-the-counter (OTC) proton-pump-inhibitors (PPIs); prescription or OTC naproxen with or without prescription or OTC PPIs) were generated. ICERs were calculated for each set of parameters, comparing generic celecoxib without PPIs to all other naproxen-containing regimens and to celecoxib with PPIs in a moderate pain cohort. The resulting cost-effectiveness acceptability curves (CEAC) were generated based on the number of times each regimen was the cost-effective option at varying willingness-to-pay (WTP) thresholds ranging from $0 to $200,000 per quality-adjusted-life-year.
Discussion
At its current annual price of $880, generic celecoxib is unlikely to be a cost-effective treatment for knee OA pain. In fact, in a high pain cohort, celecoxib did not become cost-effective under base case toxicity and discontinuation rates until costs were lowered to $360/year. At this point, celecoxib had an ICER of $70,600/QALY compared with naproxen. At a CV toxicity rate of 0.05%, (25% of base case) celecoxib could be cost-effective at a price of $600 annually while still assuming base GI toxicity and discontinuation rates. Cardiovascular toxicity had a particularly powerful effect on the cost-effectiveness of generic celecoxib, as experiencing a CV toxicity reduced quality of life by 14.4% while a GI toxicity reduced quality of life by 5.4%. Unless the CV event toxicity rate attributable to celecoxib is found to be lower than the estimated 0.2% assumed in this analysis, generic celecoxib will need substantial price reductions in order to be considered a cost-effective treatment for knee OA in populations with high pain. Results of this analysis indicate that even under the most favorable assumptions regarding its toxicity profile and discontinuation rate, generic celecoxib is unlikely to be cost-effective if priced above $600 annually. Only under the most favorable conditions for costs (less than $400) and base case discontinuation and toxicity scenarios is generic celecoxib likely to be cost-effective.
The addition of PPIs to celecoxib treatments has been shown previously to improve gastrointestinal safety, but also to increase the cost of the medication regimen.31,37 We found that the benefits of PPIs added to celecoxib do not justify the additional costs in a cohort at average risk for GI events. Brereton and colleagues evaluated the cost-effectiveness of the co-prescription of PPIs and COX-2 selective NSAIDs in patients with moderately high risk of GI events and found the addition of PPIs to be cost-effective.38 Celecoxib is already relatively safe with regard to GI toxicity compared with naproxen, and the addition of PPIs to celecoxib provides relatively little quality of life benefit despite a substantial increase in cost for those not at high risk of GI complications.14,39
We used wide ranges for toxicity and discontinuation rates in our sensitivity analyses to account for a wide variability of these parameters in the literature. The CLASS study across 386 clinical sites in the U.S. and Canada did not show a statistically significant difference in GI event rates between celecoxib and the NSAIDs studied (diclofenac and ibuprofen).13,40 However, a systematic review conducted in 2002 by Deeks and colleagues found that celecoxib had a significantly reduced risk of GI events compared with other NSAIDs.39 The CV risk associated with celecoxib is also highly variable in the published literature. One 2005 trial of 2,035 patients found celecoxib dosed at 400mg and 800mg daily was associated with increased risk of death from CV causes.41 However, another trial published in 2006 (the PreSAP trial) found no increase in CV events noted for 400mg daily celecoxib compared with placebo.42
In our sensitivity analyses, alteration of the CV toxicity rate yielded one of the most substantial changes in celecoxib cost-effectiveness, suggesting that better information on the cardiovascular risk involved in taking celecoxib could lead to more informed decision-making. Additionally, there have been few trials on the impact of 200–300mg of daily celecoxib on CV or GI events, as modeled in this analysis and used clinically. The majority of the trials we used to derive our toxicity profile were 400–500mg daily doses. However, when a probabilistic sensitivity analysis was performed varying the discontinuation, CV, and GI toxicity rates, celecoxib was only cost-effective in 1% of iterations in the high pain cohort and was never cost-effective in the moderate pain cohort at a WTP threshold of $100,000 per QALY (Figures 3a and b). These PSA results indicate that despite some initial uncertainty in our toxicity and discontinuation rates, the base case conclusion that celecoxib is not likely to be cost-effective is relatively robust.
