Keywords: nerve regeneration, Schwann cells, 3-n-butylphthalide, 3-nitrotyrosine, nitration stress, uric acid, peroxynitrite anions, diabetic peripheral neuropathy, apoptosis, proliferation, neural regeneration
Abstract
A high glucose state readily causes peripheral axon atrophy, demyelination, loss of nerve fiber function, and delayed regeneration. However, few studies have examined whether nitration is also critical for diabetic peripheral neuropathy. Therefore, this study investigated the effects of high glucose on proliferation, apoptosis, and 3-nitrotyrosine levels of Schwann cells treated with butylphthalide. In addition, we explored potential protective mechanisms of butylphthalide on peripheral nerves. Schwann cells were cultured in vitro with high glucose then stimulated with the peroxynitrite anion inhibitors uric acid and 3-n-butylphthalide for 48 hours. Cell Counting Kit-8 and flow cytometry were used to investigate the effects of uric acid and 3-n-butylphthalide on proliferation and apoptosis of Schwann cells exposed to a high glucose environment. Effects of uric acid and 3-n-butylphthalide on levels of 3-nitrotyrosine in Schwann cells were detected by enzyme-linked immunosorbent assay. The results indicated that Schwann cells cultured in high glucose showed decreased proliferation, but increased apoptosis and intracellular 3-nitrotyrosine levels. However, intervention with uric acid or 3-n-butylphthalide could increase proliferation of Schwann cells cultured in high glucose, and inhibited apoptosis and intracellular 3-nitrotyrosine levels. According to our data, 3-n-butylphthalide may inhibit cell nitrification and apoptosis, and promote cell proliferation, thereby reducing damage to Schwann cells caused by high glucose.
Chinese Library Classification No. R453; R364
Introduction
Diabetes mellitus is currently a public health problem of global concern, and its incidence increases annually. Diabetic peripheral neuropathy is the most common and complex complication in patients with type 2 diabetes. Diabetic peripheral neuropathy can affect sensory, motor, and autonomic nerves, which seriously affects patient survival and quality of life. Recent studies (Barreiro et al., 2002; Hopkins et al., 2003; Liu et al., 2009; Huang et al., 2018) found that cell nitrosative stress develops during shock, atherosclerosis, sepsis, ischemia and reperfusion, amyotrophic lateral sclerosis, Parkinson’s disease, and diabetes mellitus. In the presence of high concentrations of blood sugar, reactive oxygen species increase and react with reactive nitrogen species to generate peroxynitrite anions (ONOO–), which can react with free tyrosine or protein tyrosine residues to cause tyrosine nitration that produces 3-nitrotyrosine (3-NT; Schonfeld and Wojtczak, 2012). Subsequent research showed that 3-NT, a specific marker of ONOO–in vivo (Corpas and Barroso, 2014), is induced by increased expression of ONOO–, H2O2, OH and NO, leading to denatured proteins and enzymes, DNA damage, and cell apoptosis. Additional research indicated that 3-NT has a positive effect on the course of diabetic nephropathy (Zhang et al., 2018). Furthermore, early studies (Sefi et al., 2012; Perez-Gallardo et al., 2014) implied that NO modulation is a viable alternative strategy for rescuing diabetic renal injury. However, with regard to the complications of diabetes mellitus, it remains poorly understood if nitrosative stress has an effect on diabetic peripheral neuropathy.
3-N-butylphthalide (NBP), a component extracted from cress seeds in southern China, was approved by the Chinese State Food and Drug Administration for clinical use as an anti-cerebral ischemia agent in 2002 (Zhang et al., 2016a, b). Currently, anti-ischemic agents are widely used in clinical practice for ischemic stroke. They have shown a neuroprotective effect in Alzheimer’s disease and stroke animal models by reducing oxidative damage, improv ing mitochondrial function, reducing neuronal apoptosis, and inhibiting inflammation (Corpas and Barroso, 2014; Wang et al., 2016a). In recent studies, DL-NBP was demonstrated to improve vascular cognitive impairment caused by subcortical ischemic small vessel disease (Hu et al., 2016; Jia et al., 2016). Its multiple neuroprotective effects arise from reduced oxidative stress (Dong et al., 2002), blocked inflammatory reactions, and reduced neuronal apoptosis (Chang and Wang, 2003). NBP was also shown to exert a protective effect on endothelial cells by suppressing production of peroxynitrite, superoxide, and nitric oxide in an acute hypoxia model (Li et al., 2009). Thus, it is hypothesized that NBP may alleviate high-glucose induced cell apoptosis in rat Schwann cells (RSC96).
