Keywords: nerve regeneration, spinal cord injury, absent in melanoma 2, spatio-temporal expression, neurons, astrocytes, oligodendrocytes, infiltrated leukocytes, activated microglia, western blot assay, immunohistochemistry, neural regeneration
Abstract
In traumatic brain injury, absent in melanoma 2 (AIM2) has been demonstrated to be involved in pyroptotic neuronal cell death. Although the pathophysiological mechanism of spinal cord injury is similar to that of brain injury, the expression and cellular localization of AIM2 after spinal cord injury is still not very clear. In the present study, we used a rat model of T9 spinal cord contusive injury, produced using the weight drop method. The rats were randomly divided into 1-hour, 6-hour, 1-day, 3-day and 6-day (post-injury time points) groups. Sham-operated rats only received laminectomy at T9 without contusive injury. Western blot assay revealed that the expression levels of AIM2 were not significantly different among the 1-hour, 6-hour and 1-day groups. The expression levels of AIM2 were markedly higher in the 1-hour, 6-hour and 1-day groups compared with the sham, 3-day and 7-day groups. Double immunofluorescence staining demonstrated that AIM2 was expressed by NeuN+ (neurons), GFAP+ (astrocytes), CNPase+ (oligodendrocytes) and CD11b+ (microglia) cells in the sham-operated spinal cord. In rats with spinal cord injury, AIM2 was also found in CD45+ (leukocytes) and CD68+ (activated microglia/macrophages) cells in the spinal cord at all time points. These findings indicate that AIM2 is mainly expressed in neurons, astrocytes, microglia and oligodendrocytes in the normal spinal cord, and that after spinal cord injury, its expression increases because of the infiltration of leukocytes and the activation of astrocytes and microglia/macrophages.
Chinese Library Classification No. R456; R741
Introduction
Spinal cord injury (SCI), which can be complete or incomplete, results in varying degrees of paralysis, loss of sensation or abnormal sensation, and/or the loss of function of the associated organs. At present, there are many strategies for treating SCI, but outcomes remain unsatisfactory (Silva et al., 2014). Therefore, a better understanding of the pathogenesis of SCI is crucial for the development of more effective treatments.
Inflammasomes are macromolecular complexes involved in the innate immune system (Yogarajah et al., 2017; Wang et al., 2018). They consist of three main components: (1) intracellular pattern recognition receptors, (2) cysteine aspartate proteinase (such as caspase-1), and (3) apoptosis-associated speck-like protein containing a CARD (ASC). Inflammasomes are the first line of defense against foreign pathogens, and can identify pathogen-associated molecular patterns or damage-associated molecular patterns (Martinon et al., 2002; Zhang et al., 2015; Kesavardhana and Kanneganti, 2017; Franklin et al., 2018). Inflammasomes play a very important role in central nervous system (CNS) injury (Bernier, 2012; de Rivero Vaccari et al., 2016a, b; Slowik et al., 2018). Although not completely clear, the general mechanisms of CNS damage have been clearly defined (Kigerl et al., 2014; de Rivero Vaccari et al., 2016a, b). The damage-associated molecular patterns produced by CNS damage lead to the activation of pattern recognition receptors and the nuclear translocation of nuclear factor-κB (NF-κB), which then induces the expression of inflammatory cytokine precursors, such as pro-interleukin (IL)-1β and pro-IL-18. Opening of the channel protein pannexin-1 releases adenosine triphosphate (ATP), which then activates the ATP-gated purine receptor P2X4, resulting in an increase in the concentration of extracellular potassium ions. This reactivates the inflammasome, thereby enhancing the cleavage and activation of caspase-1. Activated caspase-1 then cleaves pro-IL-1β and pro-IL-18 to their active forms, further exacerbating inflammatory damage.
Several inflammasomes have been defined by their intracellular pattern recognition receptors, such as the nucleotide-binding oligomerization domain-like receptor (NLR) family numbers NLRP1 (NALP1), NLRP3 (NALP3) and IPAF (NLRC4) (Benko et al., 2008; Lamkanfi and Dixit, 2009). Interestingly, the absent in melanoma 2 (AIM2) inflammasome does not contain any member of the NLR family (Jin et al., 2013; Wang and Yin, 2017). Nonetheless, after activation, AIM2 can also recruit caspase-1 to form the inflammasome by interacting with ASC, resulting in IL-β and IL-18 release and a series of downstream inflammatory reactions (Tsuchiya et al., 2010; Fang et al., 2011; Hansen et al., 2011; Man and Kanneganti, 2015).
AIM2 is an important player in the innate immune system, recognizing the foreign DNA of pathogens (Jones et al., 2010; Saiga et al., 2012; Wu et al., 2013; Kigerl et al., 2014; Lugrin and Martinon, 2018). In the CNS, it was discovered that neuronal pyroptosis, an important cellular death mechanism, could be activated by AIM2 (Adamczak et al., 2014; Yogarajah et al., 2017). The AIM2 inflammasome in cerebral cortical neurons is composed of AIM2, ASC and caspase-1. The DNA released by injured brain cells can be identified by the AIM2 inflammasome, leading to cascade of downstream inflammatory reactions that trigger programmed cell death (Fernandes-Alnemri et al., 2009; Adamczak et al., 2014; Denes et al., 2015). Although the pathophysiological mechanisms of SCI and brain injury are similar, it is unknown whether AIM2 participates in the pathogenesis of SCI. The expression and localization of AIM2 after SCI are also unclear. Therefore, in the present study, we perform western blot assay and double immunofluorescence labeling to evaluate the dynamic changes in AIM2 expression and localization in the normal and injured spinal cord in an adult rat model of SCI.
Materials and Methods
Animals
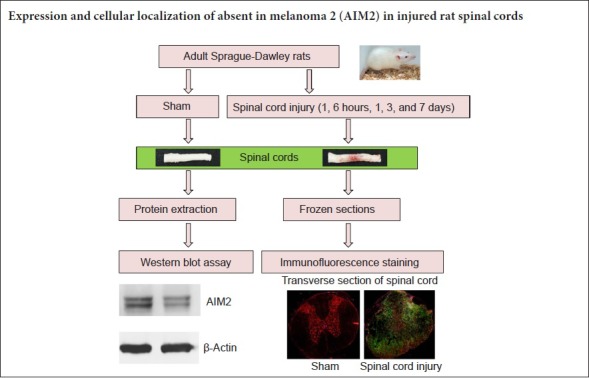
Seventy-two female Sprague-Dawley rats weighing 220–250 g and 8 weeks old were obtained from the Laboratory Animal Center of Nantong University, China (RRID: RGD_5508397; Animal license number: SCXK (Su) 2014-0001). The surgical and postoperative procedures were in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Animal Care Ethics Committee of Bengbu Medical College and Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China; revised June 2004) (approval number: 2017-037) on February 23, 2017. The rats were equally and randomly divided into six groups: sham-operated (sham), and 1 hour, 6 hours, 1 day, 3 days and 7 days post-injury.
