Abstract
Objectives:
Osteoarthritis (OA) is a chronic disease of degenerative joints. Mesenchymal stem cells (MSCs) have been used for cartilage regeneration in OA. We investigated the therapeutic potential of human umbilical cord-derived MSCs (HUCMSCs) with hyaluronic acid (HA) hydrogel transplanted into a porcine OA preclinical model.
Materials and Methods:
The HUCMSCs were characterized with respect to morphology, surface markers, and differentiation capabilities. Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) was used to examine gene expressions in a HUCMSC–HA coculture. Two healthy female minipigs weighing 30–40 kg and aged approximately 4 months were used in this large animal study. A full-thickness chondral injury was created in the trochlear groove of each of the pig's rear knees. After 3 weeks, a second osteochondral defect was created. Then, 1.5 mL of a HUCMSC (5 × 106 cells) and HA composite (4%) was transplanted into the chondral-injured area in the right knee of each pig. Using the same surgical process, an osteochondral defect (untreated) was created in the left knee as a control. The pigs were sacrificed 12 weeks after transplantation. Macroscopic and microscopic histologies, qRT-PCR, and immunostaining evaluated the degree of chondral degradation.
Results:
The HUCMSCs exhibited typical MSC characteristics, including spindle morphology, expression of surface markers (positive for CD29, CD4, CD73, CD90, and human leukocyte antigen [HLA]-ABC; negative for CD34, CD45, and HLA-DR), and multipotent differentiation (adipogenesis, osteogenesis, and chondrogenesis). More extensive proliferation of HUCMSCs was noted with 4% and 25% of HA than without HA. Expression of COL2A1 and aggrecan in the HUCMSC-derived chondrocytes was increased when HA was included. The treated knees showed significant gross and histological improvements in hyaline cartilage regeneration when compared to the control knees. The International Cartilage Repair Society histological score was higher for the treated knees than the control knees.
Conclusion:
Our findings suggest that cartilage regeneration using a mixture of HUCMSCs and HA in a large animal model may be an effective treatment for OA, and this study is a stepping stone toward the future clinical trials.
KEYWORDS: Cartilage, Human umbilical cord, Hyaluronate, Mesenchymal stem cells, Regeneration
INTRODUCTION
Osteoarthritis (OA) is a chronic disorder of degenerative joints involving mostly weight-bearing joints such as the knees and hips. More than 10% of American adults have OA, making it the fourth common cause of hospitalization and the most common cause of total knee and hip joint replacement surgeries [1]. Besides total joint arthroplasty, other management practices have been developed, including osteochondral transplantation, autologous perichondrial and periosteal grafts, and autologous chondrocyte implantation. However, these approaches may not be applicable in OA because cartilage defects are often too large [2].
Mesenchymal stem cells (MSCs) have been used for cartilage repair. Bone marrow stem cells (BMSCs) are the most common source of MSCs and can prompt cartilage regeneration when at certain levels [3,4]. However, collecting BMSCs from donors is invasive and inconvenient [5]. Human umbilical cord-derived MSCs (HUCMSCs) have recently emerged as a cell source because they are easy to obtain and store [6,7] and because they do not require a perfect human leukocyte antigen (HLA) match due to their immunomodulation ability [7,8].
Although several studies have investigated the chondrogenic potential of HUCMSCs, preclinical studies have been few and have generated inconsistent results [9,10,11,12,13]. Our previous study demonstrated that transplanting a composite of infrapatellar fat pad stem cells and a hyaluronic acid (HA) hydrogel had plausible cartilage regeneration potential in an in vitro model [14]. We further demonstrated that transplanting HUCMSCs into monosodium-iodoacetate-induced OA mice repaired injured cartilage and that this repair was dependent on the regenerative and antiapoptotic effects of the HUCMSCs [15]. Before applying our cartilage regeneration technique in a clinical trial, the results needed to be confirmed in a large animal model. Human and pig genomes are very similar [16]; therefore, biomedical studies of human diseases typically use pigs in disease models before clinical application.
The aim of the present study was to investigate whether transplanting a mixture of HUCMSCs and HA would consistently show regenerative potential in the minipig model.
MATERIALS AND METHODS
Human umbilical cord-derived mesenchymal stem cell line
The experiments using human samples were approved by the Research Ethics Committee of Buddhist Tzu Chi General Hospital, and written informed consent was obtained from all participants (Institutional Review Board 100-166).
We used the detailed derivation protocol for HUCMSCs reported in a study [6]. Briefly, one human umbilical cord sample (20 cm in length, 20 g in weight) was collected in a sterile box containing Hanks’ balanced salt solution (Gibco/BRL 14185-052, Grand Island, NY, USA), and separation of Wharton's jelly (WJ) from the vessels and amniotic membrane was performed within 24 h. The enrolled mothers provided written informed consent before the labor and delivery of their infants. All methods related to the human specimens were performed in accordance with the relevant guidelines and regulations.
Each human umbilical cord was washed three times with Ca2+-and Mg2+-free phosphate-buffered saline (PBS) (Biowest, Nuaille, France). It was then cut using scissors in a midline direction, and the vessels of the umbilical artery, vein, and outlining membrane were dissociated from the WJ. The WJ was then cut into pieces smaller than 0.5 cm3, treated with collagenase type-I (Sigma, St Louis, MO, USA), and incubated for 14–18 h at 37°C in a 95% air/5% CO2 humidified atmosphere. The explants were then cultured in low-glucose Dulbecco's Modified Eagle Medium (DMEM-LG) (Gibco) containing 10% fetal bovine serum (FBS) (Biological Ind., Kibbutz, Israel) and antibiotics at 37°C in a 95% air/5% CO2 humidified atmosphere. The explants were left undisturbed for 5–7 days to allow cells to migrate from the explants.