Results from the recently conducted PRECISION trial are unlikely to alter our principal finding that generic celecoxib is not cost-effective compared to naproxen at current prices. PRECISION, a noninferiority trial whose results were published in 2016, examined rates of CV toxicity in over 24,000 high-risk subjects randomized to celecoxib, naproxen, or ibuprofen. Celecoxib proved to be non-inferior to both naproxen and ibuprofen with regard to cardiovascular safety (relative risk <1.12). 22 However, PRECISION was conducted in patients at elevated risk for adverse cardiac events, and a CV toxicity rate attributable to NSAID use was not reported because the trial did not have a placebo arm. As such, we could not derive a CV toxicity rate attributable to celecoxib and naproxen, and published studies in our modeled patient population report contradictory results with regard to celecoxib cardiovascular safety.14,41 Furthermore, even when celecoxib had the same CV toxicity rate as naproxen, it was not cost-effective at current prices and required substantial price reduction to at least $600/year to be cost-effective when other characteristics were at base case values. At the lower price, celecoxib would be cost-effective with an ICER of $55,900/QALY using our base case toxicity and discontinuation values. However, additional data from PRECISION indicate that, contrary to prior studies, celecoxib might not have a lower discontinuation rate than naproxen.29 When celecoxib discontinuation was equivalent to naproxen, celecoxib was not cost-effective, even at the most favorable GI and CV event rates, until the cost was lowered to $360 annually.
This cost-effectiveness analysis has several limitations. First, our simulated cohort had an average age of 65 with no prevalent comorbidities, so some conclusions may not apply to a younger or less healthy population, as NSAID complication rates (GI and CV) are considerably higher in patients with more comorbidities.43,44 Additionally, studies used to determine the efficacy of the medication regimens in our analysis were short-term, so we made assumptions for long-term efficacy based on previously published methodology.6 We did not allow subjects to switch between celecoxib and naproxen regimens, or between non-PPI containing regimens and PPI containing regimens. This was done to keep the number of strategies manageable and to improve interpretability of the results. Lastly, our toxicity rates for the treatment regimens do not vary based on subject age or sex. While we derived these rates from RCTs with cohorts similar to the modeled cohort in this analysis,14 the model still misses some variability in toxicity rates.
To our knowledge, this is the first cost-effectiveness analysis for generic celecoxib in the United States. Previous evaluations of celecoxib either used brand-name or international drug prices.6 This analysis provides a framework for providers and payers evaluating the cost-effectiveness of generic celecoxib given a range of discontinuation rates, toxicity rates, and drug costs. In sum, generic celecoxib at the current price of $880/year is not cost-effective for patients with knee OA and no prevalent comorbidities in both high and moderate pain cohorts.
Supplementary Material
Acknowledgments
Role of funding
Supported by: National Institute of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases: R01 AR064320. The funding source had no role in the study design, collection, analysis and interpretation of the data, drafting of the manuscript, or decision to submit the manuscript for publication.
Support: NIAMS R01AR064320
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
None of the authors received any money or any other compensation from Pfizer for the matters related to this paper. The authors do not have any relevant conflicts of interest.
Data Statement
Data used in this analysis came from a wide variety of published literature sources. All relevant data sources are cited in this manuscript.