Contusive SCI
We used a contusion injury model, which appears to most effectively simulate human injury (Rosenzweig and McDonald, 2004). First, laminectomy was performed at T9 after intraperitoneal injection of sodium pentobarbital (50 mg/kg, Beijing Dtftchem Technology Co., Ltd., Beijing, China). The T7 and T11 vertebrae were clamped to secure the spine. Then, a 10-g rod (2.5 mm in diameter) was dropped from a height of 25 mm onto the exposed dorsal surface of the spinal cord. The sham-operated rats were only subjected to laminectomy without contusion injury. After closing the muscle and skin layers, the rat was placed in a temperature and humidity-controlled room (18–29°C, 40–70% relative humidity). The following criteria were used to assess success of the SCI model: T9 spinal cord swelling, spasmodic left and right movement of the tail, and twitching of both the lower limbs and body. Bladder massage was performed three times a day until the rat could urinate on its own. For analgesia, the rats received buprenorphine (0.05 mg/kg; Reckitt Benckiser, Hull, UK) every 12 hours for 3 days. To prevent infection, chloramphenicol (50–75 mg/kg) was provided daily in the drinking water.
Western blot assay
Western blot assay was used to evaluate protein levels of AIM2 in sham-operated and injured spinal cords. The spinal cord segment (T8–10) was quickly dissected after intracardial perfusion of 200 mL physiological saline under anesthesia with sodium pentobarbital (80 mg/kg, intraperitoneally; Beijing Dtftchem Technology Co., Ltd.). Spinal cord protein extraction and western blot assay were performed as described previously (Lin et al., 2018; Wang et al., 2018). The blots were incubated with primary antibody, rabbit polyclonal anti-β-actin (1:2000; BL005B; Biosharp, Hefei, China) or rabbit polyclonal anti-AIM2 (1:1000; 20590-1-AP; Proteintech Group, Rosemont, IL, USA), for 12 hours at 4°C. Afterwards, the blots were incubated with a horseradish peroxidase-conjugated goat anti-rabbit polyclonal secondary antibody (1:10,000; Cat# BL003A; Biosharp) for 1 hour at room temperature. Signals were detected with an enhanced chemiluminescence kit (Thermo Fisher, Waltham, MA, USA) following the manufacturer’s instructions. Finally, the signals were digitized, and densitometry was performed using ImageJ Software (http://rsb.info.nih.gov/ij/; National Institutes of Health, Bethesda, MD, USA). AIM2 protein levels were normalized to β-actin (ratio of the gray values).
Immunohistochemistry
At the indicated time points post-injury, rats were anesthetized, perfused, and fixed. The spinal cords were removed according to our previous study (Lin et al., 2018; Wang et al., 2018). After post fixation and dehydration, 8-μm frozen transverse sections were obtained using a cryostat (CM1900; Leica, Bannockburn, IL, USA). For immunohistochemistry, the sections were blocked with normal goat serum in 0.01 M phosphate-buffered saline (pH 7.4) for 1 hour. The primary rabbit anti-rat AIM2 antibody (1:200; Cat# 20590-1-AP; Proteintech Group) was incubated overnight at 4°C together with either mouse anti-rat CD68 (activated microglia/macrophage marker, 1:200; Cat# MCA341GA; RRID: AB_566872; AbD Serotech, Oxford, UK), mouse anti-rat CD45 (leukocyte marker, 1:200; Cat# MCA43R; AbD Serotech), mouse anti-rat CD11b (microglial marker, 1:200; Cat# MA181606; Thermo Fisher), mouse anti-rat glial fibrillary acidic protein (GFAP, astrocyte marker, 1:200; Cat# G3893; Sigma-Aldrich, St. Louis, MO, USA), mouse anti-rat CNPase (oligodendrocyte marker, 1:200; Cat# Ab6319; Abcam, Cambridge, UK) or mouse anti-rat NeuN (neuronal marker, 1:200; Cat# Ab104224; Abcam). Normal mouse/rabbit serum controls were used to exclude non-specific immune staining. The following day, the sections were incubated for 60 minutes at 37°C with FITC-conjugated goat anti-mouse (1:200; Cat# 115-095-003; RRID: AB_2338589; Jackson ImmunoResearch, West Grove, PA, USA) and rhodamine-conjugated goat anti-rabbit (1:200; Cat# 111-025-144; RRID: AB_2337932; Jackson Immuno Research) antibodies. The coverslips were rinsed and mounted with mounting media containing Hoechst 33342, a nuclear dye (0.5 μM; Sigma-Aldrich). The immunolabeling was examined with an Axio Observer microscope (Zeiss, Thuringen, Germany). The fluorescence density was calculated using Image J Software (Drury et al., 2011; Chen et al., 2015; Wang et al., 2018). For determining cell fluorescence density, the channels were split by running Image−>Color−>Split Channels. The images of the different channels were converted to 8-bit grayscale. Semi-automated cell density measurement was performed according to Drury’s method (Drury et al., 2011). The results were expressed as the fluorescence density (pixels/mm2), which was calculated as the integrated density divided by the area analyzed. Complete sections were analyzed per condition per cell type and the fluorescence density was calculated in a set of five slides from rostral to caudal regions containing the injury epicenter.
Statistical analysis
All statistical analyses were performed using SPSS software v22.0 (IBM, Armonk, NY, USA). Data are presented as the mean ± SD, and were analyzed using one-way analysis of variance, followed by the Bonferroni post hoc test for comparisons between groups. P < 0.05 was considered statistically significant.
Results
Expression of AIM2 protein in sham and injured spinal cords
Western blot assay was used to assess the protein expression levels of AIM2 in the sham and injured spinal cords. As shown in Figure 1A, AIM2 protein was expressed in the spinal cords of all groups. However, the expression of AIM2 significantly increased at 1 hour, 6 hours and 1 day post-injury. There were no significant differences among the sham, 3-day and 7-day post-injury groups (Figure 1B; P > 0.05). There were also no significant differences among the 1-hour, 6-hour and 1-day post-injury groups (P > 0.05). The expression levels of AIM2 were significantly higher in the 1-hour, 6-hour and 1-day groups compared with the sham, 3-day and 7-day groups (P < 0.05).
Figure 1.
