Abstract
Objective:
Vascular calcification is a cardiovascular risk factor in dialysis patients. Vascular calcification involves a complex process of biomineralization resembling osteogenesis, which leads to arterial stiffness. Osteocalcin is the most abundant noncollagenous protein in the bone matrix. It is synthesized in the bone by osteoblasts and reflects the rate of bone formation. The aim of this study was to evaluate the relationship between serum osteocalcin levels and the carotid–femoral pulse wave velocity (cfPWV) in peritoneal dialysis (PD) patients.
Materials and Methods:
Serum intact osteocalcin and cfPWV were measured in 62 PD patients. Those with CfPWV values >10 m/s were defined as the high central arterial stiffness group, while those with values ≤10 m/s were regarded as the low central arterial stiffness group, according to the European Society of Hypertension and of the European Society of Cardiology guidelines.
Results:
Seventeen of the 62 PD patients (27.4%) were in the high central arterial stiffness group. The high central arterial stiffness group were older (P = 0.002), had a longer PD vintage (P = 0.018), and had higher serum osteocalcin levels (P = 0.001) than those in the low group. Multivariate logistic regression analysis showed that the osteocalcin level (odds ratio: 1.069, 95% confidence interval (CI): 1.005–1.137, P = 0.035), PD vintage (odds ratio: 1.028, 95% CI: 1.010–1.048, P = 0.003), and age (odds ratio: 1.081, 95% CI: 1.005–1.162, P = 0.035) were independently associated with central arterial stiffness in PD patients. Among these patients, cfPWV (β: 0.216, P = 0.001) values and log-transformed intact parathyroid hormone (β: −’0.447, P < 0.001) levels were independently associated with the osteocalcin level in PD patients after multivariate forward stepwise linear regression analysis.
Conclusion:
Older PD patients with a longer PD vintage and higher serum osteocalcin levels had higher central arterial stiffness as measured by cfPWV. The serum osteocalcin level is an independent marker of central arterial stiffness in PD patients.
KEYWORDS: Carotid–femoral pulse wave velocity, Central arterial stiffness, Osteocalcin, Peritoneal dialysis
INTRODUCTION
Cardiovascular disease accounts for almost 50% of all deaths in patients with end-stage renal disease (ESRD) [1]. ESRD is associated with accelerated atherosclerosis and vascular calcification [2]. Vascular calcification involves a complex process, which is mainly due to osteoblastic differentiation from vascular smooth muscle cells to osteoblast-like cells [3]. The osteoblast-like cells secrete osteocalcin and matrix vesicles, resembling osteogenesis, which finally leads to reduced elasticity and compliance of vessels and increased arterial stiffness [3].
Carotid–femoral pulse wave velocity (cfPWV) is the gold standard for central arterial stiffness [4]. An elevated cfPWV level in ESRD patients on peritoneal dialysis (PD) or hemodialysis is an excellent predictor of fatal cardiovascular events and all-cause mortality [5,6].
Osteocalcin, also called the bone gamma-carboxyglutamic acid (Gla) protein, is a 49-amino acid secreted protein expressed primarily by osteoblasts [7]. The protein is the most abundant noncollagenous protein in the bone matrix and has been considered a marker of bone formation [7]. Evidence indicates that circulating osteocalcin is expressed by osteoblasts and is also expressed by osteoblast-like cells in vessel walls and associated with arterial stiffness [7]. The relationship between osteocalcin and arterial stiffness or cardiovascular risk had been investigated, but is still controversial due to differences in different genders, populations, and ethnicities [8,9,10]. However, evidence in dialysis patients is still unknown. Our aim is to evaluate the relationship between serum osteocalcin levels and central arterial stiffness measured by cfPWV values in PD patients.
