Abstract
Adult orthodontics has gained widespread acceptance recently with the introduction of more esthetic options for the patient. The major deterrent that remains is the prolonged treatment time associated with comprehensive orthodontic treatment. The objective of this paper is to present a review of techniques, which could be employed by the orthodontist in conjunction with a periodontist to enhance the rate of orthodontic tooth movement. The biological rationale and clinical manipulation have been discussed with a brief review of the current literature about these techniques. The interdisciplinary approach involving the orthodontist and the periodontist can benefit the patient by affording them with reduced treatment time.
Keywords: Alveolar process/surgery, cortical bone/surgery, malocclusion/therapy, osteogenesis, tooth movement techniques/methods
INTRODUCTION
The demand, as well as acceptance of orthodontic treatment by the adult population, has increased considerably, with the availability of more esthetic options for the patients to choose from, the problem of bulky steel brackets more or less has been addressed. The other reason most commonly cited for reluctance to accept the orthodontic treatment is the long duration of the treatment. A recent systematic review has indicated that a comprehensive orthodontic treatment takes <2 years to be completed.[1] Patients expect a shorter duration of treatment time of about 6–12 months.[2]
Many authors have described methods to reduce the overall treatment time for comprehensive orthodontic treatment. These methods can be broadly classified into nonsurgical methods and surgical methods.
Nonsurgical methods include the use of limited orthodontic treatment, self-ligating brackets,[3,4] customized appliance,[5,6] medication,[7,8,9,10,11] microvibrations,[12] low-intensity laser,[13] photobiomodulation,[14] electromagnetic fields,[15] and direct electrical currents.[16]
Surgical methods include micro-osteoperforations,[17] piezocision,[18] corticotomies,[19] osteotomies/periodontal ligament (PDL) distraction,[20] surgery first.[21]
Out of the surgical methods outlined, corticotomies, piezocision and micro osteoperforations can be utilized in a regular clinical setup without the need for general anesthesia. These may prove to be a clinical tool which is readily available to the clinician to accelerate the tooth movement and decrease the overall treatment time, without the need for additional inventory. Following is a biological rationale and a brief narrative about three procedures.
BIOLOGIC RATIONALE
Bone is a dynamic tissue, and it reacts to the external load put on it by modifying its internal as well as external structure.[22] The cellular components within the bone play an intricate role by complex interactions between them to bring about the above-said changes. These cellular components within the bony tissue are osteocytes, osteoblasts, and osteoclast. Osteoblasts line the bone surface and secrete the organic bone matrix (Osteoid), which then gets calcified. Mature osteoblasts which get trapped in this calcified matrix turn into osteocytes. These osteocytes function as a sensor of mechanical load on the bone and initiate bone remodeling involving both the osteoblasts and osteoclasts. This bone remodeling is an essential aspect of orthodontic tooth movement, any attempt to accelerate the orthodontic tooth movement is centered around modifying this remodeling process.
Research in the field of orthodontic tooth movement has revealed that osteoclastic activity is the rate-limiting step, which determines the rate of orthodontic tooth movement. Any attempt to increase the rate of orthodontic tooth movement should be focused around osteoclast and on the various processes by which osteoclast are recruited and differentiated to initiate bone resorption.[23]
Inflammatory markers like cytokines play an important role in osteoclast recruitment and differentiation, any process leading to an increase in the levels of these proinflammatory markers may increase the rate of tooth movement. There are different methods described in the literature to increase the levels of cytokines locally.[24,25,26]
The levels of the cytokines can be increased by locally injecting them at the site of orthodontic tooth movement, but this method is not conducive at a clinical level as these inflammatory markers have a very short half-life, when injected and disintegrate before any useful clinical tooth movement can occur.[27]
Another method to raise the levels of these cytokines locally is to induce microtrauma within the bone and the PDL. Procedures such as corticotomy, piezocision and micro-osteo perforations (MOP) are employed to induce trauma, which in turn increase the levels of pro-inflammatory markers such as tumor necrosis factor alpha, interlukin (IL)-1, IL-6 locally. These procedures can be utilized in a clinical set up to increase the rate of tooth movement temporally.[28]
The concept of the inflammatory cascade and its role in wound healing led to the development of corticotomy facilitated tooth movement by L C Bryan in 1893. It was later re-introduced by Kole in 1959 for rapid movement of the tooth.[29] His method involved osteotomy cuts being placed subapically into the full alveolus to cause bodily tooth movement after application of orthodontic forces. Due to its invasive nature and the amount of surgical trauma, the patient acceptance of this method was limited.