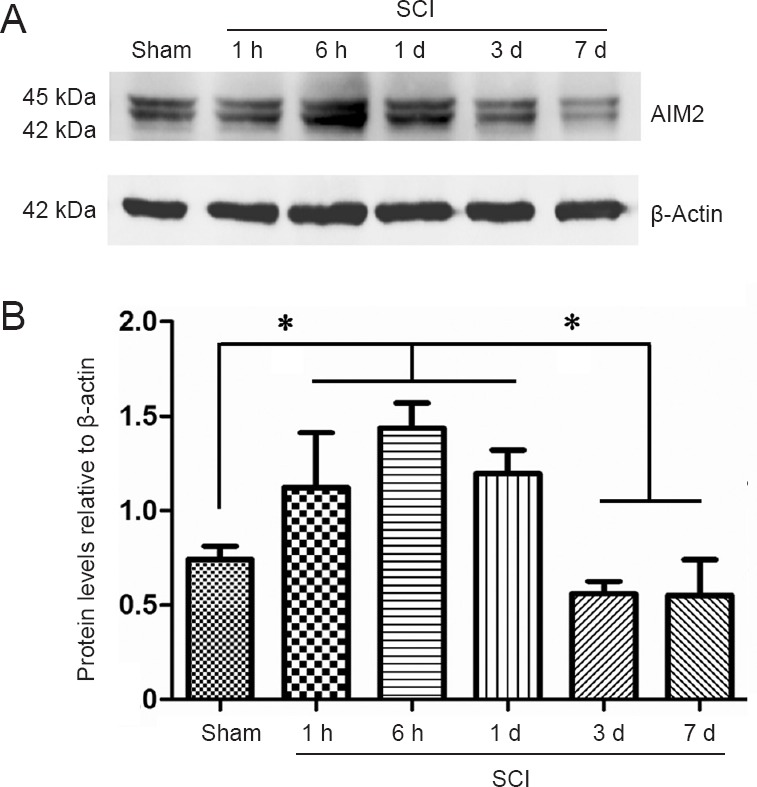
Western blot assay of AIM2 protein expression in the sham-operated and injured rat spinal cord.
(A) Representative western blots of β-actin and AIM2. AIM2 protein expression was normalized to β-actin. (B) AIM2 expression levels at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by Bonferroni post hoc test). *P < 0.05. AIM2: Absent in melanoma 2; SCI: spinal cord injury; h: hour(s); d: day(s).
Localization of AIM2 in sham and injured spinal cords
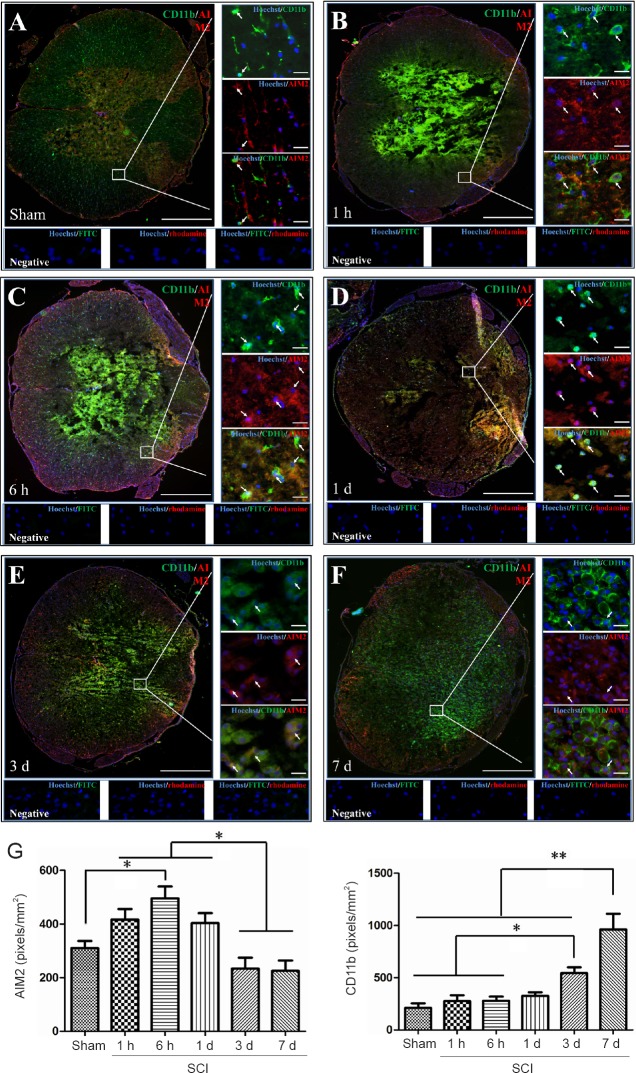
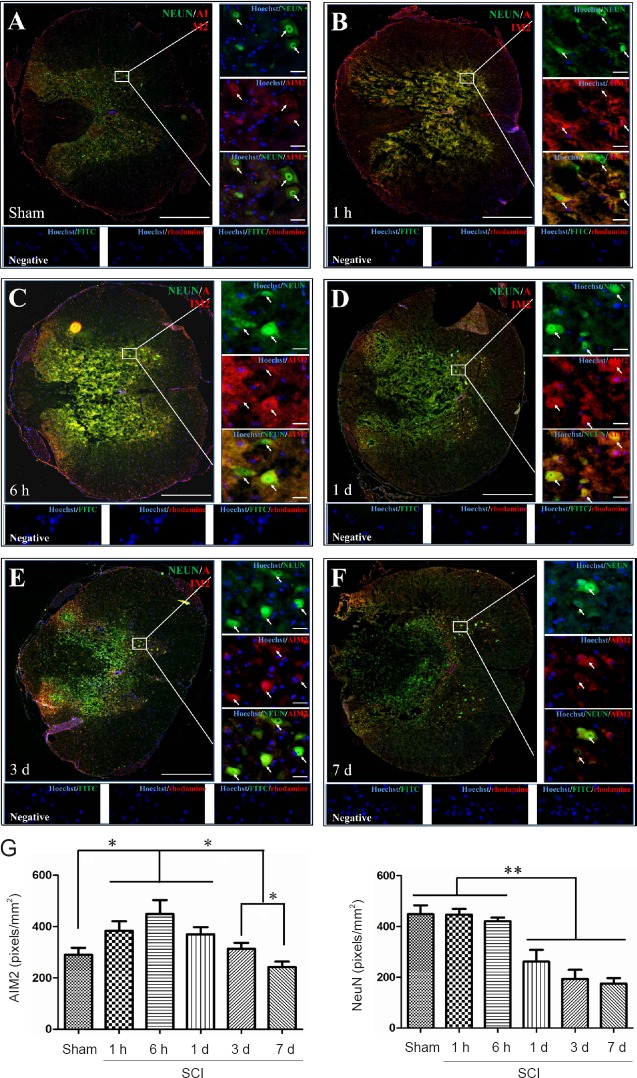
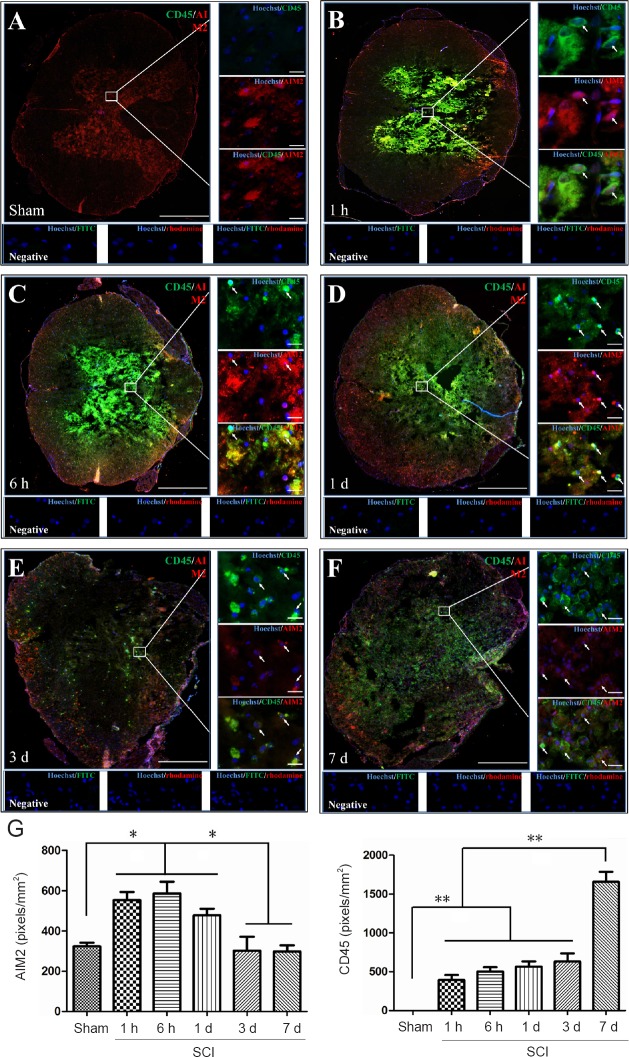
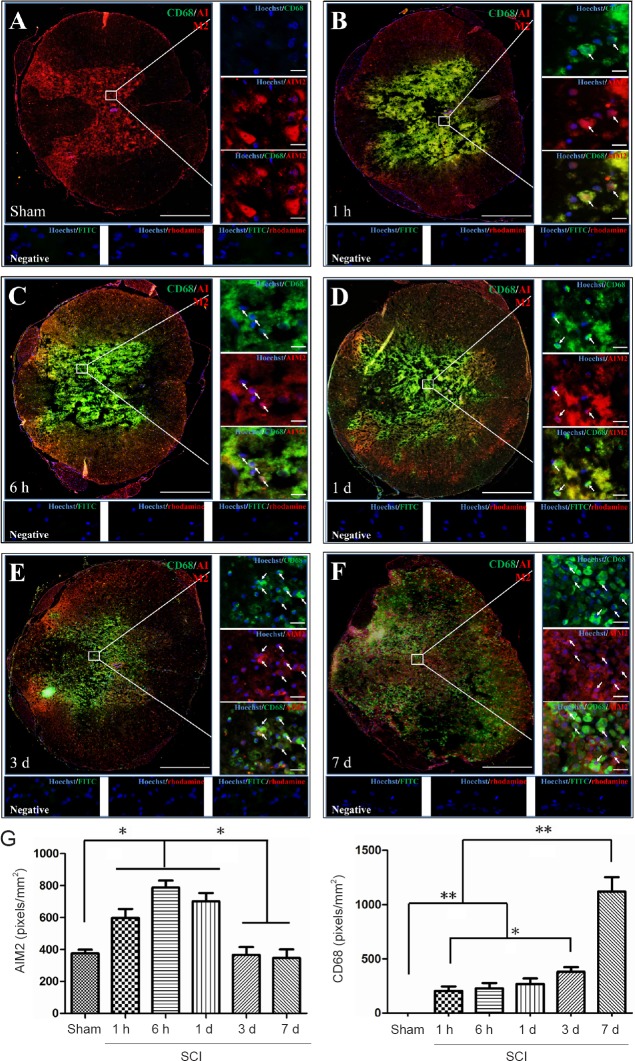
Double immunofluorescence staining for AIM2 showed massive loss of neurons in the center of the injured spinal cord. Therefore, sections 5 mm from the epicenter of injury were used for double labeling with NeuN+. Sections closer to the epicenter (within 1 mm) were used to co-localize AIM2 with the other cell types. The various types of cells were distributed in different regions of the spinal cord. GFAP and CNPase labeling were observed in the white matter. NeuN was observed in the gray matter in all groups. CD45 and CD68 were observed in the gray matter in sham-operated spinal cords, as well as in the epicenter (gray matter) in the injured spinal cord. CD11b was observed at the junction of the gray and white matter.