MATERIALS AND METHODS
Patients
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Protection of Human Subjects Institutional Review Board of Tzu Chi University and Hospital (IRB103-136-A). Sixty-two PD patients were recruited from Hualien and Dalin Tzu Chi Hospital from June 2015 to October 2016. All patients had regular PD for more than 3 months. The blood pressures (BPs) of all patients were measured by trained staff in the morning using standard mercury sphygmomanometers with appropriate cuff sizes after the patient had been sitting for at least 10 min. The systolic BP (SBP) and diastolic BP (DBP) measurements were taken 3 times at 5-min intervals and were averaged for analysis. Patients were considered to have hypertension if their SBP ≥140 mmHg, DBP ≥90 mmHg, or they had received any antihypertensive medication in the past 2 weeks. Patients were regarded as having diabetes mellitus if their fasting plasma glucose was ≥126 mg/dL or they used oral hypoglycemic medications or insulin [11]. All patients provided informed consents before participating in this study. Patients were excluded if they had an acute infection, malignancy, acute myocardial infarction, pulmonary edema, or heart failure at the time of blood sampling or they refused to sign the informed consent. Among the patients, 44 received continuous ambulatory PD (CAPD, Dianeal, Baxter Health Care, Taiwan), with 3–5 dialysate exchanges per day, while 18 other patients underwent 4–5 dialysate exchanges each night with an automated device (automated PD, APD). The weekly fractional clearance index for urea (weekly Kt/V), total clearance of creatinine, and peritoneal clearance of creatinine were provided from the medical records.
Anthropometric analysis
All anthropometric factors were measured three times, during the morning after overnight fasting without dialysate in the abdominal cavity. Body weight was measured in light clothing and without shoes to the nearest 0.5 kg; height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight (kg) divided by height-squared (m2) [12,13,14,15].
Biochemical investigations
Biochemical parameters were determined in the morning before PD dialysate exchange. Blood samples (approximately 5 mL) were immediately centrifuged at 3000 × g for 10 min. Serum samples were stored at 4°C and used for biochemical analyses within 1 h of collection. Serum levels of blood urea nitrogen, creatinine, fasting glucose, total cholesterol, triglycerides (TG), total calcium, and phosphorus were measured using an autoanalyzer (Siemens Advia 1800, Siemens Healthcare GmbH, Henkestr, Germany). Serum osteocalcin levels (eBioscience Inc., San Diego, CA, USA) and intact parathyroid hormone (iPTH) levels (Diagnostic Systems Laboratories, Webster, TX, USA) were measured using commercial available enzyme-linked immunosorbent assays [13,14,15].
Carotid–femoral pulse wave velocity measurements
CfPWV values were measured using transcutaneous recording of the pressure pulse waveform in the underlying artery using applanation tonomentry (SphygmoCor system, AtCor Medical, NSW, Australia) as previously described [13,14,15]. These measurements were performed in all patients in the morning in the supine position after a minimum 10-min rest in a quiet, temperature-controlled room. Recordings were made simultaneously with an electrocardiographic (ECG) signal, which provided an R-timing reference. Pulse wave recordings were performed consecutively at two superficial artery sites (carotid–femoral segment). Integral software (SphygmoCor system, AtCor Medical, NSW, Australia) was used to process each set of pulse waves and ECG data were used to calculate the mean time difference between the R-wave and pulse wave on a beat-to-beat basis, with an average of ten consecutive cardiac cycles. The cfPWV was calculated using the distance and mean time difference between the two recorded points. Quality indices, included in the software, were set to ensure uniformity of data. In this study, cfPWV values >10 m/s were used to define the high central arterial stiffness group according to the guidelines of the European Society of Hypertension and the European Society of Cardiology [16].
Statistical analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test. Normally distributed data were expressed as the mean ± standard deviation and comparisons between patients were performed using Student's independent t-test (two tailed). Data that were not normally distributed were expressed as medians and interquartile ranges, and comparisons between patients were performed using the Mann–Whitney U-test (TG, fasting glucose, iPTH, and osteocalcin). Data expressed as the number of patients were analyzed by the Chi-square test. Since TG, fasting glucose, iPTH, and osteocalcin were not normally distributed, they underwent base 10 logarithmic transformations to achieve normality. Clinical variables that correlated with serum logarithmically transformed osteocalcin (log-osteocalcin) levels in PD patients were evaluated using univariate linear regression analysis. Variables that were significantly associated with central arterial stiffness were tested for independence by multivariate logistic regression analysis (adapted factors: osteocalcin, age, and PD vintage). Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA), and P < 0.05 was considered statistically significant.
