Abstract
It was reported that 2,4-dichlorophenoxyacetic acid (2,4-D), a commonly used herbicide and a possible endocrine disruptor, can disturb spermatogenesis, but the precise mechanism is not understood. Since 2,4-D is a weak peroxisome proliferator in hepatocytes and peroxisome proliferator-activated receptor α (PPARα) is also expressed in Leydig cells, this study aimed to investigate the link between PPARα and 2,4-D-mediated testicular dysfunction. 2,4-D (130 mg/kg/day) was administered to wild-type and Ppara-null mice for 2 weeks, and the alterations in testis and testosterone/cholesterol metabolism in Leydig cells were examined. Treatment with 2,4-D markedly decreased testicular testosterone in wild-type mice, leading to degeneration of spermatocytes and Sertoli cells. The 2,4-D decreased cholesterol levels in Leydig cells of wild-type mice through down-regulating the expression of 3-hydroxy-3-methylglutaryl coenzyme A synthase 1 and reductase, involved in de novo cholesterogenesis. However, the mRNAs encoding the important proteins involved in testosterone synthesis were unchanged by 2,4-D except for CYP17A1, indicating that exhausted cholesterol levels in the cells is a main reason for reduced testicular testosterone. Additionally, pregnancy rate and the number of pups between 2,4-D-treated wild-type male mice and untreated female mice were significantly lower compared with those between untreated couples. These phenomena were not observed in 2,4-D-treated Ppara-null males. Collectively, these results suggest a critical role for PPARα in 2,4-D-induced testicular toxicity due to disruption of cholesterol/testosterone homeostasis in Leydig cells. This study yields novel insights into the possible mechanism of testicular dysfunction and male infertility caused by 2,4-D.
Keywords: 2,4-dichlorophenoxyacetic acid; PPARα; Cholesterol; Leydig cell; Testosterone; Testicular toxicity
Introduction
2,4-Dichlorophenoxyacetic acid (2,4-D) is a widely used herbicide for broadleaf plants in agriculture, forestry, and lawn care (Munro et al. 1992). Administration of 2,4-D results in various toxicities in rodents (Charles et al. 1996; Mattsson et al. 1997; Amer and Aly 2001; Ozaki et al. 2001), but the most notable is testicular dysfunction. The incidence of asthenospermia, necrospermia, and teratospermia is high in farmers who handle 2,4-D, and 2,4-D exposure may be associated with increased risk of early spontaneous abortion (Lerda and Rizzi 1991). Therefore, detailed studies regarding the mechanism of 2,4-D-induced testicular toxicity would be of great importance both scientifically and socially, but the mechanism is not understood.
In rodents, 2,4-D can cause peroxisome proliferation in hepatocytes (Kawashima et al. 1984) that is mediated by peroxisome proliferator-activated receptor α (PPARα) (Lee et al. 1995). PPARα is highly expressed in liver, heart, intestine, and renal proximal tubules, and its pathophysiological roles in these organs are partially elucidated (Aoyama et al. 1998; Watanabe et al. 2000; Kamijo et al. 2002). In testis, PPARα is mainly expressed in Leydig cells, and at lower levels in Sertoli cells (Braissant et al. 1996; Schultz et al. 1999). Some peroxisome proliferator chemicals decrease testosterone production (Parks et al. 2000; Kumar et al. 2000), and long-term administration of these agents results in the development of Leydig cell tumors (Biegel et al. 2001). However, the role of PPARα in testicular function and any potential link between 2,4-D-induced testicular dysfunction and PPARα remain unclear.
To address these issues, 2,4-D was administered to wild-type and Ppara-null mice for 2 weeks. The changes in testicular phenotypes and testosterone/cholesterol metabolism in Leydig cells were assessed. Additionally, the pregnancy rate and number of pups between 2,4-D-treated male mice and untreated female mice were investigated. This study demonstrates a crucial role of PPARα for 2,4-D-induced testicular toxicity likely due to disrupting cholesterol/testosterone homeostasis in Leydig cells and also suggests a possible molecular mechanism by which 2,4-D induces testicular dysfunction, as well as the constitutive role of PPARα in Leydig cells.
Methods
Mice and 2,4-D treatment
Male Sv/129 wild-type or Ppara-null mice (Lee et al. 1995) (16–20 weeks of age, 25–30 g of body weight) were used. The mice were maintained in a controlled environment at 26 °C and 60 % humidity with constant 12-h light, 12-h darkness cycle and had free access to standard laboratory chow and water. The wild-type and Ppara-null mice were divided into the two groups, vehicle-treated and 2,4-D-treated groups (n = 8/group). The 2,4-D methyl ester, purchased from Sigma-Aldrich Japan (Tokyo, Japan), was dissolved in corn oil (4 mL/kg/day) just prior to the administration and was given by gavage every day (130 mg/kg/day) for 14 days. The 2,4-D is rapidly converted to the acid form in vivo (Frantz and Kropscott 1993). For the vehicle-treated groups, the same amount of corn oil (4 mL/kg/day) was given in the same manner. Twenty-four hours after the last administration, mice were killed by carbon dioxide asphyxiation. Testes were immediately removed, weighed, and subjected to histological analyses and isolation of Leydig cells. The remaining ones were snap frozen at −80 °C for the other analyses.
Assessment of fertility
To examine the link between 2,4-D exposure and male infertility, another mouse cohort was used. Male Sv/129 wild-type mice (6–8 weeks of age, 20–25 g of body weight) were randomly divided into five groups, vehicle-treated and 2,4-D-treated groups at four different doses (13, 50, 130, or 260 mg/kg/day) (n = 10/group). Each male mouse was mated with virgin untreated female wild-type mice at the similar age and body weight (1 male vs. 3 females in each cage), and the incidence of first pregnancy and the number of pups delivered were determined. The 2,4-D was dissolved in corn oil (4 mL/kg/day) just prior to the administration and was given by gavage every day. For the vehicle-treated groups, the same amount of corn oil (4 mL/kg/day) was similarly administered. Administration of 2,4-D or vehicle was continued during mating period. The same experience was performed using male Sv/129 Ppara-null mice and untreated female wild-type mice (6–8 weeks of age, 20–25 g of body weight) that had never been pregnant (1 male vs. 3 females in each cage).