Wilcko et al.[30] reported two cases of de-crowding with the use of corticotomy cuts extending into the cortical bone plates barely entering the medullary space. The biological response to iatrogenic injury with corticotomy results from “Regionally Accelerated Phenomenon” (RAP)[31] as described by Frost, is seen as a consequence of the inflammation of the wound area. This is characterized by transient functional osteopenia followed by accelerated bone turnover over the course of time. The decreased mineral density allows for easier orthodontic movement of teeth during the remodeling and healing. RAP is said to typically peak till about 1–2 months and lasts about 4 months postsurgery. It is regulated by the RANK-RANKL/OPG axis where osteoclast accumulation takes place in the direction of the movement of the tooth and new bone formation at the tension side of the tooth.[32,33,34] New bone mineralization takes place at about 20–55 days, where the fibrous tissue is later replaced by bone.[35]
CORTICOTOMIES
Wilcko combined the refined corticotomy-facilitated orthodontic technique with alveolar augmentation and named the orthodontic and periodontal aspects of this procedure the accelerated osteogenic orthodontics (AOO) technique, and more recently, the periodontally AOO surgical technique, respectively.
Case selection
PAOO can be utilized to treat Class I cases having moderate-to-severe crowding, mild-to-moderate Class II and Class III cases and patients requiring unilateral expansion. A close interaction between the orthodontist and the periodontist is required. Areas requiring corticotomies are defined, and teeth which are going to serve as anchor teeth are delineated earlier. Any adjunct procedure which might be required along with the corticotomies, for example, free gingival grafts should also be planned beforehand to reduce any further surgical trauma to the patient.
Orthodontic strap up should be planned carefully as the application of orthodontic force should not be delayed >2 weeks after the surgery.
Procedure
Generally, the orthodontic appliance is placed 1 week before the surgical procedure and subsequent orthodontic visits are planned at a 2-week interval.
The surgical procedure could be performed under general or local anesthesia as required. Full-thickness flaps are raised labially and lingually [Figure 1]. Including or excluding the interdental papilla in the flap depends on the preference of the surgeon. Interdental papilla on the lingual side between the maxillary central incisors are usually not included in the flap as the presence of the nasopalatine foramen in that region prevents any osteotomy to be performed in that region. In addition, vertical releasing incisions can be used and are usually placed one tooth away from the intended area of the osteotomy. Adequate flap reflection should be done till area near the apices of the teeth. Sometimes, a full-thickness flap in the coronal portion and a split thickness flap in the apical portions maybe raised to ensure a tensionless closure of the flap after the surgical procedure.
Figure 1.
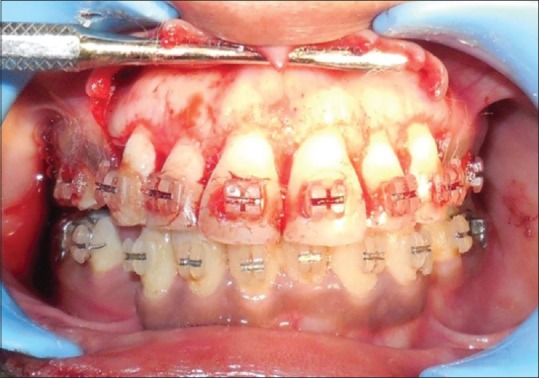
Full thickness flap reflected
A high-speed handpiece with a No 1 or No 2-round bur, physio dispenser, and piezoelectric knife can be utilized for decortication. Vertical grooves are made in the inter-radicular portion of the alveolar bone, extending from 2 to 3 mm from the crest of the alveolar bone and going 2 mm apical to the root apices. Decortication is done on both the labial/buccal and palatal/lingual aspects. The vertical osteotomies are connected with circular osteotomies [Figure 2]. Care should be taken to avoid any neurovascular structures.
Figure 2.