References
- 1.Losina E, Walensky RP, Reichmann WM, Holt HL, Gerlovin H, Solomon DH, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med 2011; 154: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum 2008; 58: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pincus T, Swearingen C, Cummins P, Callahan LF. Preference for nonsteroidal anti-inflammatory drugs versus acetaminophen and concomitant use of both types of drugs in patients with osteoarthritis. J Rheumatol 2000; 27: 1020–1027. [PubMed] [Google Scholar]
- 4.Grindrod KA, Marra CA, Colley L, Cibere J, Tsuyuki RT, Esdaile JM, et al. After patients are diagnosed with knee osteoarthritis, what do they do? Arthritis Care Res (Hoboken) 2010; 62: 510–515. [DOI] [PubMed] [Google Scholar]
- 5.Jordan KM, Sawyer S, Coakley P, Smith HE, Cooper C, Arden NK. The use of conventional and complementary treatments for knee osteoarthritis in the community. Rheumatology (Oxford) 2004; 43: 381–384. [DOI] [PubMed] [Google Scholar]
- 6.Losina E, Paltiel AD, Weinstein AM, Yelin E, Hunter DJ, Chen SP, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken) 2015; 67: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjordal JM, Klovning A, Ljunggren AE, Slordal L. Short-term efficacy of pharmacotherapeutic interventions in osteoarthritic knee pain: A meta-analysis of randomised placebo-controlled trials. Eur J Pain 2007; 11: 125–138. [DOI] [PubMed] [Google Scholar]
- 8.Bjordal JM, Ljunggren AE, Klovning A, Slordal L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: meta-analysis of randomised placebo controlled trials. BMJ 2004; 329: 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholes D, Stergachis A, Penna PM, Normand EH, Hansten PD. Nonsteroidal anti-inflammatory drug discontinuation in patients with osteoarthritis. J Rheumatol 1995; 22: 708–712. [PubMed] [Google Scholar]
- 10.Whelton A Renal and related cardiovascular effects of conventional and COX-2-specific NSAIDs and non-NSAID analgesics. Am J Ther 2000; 7: 63–74. [DOI] [PubMed] [Google Scholar]
- 11.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med 2010; 170: 1968–1976. [DOI] [PubMed] [Google Scholar]
- 12.Tramèr MR, Moore RA, Reynolds DJM, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain 2000; 85: 169–182. [DOI] [PubMed] [Google Scholar]
- 13.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000; 284: 1247–1255. [DOI] [PubMed] [Google Scholar]
- 14.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 2013; 382: 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001; 286: 954–959. [DOI] [PubMed] [Google Scholar]
- 16.Kearney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006; 332: 1302–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer M, DeLattre M, Gao X, Stephens J, Botteman M, Morreale A. Assessing the cost-effectiveness of COX-2 specific inhibitors for arthritis in the Veterans Health Administration. Curr Med Res Opin 2005; 21: 47–60. [DOI] [PubMed] [Google Scholar]
- 18.Maetzel A, Krahn M, Naglie G. The cost effectiveness of rofecoxib and celecoxib in patients with osteoarthritis or rheumatoid arthritis. Arthritis Rheum 2003; 49: 283–292. [DOI] [PubMed] [Google Scholar]
- 19.Wielage RC, Bansal M, Andrews JS, Klein RW, Happich M. Cost-utility analysis of duloxetine in osteoarthritis: a US private payer perspective. Appl Health Econ Health Policy 2013; 11: 219–236. [DOI] [PubMed] [Google Scholar]
- 20.Capel M, Tornero J, Zamorano JL, Oyagüez I, Casado MÁ, Sánchez-Covisa J, et al. Efficiency of Naproxen/Esomeprazole in Association for Osteoarthrosis Treatment in Spain. Reumatología Clínica (English Edition) 2014; 10: 210–217. [DOI] [PubMed] [Google Scholar]
- 21.Brown DW, Balluz LS, Heath GW, Moriarty DG, Ford ES, Giles WH, et al. Associations between recommended levels of physical activity and health-related quality of life: Findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev Med 2003; 37: 520–528. [DOI] [PubMed] [Google Scholar]
- 22.Nissen SE, Yeomans ND, Solomon DH, Lüscher TF, Libby P, Husni ME, et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. New England Journal of Medicine 2016; 0: null. [DOI] [PubMed] [Google Scholar]
- 23.Losina E, Michl G, Collins JE, Hunter DJ, Jordan JM, Yelin E, et al. Model-based evaluation of cost-effectiveness of nerve growth factor inhibitors in knee osteoarthritis: impact of drug cost, toxicity, and means of administration. Osteoarthritis Cartilage 2015. [DOI] [PMC free article] [PubMed]