AIM2 was co-localized in GFAP+ (Figure 2A), CNPase+ (Figure 3A), CD11b+ (Figure 4A) and NeuN+ (Figure 5A) cells in sham-operated spinal cords. No CD45+ (Figure 6A) or CD68+ (Figure 7A) cells were found in sham-operated spinal cords. After SCI, in addition to being found in GFAP+ (Figure 2B–F), CNPase+ (Figure 3B–F), CD11b+ (Figure 4B–F) and NeuN+ (Figure 5B–F) cells, AIM2 was also found in CD45+ (Figure 6B–F) and CD68+ (Figure 7B–F) cells. In the high magnification images in Figures 4–6 strong AIM2 labeling was found in neurons, infiltrated leukocytes and activated microglia/macrophages. As shown in Figures 2–7G, the fluorescence densities of AIM2 were consistent with the western blot assay results. The expression levels of AIM2 were significantly higher in the 1-hour, 6-hour and 1-day groups than those in the sham, 3-day and 7-day groups (P < 0.05). The fluorescence densities of GFAP+ (Figure 2G), CD11b+ (Figure 4G), CD45+ (Figure 6G) and CD68+ (Figure 7G) cell labeling gradually increased after SCI, peaking at 7 days post-injury (P < 0.05 or 0.01). In contrast, the fluorescence intensities of CNPase+ (Figure 3G) and NeuN+ (Figure 4G) labeling decreased gradually after injury (P < 0.05 or 0.01). These results indicate that after SCI, many neurons and oligodendrocytes are lost, while there is an increase in infiltrated leukocytes, activated astrocytes and microglia/macrophages.