RESULTS
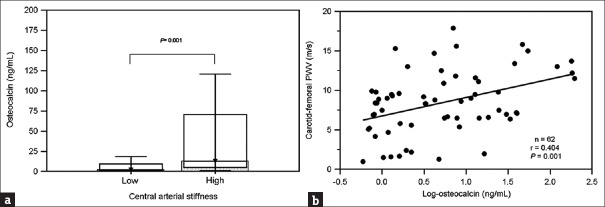
The clinical characteristics of the 62 PD patients are shown in Table 1. Their average age was 55.97 ± 14.98 years and they had received PD for a mean of 53.03 ± 43.49 months. Seventeen of the 62 PD patients (27.4%) were in the high central arterial stiffness group, with a mean cfPWV value of 13.47 ± 1.97 m/s. Compared with those in the low central arterial stiffness group, the high group were older (52.42 ± 14.30 vs. 65.35 ± 12.81 years old, P = 0.002), had a longer PD vintage (45.09 ± 39.20 vs. 74.06 ± 48.34 months, P = 0.018), and had a higher serum osteocalcin level (2.26 [range: 1.25–14.77] vs. 12.90 [range: 5.24–87.36], P = 0.001). Comparisons of serum osteocalcin levels in the high and low arterial stiffness groups in patients with PD are presented in Figure 1a. BMI, BP, lipid profiles, total calcium, phosphorus, iPTH, and weekly Kt/V levels were not significantly different between groups.
Table 1.
Clinical variables of 62 peritoneal dialysis patients with high and low arterial stiffness
| Characteristics | All participants (n=62) | Low arterial stiffness group (n=45) | High arterial stiffness group (n=17) | P |
|---|---|---|---|---|
| Age (years) | 55.97±14.98 | 52.42±14.30 | 65.35±12.81 | 0.002* |
| PD vintage (months) | 53.03±43.49 | 45.09±39.20 | 74.06±48.34 | 0.018* |
| Height (cm) | 158.89±8.37 | 159.64±8.50 | 156.88±7.92 | 0.250 |
| Body weight (kg) | 62.26±14.15 | 63.08±13.02 | 60.10±17.05 | 0.465 |
| BMI (kg/m2) | 24.69±4.30 | 24.84±4.19 | 24.32±4.68 | 0.675 |
| Carotid–femoral PWV (m/s) | 8.43±4.01 | 6.52±2.70 | 13.47±1.97 | <0.001* |
| SBP (mmHg) | 131.34±23.70 | 129.33±25.32 | 136.65±18.34 | 0.282 |
| DBP (mmHg) | 87.03±14.14 | 85.91±14.43 | 90.00±13.28 | 0.314 |
| Total cholesterol (mg/dL) | 169.35±40.66 | 168.69±40.15 | 171.12±43.20 | 0.836 |
| TG (mg/dL) | 150.00 (108.50-226.50) | 150.00 (103.00-236.50) | 159.00 (116.00-220.00) | 0.850 |
| Fasting glucose (mg/dL) | 105.00 (95.00-129.25) | 103.00 (93.50-125.00) | 112.00 (96.00-165.50) | 0.165 |
| Blood urea nitrogen (mg/dL) | 57.60±19.41 | 57.13±18.52 | 58.82±22.15 | 0.762 |
| Creatinine (mg/dL) | 10.96±3.27 | 11.26±3.38 | 10.18±2.87 | 0.248 |
| Total calcium (mg/dL) | 9.16±0.79 | 9.07±0.76 | 9.37±0.85 | 0.189 |
| Phosphorus (mg/dL) | 5.17±1.50 | 5.26±1.52 | 4.95±1.47 | 0.484 |
| Intact parathyroid hormone (pg/mL) | 231.00 (89.58-506.72) | 259.70 (104.40-572.00) | 207.70 (84.80-302.60) | 0.280 |
| Osteocalcin (ng/mL) | 4.49 (1.25-14.77) | 2.26 (0.96-10.05) | 12.90 (5.24-87.36) | 0.001* |
| Weekly Kt/V | 2.10±0.36 | 2.14±0.37 | 2.00±0.34 | 0.164 |
| Peritoneal Kt/V | 1.82±0.47 | 1.85±0.45 | 1.73±0.52 | 0.356 |
| Total clearance of creatinine (L/week) | 56.85±17.31 | 56.94±16.53 | 56.63±19.63 | 0.951 |
| Peritoneal clearance of creatinine (L/week) | 43.31±16.43 | 43.73±16.80 | 42.26±15.94 | 0.758 |
| Female, n (%) | 41 (66.1) | 30 (66.7) | 11 (64.7) | 0.884 |
| Diabetes, n (%) | 27 (43.5) | 20 (44.4) | 7 (41.2) | 0.817 |
| Hypertension, n (%) | 52 (83.9) | 38 (84.4) | 14 (82.4) | 0.842 |
| CAPD, n (%) | 44 (71.0) | 30 (66.7) | 14 (82.4) | 0.225 |
| ACE inhibitor use, n (%) | 4 (6.5) | 4 (8.9) | 0 | 0.204 |
| ARB use, n (%) | 24 (38.7) | 18 (40.0) | 6 (35.3) | 0.734 |
| β-blocker use, n (%) | 21 (33.9) | 18 (40.0) | 3 (17.6) | 0.097 |
| CCB use, n (%) | 27 (43.5) | 20 (44.4) | 7 (41.2) | 0.817 |
| Statin use, n (%) | 19 (30.6) | 15 (33.3) | 4 (23.5) | 0.455 |
| Fibrate use, n (%) | 2 (3.2) | 1 (2.2) | 1 (5.9) | 0.467 |