- 24.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second panel on cost-effectiveness in health and medicine. JAMA 2016; 316: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 25.Abell JE, Hootman JM, Zack MM, Moriarty D, Helmick CG. Physical activity and health related quality of life among people with arthritis. J Epidemiol Community Health 2005; 59: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osteoarthritis Initiative (OAI) In: University of California, San Francisco: 2013. <http://oai.epi-ucsf.org/datarelease/default.asp> Accessed [Google Scholar]
- 27.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004; 42: 851–859. [DOI] [PubMed] [Google Scholar]
- 28.Smith SR, Deshpande BR, Collins JE, Katz JN, Losina E. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthritis and Cartilage 2016; 24: 962–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Essex MN, Bhadra P, Sands GH. Efficacy and tolerability of celecoxib versus naproxen in patients with osteoarthritis of the knee: a randomized, double-blind, double-dummy trial. J Int Med Res 2012; 40: 1357–1370. [DOI] [PubMed] [Google Scholar]
- 30.Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterology 2009; 9: 41–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev 2011: CD002296. [DOI] [PubMed]
- 32.Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999; 74: 1095–1105. [DOI] [PubMed] [Google Scholar]
- 33.Levinson DR. Medicaid Drug Price Comparison: Average Sales Price to Average Wholesale Price Office of the Inspector General: Department of Health and Human Services; 2005. [Google Scholar]
- 34.Medicare Fee Schedules In: Centers for Medicare & Medicaid Services (CMS) 2012. [Google Scholar]
- 35.Consumer Price Index (CPI) In: National Bureau of Labor Statistics; 2016. [Google Scholar]
- 36.IMS Institute for Health Informatics. The Use of Medicines in the United States: Review of 2010 2011.
- 37.Rahme E, Barkun AN, Toubouti Y, Scalera A, Rochon S, Lelorier J. Do proton-pump inhibitors confer additional gastrointestinal protection in patients given celecoxib? Arthritis Rheum 2007; 57: 748–755. [DOI] [PubMed] [Google Scholar]
- 38.Brereton N, Pennington B, Ekelund M, Akehurst R. A cost-effectiveness analysis of celecoxib compared with diclofenac in the treatment of pain in osteoarthritis (OA) within the Swedish health system using an adaptation of the NICE OA model. J Med Econ 2014; 17: 677–684. [DOI] [PubMed] [Google Scholar]
- 39.Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 2002; 325: 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United States Food and Drug Administration. Medical Officer Review: Celebrex (celecoxib). NDA 20–998/S-009 In: 2000 <http://www.fda.gov/ohrms/dockets/ac/01/briefing/3677b1_03_med.pdf> Accessed
- 41.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005; 352: 1071–1080. [DOI] [PubMed] [Google Scholar]
- 42.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med 2006; 355: 885–895. [DOI] [PubMed] [Google Scholar]
- 43.Solomon DH, Gurwitz JH. Toxicity of nonsteroidal anti-inflammatory drugs in the elderly: is advanced age a risk factor? Am J Med 1997; 102: 208–215. [DOI] [PubMed] [Google Scholar]
- 44.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014; 22: 363–388. [DOI] [PubMed] [Google Scholar]
- 45.Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Common Wealth and Municipios: April 1, 2010 to July 1, 2012 In, June 2012 ed: U.S Census Bureau Population Division; 2012. <http://www.census.gov/popest/data/index.html> Accessed [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC). 2009–2010 National Health and Nutrition Examination Survey (NHANES) Data In. Hyattsville, MD: National Center for Health Statistics (NCHS), U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 47.Pope GC, Kautter J, Ellis RP, Ash AS, Ayanian JZ, Lezzoni LI, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev 2004; 25: 119–141. [PMC free article] [PubMed] [Google Scholar]
- 48.Medicare Current Beneficiary Survey In: Centers for Medicare & Medicaid Services; 2009. <http://www.cms.gov/LimitedDataSets/11_MCBS.asp> Accessed [Google Scholar]
- 49.Rostom A, Dube C, Wells G, Tugwell P, Welch V, Jolicoeur E, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev 2002: CD002296. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