Flow cytometry
Surface molecules of HUCMSCs cultured on the third or fourth passage were characterized using flow cytometry. The cells were detached using Accutase (Millipore, Billerica, MA, USA) in PBS, washed with PBS containing 2% bovine serum albumin (Sigma) and 0.1% sodium azide (Sigma), and incubated with the respective antibodies conjugated with fluorescein isothiocyanate or phycoerythrin, including CD29, CD34, CD44, CD45, CD73, CD90, HLA-ABC, and HLA-DR (BD, PharMingen, Franklin Lakes, NJ, USA). The cells were then analyzed using a flow cytometer (Becton Dickinson, San Jose, CA, USA).
Induction of adipogenesis
A total of 5 × 104 HUCMSCs were seeded in each well of a 12-well plate containing an adipogenic medium (DMEM supplemented with 10% FBS), 5 μg/mL insulin, 0.5 mmol/L isobutylmethylxanthine, 1 μmol/L dexamethasone, and 60 μmol/L indomethacin (all compounds purchased from Sigma). The HUCMSCs were grown in the adipogenic medium for 14 days; the medium was changed every 3 days. After 14 days of differentiation, the differentiated adipocytes were stained with oil red O (Sigma), and images of the staining were captured.
Induction of osteogenesis
A total of 1 × 104 HUCMSCs were then seeded in one well of a 12-well plate containing an osteogenic medium (DMEM supplemented with 10% FBS, 0.1 μmol/L dexamethasone, 10 mmol/L β-glycerol phosphate, and 50 μmol/L ascorbic acid). The medium was changed every 3 days. Following differentiation for 14 days, the differentiated osteocytes were stained with Alizarin red (Sigma), and images of the staining were captured.
Induction of chondrogenesis (pellet method)
For chondrogenic assays, micromass cultures were established. The HUCMSCs were seeded in a total volume of 30 μL at the bottom of a dry 15-mL test tube (BD) at a density of 25 × 106 cells/mL. The plate was placed in a humidified CO2 incubator at 37°C for 2 h, and a new chondrogenic medium (0.75 mL) was added to each tube. The media were changed every 48 h. Pellets were formed and retrieved at week 3. The pellets were then photographed and fixed in 4% paraformaldehyde for 24 h at 4°C. The cartilage pellets were washed in PBS, transferred to 70% ethanol, and processed for histological examination. Paraffin sections (5 μm) were assessed for cartilage using Alcian blue staining.
Proliferation and differentiation of the human umbilical cord-derived mesenchymal stem cells in hyaluronic acid (4% and 25%)
Our previous study [14] and another study [10] have shown that 4% and 25% HA are effective at promoting stem cell growth and chondrogenesis. Thus, the HUCMSCs in this study were cultured in 0%, 4%, and 25% HA (molecular weight 5000–10,000 kDa, 20 mg/2 mL) (Suplasyn; Bioniche, Galway, Ireland) and the subsequent proliferation and differentiation of the cells were evaluated. The HUCMSCs were plated at a density of 2 × 103 cells per well of a 96-well plate in a final volume of 100 μL culture medium. On culture days 0, 3, and 7, the cultured HUCMSCs were incubated with 150 μL of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide solution (Biological Industries, Kibbutz Beit Haemek, Israel) for 3 h at 37°C in accordance with the manufacturer's instructions. Absorbance was detected at 450 nm using a microplate reader (Model 3550; Bio-Rad, Hercules, CA, USA). Growth curves are represented through optical density values.
The mesoderm differentiation (adipogenesis, osteogenesis, and chondrogenesis) methods were the same as the differentiation procedures already described.
Real-time reverse-transcriptase polymerase chain reaction analyses
Total ribonucleic acid (RNA) from the pellets (n = 3) was extracted from the cultures using an RNeasy Protect Mini Kit with an on-column RNase-free DNase treatment (Qiagen, Hilden, Germany). The RNA was eluted in 30 mL of RNase-free water. Reverse transcription was performed with 8 mL of eluent using a SuperScript III One-Step RT-PCR kit (Invitrogen, Grand Island, NY, USA) for reverse-transcriptase polymerase chain reaction (RT-PCR) (Roche Applied Science, Penzberg, Bavaria, Germany). An aliquot of the cDNA product (2 μL) was amplified with RT-PCR using a FastStart SYBR Green QPCR Master (Rox) (Roche, Indianapolis, IN, USA) on a quantitative real-time PCR detection system (ABI Step One Plus system; Applied Biosystems, Foster City, CA, USA) as follows: initial incubation (95°C, 10 min), amplification for 55 cycles (denaturation at 95°C, 30 s; annealing at 55°C, 1 min; extension at 72°C, 30 s), denaturation (95°C, 1 min), and final incubation (55°C, 30 s). The primers (Invitrogen) were type-II collagen (COL2A1; chondrogenic marker) (forward, 5’-GGACTTTTCTCCCCTCT CT-3’; reverse, 5’-GACCCGAAGGTCTTACAGGA-3’); type-X collagen (COL10A1; marker of hypertrophy) (forward, 5’-CCC TCTTGTTAGTGCCAACC-3’; reverse, 5’-AGATTCCAGTCCTT GGGTCA-3’); SOX9 (chondrogenic marker) (forward, 5’-ACACACAGCTCACTCGACCTTG-3’; reverse, 5’-GGGAAT TCTGGTTGGTCCTCT-3’); aggrecan (chondrogenic marker) (forward, 50-GAGATGGAG GGTGAGGTC-30; reverse 5’-ACGCTGCCTCGGGCTTC-3’); glyceraldehyde-3-phosphate dehydrogenase (GAPDH; housekeeping gene and internal control) (forward, 5’-GAAGGTGAAGGTCGGAGTC-3’; reverse, 5’-GAAGA TGGTGATGGGATTTC-3’); and MMP13 (forward, 5’-CTT GAT GCC ATT ACC AGT C-3’; reverse, 5’-GGT TGG GAA GTT CTG GCC A-3’), all in a final concentration of 150 nM. The control conditions included PCRs using water and nonreverse-transcribed mRNA. The specificity of the products was confirmed through melting curve analysis and agarose gel electrophoresis. The threshold cycle (Ct) value for each gene of interest was measured for each amplified sample using QPCR software (Applied Biosystems), and values were normalized to GAPDH by using the 2−ΔΔCt method, as described elsewhere [17].