*Values of P<0.05 were considered statistically significant. Values for continuous variables are shown as mean±SD and analyzed by Student’s t-test; variables not normally distributed are shown as median and interquartile range and analyzed by the Mann–Whitney U-test; values presented as n (%) are analyzed by the Chi-square test. CAPD: Continuous ambulatory PD, Kt/V: Fractional clearance index for urea, ACE: Angiotensin-converting enzyme, ARB: Angiotensin receptor blocker, CCB: Calcium channel blocker, SD: Standard deviation, PD: Peritoneal dialysis, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, TG: Triglyceride, PWV: Pulse wave velocity
Figure 1.
Comparison of (a) osteocalcin levels (median and interquartile range) in the high and low central arterial stiffness groups in 62 peritoneal dialysis patients. (b) Scatter plot showing association between log-osteocalcin levels and carotid–femoral pulse wave velocity values in 62 peritoneal dialysis patients
Table 2 demonstrates the associations between clinical variables and osteocalcin levels. There were no significant differences in osteocalcin levels according to gender, diabetes, hypertension, PD model, or usage of angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, β-blockers, calcium channel blockers, and statins or fibrates [Table 2].
Table 2.
Clinical characteristics and serum osteocalcin levels of 62 peritoneal dialysis patients
| Characteristics | n (%) | Osteocalcin (ng/mL) | P |
|---|---|---|---|
| Sex | |||
| Male | 21 (33.9) | 3.26 (1.28-16.28) | 0.958 |
| Female | 41 (66.1) | 5.08 (1.11-15.18) | |
| Diabetes | |||
| No | 35 (56.5) | 7.00 (1.18-24.46) | 0.345 |
| Yes | 27 (43.5) | 3.26 (1.27-11.40) | |
| Hypertension | |||
| No | 10 (16.1) | 1.83 (0.86-11.73) | 0.228 |
| Yes | 52 (83.9) | 5.08 (1.27-22.56) | |
| PD model | |||
| CAPD | 44 (71.0) | 4.49 (1.30-24.34) | 0.271 |
| APD | 18 (29.0) | 4.41 (1.00-7.69) | |
| ACE inhibitor use | |||
| No | 58 (93.5) | 4.49 (1.67-14.77) | 0.571 |
| Yes | 4 (6.5) | 5.98 (2.49-24.80) | |
| ARB use | |||
| No | 38 (61.3) | 5.75 (1.41-14.77) | 0.254 |
| Yes | 24 (38.7) | 2.12 (1.04-16.97) | |
| β-blocker use | |||
| No | 41 (66.1) | 5.98 (1.36-19.09) | 0.174 |
| Yes | 21 (33.9) | 2.23 (1.07-11.95) | |
| CCB use | |||
| No | 35 (56.5) | 5.51 (1.32-16.43) | 0.290 |
| Yes | 27 (43.5) | 2.23 (1.00-12.90) | |
| Statin use | |||
| No | 43 (69.4) | 4.76 (1.18-30.17) | 0.187 |
| Yes | 19 (30.6) | 3.26 (1.29-7.65) | |
| Fibrate use | |||
| No | 60 (96.8) | 4.45 (1.20-14.15) | 0.343 |
| Yes | 2 (3.2) | 29.22 (4.22-54.23) |
Data are expressed as median and interquartile range and analyzed by the Mann–Whitney U-test. CAPD: Continuous ambulatory PD, APD: Automated PD, ARB: Angiotensin receptor blocker, ACE: Angiotensin-converting enzyme, CCB: Calcium channel blocker, PD: Peritoneal dialysis
The relationships between clinical variables and log-osteocalcin levels are shown in Table 3. Univariate linear regression analysis showed a significant positive correlation of the serum log-osteocalcin levels with age (r = 0.258, P = 0.043) and cfPWV values (r = 0.404, P = 0.001) and a significant negative correlation with the log-iPTH level (r = −0.461, P < 0.001) and weekly Kt/V (r = −0.310, P = 0.017). A scatter plot of log-osteocalcin and cfPWV values in PD patients is presented in Figure 1b. The cfPWV values (β: 0.377, P = 0.001) and log-iPTH level (β: −0.447, P < 0.001) were independently associated with serum log-osteocalcin levels in PD patients after multivariate forward stepwise linear regression analysis.