Histological analysis
For light microscopy, the testes were fixed in Bouin’s solution, embedded in paraffin wax, sectioned in 5 μm thickness, and stained with periodic acid–Schiff reagent and hematoxylin. For electron microscopy, the testes were fixed by whole-body perfusion using 4 % paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.4) for 5 min, cut into 2-mm cubes, and fixed in 2.5 % glutaraldehyde in PBS. The sections were washed with ethanol and PBS, post-fixed in 1 % osmium tetroxide and potassium ferrocyanide for 60 min, dehydrated with ethanol and acetone, embedded in Epon resin, and sequentially cut on an ultramicrotome. The ultrathin sections were double-stained with uranyl acetate and lead citrate and observed with a JEOL 1200 EX electron microscope (JEOL Ltd, Tokyo, Japan). To detect peroxisomes, cytochemical staining for peroxisomal catalase was conducted according to the method of Novikoff and Goldfischer (1969). After washing with PBS, the tissue blocks were incubated in alkaline 3,3′-diaminobenzidine (DAB) at 37 °C for 90 min. The incubation medium pH was 9.7, and the H2O2 concentration was 0.1 %. The blocks were then post-fixed in 1 % osmium tetroxide and processed for electron microscopy. Histochemical staining for cholesterol in Leydig cells was carried out using the method of Emeis et al. (1977). Briefly, frozen sections (10 μm thick) were incubated in 0.1 M phosphate buffer (pH 7.0) containing 1.4 U/mL cholesterol esterase (Sigma), 0.4 U/mL cholesterol oxidase (Sigma), 50 U/mL horseradish peroxidase (Sigma), 0.5 mg/mL DAB, and 0.1 % (v/v) Triton X-100 for 2 h at 37 °C. After incubation, the sections were counterstained by hematoxylin.
Isolation of Leydig cells
Leydig cells were isolated and purified by the method of Gale et al. (1982), using a procedure involving enzymatic dissociation and Percoll gradient centrifugation. Briefly, testes were decapsulated and dispersed by shaking in 25 mM HEPES buffer (pH 7.4) containing 1 mg/mL collagenase (Wako, Osaka, Japan), 1 mg/mL hyaluronidase (Sigma), and 1 mg/mL bovine serum albumin (BSA) for 20 min at 80 cycles/min at 34 °C. The dispersed tissue was immediately diluted to 40 mL with Earle’s balanced salt solution (EBSS, Sigma) containing 0.07 % BSA. The tube was allowed to settle for 5 min, and the resulting supernatant was collected and centrifuged at 250×g for 5 min. The cell pellet was re-suspended in 3 mL of EBSS/BSA. The suspension was treated with 10–65 % Percoll (GE Health-care, Piscataway, NJ) and centrifuged at 800×g for 20 min. The Leydig cells contained in a diffuse band at a density corresponding to 35–50 % Percoll (1.050–1.070 g/mL) were collected and washed. The purity of Leydig cells was assessed by histochemical 3β-hydroxysteroid dehydrogenase reaction (Steinberger et al. 1966). More than 80 % of cells were found to be positive for 3β-hydroxysteroid dehydrogenase. Cell numbers were determined by hemocytometer as the mean of three estimates.
mRNA analysis
Purified Leydig cells were homogenized (Aoyama et al. 1989), and total RNA was extracted using RNeasy Mini Kit (QIAGEN, Tokyo, Japan). One μg of RNA was reverse-transcribed with SuperScript II reverse transcriptase (GIBCO BRL, Paisley, Scotland). Real-time quantitative polymerase chain reaction (qPCR) was performed and analyzed with the ABI PRISM 7700 Sequence Detection System (PerkinElmer Applied Biosystems, Foster City, CA). The detection was performed by measuring the binding of the fluorescence dye SYBR Green I to double-stranded DNA. The presence of a single-PCR product was verified by agarose gel electrophoresis, and only the primers that amplified a unique band of the correct size were used for the assay. Relative expression levels were calculated by the comparative CT (cycle of threshold detection) method as outlined in the manufacturer’s technical bulletin. The primer sequences were selected with Primer Express software (PerkinElmer Applied Biosystems) as shown in Supplementary Table 1. The mRNA levels of target genes were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (Gapdh).
Other methods
Serum and testicular testosterone levels were measured by radioimmunoassay using DPC total testosterone kit (Mitsubishi Kagaku Iatron, Tokyo, Japan). For testicular testosterone measurements, testes were treated using the extraction procedure provided by Diagnostic Products Corporation (Gwynedd, UK). Serum luteinizing hormone (LH) level was assayed by LH EIA system (Amersham Biosciences, Piscataway, NJ, USA). The contents of cholesterol in serum and isolated Leydig cells were determined by cholesterol C-test kit (Wako).
Statistical analysis
Quantitative data were expressed as mean ± SEM. Statistical analysis was conducted using two-way ANOVA method with Bonferroni’s correction. A P value of <0.05 was considered to be statistically significant.
Results
2,4-D treatment decreases serum/testicular testosterone levels in a PPARα-dependent manner
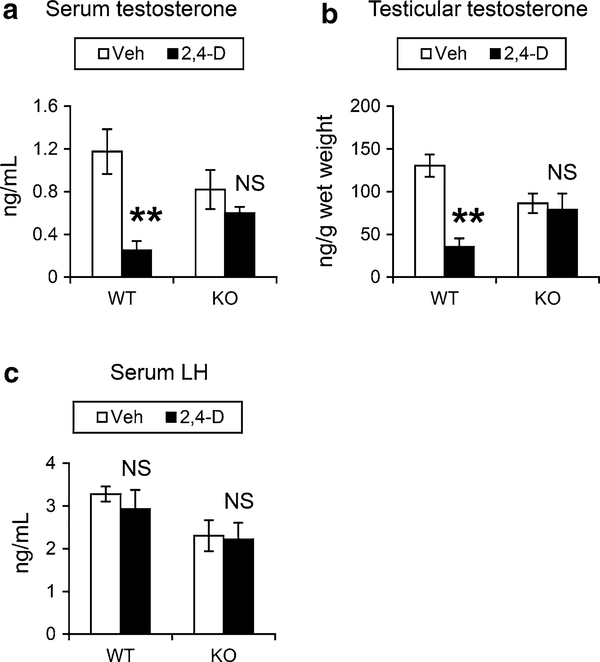
The effect of 2,4-D on testosterone, a representative sex hormone maintaining sperm production, was examined. 2,4-D treatment significantly decreased serum testosterone levels in the wild-type mice, but not in the Ppara-null mice (Fig. 1a). Serum testosterone levels in the vehicle-treated Ppara-null mice were lower than that in the similarly treated wild-type mice (Fig. 1a), which was in agreement with the results from a previous study (Gazouli et al. 2002), but this change did not reach statistical significance. Similar decreases in testicular testosterone levels were observed after the 2,4-D administration only in the wild-type mice (Fig. 1b). Serum LH concentrations were also measured since LH is one of the principal regulators of testicular testosterone synthesis, but no significant differences were found between the mouse groups (Fig. 1c). These results demonstrate that the 2,4-D treatment decreases serum/testicular testosterone levels in a PPARα-dependent manner.
Fig. 1.