Figure 2.
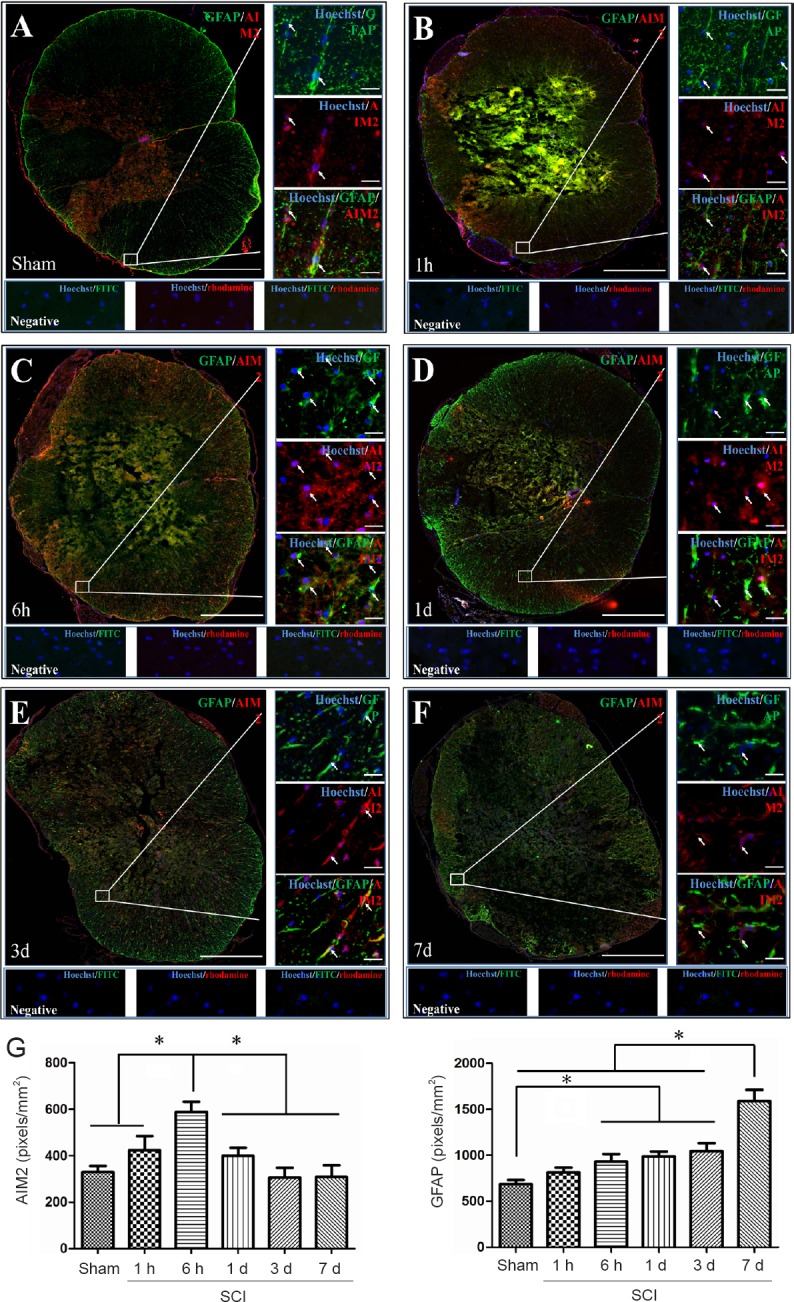
Co-localization of AIM2 with GFAP in the spinal cords of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for GFAP (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm at the original magnification (100×). Arrows show double-positive cells. (G) The expression levels of AIM2 and GFAP at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance followed by Bonferroni post hoc test). *P < 0.05. AIM2: Absent in melanoma 2; GFAP: glial fibrillary acidic protein; SCI: spinal cord injury; h: hour(s); d: day(s).
Figure 3.
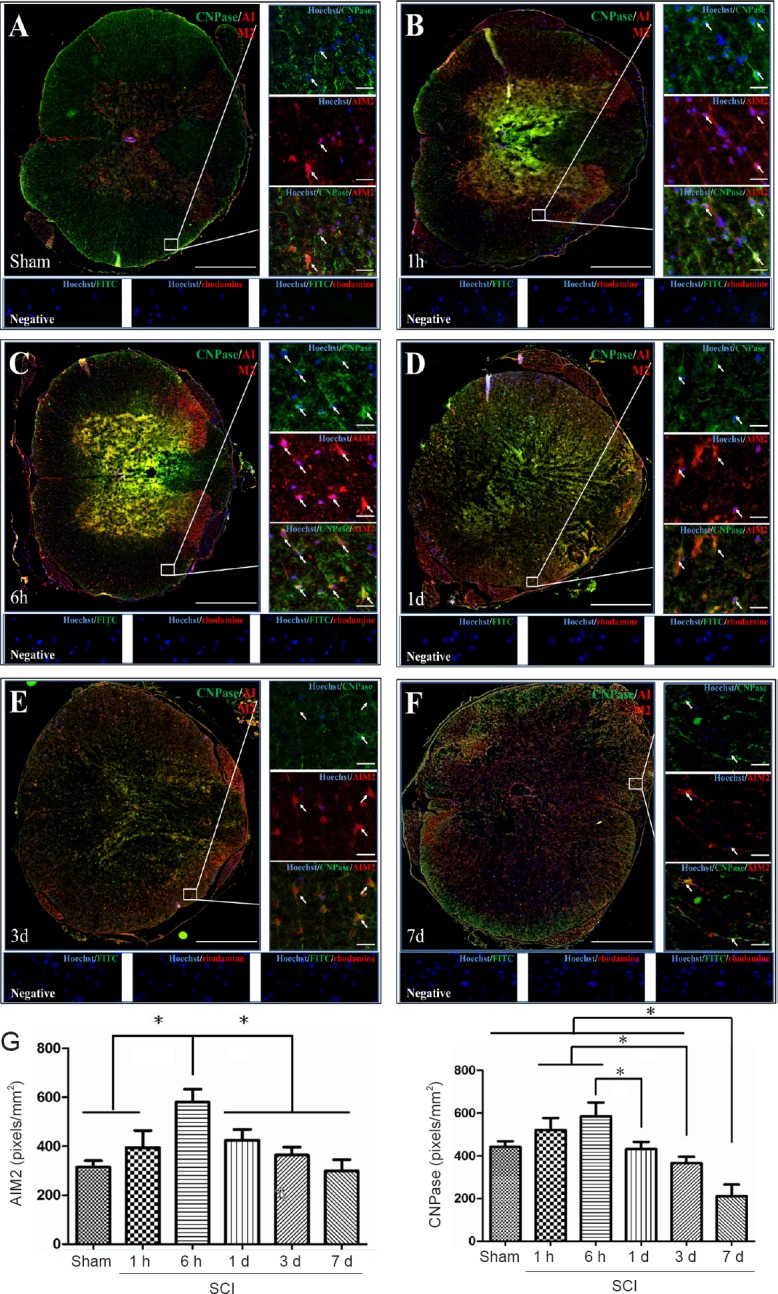
Co-localization of AIM2 with CNPase in the spinal cords of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for CNPase (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm at the original magnification (100×). Arrows show double-positive cells. The graph (G) shows the expression levels of AIM2 and CNPase at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance, followed by Bonferroni post hoc test). *P < 0.05. AIM2: Absent in melanoma 2; CNPase: 2′,3′-cyclic nucleotide 3′ phosphodiesterase; SCI: spinal cord injury; h: hour(s); d: day(s).