Table 3.
Correlation between serum osteocalcin levels and clinical variables in 62 peritoneal dialysis patients
| Variables | Log-osteocalcin (ng/mL) | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| r | P | β | P | |
| Age (years) | 0.258 | 0.043* | ||
| PD vintage (months) | −0.147 | 0.254 | ||
| Height (cm) | −0.102 | 0.429 | ||
| Body weight (kg) | −0.136 | 0.292 | ||
| BMI (kg/m2) | −0.100 | 0.441 | ||
| Carotid–femoral PWV (m/s) | 0.404 | 0.001* | 0.377 | 0.001* |
| SBP (mmHg) | 0.021 | 0.873 | ||
| DBP (mmHg) | 0.088 | 0.497 | ||
| Total cholesterol (mg/dL) | 0.215 | 0.093 | ||
| Log-TG (mg/dL) | −0.185 | 0.150 | ||
| Log-glucose (mg/dL) | −0.040 | 0.756 | ||
| Blood urea nitrogen (mg/dL) | 0.079 | 0.542 | ||
| Creatinine (mg/dL) | −0.186 | 0.147 | ||
| Total calcium (mg/dL) | 0.153 | 0.235 | ||
| Phosphorus (mg/dL) | −0.136 | 0.292 | ||
| Log-intact parathyroid | −0.461 | <0.001* | −0.447 | <0.001* |
| hormone (pg/mL) | ||||
| Weekly Kt/V | −0.310 | 0.017* | ||
| Peritoneal Kt/V | 0.189 | 0.152 | ||
| Total clearance of creatinine | −0.091 | 0.492 | ||
| (L/week) | ||||
| Peritoneal clearance of | −0.098 | 0.459 | ||
| creatinine (L/week) | ||||
*Values of P<0.05 were considered statistically significant. Data for the triglyceride, glucose, intact parathyroid hormone, and osteocalcin levels showed skewed distributions and were log-transformed before analysis. Analysis was done using univariate linear regression analyses or multivariate stepwise linear regression analysis. Kt/V: Fractional clearance index for urea, PWV: Pulse wave velocity, PD: Peritoneal dialysis, BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, TG: Triglyceride
Multivariate logistic regression analysis of the factors significantly associated with central arterial stiffness (osteocalcin, age, and PD vintage) showed that the osteocalcin level (odds ratio: 1.069, 95% confidence interval (CI): 1.005–1.137, P = 0.035), PD vintage (odds ratio: 1.028, 95% CI: 1.010–1.048, P = 0.003), and age (odds ratio: 1.081, 95% CI: 1.005–1.162, P = 0.035) were independently associated with central arterial stiffness in PD patients [Table 4].
Table 4.
Multivariate logistic regression analysis of factors correlated with arterial stiffness in 62 peritoneal dialysis patients
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Osteocalcin (ng/mL) | 1.069 | 1.005-1.137 | 0.035* |
| (each increase of 1 ng/mL) | |||
| PD vintage (months) | 1.028 | 1.010-1.048 | 0.003* |
| (each increase of 1 month) | |||
| Age (years) | 1.081 | 1.005-1.162 | 0.035* |
| (each increase of 1 year) |
*P<0.05 was considered statistically significant in multivariate logistic regression analysis (adopted factors: osteocalcin, age, and PD vintage). OR: Odds ratio, CI: Confidence interval, PD: Peritoneal dialysis
DISCUSSION
Our results showed that osteocalcin, PD vintage, and age were independently associated with central arterial stiffness in PD patients. Furthermore, iPTH levels were negatively correlated, while cfPWV values were positively correlated with the osteocalcin level in these patients.