2,4-D treatment decreased serum/testicular testosterone levels in a PPARα-dependent manner. 2,4-D methyl ester was dissolved in corn oil (4 mL/kg/day) just prior to administration to male Sv/129 wild-type (WT) or Ppara-null (KO) mice (16–20 weeks of age, 25–30 g of body weight) by daily gavage at 130 mg/kg/day. For the control groups, the same volume of corn oil was given as a vehicle (Veh). After treatment for 14 days, the mice were killed and serum/testicular testosterone (a, b) and serum luteinizing hormone (LH) levels (c) measured. Values were expressed as mean ± SEM (n = 5–8). Statistical analysis was performed using ANOVA test with Bonferroni’s correction. **P < 0.01; NS not significant between the 2,4-D-treated and Veh-treated mice in the same genotype
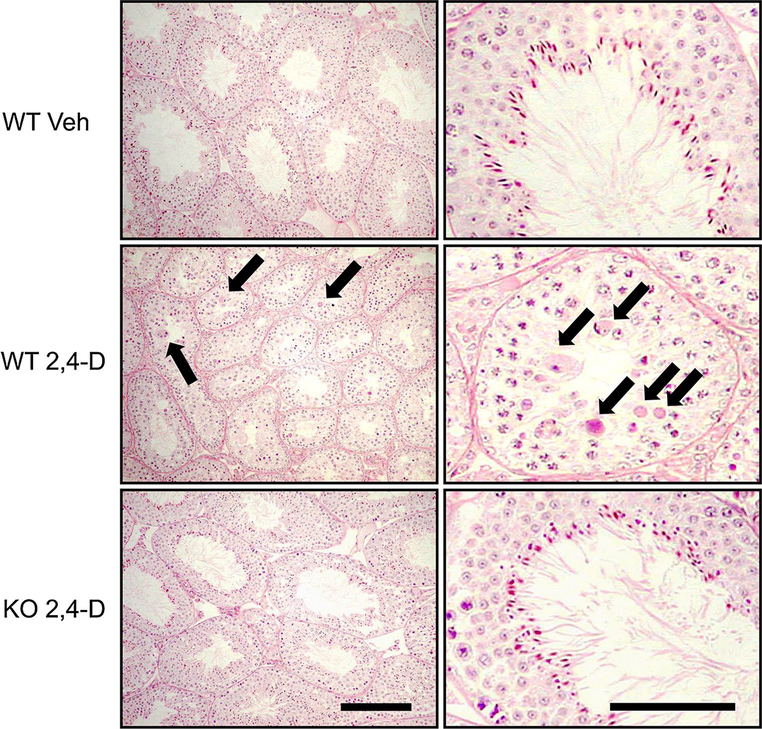
2,4-D treatment causes atrophy of seminiferous tubules and injury of seminiferous epithelium in a PPARα-dependent manner
Pathological changes in testes caused by 2,4-D exposure were assessed using light microscopy. In the control wild-type mice, germ cells were orderly arranged in the seminiferous epithelium, and no degeneration was found (Fig. 2, top). Similar findings were also seen in the control Ppara-null mice (data not shown). However, in the 2,4-D-treated wild-type mice, almost all of tubules were atrophic, and germ cell degeneration and disorganization of seminiferous epithelium were observed (Fig. 2, middle). The number of spermatozoa looked fewer in the 2,4-D-treated wild-type mice compared with the vehicle-treated wild-type mice (Fig. 2, middle, right). Two of the six mice showed severe disorganization of seminiferous epithelium, and three exhibited marked exfoliation of elongated and/or round spermatids. On the other hand, no apparent tubular atrophy was observed in the 2,4-D-treated Ppara-null mice (Fig. 2, bottom). There were a few tubules with exfoliation of spermatids, but germ cell degeneration was rarely observed in these mice (Fig. 2, bottom). Leydig cell degeneration could not be detected in any of the 2,4-D-treated mice by light microscopy.
Fig. 2.
2,4-D treatment caused atrophy of seminiferous tubules and injury of seminiferous epithelium in a PPARα-dependent manner. The paraffin-embedded sections were stained with periodic acid–Schiff and hematoxylin and subjected to light microscopy. Representative photomicrographs obtained from vehicle (Veh)- or 2,4-D-treated Sv/129 wild-type (WT) or Ppara-null (KO) mice were shown. Arrows indicate degenerated germ cells. Bars represent 100 μm in left photos and 50 μm in right ones
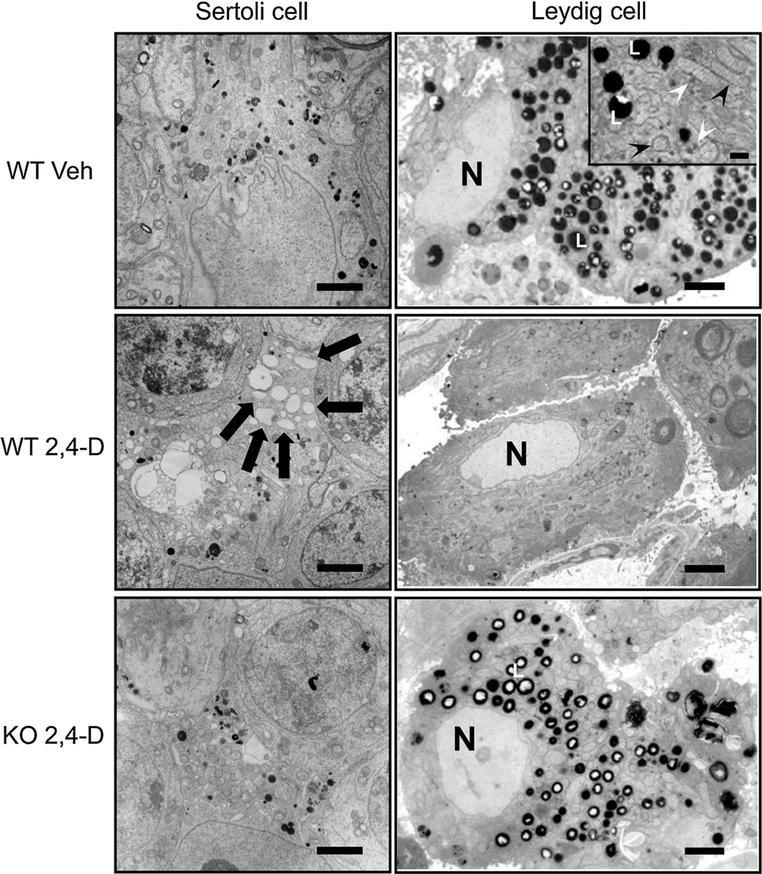
Electron microscopic examination revealed that Sertoli cells from the control wild-type and Ppara-null mice had no histological abnormalities (Fig. 3, left top, and data not shown, respectively). In the 2,4-D-treated wild-type mice, many vacuoles in various sizes were detected in the cytoplasm of Sertoli cells (Fig. 3, left middle), but the corresponding vacuoles were less abundant in the 2,4-D-treated Ppara-null mice (Fig. 3, left bottom). Sertoli cells support the architecture of seminiferous tubules and maintain testicular microenvironment required for development and maturation of spermatogenic cells (Maqdasy et al. 2013). Therefore, various morphological abnormalities in seminiferous tubules observed in the 2,4-D-treated wild-type mice are likely associated with Sertoli cell abnormalities.
Fig. 3.