Figure 4.
Co-localization of AIM2 with CD11b in the spinal cords of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for CD11b (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm at the original magnification (100×). Arrows show double-positive cells. The graph (G) shows the expression levels of AIM2 and CD11b at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance, followed by Bonferroni post hoc test). *P < 0.05, **P < 0.01. AIM2: Absent in melanoma 2; CD11b: cluster of differentiation 11b; SCI: spinal cord injury; h: hour(s); d: day(s).
Figure 5.
Co-localization of AIM2 with NeuN in the spinal cords of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for NeuN (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm in the original magnification (100×). Arrows show double-positive cells. The graph (G) shows the expression levels of AIM2 and NeuN at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance, followed by Bonferroni post hoc test). *P < 0.05, **P < 0.01. AIM2: Absent in melanoma 2; NeuN: neuronal nuclei antigen/Fox-3; SCI: spinal cord injury; h: hour(s); d: day(s).
Figure 6.
Co-localization of AIM2 with CD45 in the spinal cord of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for CD45 (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm at the original magnification (100×). Arrows show double-positive cells. The graph (G) shows the expression levels of AIM2 and CD45 at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance, followed by Bonferroni post hoc test). *P < 0.05, **P < 0.01. AIM2: Absent in melanoma 2; CD45: cluster of differentiation 45; SCI: spinal cord injury; h: hour(s); d: day(s).
Figure 7.
Co-localization of AIM2 with CD68 in the spinal cords of sham-operated and injured rats.
Representative immunofluorescence staining (< 1 mm from the injury epicenter) for CD68 (green) and AIM2 (red) in the sham-operated spinal cord (A) and in the injured spinal cord in the 1-h (B), 6-h (C), 1-d (D), 3-d (E) and 7-d (F) post-injury groups. Hoechst 33342 (blue) was used to counterstain the nuclei. Labeling was observed under a ZEISS Axio Observer microscope. Scale bars: 20 μm in insets (400×); 500 μm at the original magnification (100×). Arrows show double-positive cells. The graph (G) shows the expression levels of AIM2 and CD68 at each time point after SCI. Data are expressed as the mean ± SD (n = 6; one-way analysis of variance, followed by Bonferroni post hoc test). *P < 0.05, **P < 0.01. AIM2: Absent in melanoma 2; CD68: cluster of differentiation 68; SCI: spinal cord injury; h: hour(s); d: day(s).
Discussion
Although AIM2 has been shown to be involved in pyroptotic neuronal cell death in brain injury (Adamczak et al., 2014; Denes et al., 2015; Freeman and Ting, 2016), its role in SCI was unclear. In this study, the spatio-temporal expression of AIM2 in the normal and injured spinal cord was investigated in an adult rat model of contusive SCI. Western blot assay revealed that AIM2 expression markedly increased at 1 hour, 6 hours and 1 day post-injury compared with sham-operated rats, but there were no significant differences among the other three groups. This suggests that AIM2 may participate in the pathogenesis of SCI at an early stage. To our knowledge, this is the first systematic report on AIM2 expression after SCI.
We also examined the cell types expressing AIM2 after SCI. The microenvironment of the spinal cord changes after SCI, with the development of an inflammatory reaction involving the activation of microglia and astrocytes, as well as leukocyte infiltration into the injury site (Chen et al., 2015; Cruz et al., 2015; Ma et al., 2015; Hu et al., 2016; Saghazadeh and Rezaei, 2017; Wu et al., 2017). Double immunofluorescence showed that in the sham-operated spinal cord, AIM2 was expressed in GFAP+, NeuN+, CNPase+ and CD11b+ cells. GFAP is often used as a specific marker of astrocytes in the CNS (Eng et al., 2000; Filous and Silver, 2016). NeuN is a marker for neurons (Gusel’nikova and Korzhevskiy, 2015; Sarnat, 2015). CNPase is an enzyme that can be used as a marker of oligodendrocytes (Sprinkle, 1989; Barradas and Cavalcante, 1998). CD11b is a specific marker of microglia (Korzhevskiy and Kirik, 2015). Our findings indicate that AIM2 is indeed expressed in astrocytes, neurons, oligodendrocytes and microglia in normal spinal cord tissue. Wu et al. (2016) showed that AIM2 is expressed in neurons, and Cox et al. (2015) reported that AIM2 is likely expressed in astrocytes and microglia. Our results are consistent with these reports. Moreover, our current findings extend our knowledge of the dynamic changes in cell-type specific AIM2 expression after SCI. After SCI, AIM2 was found in CD45+, CD68+, GFAP+, CD11b+, CNPase+ and NeuN+ cells. CD45 is a specific surface antigen of leukocytes (Patarroyo et al., 1990; Trowbridge et al., 1991). Usually, CD68 is not observed in microglia, but is expressed by activated microglia and macrophages (Greaves and Gordon, 2002; Hendrickx et al., 2017). Therefore, AIM2 is mainly expressed in astrocytes, neurons, microglia and oligodendrocytes in the normal spinal cord. After SCI, infiltrated leukocytes and activated microglia/macrophages also express AIM2. Because many neurons and oligodendrocytes are lost in the injured spinal cord, the increase in AIM2 expression is likely from infiltrated leukocytes, and by activated microglia/macrophages and astrocytes. Our novel results suggest that AIM2 may play an important role in the pathophysiology of SCI.