With aging, the endothelium, vascular wall, and adventitia undergo functional and structural changes. Endothelial function is impaired and the vascular media is thickened. The adventitial extracellular matrix undergoes remodeling, with increased collagen deposition, reduced elastin content, and increased pro-inflammatory cells. These processes contribute to increased arterial stiffness, reduced elasticity, impaired distensibility, increased endothelial dysfunction, and increased vascular tone, vascular fibrosis, and stiffening [17]. Most PD solutions contain glucose. The breakdown of glucose into glucose degradation products and advanced glycation end products has the ability to alter cell viability and cause premature apoptosis and is strongly correlated with interstitial fibrosis and microvascular sclerosis [18]. The concentration of plasma advanced glycation end products was significantly associated with cfPWV levels in patients and in subgroups with diabetes and prediabetes [19]. Many authors have noted that cfPWV levels are positively associated with age in PD patients [20,21,22,23]. Lu et al. also noted that cfPWV levels were positively associated with PD duration [21]. Our study also found that older age and PD vintage were independently associated with central arterial stiffness in PD patients.
The process of vascular calcification resembles bone formation. Vascular smooth muscle cells undergo osteogenic differentiation into osteoblast-like cells, and then secrete mineralized vesicles and express osteocalcin, which leads to vascular calcification [3]. Therefore, higher circulating osteocalcin levels reflect more severe arterial stiffness. Ideleviech et al. found that osteocalcin functions as a stimulator of differentiation and mineralization and upregulates Sox9, RunX2, collagen type X, alkaline phosphatase, proteoglycan, and mineral content on vascular smooth muscle cells, which further increase vascular calcification [24]. In addition, osteocalcin is mainly cleared by the kidney. The circulating osteocalcin level is higher in patients with impaired renal function, especially dialysis patients [25,26]. The relationship between osteocalcin and arterial stiffness or cardiovascular risk is still controversial and inconclusive. Many researchers have found inverse correlations between osteocalcin and calcification [8,27,28,29,30]; however, some have also found positive relationships between osteocalcin and vascular calcification or atherosclerosis [9,31]. Recently, three studies with large populations indicated that not only reduced, but also increased osteocalcin levels predict cardiovascular mortality in a U-shaped relationship [10,32] or predict arterial stiffness by pulse wave velocity in an inverse J-shaped relationship [33]. In our study, the osteocalcin level was independently associated with central arterial stiffness after multivariate logistic regression analysis.
Osteocalcin is an important noncollangeous protein in bone; iPTH stimulates bone turnover and increases bone formation [26]. However, our study showed that the iPTH level was negatively correlated with the osteocalcin level in PD patients. One explanation for this is that increased osteocalcin levels in PD patients provide negative feedback to inhibit iPTH production. Moreover, many factors can affect serum iPTH levels, such as a parathyroidectomy, use of phosphate-binding drugs, and use of calcitriol in dialysis patients. The association of osteocalcin levels and iPTH levels in dialysis patients needs further investigation to draw solid conclusions.
There were some limitations in our study. First, the case number was small. Larger number of case studies are needed to prove the association of serum osteocalcin levels and central arterial stiffness in PD patients. Second, this was a cross-sectional observational study. A causative relationship between osteocalcin and central arterial stiffness could not be well established. Third, we did not include other confounding factors that possibly affect the osteocalcin level, such as Vitamin D status and warfarin use. We also did not check bone mineral density and other bone formation markers (bone-specific alkaline phosphatase, procollagen type 1 amino-terminal propeptide) or bone resorption markers (collagen type 1 cross-linked C-telopeptide) to identify the bone status in PD patients.
In conclusion, our study showed that a higher osteocalcin level, longer PD vintage, and older age were independently associated with central arterial stiffness in PD patients. In addition, the serum iPTH level was negatively associated with the serum osteocalcin level in PD patients.