To our knowledge, few studies have addressed the possible effects of NBP in development of diabetic peripheral neuropathy. Thus, we hypothesized that high glucose increases 3-NT levels, and treatment with NBP could modulate 3-NT levels in RSC96, further demonstrating the importance of nitration stress. This hypothesis was tested with in vitro exposure of RSC96 to a high glucose condition.
Materials and Methods
Cell culture, inheritance, and experimental groups
Cells (RSC96) purchased from Boster Biological Technology (Wuhan, China) were resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM; Hyclone, South Logan, UT) containing 10% fetal bovine serum (BI, Tel Aviv-Yafo, Israel) and 1% double antibiotic (penicillin 100 IU/mL and streptomycin 100 μg/mL; Biosharp, Shanghai, China), and incubated in a 37°C, 5% CO2 incubator. After 24 hours of cell adherence, the effect of the ingredient of freezing medium on cells was halted by replacing the solution, which was replaced again 2 days later. When 80% of cells had adhered, 800 μL of 0.25% trypsin (HyClone) was applied for approximately 2 minutes to digest cells, which were subcultured at a ratio of 1:4.
To establish an in vitro cell model of high glucose, RSC96 were treated with DMEM containing 100 mM glucose for 48 hours. Cells in logarithmic growth phase were cultured overnight for adherence and grouped as follows: normal control group (DMEM containing 25 mM glucose), high-glucose group (DMEM containing 100 mM glucose), mannitol group (DMEM containing 25 mM glucose and 75 mM mannitol), uric acid (UA) (Biosharp, Shanghai, China) group (DMEM containing 100 mM glucose and 0.1, 1, 10 or 100 μM of UA), and NBP (CSPC, Hebei, China) group (DMEM containing 100 mM glucose and 0.1, 1, 10 or 100 μM NBP).
Detection of cell proliferation with Cell Counting Kit-8
RSC96 cells were cultured in complete DMEM containing 25 mM glucose. Cells in logarithmic growth phase were used to prepare cell suspensions (3 × 104 cells/mL) in complete medium. After seeding 200 μL of cell suspension per well in 96-well plates (four wells per group), they were incubated overnight. After the medium was changed, the cell suspension was divided into groups according to different experimental conditions for 48 hours. The culture medium was discarded and replaced with 100 μL of standard medium (DMEM without 10% fetal bovine serum) and 10 μL of Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), which were mixed in each well. After incubation for 2 hours at 37°C, a microplate reader (Thermo Fisher, Waltham, MA, USA) was used to detect the absorbance at 450 nm. This process was repeated three times, and an appropriate concentration of each drug was used in subsequent experimental treatment groups.
Detection of apoptosis with Annexin V-FITC
RSC96 cells were cultured in complete DMEM containing 25 mM glucose. Cells in logarithmic growth phase were used to prepare cell suspensions (5 × 104) cells/mL in complete culture medium, which were seeded into 6-well plates (3 mL/well). After incubating cells overnight, the medium was replaced and the cell suspension was divided into normal control, high-glucose, UA (1 μM final concentration), and NBP (10 μM final concentration) groups. After 48 hours, cells were harvested with 0.25% trypsin digestion and washed twice with phosphate-buffered saline (PBS). Cells were collected and diluted to 1 × 106 cells/mL in 1× binding buffer. To 100 μL of this cell suspension, 5 μL of Annexin V-FITC and 5 μL of propidium iodide (BD Biosciences, Franklin Lakes, NJ, USA) were added, mixed, and incubated for 15 minutes at room temperature in the dark. Next, 400 μL of 1× binding buffer was added, and the percentage of apoptosis was analyzed by flow cytometry (BD FACSCanto II). This process was repeated three times. The upper left quadrant (FITC–/7-AAD+) contains necrotic cells, while the bottom left quadrant (FITC+/7-AAD–) contains normal living cells, the upper right quadrant (FITC+/7-AAD+) contains cells in late apoptosis, and the lower right quadrant (FITC+/7-AAD–) contains early apoptotic cells.