Human umbilical cord-derived mesenchymal stem cell conditioned medium collection
A HUCMSC conditioned medium (CM) was generated as follows: 80% confluent, passage 4–6 HUCMSCs in a 15-cm culture dish were washed three times with PBS and transferred to serum-free DMEM-LG (Sigma) for 48 h. The CM was collected and centrifuged at 300 × g for 5 min, and then the supernatant was aspirated into a new centrifuge tube. To remove cell debris, the collected CM was centrifuged at 2000 × g for 20 min. The CMs from different dishes were harvested and pooled. These CMs were filtered through a 0.22-μm filter before storage at −80°C.
Preparation of a human umbilical cord-derived mesenchymal stem cell and hyaluronic acid composite
The third and fourth passages of the HUCMSCs were used in this experiment. The HA concentration used was 4%, which was based on related in vitro studies. After being cultured for 1 week, the HUCMSCs reached 80% confluence; the cells were trypsinized (0.25% trypsin, Sigma), washed, and resuspended in a culture medium (DMEM-LG supplemented with 10% FBS). One HUCMSC cell line with 0.5 × 107 cells/mL was mixed with 4% HA (molecular weight 5000–10,000 kDa, 20 mg/2 mL) (Suplasyn) to create a HUCMSC–HA composite. The final volume was 1.5 mL.
Animals
Two healthy female minipigs weighing 30–40 kg and aged approximately 4 months were used in this experiment. The minipigs arrived at our animal center 1 week before the experiment and were raised under the same environmental conditions. The Institutional Animal Care and Use Committee of Buddhist Tzu Chi General Hospital approved the animal experiments (Approval No. 105-04). All methods for the pigs were performed in accordance with relevant guidelines and regulations [18].
Animal experiment
The experiment was performed using one HUCMSC cell line. The HUCMSC and 4% HA composite was injected into the pigs’ knee joints. General anesthesia was induced through inhalation of isoflurane combined with an intramuscular injection of xylazine (5 mg/kg) and ketamine (35 mg/kg). In each pig, the knee joint area of both legs was shaved, cleaned with 10% betadine solution, and draped. A medial parapatellar approach was adopted to open the knee joint. We everted the patella laterally and thoroughly inspected the intra-articular structures. After confirming a typical intra-articular structure, the knee joint was flexed, and a full-thickness chondral injury (8 mm in diameter) was created in the trochlear groove by using an arthroscopic burr.
Three weeks later, the cartilage-injured area was reinspected. Any fibrous scar tissue in the injured area was removed. A 4-mm drill was employed to make a defect to a depth of 10 mm in the middle of the cartilage-(chondral) injured area. A defect to a depth of 5 mm was made using a 6-mm drill in the same region. Gross visualization checked the deep drilling into the subchondral bone. After the cartilage, bone debris, and thrombi were cleaned out, a 1.5-mL mixture of HUCMSCs (dose: 5 × 106) and HA (4%) was transplanted into the chondral-injured area of the right rear knee of each pig. An osteochondral defect was created on the left rear knee with the same method but was left untreated as the control.
For the next 7 days, antibiotics (amikacin 12.5 mg/kg) were administered daily. The pigs were allowed to move their knee joints freely in the room. Using the same anesthetic procedure as used before the pigs were injured, the pigs were sacrificed, 12 weeks after transplantation, by an overdose intravenous injection of pentobarbital (100 mg/kg).
Macroscopic evaluation
Arthrotomy was performed to inspect the intra-articular structure in the same manner. Abnormal findings suggesting rejection or infection included severe inflammation and extensive fibrosis. The degree of cartilage repair was grossly evaluated. Coloring, luster, irregularity, repaired tissue in the defect area, and state of the border with the surrounding normal cartilage tissues were carefully evaluated.
Microscopic evaluation
Full-thickness samples (including cartilage and bone) were taken from both knee joints of the pigs. The specimens were fixed with 10% formaldehyde, decalcified using 10% nitric acid for 3 days, dehydrated in graded ethanol, and embedded in paraffin wax. Paraffin-embedded blocks were cut at a thickness of 4 μm and deparaffinized. The slices were then stained with hematoxylin and counterstained with eosin (Sigma). To detect cartilage repair, the sections were stained with a 0.1% safranin O solution (Sigma). A type-II collagen monoclonal antibody (EMD; Millipore) was used for immunohistochemistry. A diaminobenzidine tetrahydrochloride substrate was used to detect reactivity. Images of the stained sections were captured using a digital camera under a light microscope (Nikon, Tokyo, Japan).
The sections were semiquantitatively analyzed using the International Cartilage Repair Society (ICRS) scoring system [19]. The surface, matrix, cell distribution, cell population viability, subchondral bone, and cartilage mineralization were evaluated. The score consists of evaluation in six categories; scores ranged from 0 to 18.