Financial support and sponsorship
This study was supported by a grant from the Ministry of Science and Technology, Taiwan (MOST-104-2314-B-303-010).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–9. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 2.Fischer EC, Zócalo Y, Galli C, Wray S, Bia D. Arterial stiffness and renal replacement therapy: A Controversial topic. Int J Nephrol 2015. 2015:729609. doi: 10.1155/2015/729609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evrard S, Delanaye P, Kamel S, Cristol JP, Cavalier E SFBC/SN joined working group on vascular calcifications. Vascular calcification: From pathophysiology to biomarkers. Clin Chim Acta. 2015;438:401–14. doi: 10.1016/j.cca.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 5.Tang M, Romann A, Chiarelli G, Djurdjev O, Beaulieu M, Sigrist M, et al. Vascular stiffness in incident peritoneal dialysis patients over time. Clin Nephrol. 2012;78:254–62. doi: 10.5414/CN107398. [DOI] [PubMed] [Google Scholar]
- 6.Speer G, Fekete BC, El Hadj Othmane T, Szabó T, Egresits J, Fodor E, et al. Serum osteoprotegerin level, carotid-femoral pulse wave velocity and cardiovascular survival in haemodialysis patients. Nephrol Dial Transplant. 2008;23:3256–62. doi: 10.1093/ndt/gfn242. [DOI] [PubMed] [Google Scholar]
- 7.Yamanouchi D, Takei Y, Komori K. Balanced mineralization in the arterial system: Possible role of osteoclastogenesis/osteoblastogenesis in abdominal aortic aneurysm and stenotic disease. Circ J. 2012;76:2732–7. doi: 10.1253/circj.cj-12-1240. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa-Furuya N, Yamaguchi T, Yamamoto M, Kanazawa I, Sugimoto T. Serum osteocalcin levels are inversely associated with abdominal aortic calcification in men with type 2 diabetes mellitus. Osteoporos Int. 2013;24:2223–30. doi: 10.1007/s00198-013-2289-6. [DOI] [PubMed] [Google Scholar]
- 9.Reyes-Garcia R, Rozas-Moreno P, Jimenez-Moleon JJ, Villoslada MJ, Garcia-Salcedo JA, Santana-Morales S, et al. Relationship between serum levels of osteocalcin and atherosclerotic disease in type 2 diabetes. Diabetes Metab. 2012;38:76–81. doi: 10.1016/j.diabet.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Hankey GJ, et al. Associations of total osteocalcin with all-cause and cardiovascular mortality in older men. The health in men study. Osteoporos Int. 2012;23:599–606. doi: 10.1007/s00198-011-1586-1. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 12.Lin YL, Lai YH, Wang CH, Kuo CH, Liou HH, Hsu BG, et al. Triceps skinfold thickness is associated with lumbar bone mineral density in peritoneal dialysis patients. Ther Apher Dial. 2017;21:102–7. doi: 10.1111/1744-9987.12482. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Lee CJ, Chen ML, Yang CF, Chen YC, Hsu BG, et al. Association of serum osteoprotegerin levels with carotid-femoral pulse wave velocity in hypertensive patients. J Clin Hypertens (Greenwich) 2014;16:301–8. doi: 10.1111/jch.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai JP, Wang JH, Lee CJ, Chen YC, Hsu BG. Positive correlation of serum adipocyte fatty acid binding protein levels with carotid-femoral pulse wave velocity in geriatric population. BMC Geriatr. 2015;15:88. doi: 10.1186/s12877-015-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG, et al. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 17.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–68. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grantham CE, Hull KL, Graham-Brown MPM, March DS, Burton JO. The potential cardiovascular benefits of low-glucose degradation product, biocompatible peritoneal dialysis fluids: A review of the literature. Perit Dial Int. 2017;37:375–83. doi: 10.3747/pdi.2016.00228. [DOI] [PubMed] [Google Scholar]