Electron microscopic evaluation of Sertoli cells and Leydig cells. The testes were fixed by whole-body perfusion using 4 % paraformaldehyde and ultrathin sections were double-stained with uranyl acetate and lead citrate after cytochemical staining for catalase using alkaline DAB. Representative photomicrographs obtained from vehicle (Veh)- or 2,4-D-treated Sv/129 wild-type (WT) or Ppara-null (KO) mice were demonstrated. Left column Sertoli cells. Arrows indicate vacuoles which were observed only in 2,4-D-treated WT mice. Bars represent 2 μm. Right column Leydig cells. An inset in the top panel is a magnified photomicrograph showing organelles and lipid droplets in a Leydig cell from the control WT mice. Black and white arrowheads in the inset indicate mitochondria and peroxisomes, respectively. L lipid droplet, N nucleus of Leydig cell. Bars represent 2 μm. in regular photos and 0.5 μm in the inset
2,4-D treatment decreases lipid droplets in Leydig cells in a PPARα-dependent manner
Since maintenance of Sertoli cells is highly dependent on testosterone synthesized in Leydig cells (Skinner et al. 1991), pathological changes in Leydig cells were investigated. Based on the fact that 2,4-D possesses peroxisome-proliferating properties (Abdellatif et al. 1990), cytochemical staining for peroxisomal catalase was performed in Leydig cells to identify peroxisome proliferation. Electron microscopic analysis revealed that the number of peroxisomes seemed to be similar between the control and 2,4-D-treated wild-type mice (Fig. 3). However, many highly stained particles were found in the cytoplasm of Leydig cells in the control wild-type mice (Fig. 3, right top, indicated as L) and Ppara-null mice (data not shown). These particles were much larger in size than peroxisomes and exhibited a clearly different staining pattern from peroxisomes (the inset in Fig. 3, right top, indicated as white arrowheads). According to other reports (Reddy and Svoboda 1972; Mendis-Handagama et al. 1990), these particles were considered to be lipid droplets. The number of these particles was significantly decreased in the 2,4-D-treated wild-type mice (Fig. 3, right middle) compared with the controls, whereas such decreases were not seen in the 2,4-D-treated Ppara-nulls (Fig. 3, right bottom).
2,4-D treatment decreases cholesterol contents in Leydig cells in a PPARα-dependent manner
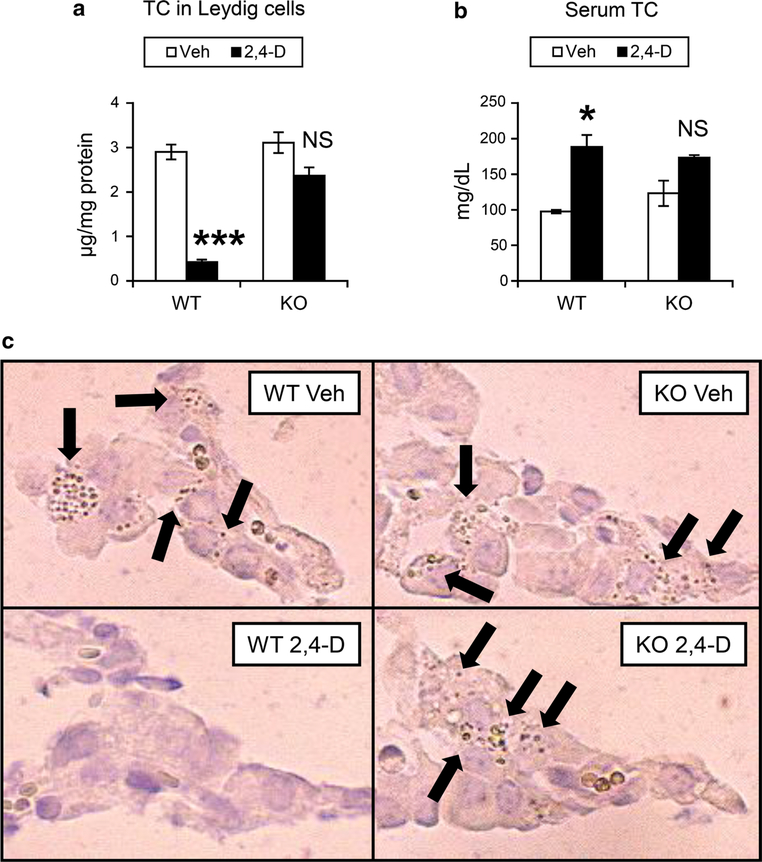
Since cholesterol and cholesterol ester are major components of lipid droplets in Leydig cells (Mrotek et al. 1981), the cholesterol contents were quantified using Leydig cells isolated from the mice. The levels of total cholesterol were significantly decreased in Leydig cells isolated from the 2,4-D-treated wild-type mice compared with the control wild-type mice (Fig. 4a). The reductions in total cholesterol in Leydig cells were not detected in the 2,4-D-treated Ppara-null mice (Fig. 4a). Serum total cholesterol concentrations were increased only in the 2,4-D-treated wild-type mice (Fig. 4b).
Fig. 4.
2,4-D treatment depleted cholesterol contents in Leydig cells in a PPARα-dependent manner. a, b Total cholesterol (TC) levels in isolated Leydig cells (a) and serum (b) were determined using the samples obtained from vehicle (Veh)- or 2,4-D-treated Sv/129 wild-type (WT) or Ppara-null (KO) mice. Values were expressed as mean ± SEM (n = 5). Statistical analysis was performed using ANOVA test with Bonferroni’s correction. *P < 0.05; ***P < 0.001; NS not significant between the 2,4-D-treated and Veh-treated mice in the same genotype. c The abundance of cholesterol ester in Leydig cells was assayed using frozen testis sections obtained from vehicle (Veh)- or 2,4-D-treated Sv/129 wild-type (WT) or Ppara-null (KO) mice and cytochemical staining according to the method of Emeis et al. Arrows indicate cholesterol-rich particles
Additionally, cholesterol esters in Leydig cells were stained using the method of Emeis et al. (1977) in which cholesterol esters preferentially stain brown. Some stained particles were observed in Leydig cells of the control wild-type mice, which were similarly seen in the control Ppara-null mice (Fig. 4c). These stained particles were rarely detected in the 2,4-D-treated wild-type mice (Fig. 4c), whereas many particles were found in the 2,4-D-treated Ppara-null mice (Fig. 4c). Collectively, these results indicate that the 2,4-D treatment significantly decreases cholesterol contents in Leydig cells in a PPARα-dependent manner. Since testosterone is synthesized from cholesterol, PPARα-mediated disruption of cholesterol metabolism in Leydig cells by 2,4-D administration presumably led to marked decreases in testicular testosterone and abnormalities in seminiferous epithelium and Sertoli cells.