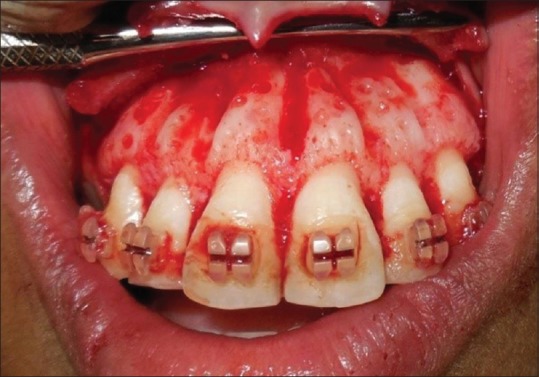
Corticotomy cuts placed
As recommended by Wilcko, all the areas undergoing decortication require a bone graft, with the decorticated surface acting as a scaffold for the graft material. The amount and direction of tooth movement anticipated and pretreatment thickness of existing alveolar bone determine the amount of graft. Usually, 0.25–0.5 ml of graft material is required per tooth. Autogenous bone, allograft, xenograft, or a combination of these can be utilized with barrier membranes usually not being required. The flaps are sutured back making sure that there is no tension present. Interrupted sutures with a nonresorbable material are preferred [Figure 3].
Figure 3.
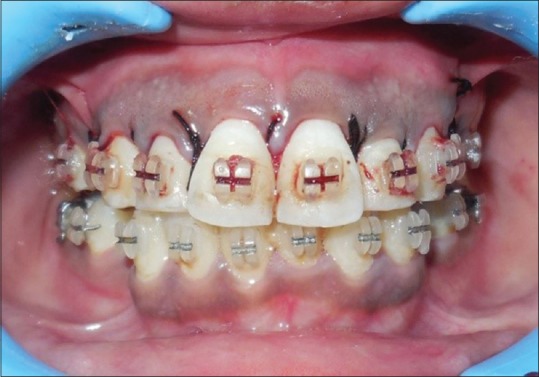
Interrupted sutures placed
Various studies in orthodontic patients have addressed the efficacy of corticotomy in accelerating the tooth movement in an orthodontic patient. Bhat et al. have demonstrated the effectiveness of this procedure in published case reports.[36] The studies by Mohammed et al.[37] in 2011 and Al-Naoum et al.[38] in 2014 both have concluded that corticotomy-assisted accelerated tooth movement may be a viable treatment option for adults seeking orthodontic treatment. A systematic review[39] in 2013 compared five different methods used for accelerating orthodontic tooth movement (low-level laser therapy, corticotomy, electrical current, pulsed electromagnetic fields, and dentoalveolar or periodontal distraction). The authors concluded that among the five methods, corticotomy was the most reliable and safe method to accelerate tooth movement hence reducing the overall treatment time. Two systematic reviews one in 2014[40] and another in 2018[41] corroborated with the above-mentioned statement; however, more recently the Cochrane review[42] and a meta-analysis[43] on the same subject suggested that the level of evidence in support of corticotomy and surgically-assisted accelerated tooth movement was low and well-designed studies were required to make definitive evidence-based decisions. Therefore, to summarize, while there are suggestions regarding corticotomy in being effective in accelerating orthodontic tooth movement; further research is needed to justify it as a clinical tool which could be used regularly.
Piezocision
An alternative to the conventional corticotomies is piezocision, which is a conservative procedure that does not involve raising a full-thickness flap. Due to this reason, patients readily accept this procedure in place of a conventional corticotomy. An animal histological study conducted has revealed that RAP due to piezocision may be greater than conventional corticotomy because of the use of Piezosurgery knife at specific vibrations.[44] This could be due to the additive effect of the osteocytes’ response to micro-vibrations created by the ultrasonic handpiece at specific settings.
Procedure
Similar to corticotomy, the orthodontic appliance should be in position 1 week before the piezocision. A mid-level vertical incision is placed on the buccal and the interdental aspect to facilitate the insertion of the piezoelectric knife. A 3-mm deep piezoelectric corticotomy is done through the vertical incision. It is essential that the bony cut should pass through the cortical bone and be deep enough to reach the medullary bone. The incision is closed using interrupted sutures of a nonresorbable material. The patient is then seen by the orthodontist after every 2 weeks.