During CNS injury, caspase-1 is activated after inflammasomal activation, leading to further inflammatory cascades (Kigerl et al., 2014; Walsh et al., 2014; de Rivero Vaccari et al., 2016b; Barclay and Shinohara, 2017). In the CNS, AIM2 is an important component of the inflammasome. During CNS damage, DNA released from injured cells can be identified by AIM2, triggering inflammasome-mediated programmed cell death (Adamczak et al., 2014; Hanamsagar et al., 2014; Denes et al., 2015; Lang et al., 2018). Accordingly, AIM2 may be disadvantageous for the repair of SCI. Therefore, drugs that inhibit AIM2 function or expression may have potential for the clinical treatment of SCI.
Although we systematically explored the expression and localization of AIM2 after SCI in the present study, the mechanisms underlying the increase in AIM2 expression remain unclear, as are the roles of the protein in the pathophysiology of SCI. Further study is needed to resolve these issues.
In summary, we found that AIM2 is elevated in the early phase after SCI, and that infiltrated leukocytes, microglia/macrophages and astrocytes are primarily responsible for this increase. Our findings suggest that AIM2 may be involved in the pathogenesis of SCI, and is therefore a potential therapeutic target.
Additional file: Open peer review report 1 (61.7KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that there is no duality of interest associated with this manuscript.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81772321 (to HZL), 81571194 (to HZL), 81471277 (to JGH); a grant from the Key Program of Anhui Province for Outstanding Talents in Universities in China, No. gxbjZD2016071 (to HZL), 2014H012 (to HZL). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The Guidelines for the Care and Use of Laboratory Animals and Animal Care Ethics Committee of Bengbu Medical College and Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China; revised in June 2004) were used for animal surgery and postoperative care (ethics approval number: 2017-037). All experimental procedures described here were in accordance with the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Kim C-Yoon, Seoul National University, Republic of Korea.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81772321 (to HZL), 81571194 (to HZL), 81471277 (to JGH); a grant from the Key Program of Anhui Province for Outstanding Talents in Universities in China, No. gxbjZD2016071 (to HZL), 2014H012 (to HZL).
P-Reviewer: C-Ywn K; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Raye W, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, 3rd, Nonner D, Bullock MR, Dahl GP, Dietrich WD, Keane RW. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barclay W, Shinohara ML. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Brain Pathol. 2017;27:213–219. doi: 10.1111/bpa.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barradas PC, Cavalcante LA. Proliferation of differentiated glial cells in the brain stem. Braz J Med Biol Res. 1998;31:257–270. doi: 10.1590/s0100-879x1998000200009. [DOI] [PubMed] [Google Scholar]
- 4.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine. 2008;43:368–373. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Bernier LP. Purinergic regulation of inflammasome activation after central nervous system injury. J Gen Physiol. 2012;140:571–575. doi: 10.1085/jgp.201210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YJ, Zhu H, Zhang N, Shen L, Wang R, Zhou JS, Hu JG, Lu HZ. Temporal kinetics of macrophage polarization in the injured rat spinal cord. J Neurosci Res. 2015;93:1526–1533. doi: 10.1002/jnr.23612. [DOI] [PubMed] [Google Scholar]
- 7.Cox DJ, Field RH, Williams DG, Baran M, Bowie AG, Cunningham C, Dunne A. DNA sensors are expressed in astrocytes and microglia in vitro and are upregulated during gliosis in neurodegenerative disease. Glia. 2015;63:812–825. doi: 10.1002/glia.22786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz CD, Coelho A, Antunes-Lopes T, Cruz F. Biomarkers of spinal cord injury and ensuing bladder dysfunction. Adv Drug Deliv Rev. 2015;82-83:153–159. doi: 10.1016/j.addr.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 9.de Rivero Vaccari JP, Dietrich WD, Keane RW. Therapeutics targeting the inflammasome after central nervous system injury. Transl Res. 2016a;167:35–45. doi: 10.1016/j.trsl.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Rivero Vaccari JP, Brand F, 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD, Keane RW. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016b;136(Suppl 1):39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denes A, Coutts G, Lenart N, Cruickshank SM, Pelegrin P, Skinner J, Rothwell N, Allan SM, Brough D. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A. 2015;112:4050–4055. doi: 10.1073/pnas.1419090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drury JA, Nik H, van Oppenraaij RH, Tang AW, Turner MA, Quenby S. Endometrial cell counts in recurrent miscarriage: a comparison of counting methods. Histopathology. 2011;59:1156–1162. doi: 10.1111/j.1365-2559.2011.04046.x. [DOI] [PubMed] [Google Scholar]
- 13.Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAPthirty-one years (1969-2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- 14.Fang R, Tsuchiya K, Kawamura I, Shen Y, Hara H, Sakai S, Yamamoto T, Fernandes-Alnemri T, Yang R, Hernandez-Cuellar E, Dewamitta SR, Xu Y, Qu H, Alnemri ES, Mitsuyama M. Critical roles of ASC inflammasomes in caspase-1 activation and host innate resistance to Streptococcus pneumoniae infection. J Immunol. 2011;187:4890–4899. doi: 10.4049/jimmunol.1100381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filous AR, Silver J. “Targeting astrocytes in CNS injury and disease: a translational research approach”. Prog Neurobiol. 2016;144:173–187. doi: 10.1016/j.pneurobio.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franklin BS, Latz E, Schmidt FI. The intra- and extracellular functions of ASC specks. Immunol Rev. 2018;281:74–87. doi: 10.1111/imr.12611. [DOI] [PubMed] [Google Scholar]
- 18.Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136(Suppl 1):29–38. doi: 10.1111/jnc.13217. [DOI] [PubMed] [Google Scholar]
- 19.Greaves DR, Gordon S. Macrophage-specific gene expression: current paradigms and future challenges. Int J Hematol. 2002;76:6–15. doi: 10.1007/BF02982713. [DOI] [PubMed] [Google Scholar]