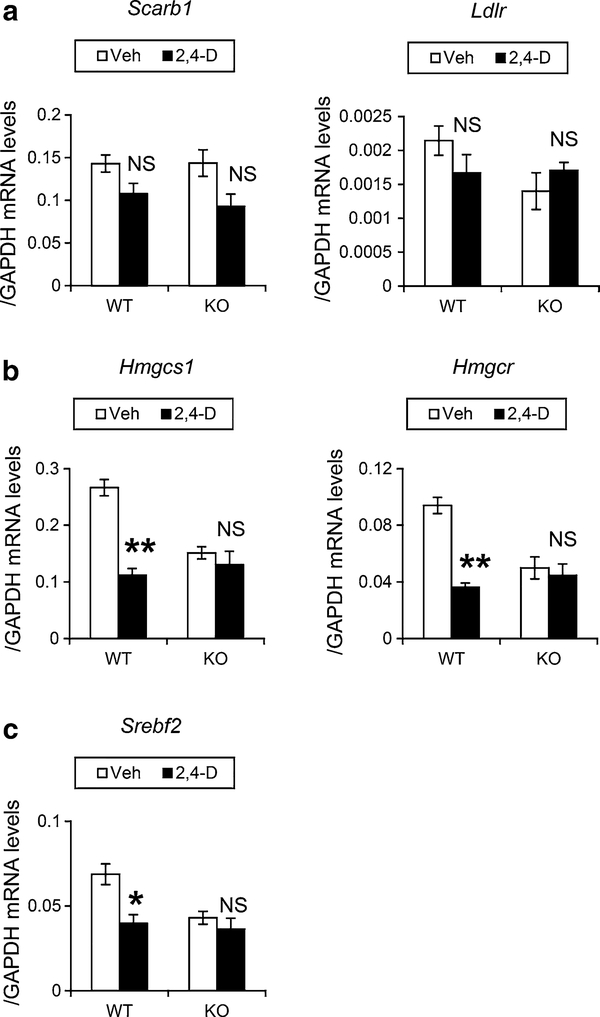
2,4-D treatment down-regulates the expression of genes involved in de novo cholesterol synthesis in Leydig cells in a PPARα-dependent manner
To understand the mechanism of PPARα-dependent disruption of cholesterol/testosterone metabolism in Leydig cells, the mRNA levels of genes associated with cholesterol/testosterone metabolism were determined. No significant changes were observed in the mRNAs encoding scavenger receptor B1 (Scarb1) and low-density-lipoprotein receptor (Ldlr) in the wild-type mice after the 2,4-D treatment (Fig. 5a). Very low levels of Ldlr mRNA suggest that Scarb1 plays a major role in cholesterol uptake on the surface of Leydig cells. The 2,4-D treatment significantly decreased the levels of mRNA encoding 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase 1 (Hmgcs1) and reductase (Hmgcr), rate-limiting enzymes in de novo cholesterol synthesis, in the wild-type mice, but not in the similarly treated Ppara-null mice (Fig. 5b). The expression of mRNA encoding sterol-responsive element-binding protein 2 (SREBP2, encoded by Srebf2), one of the master regulators of Hmgcs1 and Hmgcr mRNA levels, was also decreased (Fig. 5c). These results indicate that cholesterol synthesis is suppressed by 2,4-D administration through PPARα.
Fig. 5.
Quantification of mRNA levels of genes associated with cholesterol metabolism in Leydig cells. Leydig cells were isolated and purified from vehicle (Veh)- or 2,4-D-treated Sv/129 wild-type (WT) or Ppara-null (KO) mice and were subjected to qPCR analysis. a The mRNA levels of genes encoding scavenger receptor BI (Scarb1) and low-density-lipoprotein receptor (Ldlr). b The mRNA levels of genes encoding HMG-CoA synthase 1 (Hmgcs1) and reductase (Hmgcr), involved in de novo cholesterol synthesis. c The mRNA levels of genes encoding sterol-responsive element-binding protein 2 (Srebf2). The mRNA levels of these mRNAs were normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA and expressed as mean ± SEM (n = 3). Statistical analysis was performed using ANOVA test with Bonferroni’s correction. *P < 0.05; **P < 0.01; NS not significant between the 2,4-D-treated and Veh-treated mice in the same genotype
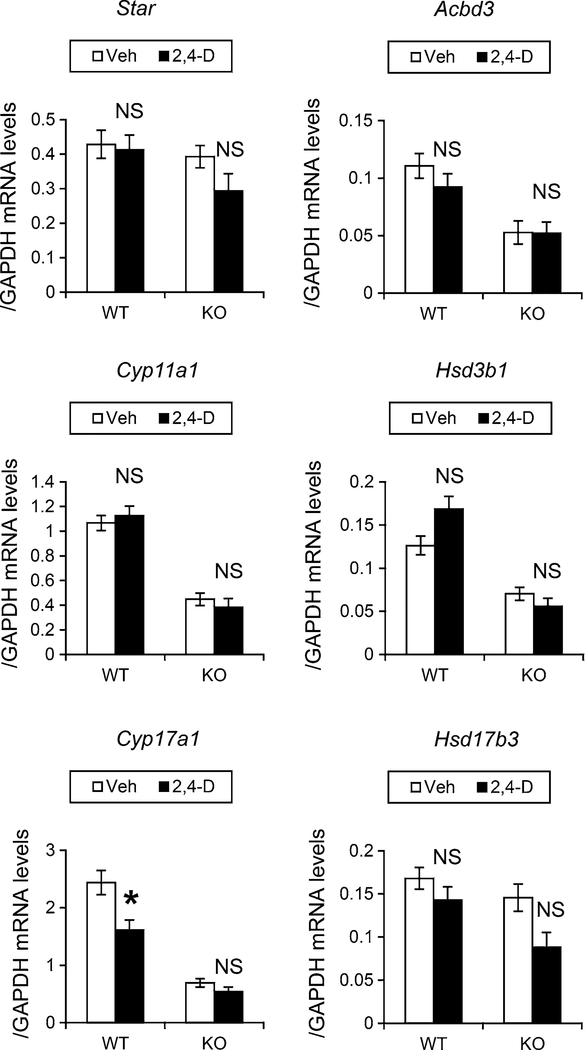
Next, the mRNA levels of the six key genes involved in testosterone synthesis from cholesterol in Leydig cells were examined. After the 2,4-D treatment, no significant changes were observed in the mRNAs encoding steroidogenic acute regulatory protein (Star), peripheral-type benzodiazepine receptor (Acbd3), cytochrome P450 (CYP) 11A1 (Cyp11a1), hydroxy-delta-5-steroid dehydrogenase, 3-beta and steroid delta-isomerase 1 (Hsd3b1), and hydroxysteroid (17-beta) dehydrogenase 3 (Hsd17b3) between the two mouse groups (Fig. 6). CYP17A1 (Cyp17a1) mRNA expression was decreased in the wild-type mice after the 2,4-D treatment, while no change was observed in the Ppara-null mice (Fig. 6). The decrease in Cyp17a1 mRNA by the 2,4-D treatment may faintly influence whole testosterone synthesis ability.
Fig. 6.