While the literature regarding the efficacy of piezocision is limited, a few case reports[45] (Pakhare VV) and well-designed randomized controlled trials[46] have given a favorable view for the procedure, with a reduction of about 43% in the total treatment time being reported. Similarly, another study[47] reported that piezocision-assisted distalization accelerates tooth movement and decreases anchorage loss. However, a few authors have compared corticotomy and piezocision, with the results favoring corticotomy in being the more effective method.[48] As with corticotomies, a recent systematic review,[49] indicated weak evidence in support of piezocision.
MICRO-OSTEOPERFORATIONS
MOP is the least invasive procedure which is undertaken to increase the rate of orthodontic tooth movement. Without the need of reflecting a full thickness flap, as required in conventional corticotomy or incisions as required in piezocision, the MOPs can be used to induce microtrauma to the alveolar bone, thus producing RAP which in turn accelerates the rate of tooth movement. The MOPs can be done by the treating orthodontist without the requirement of additional health-care professional, thus reducing the overall cost of the procedure for the patient.
A Consortium for Translational Orthodontic Research, New York University College of Dentistry, New York, in collaboration with the Department of Developmental Biology, Harvard School of Dental Medicine, Boston has advocated this technique. The New York University (NYU) filled a patent on micro-perforations. Propel orthodontics Inc., licensed this patent from NYU and developed a tool to facilitate the procedure.[50]
Procedure
MOPs are done only before the initiation of the intended tooth movement. After informed consent has been signed, the area where the MOPs are to be done is infiltrated by a local anesthetic agent. The location of the MOPs is very critical to get the maximum benefit. The MOPs should be close to the target tooth. To get increased recruitment of osteoclasts (catabolic effect), deep perforations are required (5–7 mm), whereas if increased recruitment of osteoblasts is required (Anabolic effect) then shallow perforations (1 mm) spread over a large area is required.
Positioning of the micro-osteoperforations
The MOPs should be placed in the attached gingiva to 1 mm apical to the mucogingival junction. Clinically, the height of the MOPs is dependent on the required tooth movement, movements like torquing and intrusion might require MOPs higher up in alveolar ridges near the apices of the teeth, this might require vertical stab incisions in areas above the mucogingival junction to avoid incisions in the movable mucosa the MOPs can be placed obliquely so that the perforation start in the attached gingiva but move apically in an oblique fashion.
For deeper perforation, which is required for a catabolic effect, the position of the root is very important to avoid any trauma to the root surface. MOPs are required on both the mesial and the distal portion of the target tooth.
In general, the MOPs are made on the buccal aspect avoiding the lingual mucosa which is generally thinner than the buccal mucosa. MOPs are required on the lingual aspect; if the clinical situation requires so, contra angled handpieces can be employed. In some cases where the residual ridge has atrophied, MOPs will be required on the crest of the residual alveolar ridge in addition to the buccal and the lingual aspect.
Number and depth of the micro-osteoperforations
Increasing the number of the MOPs is a potent method to increase the catabolic effect of the MOPs. In a clinical scenario placing 2-4 MOPs per site is enough to initiate the catabolic effect. Some sites may not permit increasing the number of MOPs, in such cases increasing the depth of the MOPs can compensate. MOPs of 5–7 mm can be placed to increase the catabolic effect.
Clinical manipulation
The patient is asked to rinse with 15 ml of chlorhexidine for 30 s
The area intended for MOPs is identified, and the area is anesthetized by local infiltration
A sterile MOP device (PROPEL, Excel orthodontics) [Figure 4] is used to perform the perforations. The PROPEL device has markings for 3 mm, 5 mm, and 7 mm of lengths for varying lengths of disposable tips that would be used according to the depth of the MOP required
The desired length is set, and perforations are performed on the cortical plate by gentle rotational movement until the desired depth has been reached [Figure 5]
The MOP device is removed by rotational movements
Ideally, bleeding is not observed after MOP, but slight bleeding might occur for some patients. If bleeding does occur, normal hemostatic measures are enough to control the bleeding
The treating orthodontist should inform the patient, that slight discomfort is normal after the perforations. Analgesics are usually not required after MOP but if pain control is required acetaminophen can be prescribed. Nonsteroidal anti-inflammatory drugs are usually contraindicated as their effect might inhibit the catabolic effect of the MOP
Chlorhexidine rinses are not required after the perforations, but if the patient has poor oral hygiene, chlorhexidine rinses can be initiated to prevent any postoperative complications.
Figure 4.

PROPEL device
Figure 5.