ELISA detection of 3-NT levels in cell culture supernatants
RSC96 cells were cultured in complete DMEM containing 25 mM glucose. Cells in logarithmic growth phase were used to prepare cells suspensions with a density of 5 × 104 cells/mL in complete medium, which were inoculated in 10-cm cell culture plates (10 mL of cell suspension/dish). Cells were incubated for 48 hours under different conditions, then scraped with a cell scratch, diluted to 1 × 106 cells/mL in 1× PBS, and frozen at −20°C overnight. After two rounds of freezing and thawing to destroy cell membranes, cell suspensions were centrifuged at 1000 × g and the supernatant was collected for detection. A 3-NT ELISA kit (CUSABIO, China) was used for detection in strict accordance with the manufacturer’s instructions and repeated three times.
Statistical analysis
SPSS 17.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Data are expressed as the mean ± SD. Inter-group comparisons were performed by one-way analysis of variance followed by the least significant difference post hoc test. A value of P < 0.05 was considered statistically significant.
Results
NBP protects RSC96 cell proliferation in a high glucose environment
Different concentrations of NBP were added to medium containing a final concentration of 100 mM glucose. After 48 hours, the optical density (OD) of the high-glucose group was significantly lower than that of the normal control group (P < 0.01). However, there was no significant difference in OD value between mannitol and normal control groups (P > 0.05); thus, the effect of osmotic pressure on RSC96 cells cultured in high glucose was excluded. OD values for 0.1 and 1 μM were higher in the NBP group compared with the high glucose group (P < 0.05), while OD values for 10 and 100 μM in the NBP group were significantly higher than the high glucose group (P < 0.01). According to Figure 1, NBP promotes proliferation of RSC96 cells in a high glucose environment, but the higher concentration of NBP was stronger; thus, 10 μM NBP was chosen as the most effective drug concentration.
Figure 1.
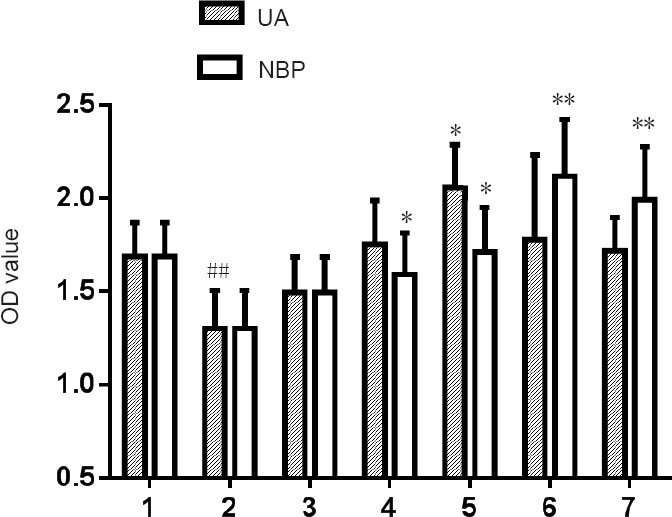
Effects of different concentrations of NBP or UA on OD values of RSC96 cells cultured in a high glucose environment.
Data are presented as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, **P < 0.01, vs. high glucose group; ##P < 0.01, vs. normal control group. 1: Normal control group; 2: high glucose group; 3: mannitol group; 4: 0.1 μM UA/NBP group; 5: 1 μM UA/NBP group; 6: 10 μM UA/NBP group; 7: 100 μM UA/NBP group. OD: Optical density; UA: uric acid; NBP: 3-N-butylphthalide.