Porcine chondrocyte derivation
Cartilage samples were collected from the pigs’ knees after sacrifice at 12 weeks after HUCMSC transplantation. Cartilage fragments were minced into 1 mm3 pieces and digested with type-II collagenase (Worthington, Lakewood, NJ, USA) solution (0.1%) overnight at 37°C. The digested contents were then filtered through a 100-μm filter and washed with PBS. Subsequently, the isolated chondrocytes were plated at 5000 cells per cm2 and grown to confluence with DMEM:F12 (Gibco) containing 2 mM L-glutamine and 10% FBS (Gibco), 1 × penicillin/streptomycin, 50 μg/mL ascorbic acid, and 0.1M nonessential amino acids (Gibco).
Statistical analysis
Statistical analysis was performed using the two-tailed Mann–Whitney U-test to compare histological evaluations; SPSS software was employed for this purpose (version 20, IBM, NY, USA). P < 0.05 was considered statistically significant.
RESULTS
The human umbilical cord-derived mesenchymal stem cells displayed typical mesenchymal stem cell characteristics
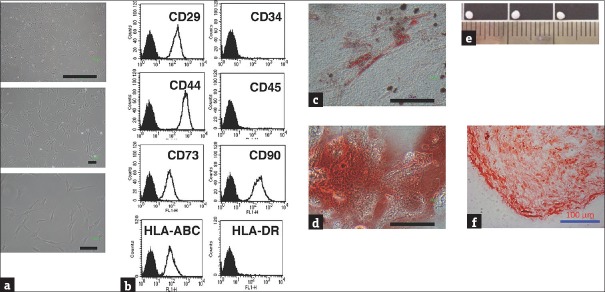
The HUCMSCs were isolated from human umbilical cord stroma. The MSC characteristics of the HUCMSCs were evaluated with respect to the morphology, surface markers, and differentiation capability of the HUCMSCs. The HUCMSCs were characterized through fibroblastic morphology [Figure 1a] and flow cytometry analysis [Figure 1b]. The HUCMSCs were negative for CD34, CD45, and HLA-DR and positive for CD29, CD44, CD90, CD105, and HLA-ABC. Following induction of differentiation, the HUCMSCs readily differentiated into fat and bone. By 14 days postinduction under adipogenic and osteogenic conditions, the differentiated HUCMSCs exhibited large oil red O-positive lipid droplets within the cytoplasm [Figure 1c] and became positive for Alizarin red staining, with a change of cell morphology to a cuboid shape [Figure 1d]. These findings indicated that the HUCMSCs could differentiate into adipocytes [Figure 1c] and osteocytes [Figure 1d]. The HUCMSCs conglobulated into a pellet after chondrogenic induction for 21 days; [Figure 1e] displays the sizes of the pellets, and [Figure 1f] shows the safranin O staining of chondrogenic proteoglycan expressed in the HUCMSCs. The findings indicated that the HUCMSCs could differentiate into chondrocytes in chondrogenic medium.
Figure 1.
Characterization and mesosderm differentiation of human umbilical cord mesenchymal stem cells. (a) Fibroblastic morphology of human umbilical cord mesenchymal stem cells with different degrees of magnification. (b) Representative flow cytometry histograms of human umbilical cord mesenchymal stem cells at passage 3 were negative for CD34, CD45, and human leukocyte antigen-DR but positive for CD29, CD44, CD73, CD90, and human leukocyte antigen-ABC. (c) Human umbilical cord mesenchymal stem cells were positive for Oil Red O staining after 14 days, indicating adipogenesis. (d) Osteogenesis of human umbilical cord mesenchymal stem cells for 14 days showed positive for Alizarin Red staining. (e) Human umbilical cord mesenchymal stem cells cultured in achondrogenesis medium for 3 weeks. The formation of pellets was noted. (f) The pellets tested positive for safranin O staining. Scale bar = 100 μm
Collectively, these results [Figure 1] confirmed that the HUCMSCs fulfilled the criteria of MSCs and exhibited mesoderm differentiation potential (ability to differentiate into adipocytes, osteocytes, and chondrocytes).
The human umbilical cord-derived mesenchymal stem cells proliferated faster in hyaluronic acid
We examined the proliferation of the HUCMSCs under various concentrations of HA. Figure 2 shows that the HUCMSCs in either 4% or 25% HA proliferated significantly faster than those without HA. This experiment confirmed that HA enhanced the proliferation of the HUCMSCs.
Figure 2.

Proliferation of human umbilical cord mesenchymal stem cells under different concentrations of hyaluronic acid. Human umbilical cord mesenchymal stem cells in 4% and 25% hyaluronic acid proliferated significantly faster than those without hyaluronic acid. *P < 0.05
Four percent hyaluronic acid increased the chondrogenic gene expression of the human umbilical cord-derived mesenchymal stem cell-derived chondrocytes
The HUCMSCs were treated with different concentrations (0%, 4%, and 25%) of HA [Figure 3]. The quantitative RT-PCR analysis revealed that the HA (4%)-treated HUCMSCs-derived chondrocytes had significantly increased expression of chondrogenic markers, type-II collagen (COL2A1) and aggrecan, and SOX9 [P < 0.001, Figure 3a–c]. Both 4% and 25% HA significantly decreased the expression of the catabolic marker MMP13 in the HUCMSC-derived chondrocytes [P < 0.001, Figure 3d].
Figure 3.