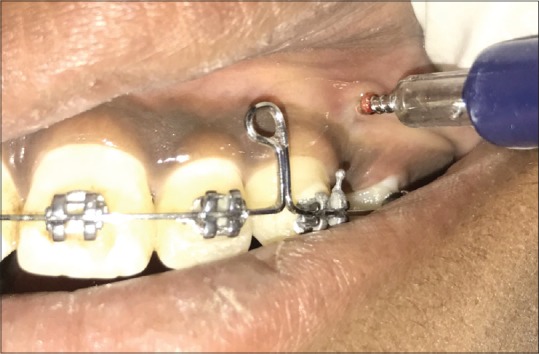
Micro-osteoperforations being done
Frequency of micro-osteoperforation
For catabolic effect 3–5 repetitions of deep perforations are required and for anabolic effect shallow perforations are continued to enhance bone formation until the tooth movement has been completed.
Orthodontic forces in a conventional set up are activated after every 21 days, but in patients receiving MOPs to enhance the rate of tooth movement, reactivation of the orthodontic appliance is done after 2 weeks only. Application of orthodontic forces leads to the secretion of cytokines locally; frequent reactivation prevents any drop in the levels of cytokines which are already saturated after the MOPs.
Saturation of the biological response
The accelerated response seen after inducing trauma to the bone remains for a limited period, and saturation of the biological response occurs after which the bone has to be traumatized again to elicit the acceleration in tooth movement. With procedures such as corticotomy and piezocision there is a period of 4–6 months after which the saturation of the biological response occurs. With MOP, this saturation period is of around 28 days. The procedure thus has to be repeated because of this reduced window of accelerated tooth movement with MOP.
Force Magnitudes with micro-osteoperforation
As seen earlier the perforations have to be repeated after 28 days to keep the RAP activated with MOP. Repeated perforations lead to a decrease in the bone density of that particular area. As a result, the saturation point increases thus orthodontic forces of heavier magnitude can be tolerated by the patient, which lead to increase in the rate of tooth movement.
The status of the literature regarding the effectiveness of MOPs as a procedure to increase the rate of tooth movement is not very conclusive. The initial few studies, which discuss the effectiveness of the procedure, have been animal studies.[28,51]
Human trials on this have been done by the researchers at the university where this procedure was patented and licensed to a company for the development of a commercial product.[17] Bias cannot be ruled out when discussing the results of the study. A split-mouth micro-computed tomography study conducted recently has shown that patients undergoing MOPs might have a greater mean volumetric root loss when compared to the control side.[52] Recently, a controlled trial assessing the efficacy of MOPs as a technique was done, it concluded that MOPs were not effective in accelerating orthodontic tooth movement.[53] Further research is needed to clear the ambiguity regarding the effectiveness of MOPs in accelerating orthodontic tooth movement.
Disadvantages of the surgical methods
Surgical morbidity associated with the procedures
Increased cost of the procedure has to be borne by the patient
If not carried out properly chances of damage to the roots of adjacent teeth
Chances of pain, infection, and swelling after the procedure if proper hygiene is not maintained.