- 20.Gusel’nikova VV, Korzhevskiy DE. NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Nat. 2015;7:42–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Hanamsagar R, Aldrich A, Kielian T. Critical role for the AIM2 inflammasome during acute CNS bacterial infection. J Neurochem. 2014;129:704–711. doi: 10.1111/jnc.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JD, Vojtech LN, Laing KJ. Sensing disease and danger: a survey of vertebrate PRRs and their origins. Dev Comp Immunol. 2011;35:886–897. doi: 10.1016/j.dci.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickx DAE, van Eden CG, Schuurman KG, Hamann J, Huitinga I. Staining of HLA-DR, Iba1 and CD68 in human microglia reveals partially overlapping expression depending on cellular morphology and pathology. J Neuroimmunol. 2017;309:12–22. doi: 10.1016/j.jneuroim.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Hu JG, Shi LL, Chen YJ, Xie XM, Zhang N, Zhu AY, Jiang ZS, Feng YF, Zhang C, Xi J, Lu HZ. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp Neurol. 2016;277:190–201. doi: 10.1016/j.expneurol.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Jin T, Perry A, Smith P, Jiang J, Xiao TS. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem. 2013;288:13225–13235. doi: 10.1074/jbc.M113.468033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, O’Rourke K, Chan S, Dong J, Qu Y, Roose-Girma M, Dixit VM, Monack DM. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–9776. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kesavardhana S, Kanneganti TD. Mechanisms governing inflammasome activation, assembly and pyroptosis induction. Int Immunol. 2017;29:201–210. doi: 10.1093/intimm/dxx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 2014;258:5–16. doi: 10.1016/j.expneurol.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korzhevskiy DE, Kirik OV. Cerebral microglia and microglial markers. Morfologiia. 2015;147:37–44. [PubMed] [Google Scholar]
- 30.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunol Rev. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 31.Lang Y, Chu F, Shen D, Zhang W, Zheng C, Zhu J, Cui L. Role of inflammasomes in neuroimmune and neurodegenerative diseases: a systematic review. Mediators Inflamm. 2018;2018:1549549. doi: 10.1155/2018/1549549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin YH, Wu Y, Wang Y, Yao ZF, Tang J, Wang R, Shen L, Ding SQ, Hu JG, Lu HZ. Spatio-temporal expression of Hexokinase-3 in the injured female rat spinal cords. Neurochem Int. 2018;113:23–33. doi: 10.1016/j.neuint.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Lugrin J, Martinon F. The AIM2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol Rev. 2018;281:99–114. doi: 10.1111/imr.12618. [DOI] [PubMed] [Google Scholar]
- 34.Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lu HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 37.Patarroyo M, Prieto J, Rincon J, Timonen T, Lundberg C, Lindbom L, Asjo B, Gahmberg CG. Leukocyte-cell adhesion: a molecular process fundamental in leukocyte physiology. Immunol Rev. 1990;114:67–108. doi: 10.1111/j.1600-065x.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17:121–131. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Saghazadeh A, Rezaei N. The role of timing in the treatment of spinal cord injury. Biomed Pharmacother. 2017;92:128–139. doi: 10.1016/j.biopha.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 40.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, Yamamoto M, Takeda K. Critical role of AIM2 in mycobacterium tuberculosis infection. Int Immunol. 2012;24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 41.Sarnat HB. Immunocytochemical markers of neuronal maturation in human diagnostic neuropathology. Cell Tissue Res. 2015;359:279–294. doi: 10.1007/s00441-014-1988-4. [DOI] [PubMed] [Google Scholar]
- 42.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: a comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Slowik A, Lammerding L, Hoffmann S, Beyer C. Brain inflammasomes in stroke and depressive disorders: regulation by oestrogen. Neuroendocrinol. 2018 doi: 10.1111/jne.12482. doi: 10.1111/jne.12482. [DOI] [PubMed] [Google Scholar]
- 44.Sprinkle TJ. 2’,3’-cyclic nucleotide 3’-phosphodiesterase, an oligodendrocyte-Schwann cell and myelin-associated enzyme of the nervous system. Crit Rev Neurobiol. 1989;4:235–301. [PubMed] [Google Scholar]
- 45.Trowbridge IS, Ostergaard HL, Johnson P. CD45: a leukocyte-specific member of the protein tyrosine phosphatase family. Biochim Biophys Acta. 1991;1095:46–56. doi: 10.1016/0167-4889(91)90043-w. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchiya K, Hara H, Kawamura I, Nomura T, Yamamoto T, Daim S, Dewamitta SR, Shen Y, Fang R, Mitsuyama M. Involvement of absent in melanoma 2 in inflammasome activation in macrophages infected with Listeria monocytogenes. J Immunol. 2010;185:1186–1195. doi: 10.4049/jimmunol.1001058. [DOI] [PubMed] [Google Scholar]
- 47.Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 48.Wang B, Yin Q. AIM2 inflammasome activation and regulation: a structural perspective. J Struct Biol. 2017;200:279–282. doi: 10.1016/j.jsb.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Xu X, Wu YG, Lyu L, Zhou ZW, Zhang JN. Dexmedetomidine attenuates traumatic brain injury: action pathway and mechanisms. Neural Regen Res. 2018;13:819–826. doi: 10.4103/1673-5374.232529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Lin YH, Wu Y, Yao ZF, Tang J, Shen L, Wang R, Ding SQ, Hu JG, Lu HZ. Expression and cellular localization of IFITM1 in normal and injured rat spinal cords. J Histochem Cytochem. 2018;66:175–187. doi: 10.1369/0022155417749491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu DL, Xu GH, Lu SM, Ma BL, Miao NZ, Liu XB, Cheng YP, Feng JH, Liu ZG, Feng D, Na L, Li WQ, Zhao YR. Correlation of AIM2 expression in peripheral blood mononuclear cells from humans with acute and chronic hepatitis B. Hum Immunol. 2013;74:514–521. doi: 10.1016/j.humimm.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 52.Wu PJ, Liu HY, Huang TN, Hsueh YP. AIM 2 inflammasomes regulate neuronal morphology and influence anxiety and memory in mice. Sci Rep. 2016;6:32405. doi: 10.1038/srep32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Y, Lin YH, Shi LL, Yao ZF, Xie XM, Jiang ZS, Tang J, Hu JG, Lu HZ. Temporal kinetics of CD8(+) CD28(+) and CD8(+) CD28(-) T lymphocytes in the injured rat spinal cord. J Neurosci Res. 2017;95:1666–1676. doi: 10.1002/jnr.23993. [DOI] [PubMed] [Google Scholar]
- 54.Yogarajah T, Ong KC, Perera D, Wong KT. AIM2 inflammasome-mediated pyroptosis in enterovirus A71-infected neuronal cells restricts viral replication. Sci Rep. 2017;7:5845. doi: 10.1038/s41598-017-05589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang A, Wang P, Ma X, Yin X, Li J, Wang H, Jiang W, Jia Q, Ni L. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol Immunol. 2015;66:253–262. doi: 10.1016/j.molimm.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Shen CL, Dong FL, Zhang RJ, Ge P. Correlation of cytokine levels in the peripheral blood within 24 hours after cervical spinal cord injury with the American Spinal Injury Association impairment scale: a comparative study. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:3824–3830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.