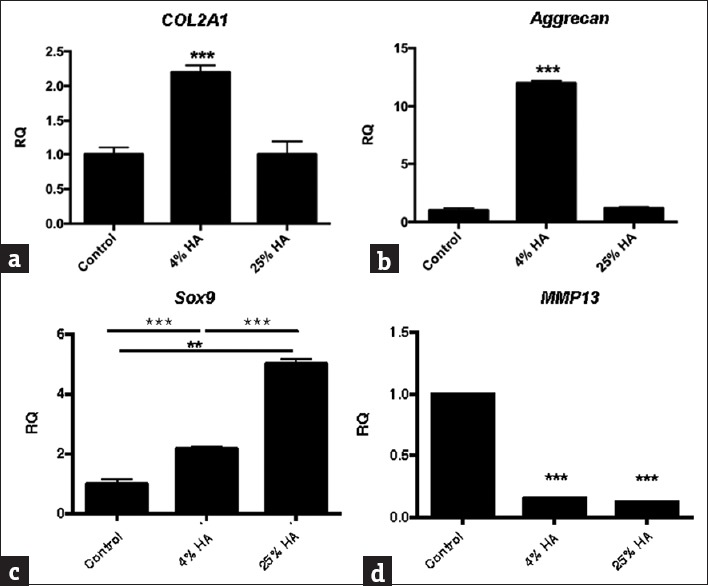
Expression of chondrogenic genes in human umbilical cord mesenchymal stem cells treated with different concentrations (0%, 4%, and 25%) of hyaluronic acid. After extracting mRNA from the human umbilical cord mesenchymal stem cells, we conducted quantitative reverse-transcriptase polymerase chain reaction analysis of the gene expression of chondrogenic markers including transcription factor (a) type-II collagen (COL2A1), (b) aggrecan, (c) SOX9, and (d) MMP13, with glyceraldehyde-3-phosphate dehydrogenase serving as an internal control. Threshold cycle (Ct) values were obtained for each target gene and glyceraldehyde-3-phosphate dehydrogenase as a control for normalization, and fold expression (relative to control [without hyaluronic acid]) was measured using the 2−ΔΔCt method. **P < 0.01, ***P < 0.001. RQ: relative quantification
Macroscopic findings
At 12 weeks after HUCMSC transplantation, no evidence of infection or rejection was noted in either pig. In contrast to the control knee, the articular surface in the transplanted knee (right knee) of both pigs was relatively smooth, with the same coloration as the surrounding normal cartilage [Figure 4].
Figure 4.
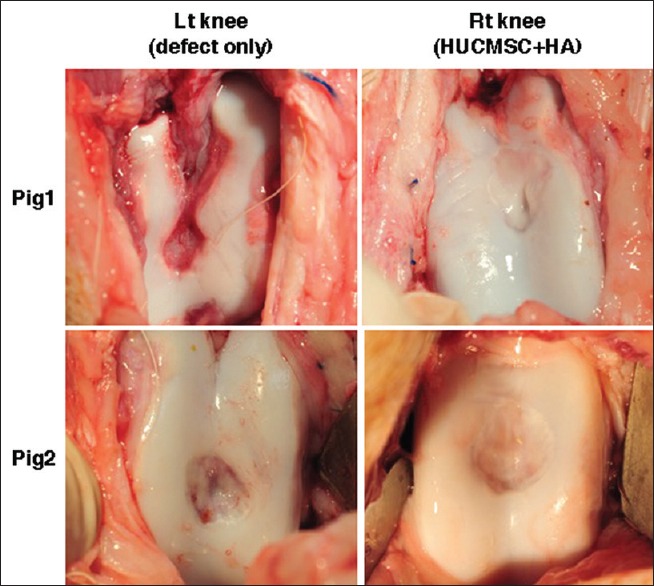
Macroscopic findings of the osteochondral defects in knees. The defects of both the left and right knees induced production of regenerated tissues that were pearly white and firm at 12 weeks post operation. After treatment with human umbilical cord mesenchymal stem cells and hyaluronic acid (right knee), the regenerated tissues were adherent to the adjacent cartilage and restored the appearance of the femoral condyles (smooth articular surface). The control knee (left knee) showed that the regenerated tissues were fibrillated and that the hole was obviously deepening
Transplanting the human umbilical cord-derived mesenchymal stem cells and hyaluronic acid increased chondrogenic gene expression
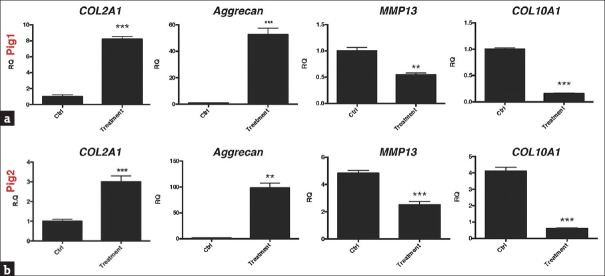
Figure 5 presents the gene expression profiles of the cartilage in the treated (transplanted) and control knees at 12 weeks after transplantation of the HUCMSCs and HA into the pigs. The treated cartilage of both pigs had increased expression of chondrogenic markers, type-II collagen (COL2A1), and aggrecan [Figure 5a and b] but decreased expression of hypertrophic and catabolic markers, type-X collagen (COL10A1), and MMP13 [Figure 5a and b].
Figure 5.