CONCLUSION
Increase in the rate of orthodontic tooth movement facilitated surgically could be used to reduce the overall treatment time for comprehensive Orthodontic treatment. Though the increase is temporary in nature because of the biological saturation point (after which the RAP reduces), a significant reduction in the treatment time could be brought about. Procedures such as corticotomy and piezocision may have a RAP for around 4–6 months during which acceleration in the tooth movement might occur. As for MOP, the RAP lasts around 28 days after which the procedure has to be repeated. The current literature indicates that these procedures might be capable of reducing the overall treatment time, further controlled trials are required to raise the level of the evidence in support so that these procedures could be used routinely with prescribed practice guidelines.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tsichlaki A, Chin SY, Pandis N, Fleming PS. How long does treatment with fixed orthodontic appliances last? A systematic review. Am J Orthod Dentofacial Orthop. 2016;149:308–18. doi: 10.1016/j.ajodo.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Uribe F, Padala S, Allareddy V, Nanda R. Patients’, parents’, and orthodontists’ perceptions of the need for and costs of additional procedures to reduce treatment time. Am J Orthod Dentofacial Orthop. 2014;145:S65–73. doi: 10.1016/j.ajodo.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Harradine NW. Self-ligating brackets and treatment efficiency. Clin Orthod Res. 2001;4:220–7. doi: 10.1034/j.1600-0544.2001.40406.x. [DOI] [PubMed] [Google Scholar]
- 4.Eberting JJ, Straja SR, Tuncay OC. Treatment time, outcome, and patient satisfaction comparisons of Damon and conventional brackets. Clin Orthod Res. 2001;4:228–34. doi: 10.1034/j.1600-0544.2001.40407.x. [DOI] [PubMed] [Google Scholar]
- 5.Saxe AK, Louie LJ, Mah J. Efficiency and effectiveness of SureSmile. World J Orthod. 2010;11:16–22. [PubMed] [Google Scholar]
- 6.Alford TJ, Roberts WE, Hartsfield JK, Jr, Eckert GJ, Snyder RJ. Clinical outcomes for patients finished with the SureSmile™ method compared with conventional fixed orthodontic therapy. Angle Orthod. 2011;81:383–8. doi: 10.2319/071810-413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartzela T, Türp JC, Motschall E, Maltha JC. Medication effects on the rate of orthodontic tooth movement: A systematic literature review. Am J Orthod Dentofacial Orthop. 2009;135:16–26. doi: 10.1016/j.ajodo.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Yamasaki K, Miura F, Suda T. Prostaglandin as a mediator of bone resorption induced by experimental tooth movement in rats. J Dent Res. 1980;59:1635–42. doi: 10.1177/00220345800590101301. [DOI] [PubMed] [Google Scholar]
- 9.Yamasaki K, Shibata Y, Imai S, Tani Y, Shibasaki Y, Fukuhara T, et al. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am J Orthod. 1984;85:508–18. doi: 10.1016/0002-9416(84)90091-5. [DOI] [PubMed] [Google Scholar]
- 10.Spielmann T, Wieslander L, Hefti AF. Acceleration of orthodontically induced tooth movement through the local application of prostaglandin (PGE1) Schweiz Monatsschr Zahnmed. 1989;99:162–5. [PubMed] [Google Scholar]
- 11.Patil A, Keluskar KM, Gaitonde SD. The clinical application of Prostaglandin E1 on orthodontic movement – A clinical trial. J Ind Ortho Soc. 2005;38:91–8. [Google Scholar]
- 12.Proffit WR. Contemporary Orthodontics. 4th ed. St Louis: Mosby; 2007. pp. 331–8. [Google Scholar]
- 13.Gkantidis N, Mistakidis I, Kouskoura T, Pandis N. Effectiveness of non-conventional methods for accelerated orthodontic tooth movement: A systematic review and meta-analysis. J Dent. 2014;42:1300–19. doi: 10.1016/j.jdent.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Shaughnessy T, Kantarci A, Kau CH, Skrenes D, Skrenes S, Ma D, et al. Intraoral photobiomodulation-induced orthodontic tooth alignment: A preliminary study. BMC Oral Health. 2016;16:3. doi: 10.1186/s12903-015-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Showkatbakhsh R, Jamilian A, Showkatbakhsh M. The effect of pulsed electromagnetic fields on the acceleration of tooth movement. World J Orthod. 2010;11:e52–6. [PubMed] [Google Scholar]
- 16.Davidovitch Z, Finkelson MD, Steigman S, Shanfeld JL, Montgomery PC, Korostoff E, et al. Electric currents, bone remodeling, and orthodontic tooth movement. II. Increase in rate of tooth movement and periodontal cyclic nucleotide levels by combined force and electric current. Am J Orthod. 1980;77:33–47. doi: 10.1016/0002-9416(80)90222-5. [DOI] [PubMed] [Google Scholar]