ONOO_ inhibitor UA protects RSC96 cell proliferation in a high glucose environment
Various concentrations of UA were added to medium containing a final concentration of 100 mM glucose. After 48 hours, the OD value of the UA group was higher than that of the high glucose group, and only the 1 μM UA group was significantly different (P < 0.05). According to Figure 1, 1 μM UA promoted proliferation of RSC96 cells cultured in high glucose, so the final concentration of 1 μM UA was selected as the best drug concentration.
ONOO– inhibitors UA and NBP inhibit cell apoptosis
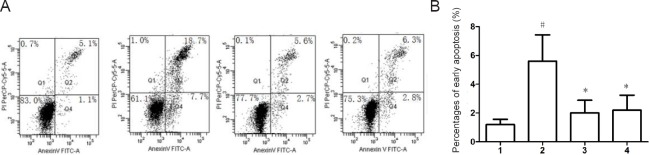
Compared with the normal control group, the high glucose group exhibited an increased percentage of early apoptotic cells (P < 0.05). Moreover, compared with the high glucose group, UA and NBP groups exhibited decreased percentages of early apoptotic cells (P < 0.05). Therefore, the ONOO– inhibitors UA and NBP can inhibit apoptosis of RSC96 cells in response to a high glucose concentration (Figure 2).
Figure 2.
Effects of UA and NBP on apoptosis of RSC96 cells cultured in high glucose.
(A) Apoptosis of RSC96 cells cultured under different conditions for 48 hours was detected by measuring Annexin V and propidium iodide (PI) with flow cytometry. (B) Percentages of early apoptosis in each group. Data are presented as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. high glucose group; #P < 0.05, vs. normal control group. 1: Normal control group; 2: high-glucose group; 3: UA group (1 μM); 4: NBP group (10 μM); UA: uric acid; NBP: 3-n-butylphthalide.
High glucose increases 3-NT levels, while UA and NBP decrease 3-NT levels
Levels of 3-NT in RSC96 cells cultured in a high glucose condition were significantly higher than in the normal control group (P < 0.01), suggesting that levels of nitration were increased in response to high glucose. Compared with the high glucose group, UA and NBP groups exhibited decreased 3-NT levels (P < 0.05, P < 0.01, respectively) (Figure 3). These results suggested that high glucose could promote apoptosis of RSC96 cells through a mechanism involving nitration within cells. Consequently, UA and NBP could inhibit high glucose-induced ONOO– expression in RSC96 cells.
Figure 3.
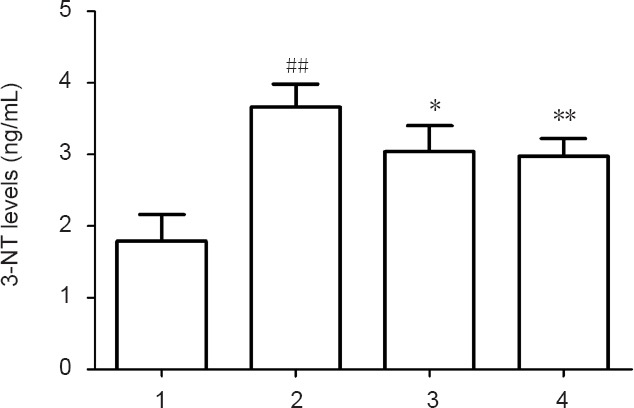
Intracellular 3-NT levels of RSC96 cells from each group.
Data are presented as the mean ± SD (n = 6; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, **P < 0.01, vs. high glucose group; ##P < 0.01, vs. normal control group. 1: Normal control group; 2: high glucose group; 3: UA group (1 μM); 4. NBP group (10 μM). UA: Uric acid; NBP: 3-n-butylphthalide; 3-NT: 3-nitrotyrosine.