Gene expression profiles of cartilage in treated and control porcine knees. At 12 weeks after transplantation of the human umbilical cord mesenchymal stem cells and hyaluronic acid, mRNA extractions from treated or nontreated cartilages of the two pigs were examined for their gene expression profiles by using real-time polymerase chain reaction. (a) Pig 1; (b) pig 2. The genes included the transcription factor aggrecan, type-II collagen (COL2A1), type-X collagen (COL10A1), and the stress-related gene MMP13, with glyceraldehyde-3-phosphate dehydrogenase serving as an internal control. Threshold cycle (Ct) values were obtained for each target gene and glyceraldehyde-3-phosphate dehydrogenase as a control for normalization, and fold expression (relative to control knees) was measured using the 2−ΔΔCt method. **P < 0.01, ***P < 0.001. RQ: relative quantification
Microscopic findings of the osteochondral defects after the transplantation
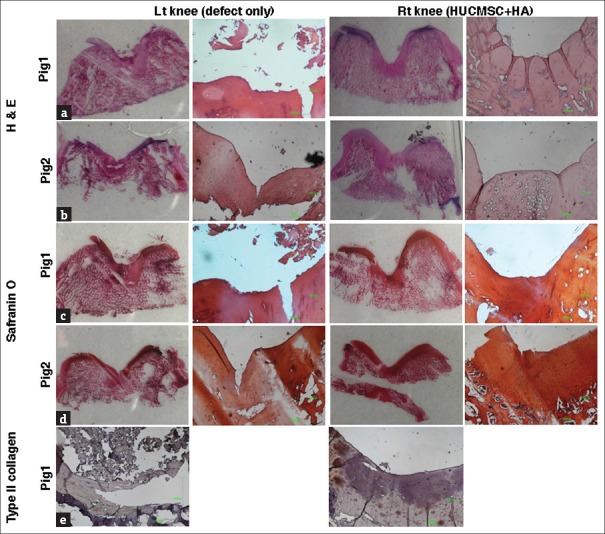
Figure 6 shows that the osteochondral defect in the two pigs at 12 weeks after transplantation of the HUCMSCs and HA became nearly normal cartilage with a smooth surface and the same thickness (right rear knee). However, in the osteochondral defect of the left rear knee, the cartilage had a slightly irregular surface. The cartilage stained with hematoxylin and eosin revealed microscopically that more cartilaginous tissue had regenerated with the surface in the transplanted knee and the tissue was produced more smoothly with the surrounding normal cartilage [Figure 6a and b]. The specimens stained with safranin O demonstrated that compared with the control knees, the transplanted knees had more cartilaginous substances that were more densely stained over a broader area with the presence of lacunae. The cells were more crowded and resembled the normal chondrocytes in the regenerated tissues [Figure 6c and d]. The differences in cell arrangement between the deep and superficial layers were more apparent in the transplanted knees and the same as in the normal cartilage.
Figure 6.
Microscopic findings of the tissue regeneration in osteochondral defects of pig articular cartilage. After 12 weeks posttreatment, the pig articular cartilages were stained with hematoxylin and eosin (a and b), safranin O (c and d), and type-II collagen (e). Scale bar: 1000 μm
Transplantation of human umbilical cord-derived mesenchymal stem cells and hyaluronic acid increased type-II collagen expression
In the immunohistochemical analysis of pig 1 for type-II collagen, the defect area in the control knees appeared as nearly pale staining, indicating minimal production or absence of hyaline cartilage; by contrast, that in the transplanted group showed a more even distribution of staining, which had expanded and was darker, indicating the presence of hyaline cartilage in the regenerated tissue [Figure 6e].
Transplantation of human umbilical cord-derived mesenchymal stem cells and hyaluronic acid increased the International Cartilage Repair Society histological score
In semiquantitative analysis of the sections, the ICRS visual histological assessment scores of the two pigs revealed that the repaired tissue in the treated knees was histologically superior to that in the control knees [Figure 7, P = 0.02].
Figure 7.
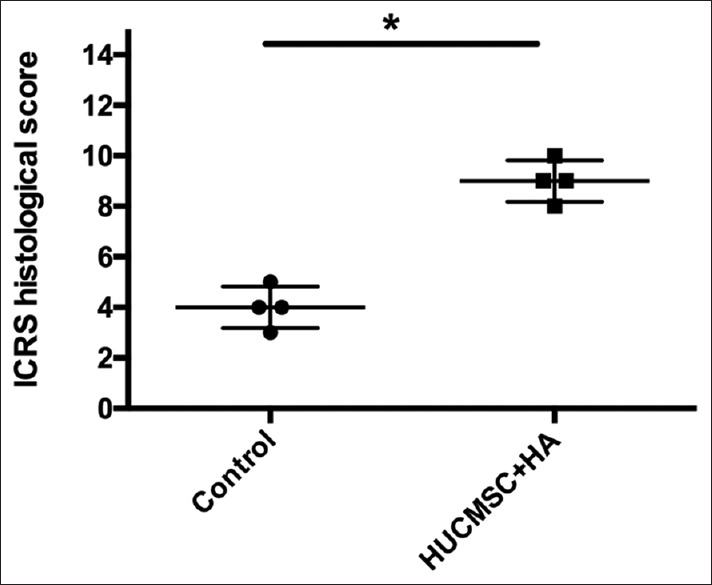
International Cartilage Repair Society histological score evaluating tissue regeneration in osteochondral defects in porcine articular cartilage at 12 weeks posttreatment. The International Cartilage Repair Society histological scores in the treatment group (human umbilical cord mesenchymal stem cell + hyaluronic acid) were significantly higher than those in the control group. *P = 0.02
DISCUSSION
According to our review of the relevant literature, this is the first paper to report that HUCMSCs can effectively treat cartilage defects in a large animal model. We demonstrated that HUCMSCs are a potential therapy for repairing cartilage in OA. The HUCMSCs in this study fulfilled the characteristics of MSCs, including their morphology, surface marker expression, and differentiation capability. When combined with 4% HA, the HUCMSCs proliferated faster and differentiated into chondrocytes efficiently. In the pig model in this study, transplanting HUCMSCs with 4% HA not only resulted in macroscopic and microscopic histological improvements but also enhanced the chondrogenesis of the treated joints. On the basis of the results from this study, future studies can include human clinical trials.