- 17.Alikhani M, Raptis M, Zoldan B, Sangsuwon C, Lee YB, Alyami B, et al. Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop. 2013;144:639–48. doi: 10.1016/j.ajodo.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Dilbart S, Keser E, Nelson D. Piezocision™ – Assisted orthodontics: Past, present & future. Semin Orthod. 2015;21:170–5. [Google Scholar]
- 19.Wilcko MT, Wilcko WM, Pulver JJ, Bissada NF, Bouquot JE. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofac Surg. 2009;67:2149–59. doi: 10.1016/j.joms.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 20.Liou E. Accelerated orthodontic tooth movement. In: Miles PG, Rinchuse DR, Rinchuse DR, editors. Evidence-Based Clinical Orthodontics. 1st ed. Illinois: Quintessence Publishing Co Inc; 2012. pp. 179–200. [Google Scholar]
- 21.Liou EJ, Chen PH, Wang YC, Yu CC, Huang CS, Chen YR, et al. Surgery- first accelerated orthognathic surgery: Orthodontic guidelines and setup for model surgery. J Oral Maxillofac Surg. 2011;69:771–80. doi: 10.1016/j.joms.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Wolff J. The Law of Bone Remodelling. Berlin, Germany: Springer-Verlag; 1986. [Google Scholar]
- 23.Alansari S, Sangsuwon C, Vongthongleur T, Kwal R, Teo MC, Lee Y, et al. Biological principles behind accelerated tooth movement. Semin Orthod. 2015;21:151–61. [Google Scholar]
- 24.Kale S, Kocadereli I, Atilla P, Aşan E. Comparison of the effects of 1,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125:607–14. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Sekhavat AR, Mousavizadeh K, Pakshir HR, Aslani FS. Effect of misoprostol, a prostaglandin E1 analog, on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2002;122:542–7. doi: 10.1067/mod.2002.126153. [DOI] [PubMed] [Google Scholar]
- 26.Gurton AU, Akin E, Sagdic D, Olmez H. Effects of PGI2 and txA2 analogs and inhibitors in orthodontic tooth movement. Angle Orthod. 2004;74:526–32. doi: 10.1043/0003-3219(2004)074<0526:EOPTAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Leiker BJ, Nanda RS, Currier GF, Howes RI, Sinha PK. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 1995;108:380–8. doi: 10.1016/s0889-5406(95)70035-8. [DOI] [PubMed] [Google Scholar]
- 28.Teixeira CC, Khoo E, Tran J, Chartres I, Liu Y, Thant LM, et al. Cytokine expression and accelerated tooth movement. J Dent Res. 2010;89:1135–41. doi: 10.1177/0022034510373764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12:515–29. doi: 10.1016/0030-4220(59)90153-7. [DOI] [PubMed] [Google Scholar]
- 30.Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: Two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9–19. [PubMed] [Google Scholar]
- 31.Frost HM. The regional acceleratory phenomenon: A review. Henry Ford Hosp Med J. 1983;31:3–9. [PubMed] [Google Scholar]
- 32.Taddei SR, Andrade I, Jr, Queiroz CM, Junior, Garlet TP, Garlet GP, Cunha Fde Q, et al. Role of CCR2 in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2012;141:153–60. doi: 10.1016/j.ajodo.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Garlet TP, Coelho U, Silva JS, Garlet GP. Cytokine expression pattern in compression and tension sides of the periodontal ligament during orthodontic tooth movement in humans. Eur J Oral Sci. 2007;115:355–62. doi: 10.1111/j.1600-0722.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 34.Uematsu S, Mogi M, Deguchi T. Interleukin (IL)-1 beta, IL-6, tumor necrosis factor-alpha, epidermal growth factor, and beta 2-microglobulin levels are elevated in gingival crevicular fluid during human orthodontic tooth movement. J Dent Res. 1996;75:562–7. doi: 10.1177/00220345960750010801. [DOI] [PubMed] [Google Scholar]
- 35.Shih MS, Norrdin RW. Regional acceleration of remodeling during healing of bone defects in beagles of various ages. Bone. 1985;6:377–9. doi: 10.1016/8756-3282(85)90336-9. [DOI] [PubMed] [Google Scholar]