Discussion
Diabetic peripheral neuropathy includes a group of heterogeneous diseases with complex pathophysiological mechanisms that can affect the autonomic nervous system and entire body (Wang et al., 2017). Metabolic disorder of the peripheral nervous system plays a key role in development and progression of diabetic neuropathy, including changes in protein kinase C activity and the polyol pathway in neuronal and RSC96 cells in response to high glucose (Singh et al., 2014; Yang et al., 2016). Recently, increased attention has been paid to nitration stress because it has been observed in diabetic complications (Yin et al., 2010; Xu et al., 2013). Nitric oxide is the body’s physiological gas and, even at low concentrations, it can combine with many molecules to play physiological or protective roles. However, under pathological conditions, nitric oxide concentrations remarkably increase the role of inducible nitric oxide synthase, which can react with surrounding oxygen free radicals to generate reactive oxygen-nitrogen compounds (reactive nitrogen species) that are strongly toxic to the body (Miranda et al., 2001; Kim et al., 2006; Coughlan et al., 2009; Drummond et al., 2011). ONOO– can effectively oxidize protein thiols, zinc-finger structures, and nitro-protein tyrosine residues to inactivate many important proteins, thus affecting cell metabolism by inhibiting respiratory chain enzymes, destroying mitochondrial structures, damaging DNA, and initiating lipid peroxidation; thus leading to tissue damage (Szabo et al., 2007). ONOO– is not only a potent oxidant, but also undergoes nitration, which can cause nitration of protein tyrosine residues to produce 3-NT; such protein nitration can ultimately lead to structural and functional changes (Kuo et al., 2000; Turko and Murad, 2002; Yin et al., 2010). Negi et al. (2010) and others found that mediating DNA damage and PARP overactivation in high glucose environments can reduce cell damage; accordingly, peroxynitrite catalysts and PARP inhibitors are protective against diabetic nephropathy. The present study shows that in previous reports, nitration participated in myocardial ischemia-reperfusion injury and diabetic nephropathy (Yin et al., 2010; Xu et al., 2013). Moreover, the results of this study showed that compared with the normal control group, the high glucose group exhibited decreased cell proliferation and increased percentages of apoptotic cells, indicating that high glucose inhibits proliferation of RSC96 cells and promotes their apoptosis. Thus, high concentrations of glucose damage Schwann cells at the cellular level. Importantly, increased 3-NT levels in the high glucose group compared with the normal control group indicated that high glucose could induce apoptosis via nitration stress. However, the related molecular mechanisms require further investigation.
NBP was developed and is produced by the China Hebei Pharmaceutical Group, which has proprietary intellectual property rights. NBP is classified as a National Class I new drug. After years of clinical trials, NBP has been shown to be effective and safe for treating ischemic stroke (Cui et al., 2013). Therefore, its mechanism is worth in-depth study to provide a theoretical basis for its wider clinical application. Its protective mechanisms include reconstruction of ischemic circulation, reduction of infarct area (Liao et al., 2009), protection of mitochondria, inhibition of neural cell apoptosis (Chang and Wang, 2003), inhibition of calcium overload, free radical scavenging, anti-platelet aggregation, inhibition of thrombosis (Peng et al., 2004), inhibition of the inflammatory response, and protection of vascular endothelial cells (Thored et al., 2007). In-depth study of NBP has also demonstrated protection in other areas; namely, it can improve cognitive function in Alzheimer’s disease and stroke by partially reversing the toxicity of amyloid in neuronal cells (Lei et al., 2014; Zhao et al., 2014). Moreover, administration of NBP resulted in upregulation of N-methyl-D-aspartic acid receptor 2B expression in streptozotocin-induced diabetic rats and improved cognitive function (Li et al., 2015). Furthermore, NBP was shown to slow the progression of hypertensive nephropathy and diabetic cataracts. Potentially, this effect is associated with nuclear factor kappa B and tumor necrosis factor alpha signaling, as Feng et al. (2012a, b) reported increased expression of these factors in spinal cord tissues of amyotrophic lateral sclerosis model mice, and nuclear factor kappa B is an important transcription factor involved in inflammatory reactions capable of regulating inducible nitric oxide synthase expression. A high level of inducible nitric oxide synthase expression can promote production of nitric oxide and ONOO–, thus eliciting a series of important signal activations. Therefore, NBP can protect motor neurons in the anterior horn by inhibiting expression of nuclear factor kappa B and tumor necrosis factor alpha in the spinal cord tissue of lateral sclerosis mice. In this study, NBP was used to treat RSC96 cells cultured in a high glucose environment, and the results indicated increased cell proliferation, whereas apoptosis and intracellular 3-NT levels were reduced; thus, NBP had a protective effect on RSC96 cells exposed to a high glucose environment.