Although the chondrogenic potential of HUCMSCs (including cord blood MSCs [hUCBMSCs]) has been widely investigated, preclinical studies have been limited and have generated inconsistent results [9,10,11,12,13] xs. Yan and Yu compared chondrocytes, MSCs, hUCBMSCs, and fibroblasts in a rabbit OA model. They discovered that chondrocytes and MSCs were better than hUCBMSCs and fibroblasts at repairing a full-thickness cartilage defect [9]. Ha et al. reported cartilage regeneration with composites of hUCBMSCs and 4% HA hydrogel in a porcine model [10]. Lee et al. reported that the use of intra-articular injections of porcine bone marrow MSCs suspended in 2 ml HA is a viable option for treating large cartilage defects in a pig model [11]. In our study, we used human fetal MSC and mixed with 4% of HA intra-articular injection (total volume: 1.5 mL) to regenerate cartilage in pigs. Our results were consistent with theirs regarding the cartilage regeneration.
Fisher et al. reported the use of autologous cartilage or HA hydrogel to repair cartilage defects and found a different healing process between full- and partial-thickness defects in a large animal model [12]. Experiments have also shown that HUCMSCs seeded in poly(lactic-co-glycolic acid) scaffolds facilitated cartilage regeneration in a rabbit model with a chondral defect [13]. Cartilage regeneration using HUCMSCs mixed with 4% HA in a porcine model in this study was consistent with that reported by Ha et al. [10]. The cell dosage also correlated with successful chondrogenesis. We used 5 × 106 cell/mL in each joint, the same as in Yan and Yu [9] and Ha et al. [10].
In vitro studies of HUCMSCs have also obtained different results regarding chondrogenesis. HUCMSCs highly express HA, sulfated glycosaminoglycans, and collagen [20], resembling native cartilage. Cartilage regeneration is reportedly extensive during the chondrogenic differentiation process of HUCMSCs [21]. Mara et al. reported that transforming growth factor-ss3 used in a micromass culture was the leading growth factor for promoting the proliferation and differentiation of hUCBMSCs during chondrogenesis [22]. Choi et al. reported that the chondrogenic potential of MSCs in atelocollagen (a low-immunogenic derivative of collagen obtained by removal of N- and C-terminal telopeptide components [23]) could be suitable for cartilage tissue engineering [24]. Hildner et al. reported that adipose-derived stem cells might be more suitable than HUCMSCs for cartilage regeneration [25]. HUCMSCs are immune privileged because of persistent expression of an immune-related molecule (CD276) in undifferentiated HUCMSCs and HUCMSC-differentiated chondrocytes. This feature is maintained even after HUCMSCs differentiate [26]. Three-dimensional culture systems such as nanofibrous scaffolds [27], collagen hydrogels [28], and polycaprolactone/collagen nanoscaffolds [29] enhance the propensity of HUCMSCs to differentiate into chondrocytes. In this study, we demonstrated that chondrogenesis is more pronounced in HUCMSCs combined with HA than in HUCMSCs without HA.
HA is a natural, nonsulfated glycosaminoglycan that is found in articular cartilage and is widely scattered over the extracellular membrane of all connective tissues in humans and other animals. HA (and its derivatives) has been used as a hydrogel in tissue engineering because of its biocompatibility, biodegradability, and gel-forming properties [30]. The therapeutic mechanisms of HA include chondroprotection, synthesis of proteoglycan and glycosaminoglycan, and anti-inflammatory, mechanical, subchondral, and analgesic actions [31]. We discovered that HA supports the proliferation of HUCMSCs and promotes their chondrogenic differentiation. This result suggests that HA plays the role of a scaffold and thus provides a three-dimensional structure, mimicking the niche of prechondrocytes. Therefore, the combination of HA and HUCMSCs may provide superior therapeutic effects in articular regeneration. Moreover, HA concentrates HUCMSCs in a transplanted joint space and prevents the cells from spreading.
This study had several limitations. First, xenogeneic HUCMSC transplantation is an unlikely physiological condition. Moreover, male pigs were not included in this study. Therefore, the rejection possibility of transplanted HUCMSCs in male pigs is unknown. Nevertheless, HUCMSCs have immunomodulation ability [8]. In the present study, there was no local inflammation, joint effusion, or unloading of the joint resulting from a rejection response. Second, HA treatment only was not included as a control. HA hydrogel has been shown to support and promote the chondrogenic differentiation of MSCs [14,32]. Studies have revealed considerable cartilage repair between HA-only and MSC-seeded HA, but no differences between defect-only and HA-only controls in the minipig model [11,12]. Third, the mechanism of HUCMSCs in articular cartilage regeneration is only partially known [33]. The underlying mechanisms should be investigated in the future studies. Fourth, a functional assessment of the knees was not performed. Magnetic resonance imaging of the pigs (such as T2-weighted mapping) is technically demanding and expensive.
CONCLUSION
Our study shows that HUCMSCs combined with HA can be used for the regenerative treatment of full-thickness cartilage degradation. The transplantation of HUCMSCs combined with HA may be considered in the future human clinical trials.
Financial support and sponsorship
We thank the intramural funding of Hualien Tzu Chi Hospital (TCRD 105-54-D).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank Dr. Jon-Son Kuo for English editing. This manuscript was edited by Wallace Academic Editing.