- 36.Bhat SG, Singh V, Bhat MK. PAOO technique for the bimaxillary protrusion: Perio-ortho interrelationship. J Indian Soc Periodontol. 2012;16:584–7. doi: 10.4103/0972-124X.106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aboul-Ela SM, El-Beialy AR, El-Sayed KM, Selim EM, El-Mangoury NH, Mostafa YA, et al. Miniscrew implant-supported maxillary canine retraction with and without corticotomy-facilitated orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:252–9. doi: 10.1016/j.ajodo.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Al-Naoum F, Hajeer MY, Al-Jundi A. Does alveolar corticotomy accelerate orthodontic tooth movement when retracting upper canines? A split-mouth design randomized controlled trial. J Oral Maxillofac Surg. 2014;72:1880–9. doi: 10.1016/j.joms.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Long H, Pyakurel U, Wang Y, Liao L, Zhou Y, Lai W, et al. Interventions for accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2013;83:164–71. doi: 10.2319/031512-224.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogeveen EJ, Jansma J, Ren Y. Surgically facilitated orthodontic treatment: A systematic review. Am J Orthod Dentofacial Orthop. 2014;145:S51–64. doi: 10.1016/j.ajodo.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Gil APS, Haas OL, Jr, Méndez-Manjón I, Masiá-Gridilla J, Valls-Ontañón A, Hernández-Alfaro F, et al. Alveolar corticotomies for accelerated orthodontics: A systematic review. J Craniomaxillofac Surg. 2018;46:438–45. doi: 10.1016/j.jcms.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 42.Fleming PS, Fedorowicz Z, Johal A, El-Angbawi A, Pandis N. Surgical adjunctive procedures for accelerating orthodontic treatment. Cochrane Database Syst Rev. 2015;6:CD010572. doi: 10.1002/14651858.CD010572.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alfawal AM, Hajeer MY, Ajaj MA, Hamadah O, Brad B. Effectiveness of minimally invasive surgical procedures in the acceleration of tooth movement: A systematic review and meta-analysis. Prog Orthod. 2016;17:33. doi: 10.1186/s40510-016-0146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dibart S, Yee C, Surmenian J, Sebaoun JD, Baloul S, Goguet-Surmenian E, et al. Tissue response during Piezocision-assisted tooth movement: A histological study in rats. Eur J Orthod. 2014;36:457–64. doi: 10.1093/ejo/cjt079. [DOI] [PubMed] [Google Scholar]
- 45.Pakhare VV, Khandait CH, Shrivastav SS, Dhadse PV, Baliga VS, Seegavadi VD, et al. Piezosurgery®-assisted periodontally accelerated osteogenic orthodontics. J Indian Soc Periodontol. 2017;21:422–6. doi: 10.4103/jisp.jisp_255_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charavet C, Lecloux G, Bruwier A, Rompen E, Maes N, Limme M, et al. Localized piezoelectric alveolar decortication for orthodontic treatment in adults: A Randomized controlled trial. J Dent Res. 2016;95:1003–9. doi: 10.1177/0022034516645066. [DOI] [PubMed] [Google Scholar]
- 47.Aksakalli S, Calik B, Kara B, Ezirganli S. Accelerated tooth movement with Piezocision and its periodontal-transversal effects in patients with class II malocclusion. Angle Orthod. 2016;86:59–65. doi: 10.2319/012215-49.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas NH, Sabet NE, Hassan IT. Evaluation of corticotomy-facilitated orthodontics and Piezocision in rapid canine retraction. Am J Orthod Dentofacial Orthop. 2016;149:473–80. doi: 10.1016/j.ajodo.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Yi J, Xiao J, Li Y, Li X, Zhao Z. Efficacy of Piezocision on accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2017;87:491–8. doi: 10.2319/01191-751.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alikhani M, Alansari S, Sangsuwon C, Alikhani M, Yuching M, Alyami B, et al. Micro-osteoperforations: Minimally invasive accelerated tooth movement. Semin Orthod. 2015;21:162–9. [Google Scholar]
- 51.Tsai CY, Yang TK, Hsieh HY, Yang LY. Comparison of the effects of micro-osteoperforation and corticision on the rate of orthodontic tooth movement in rats. Angle Orthod. 2016;86:558–64. doi: 10.2319/052015-343.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan E, Dalci O, Petocz P, Papadopoulou AK, Darendeliler MA. Physical properties of root cementum: Part 26. Effects of micro-osteoperforations on orthodontic root resorption: A microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2018;153:204–13. doi: 10.1016/j.ajodo.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Alkebsi A, Al-Maaitah E, Al-Shorman H, Abu Alhaija E. Three-dimensional assessment of the effect of micro-osteoperforations on the rate of tooth movement during canine retraction in adults with class II malocclusion: A randomized controlled clinical trial. Am J Orthod Dentofacial Orthop. 2018;153:771–85. doi: 10.1016/j.ajodo.2017.11.026. [DOI] [PubMed] [Google Scholar]