Quantification of mRNA levels of genes associated with testosterone metabolism in Leydig cells. The cDNA samples used in Fig. 5 were adopted. The mRNA levels of genes encoding steroidogenic acute regulatory protein (Star), peripheral-type benzodiazepine receptor (Acbd3), cytochrome P450 (CYP) 11A1 (Cyp11a1), hydroxy-delta-5-steroid dehydrogenase, 3 beta- and steroid delta-isomerase 1 (Hsd3b1), CYP17A1 (Cyp17a1), and hydroxysteroid (17-beta) dehydrogenase 3 (Hsd17b3) were measured, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) mRNA, and expressed as mean ± SEM (n = 3). Statistical analysis was performed using ANOVA test with Bonferroni’s correction. *P < 0.05; NS not significant between the 2,4-D-treated and vehicle (Veh)-treated mice in the same genotype. WT wild-type mice, KO Ppara-null mice
Furthermore, the constitutive mRNA levels of these genes involved in cholesterol/testosterone synthesis in Leydig cells were compared between the control wild-type mice and the Ppara-null counterparts. Interestingly, the mRNA levels of Hmgcs1, Hmgcr, Acbd3, Cyp11a1, Hsd3b1, and Cyp17a1 in the control Ppara-null mice were approximately a half of those in the control wild-type mice (Figs. 5, 6, P = 0.006, 0.008, 0.028, 0.001, 0.038, and 0.001 for Hmgcs1, Hmgcr, Acbd3, Cyp11a1, Hsd3b1, and Cyp17a1, respectively, calculated by Bonferroni’s post hoc analysis). These decreases were correlated with tendencies to lower serum/testicular testosterone levels (Fig. 1a, b).
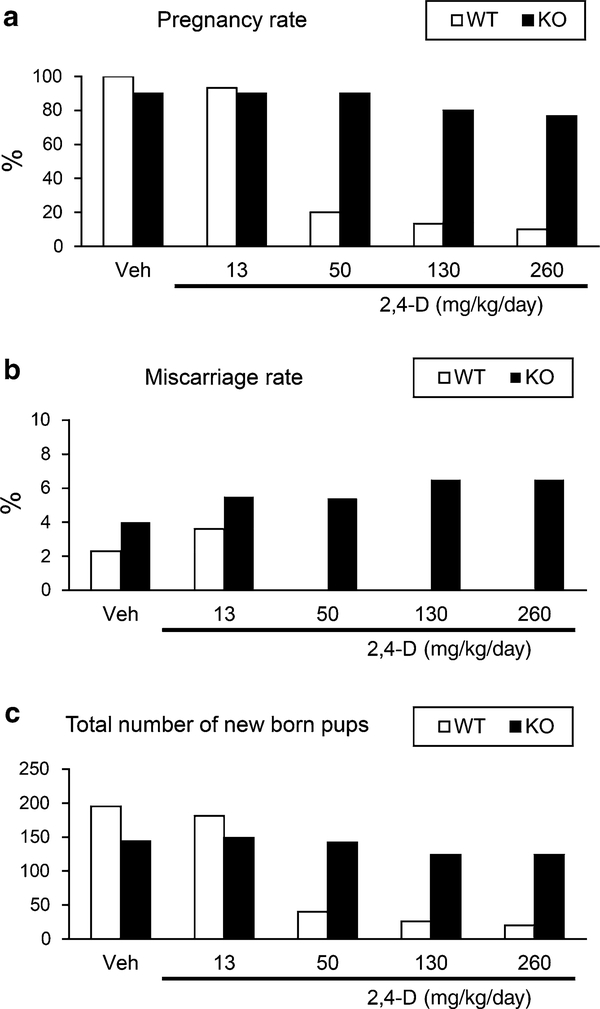
2,4-D treatment reduces pregnancy rate in a PPARα-dependent manner
Lastly, in order to assess the influence of 2,4-D for male fertility, the male wild-type mice treated with 2,4-D were mated to the naïve female wild-type mice, and the incidence of first pregnancy and the number of total pups was evaluated. The pregnancy rate was reduced by the 2,4-D treatment in a dose-dependent manner between the wild-type males and females, but this reduction was not found in the Ppara-null males and wild-type females (Fig. 7a). A similar tendency was detected in the number of total pups obtained in the first childbirth (Fig. 7c). Therefore, PPARα-mediated testicular toxicity by the 2,4-D administration may lead to impaired male fertility in mice. Interestingly, the miscarriage rates tended to be high in the Ppara-null mice despite the absence or presence of the 2,4-D treatment under unknown reasons (Fig. 7b).
Fig. 7.
2,4-D treatment reduced pregnancy rate and new born pup number in a PPARα-dependent manner. Male Sv/129 wild-type (WT) or Ppara-null (KO) mice (6–8 weeks of age) were mated with untreated female wild-type mice at the similar age and body weight that has never experienced pregnancy (1 male vs. 3 females in each cage). During mating, male mice were treated with vehicle (Veh) or 2,4-D at four different doses (13, 50, 130, or 260 mg/kg/day) every day (n = 10 males/treatment group/genotype). The incidence of first pregnancy rate (a), miscarriage rate (b), and the total number of pups delivered (c) were assessed for 12 weeks
Discussion
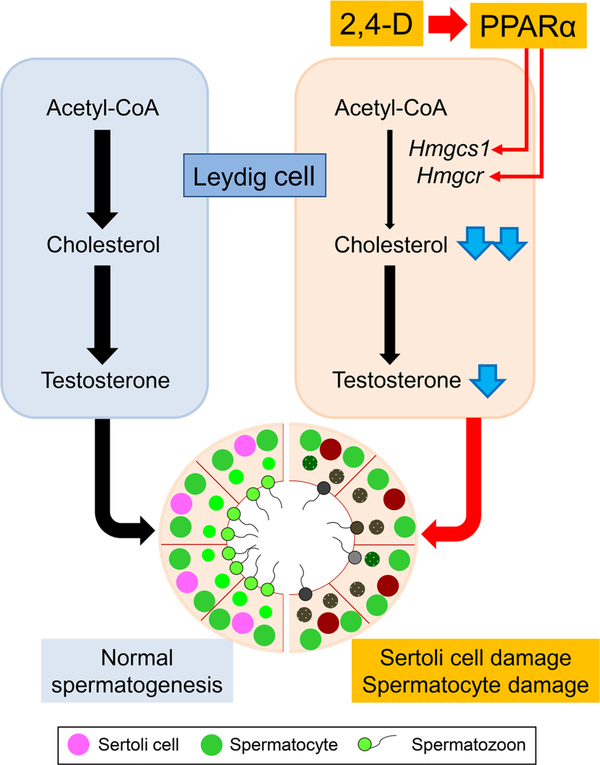
2,4-D, a possible endocrine disruptor, is widely used as a herbicide, and while role in its testicular toxicity has been reported (Lerda and Rizzi 1991; Munro et al. 1992; Charles et al. 1996), the precise mechanism has not been clarified. This study revealed that 2,4-D decreased cholesterol contents in testosterone-synthesizing Leydig cells likely due to suppressed mRNAs of Hmgcs1/Hmgcr, rate-limiting enzymes in de novo cholesterol-synthesizing pathway. However, the mRNAs encoding the major proteins involved in testosteronogenesis were unchanged by 2,4-D, except for CYP17A1, indicating that 2,4-D caused exhausted cholesterol in the cells and subsequent decreases in testicular testosterone synthesis. These changes were associated with spermatocyte/Sertoli cell damages, leading to reduced pregnancy rate and the number of total pups in the first childbirth. Importantly, these abnormalities induced by the 2,4-D treatment were not detected in Ppara-null mice. Therefore, the 2,4-D treatment impaired normal spermatogenesis due to disrupting cholesterol/testosterone homeostasis in Leydig cells through PPARα. These findings propose a possible mechanism by which 2,4-D causes testicular dysfunction (Fig. 8).