REFERENCES
- 1.Murphy L, Helmick CG. The impact of osteoarthritis in the United States: A population-health perspective. Am J Nurs. 2012;112:S13–9. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 2.Versier G, Dubrana F French Arthroscopy Society. Treatment of knee cartilage defect in 2010. Orthop Traumatol Surg Res. 2011;97:S140–53. doi: 10.1016/j.otsr.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Review: Mesenchymal stem cells: Cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 4.Chang YH, Liu HW, Wu KC, Ding DC. Mesenchymal stem cells and their clinical applications in osteoarthritis. Cell Transplant. 2016;25:937–50. doi: 10.3727/096368915X690288. [DOI] [PubMed] [Google Scholar]
- 5.Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B, et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–60. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 6.Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, et al. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339–53. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplant. 2015;24:339–47. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 8.Ding DC, Chou HL, Chang YH, Hung WT, Liu HW, Chu TY, et al. Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant. 2016;25:217–28. doi: 10.3727/096368915X688182. [DOI] [PubMed] [Google Scholar]
- 9.Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178–87. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Ha CW, Park YB, Chung JY, Park YG. Cartilage repair using composites of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel in a minipig model. Stem Cells Transl Med. 2015;4:1044–51. doi: 10.5966/sctm.2014-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects – A porcine model. Stem Cells. 2007;25:2964–71. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: Implications for tissue engineering. Tissue Eng Part A. 2015;21:850–60. doi: 10.1089/ten.tea.2014.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin YX, Ding ZY, Zhou XB, Li ST, Xie de M, Li ZZ, et al. In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed Environ Sci. 2015;28:1–2. doi: 10.3967/bes2015.001. [DOI] [PubMed] [Google Scholar]
- 14.Ding DC, Wu KC, Chou HL, Hung WT, Liu HW, Chu TY, et al. Human infrapatellar fat pad-derived stromal cells have more potent differentiation capacity than other mesenchymal cells and can be enhanced by hyaluronan. Cell Transplant. 2015;24:1221–32. doi: 10.3727/096368914X681937. [DOI] [PubMed] [Google Scholar]
- 15.Chang YH, Wu KC, Liu HW, Chu TY, Ding DC. Human umbilical cord-derived mesenchymal stem cells reduces monosodium iodoacetate-induced apoptosis of cartilage. Tzu Chi Med J. 2018;30:71–80. doi: 10.4103/tcmj.tcmj_23_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphray SJ, Scott CE, Clark R, Marron B, Bender C, Camm N, et al. A high utility integrated map of the pig genome. Genome Biol. 2007;8:R139. doi: 10.1186/gb-2007-8-7-r139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. 8th ed. Washington DC: The National Academic Press; 2011. [Google Scholar]
- 19.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: A report by the histology endpoint committee of the international cartilage repair society (ICRS) J Bone Joint Surg Am. 2003;85-A(Suppl 2):45–57. [PubMed] [Google Scholar]
- 20.Jadalannagari S, Converse G, McFall C, Buse E, Filla M, Villar MT, et al. Decellularized Wharton's jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS One. 2017;12:e0172098. doi: 10.1371/journal.pone.0172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Detamore MS. Human umbilical cord mesenchymal stromal cells in regenerative medicine. Stem Cell Res Ther. 2013;4:142. doi: 10.1186/scrt353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mara CS, Duarte AS, Sartori A, Luzo AC, Saad ST, Coimbra IB, et al. Regulation of chondrogenesis by transforming growth factor-beta 3 and insulin-like growth factor-1 from human mesenchymal umbilical cord blood cells. J Rheumatol. 2010;37:1519–26. doi: 10.3899/jrheum.091169. [DOI] [PubMed] [Google Scholar]
- 23.Ochiya T, Takahama Y, Nagahara S, Sumita Y, Hisada A, Itoh H, et al. New delivery system for plasmid DNA in vivo using atelocollagen as a carrier material: The minipellet. Nat Med. 1999;5:707–10. doi: 10.1038/9560. [DOI] [PubMed] [Google Scholar]
- 24.Choi YS, Im MW, Kim CS, Lee MH, Noh SE, Lim SM, et al. Chondrogenic differentiation of human umbilical cord blood-derived multilineage progenitor cells in atelocollagen. Cytotherapy. 2008;10:165–73. doi: 10.1080/14653240701817002. [DOI] [PubMed] [Google Scholar]
- 25.Hildner F, Wolbank S, Redl H, van Griensven M, Peterbauer A. How chondrogenic are human umbilical cord matrix cells? A comparison to adipose-derived stem cells. J Tissue Eng Regen Med. 2010;4:242–5. doi: 10.1002/term.236. [DOI] [PubMed] [Google Scholar]
- 26.La Rocca G, Lo Iacono M, Corsello T, Corrao S, Farina F, Anzalone R, et al. Human Wharton's jelly mesenchymal stem cells maintain the expression of key immunomodulatory molecules when subjected to osteogenic, adipogenic and chondrogenic differentiation in vitro: New perspectives for cellular therapy. Curr Stem Cell Res Ther. 2013;8:100–13. doi: 10.2174/1574888x11308010012. [DOI] [PubMed] [Google Scholar]
- 27.Gauthaman K, Fong CY, Venugopal JR, Biswas A, Ramakrishna S, Bongso A, et al. Propagation and differentiation of human Wharton's jelly stem cells on three-dimensional nanofibrous scaffolds. Methods Mol Biol. 2013;1058:1–23. doi: 10.1007/7651_2012_1. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Zhang Y, Yang Z, Zhang H. Human umbilical cord Wharton's jelly-derived oligodendrocyte precursor-like cells for axon and myelin sheath regeneration. Neural Regen Res. 2013;8:890–9. doi: 10.3969/j.issn.1673-5374.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fong CY, Subramanian A, Gauthaman K, Venugopal J, Biswas A, Ramakrishna S, et al. Human umbilical cord Wharton's jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012;8:195–209. doi: 10.1007/s12015-011-9289-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–26. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet Disord. 2015;16:321. doi: 10.1186/s12891-015-0775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim IL, Mauck RL, Burdick JA. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials. 2011;32:8771–82. doi: 10.1016/j.biomaterials.2011.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136–48. doi: 10.1002/stem.1471. [DOI] [PubMed] [Google Scholar]