- 19.Liu CY, Huang QF, Cheng YB, Guo QH, Chen Q, Li Y, et al. Acomparative study on skin and plasma advanced glycation end products and their associations with arterial stiffness. Pulse (Basel) 2017;4:208–18. doi: 10.1159/000453581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhe XW, Zeng J, Tian XK, Chen W, Gu Y, Cheng LT, et al. Pulse wave velocity is associated with metabolic syndrome components in CAPD patients. Am J Nephrol. 2008;28:641–6. doi: 10.1159/000117813. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q, Cheng LT, Wang T, Wan J, Liao LL, Zeng J, et al. Visceral fat, arterial stiffness, and endothelial function in peritoneal dialysis patients. J Ren Nutr. 2008;18:495–502. doi: 10.1053/j.jrn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Tatar E, Demirci MS, Kircelli F, Gungor O, Turan MN, Sevinc Ok E, et al. Association of insulin resistance with arterial stiffness in nondiabetic peritoneal dialysis patients. Int Urol Nephrol. 2012;44:255–62. doi: 10.1007/s11255-011-9984-z. [DOI] [PubMed] [Google Scholar]
- 23.Xu T, Xie J, Zong X, Wang W, Ren H, Chen N, et al. Pulse wave velocity: A Valuable predictor for cardio-cerebrovascular disease and death in PD patients. Blood Purif. 2015;40:203–8. doi: 10.1159/000433516. [DOI] [PubMed] [Google Scholar]
- 24.Idelevich A, Rais Y, Monsonego-Ornan E. Bone gla protein increases HIF-1alpha-dependent glucose metabolism and induces cartilage and vascular calcification. Arterioscler Thromb Vasc Biol. 2011;31:e55–71. doi: 10.1161/ATVBAHA.111.230904. [DOI] [PubMed] [Google Scholar]
- 25.Osorio A, Ortega E, Torres JM, Sanchez P, Ruiz-Requena E. Biochemical markers of vascular calcification in elderly hemodialysis patients. Mol Cell Biochem. 2013;374:21–7. doi: 10.1007/s11010-012-1500-y. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y, Inaba M, Imanishi Y, Okazaki H, Yamada S, Mori K, et al. Increased undercarboxylated osteocalcin/intact osteocalcin ratio in patients undergoing hemodialysis. Osteoporos Int. 2015;26:1053–61. doi: 10.1007/s00198-014-2954-4. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Ma X, Dou J, Wang F, Luo Y, Li D, et al. Relationship between serum osteocalcin levels and carotid intima-media thickness in Chinese postmenopausal women. Menopause. 2013;20:1194–9. doi: 10.1097/GME.0b013e31828aa32d. [DOI] [PubMed] [Google Scholar]
- 28.Ma H, Lin H, Hu Y, Li X, He W, Jin X, et al. Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in chinese middle-aged and elderly male adults: The Shanghai Changfeng Study. Eur J Intern Med. 2014;25:259–64. doi: 10.1016/j.ejim.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Holvik K, van Schoor NM, Eekhoff EM, den Heijer M, Deeg DJ, Lips P, et al. Plasma osteocalcin levels as a predictor of cardiovascular disease in older men and women: A population-based cohort study. Eur J Endocrinol. 2014;171:161–70. doi: 10.1530/EJE-13-1044. [DOI] [PubMed] [Google Scholar]
- 30.Confavreux CB, Szulc P, Casey R, Boutroy S, Varennes A, Vilayphiou N, et al. Higher serum osteocalcin is associated with lower abdominal aortic calcification progression and longer 10-year survival in elderly men of the MINOS cohort. J Clin Endocrinol Metab. 2013;98:1084–92. doi: 10.1210/jc.2012-3426. [DOI] [PubMed] [Google Scholar]
- 31.Choi BH, Joo NS, Kim MJ, Kim KM, Park KC, Kim YS, et al. Coronary artery calcification is associated with high serum concentration of undercarboxylated osteocalcin in asymptomatic Korean men. Clin Endocrinol (Oxf) 2015;83:320–6. doi: 10.1111/cen.12792. [DOI] [PubMed] [Google Scholar]
- 32.Lerchbaum E, Schwetz V, Pilz S, Grammer TB, Look M, Boehm BO, et al. Association of bone turnover markers with mortality in men referred to coronary angiography. Osteoporos Int. 2013;24:1321–32. doi: 10.1007/s00198-012-2076-9. [DOI] [PubMed] [Google Scholar]
- 33.Yun SH, Kim MJ, Choi BH, Park KC, Park KS, Kim YS, et al. Low level of osteocalcin is related with arterial stiffness in Korean adults: An inverse J-shaped relationship. J Clin Endocrinol Metab. 2016;101:96–102. doi: 10.1210/jc.2015-2847. [DOI] [PubMed] [Google Scholar]