Fig. 8.
Proposed mechanism of PPARα-mediated 2,4-D-induced testicular toxicity. Testosterone synthesized in Leydig cells plays an important role for the maintenance of normal spermatogenesis. Source of testosterone is cholesterol contained in circulating lipoproteins and newly synthesized from acetyl-CoA. 2,4-D mainly suppresses mRNAs of Hmgcs1/Hmgcr, rate-limiting enzymes of de novo cholesterogenesis, via PPARα signaling and reduces testicular cholesterol levels. PPARα-mediated disruption of cholesterol/testosterone homeostasis in Leydig cells causes Sertoli cell/spermatocyte damage and testicular dysfunction
2,4-D is also a weak peroxisome proliferator in hepatocytes. While some peroxisome proliferators, such as di2-ethylhexyl phthalate (DEHP) (Parks et al. 2000), trichloroethylene (Kumar et al. 2000), Wy-14,643, and clofibric acid (Gazouli et al. 2002) are known to lower serum testosterone levels causing testicular atrophy and impaired spermatogenesis, the mechanistic link between decreased testosterone and peroxisome proliferation or PPARα was not understood. The previous study demonstrated that the treatment of isolated rat Leydig cells with bezafibrate, a typical peroxisome proliferator, decreased intracellular testosterone contents, but the treatment of MA-10 and R2C Leydig cell lines with the same concentration of bezafibrate did not increase catalase activity, an indicator of peroxisome proliferation, suggesting that bezafibrate can directly inhibit Leydig cell testosterone synthesis independently of peroxisome proliferation (Gazouli et al. 2002). This is in agreement with the result of the present study that no remarkable peroxisome proliferation was detected in Leydig cells of the 2,4-D-treated wild-type mice using electron microscopy and cytochemical staining for catalase. While it was also documented that the treatment of mice with DEHP or Wy-14,643 led to a PPARα-dependent decrease in serum testosterone levels that was associated with lower testicular Acbd3 mRNA levels (Gazouli et al. 2002), metabolic alterations other than Acbd3 that occurred in Leydig cells have not been accessed. The present study conducted comprehensive analysis of cholesterol/testosterone metabolism using isolated Leydig cells from the 2,4-D-treated wild-type and Ppara-null mice and uncovered PPARα-mediated disruption in Leydig cholesterol/testosterone-synthesizing pathway due to 2,4-D. This study offers a useful strategy for analyzing the mechanism of testicular toxicity induced by endocrine disruptors and the contribution of PPARα. It is intriguing to examine whether other endocrine disruptors and peroxisome proliferators similarly disrupt cholesterol/testosterone metabolism in Leydig cells.
The mechanism by which 2,4-D suppressed the expression of Hmgcs1 and Hmgcr via PPARα in Leydig cells needs further investigation. In liver, bezafibrate increased mRNAs of Srebf2 and its downstream genes, such as Hmgcs1 and Hmgcr, through PPARα activation (Nakajima et al. 2008). It was documented that after the Wy-14,643 treatment, Acbd3 mRNA was induced in liver but decreased in testis via PPARα, suggesting the presence of reciprocity in PPARα-mediated gene regulation between liver and testis (Gazouli et al. 2002). Future studies using hepatocyte- or Leydig cell-specific Ppara-disrupted mice might address this question.
Another intriguing result was suppressed Hmgcs1, Hmgcr, Acbd3, Cyp11a1, Hsd3b1, and Cyp17a1 mRNAs in Leydig cells of the control Ppara-null mice. A trend of lower serum/testicular testosterone was also found in the control Ppara-null mice compared with the wild-type mice. Similarly, the previous study reported decreased circulating testosterone in the control Ppara-null mice compared with the wild-type counterparts (Gazouli et al. 2002). These findings suggest that the presence of PPARα is required for maintaining constitutive testosterone synthesis in Leydig cells, but the control Ppara-null mice did not exhibit cholesterol depletion in Leydig cells and male infertility. Testosterone synthesis in Leydig cells is regulated by several transcription factors other than PPARα, such as steroidogenic factor-1, liver receptor homologue-1, and liver X receptor α (Maqdasy et al. 2013). In the absence of PPARα, a suitable adaptation mechanism might exist in cholesterol/testosterone homeostasis to maintain spermatogenesis, so these abnormalities might not occur in Ppara-null mice.
To maintain normal reproductive function, a feedback mechanism is present, where decreased circulating testosterone increases circulating LH that stimulates testosterone synthesis. In this study, there were no significant increases either in LH levels or those in the mRNA expression of genes induced by LH, such as Scarb1 and Ldlr, after the 2,4-D administration. The feedback mechanism did not work, possibly due to the short-term treatment.
The results in the present study let us to consider human risk of PPARα-mediated testicular toxicity. PPARα was reported to be abundantly expressed in human Leydig cells and spermatocytes (Schultz et al. 1999). Mono-2-ethyl-hexyl phthalate, a monoester of DEHP, interacted with human PPARα and reduced testosterone in adult human testis (Lapinskas et al. 2005; Corton and Lapinskas 2005; Desdoits-Lethimonier et al. 2012). Although there are no studies corroborating the direct relationship between 2,4-D-mediated testicular toxicity and PPARα in humans, human testicular toxicity induced by long-term exposure of endocrine disruptors/herbicides might be associated with PPARα.
In conclusion, these results indicate a critical role for PPARα in 2,4-D-induced testicular dysfunction due to disrupted cholesterol/testosterone homeostasis in Leydig cells. These findings propose a possible mechanism on how 2,4-D, a widely used herbicide, disrupts male reproductive system.
Supplementary Material
Acknowledgments
The authors thank Drs. Tamie Nakajima, Osamu Yamanoshita, and Minoru Omura for advice in support of this study.
Abbreviations
- 2,4-D
2,4-Dichlorophenoxyacetic acid
- PPARα
Peroxisome proliferator-activated receptor α
- HMG-CoA
3-Hydroxy-3-methylglutaryl coenzyme A
- PBS
Phosphate-buffered saline
- DAB
3,3′-Diaminobenzidine
- BSA
Bovine serum albumin
- EBSS
Earle’s balanced salt solution
- qPCR
Quantitative polymerase chain reaction
- LH
Luteinizing hormone
- SD
Standard deviation
- CYP
Cytochrome P450
- DEHP
Di-2-ethylhexyl phthalate
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s00204-016-1669-z) contains supplementary material, which is available to authorized users.
References
- Abdellatif AG, Préat V, Vamecq J, Nilsson R, Roberfroid M (1990) Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis 11:1899–1902 [DOI] [PubMed] [Google Scholar]
- Amer SM, Aly FA (2001) Genotoxic effect of 2,4-dichlorophenoxy acetic acid and its metabolite 2,4-dichlorophenol in mouse. Mutat Res 494:1–12 [DOI] [PubMed] [Google Scholar]
- Aoyama T, Yamano S, Waxman DJ, Lapenson DP, Meyer UA, Fischer V, Tyndale R, Inaba T, Kalow W, Gelboin HV et al. (1989) Cytochrome P-450 hPCN3, a novel cytochrome P-450 IIIA gene product that is differentially expressed in adult human liver. cDNA and deduced amino acid sequence and distinct specificities of cDNA-expressed hPCN1 and hPCN3 for the metabolism of steroid hormones and cyclosporine. J Biol Chem 264:10388–10395 [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ (1998) Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem 273:5678–5684 [DOI] [PubMed] [Google Scholar]
- Biegel LB, Hurtt ME, Frame SR, O’Connor JC, Cook JC (2001) Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 60:44–55 [DOI] [PubMed] [Google Scholar]
- Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137:354–366 [DOI] [PubMed] [Google Scholar]
- Charles JM, Cunny HC, Wilson RD, Bus JS (1996) Comparative subchronic studies on 2,4-dichlorophenoxyacetic acid, amine, and ester in rats. Fundam Appl Toxicol 33:161–165 [DOI] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ (2005) Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83:4–17 [DOI] [PubMed] [Google Scholar]
- Desdoits-Lethimonier C, Albert O, Le Bizec B, Perdu E, Zalko D, Courant F, Lesné L, Guillé F, Dejucq-Rainsford N, Jégou B (2012) Human testis steroidogenesis is inhibited by phthalates. Hum Reprod 27:1451–1459 [DOI] [PubMed] [Google Scholar]
- Emeis JJ, Van Gent CM, Van Sabben CM (1977) An enzymatic method for the histochemical localization of free and esterified cholesterol separately. Histochem J 9:197–204 [DOI] [PubMed] [Google Scholar]
- Frantz SW, Kropscott BE (1993) Pharmacokinetic evaluation of a single oral administration of the 2-ethylhexyl (isooctyl) ester of 2,4-D to Fischer 344 rats. J Occup Med Toxicol 2:75–85 [Google Scholar]
- Gale JS, Wakefield JS, Ford HC (1982) Isolation of rat Leydig cells by density gradient centrifugation. J Endocrinol 92:293–302 [DOI] [PubMed] [Google Scholar]
- Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V (2002) Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology 143:2571–2583 [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Hora K, Tanaka N, Usuda N, Kiyosawa K, Nakajima T, Gonzalez FJ, Aoyama T (2002) Identification of functions of peroxisome proliferator-activated receptor alpha in proximal tubules. J Am Soc Nephrol 13:1691–1702 [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Katoh H, Nakajima S, Kozuka H, Uchiyama M (1984) Effects of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid on peroxisomal enzymes in rat liver. Biochem Pharmacol 33:241–245 [DOI] [PubMed] [Google Scholar]
- Kumar P, Prasad AK, Dutta KK (2000) Steroidogenic alterations in testes and sera of rats exposed to trichloroethylene (TCE) by inhalation. Hum Exp Toxicol 19:117–121 [DOI] [PubMed] [Google Scholar]
- Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard S, Swanson C, Cattley RC, Corton JC (2005) Role of PPARalpha in mediating the effects of phthalates and metabolites in the liver. Toxicology 207:149–163 [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ (1995) Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15:3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerda D, Rizzi R (1991) Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D). Mutat Res 262:47–50 [DOI] [PubMed] [Google Scholar]
- Maqdasy S, Baptissart M, Vega A, Baron S, Lobaccaro JM, Volle DH (2013) Cholesterol and male fertility: what about orphans and adopted? Mol Cell Endocrinol 368:30–46 [DOI] [PubMed] [Google Scholar]
- Mattsson JL, Charles JM, Yano BL, Cunny HC, Wilson RD, Bus JS (1997) Single-dose and chronic dietary neurotoxicity screening studies on 2,4-dichlorophenoxyacetic acid in rats. Fundam Appl Toxicol 40:111–119 [DOI] [PubMed] [Google Scholar]
- Mendis-Handagama SM, Zirkin BR, Scallen TJ, Ewing LL (1990) Studies on peroxisomes of the adult rat Leydig cell. J Androl 11:270–278 [PubMed] [Google Scholar]
- Mrotek JJ, Mathew JK, Curtis JC, Johansson KR (1981) A method for the isolation of lipid droplet fractions from decapsulated rat adrenals. Steroids 38:229–241 [DOI] [PubMed] [Google Scholar]
- Munro IC, Carlo GL, Orr JC, Sund KG, Wilson RM, Kennepohl E, Lynch BS, Jablinske M (1992) A comprehensive, integrated review and evaluation of scientific evidence relating to the safety of the herbicide 2,4-D. Int J Toxicol 11:559–664 [Google Scholar]
- Nakajima T, Tanaka N, Sugiyama E, Kamijo Y, Hara A, Hu R, Li G, Li Y, Nakamura K, Gonzalez FJ, Aoyama T (2008) Cholesterol-lowering effect of bezafibrate is independent of peroxisome proliferator-activated receptor activation in mice. Biochem Pharmacol 76:108–119 [DOI] [PubMed] [Google Scholar]
- Novikoff AB, Goldfischer S (1969) Visualization of peroxisomes (microbodies) and mitochondria with diaminobenzidine. J Histo-chem Cytochem 17:675–680 [DOI] [PubMed] [Google Scholar]
- Ozaki K, Mahler JF, Haseman JK, Moomaw CR, Nicolette ML, Nyska A (2001) Unique renal tubule changes induced in rats and mice by the peroxisome proliferators 2,4-dichlorophenoxyacetic acid (2,4-D) and WY-14643. Toxicol Pathol 29:440–450 [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE Jr (2000) The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58:339–349 [DOI] [PubMed] [Google Scholar]
- Reddy J, Svoboda D (1972) Microbodies (peroxisomes) in the interstitial cells of rodent testes. Lab Investig 26:657–665 [PubMed] [Google Scholar]
- Schultz R, Yan W, Toppari J, Völkl A, Gustafsson JA, Pelto-Huikko M (1999) Expression of peroxisome proliferator-activated receptor alpha messenger ribonucleic acid and protein in human and rat testis. Endocrinology 140:2968–2975 [DOI] [PubMed] [Google Scholar]
- Skinner MK, Norton JN, Mullaney BP, Rosselli M, Whaley PD, Anthony CT (1991) Cell-cell interactions and the regulation of testis function. Ann N Y Acad Sci 637:354–363 [DOI] [PubMed] [Google Scholar]
- Steinberger E, Steinberger A, Vilar O (1966) Cytochemical study of delta-5–3-beta-hydroxysteroid dehydrogenase in testicular cells grown in vitro. Endocrinology 79:406–410 [DOI] [PubMed] [Google Scholar]
- Watanabe K, Fujii H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, Kamijo Y, Gonzalez FJ, Aoyama T (2000) Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem 275:22293–22299 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.