With increased detection of nitration stress, interest in methods to reduce cellular and tissue damage caused by ONOO– has gradually increased. Some studies have demonstrated that application of a protein nitration or ONOO– inhibitor can improve diabetic peripheral neuropathy (Cassuto et al., 2014; Stavniichuk et al., 2014). At physiological concentrations, ONOO– can dissociate into the highly reactive intermediates NO2 and CO3, which can be inactivated by UA, thereby inhibiting ONOO– activity to reduce protein nitration. Consequently, UA is considered a specific endogenous scavenger of ONOO– (Whiteman et al., 2002). In this experiment, UA-stimulated RSC96 cells cultured in high glucose condition exhibited reduced effects of high glucose on cell proliferation and apoptosis; thus, ONOO– inhibition could mitigate the damage of high glucose levels on RSC96 cells. In addition, NBP could increase cell proliferation, reduce apoptosis, and decrease 3-NT levels of RSC96 cells cultured in high glucose, indicating that NBP had anti-nitrosylation effects. Simultaneously, these results provide a theoretical basis for clinical treatment of diabetic peripheral neuropathy by NBP, which had good effects for reducing in vitro damage of RSC96 cells. The protective effects of NBP on RSC96 cells cultured in high glucose conditions may occur through a mechanism involving inhibition of inducible nitric oxide synthase activity to decrease production of nitric oxide and ONOO–, which subsequently reduces protein over-nitration. However, nitration stress must be further studied with regard to molecular mechanisms and signal transduction pathways related to diabetic peripheral neuropathy to provide a stronger theoretical basis for identifying suitable effective drugs for prevention and treatment. As a first step, follow up of this study will include identifying the molecular pathway by which nitration was induced in RSC96 cells by high glucose.
In conclusion, nitration stress plays an important role in apoptosis of RSC96 cells under high glucose conditions. Moreover, NBP can protect RSC96 cells by inhibiting nitration stress in response to a high glucose environment. NBP has few adverse reactions, and is widely used in the clinic for stroke. Thus, if a conclusive protective mechanism of NBP in diabetic peripheral neuropathy is found, NBP can readily be applied for clinical treatment.
Additional file: Open peer review report 1 (98.7KB, pdf) .
Acknowledgments:
In the experiments, Quan Wu from clinical laboratory of The First Affiliated Hospital of University of Science and Technology of China has conducted technology.
Footnotes
Conflicts of interest: The authors declare that there is no duality of interest associated with this manuscript.
Financial support: This study was supported by the Natural Science Foundation of Anhui Province, China, No. 1608085MH209 (to YBW); New Medicine of University of Science and Techology of China, No. WK110000036 (to YBW). Funders had no involvement in the study design; data collection, analysis, and interpretation; paper writing; or decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Gentian Vyshka, University of Medicine in Tirana, Albania.
Funding: This study was supported by the Natural Science Foundation of Anhui Province, China, No. 1608085MH209 (to YBW); New Medicine of University of Science and Techology of China, No. WK110000036 (to YBW).
P-Reviewer: Vyshka G; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Deusen AV, Pack M, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Barreiro E, Comtois AS, Gea J, Laubach VE, Hussain SN. Protein tyrosine nitration in the ventilatory muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol. 2002;26:438–446. doi: 10.1165/ajrcmb.26.4.4634. [DOI] [PubMed] [Google Scholar]
- 2.Cassuto J, Dou H, Czikora I, Szabo A, Patel VS, Kamath V, Belin de Chantemele E, Feher A, Romero MJ, Bagi Z. Peroxynitrite disrupts endothelial caveolae leading to eNOS uncoupling and diminished flow-mediated dilation in coronary arterioles of diabetic patients. Diabetes. 2014;63:1381–1393. doi: 10.2337/db13-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang Q, Wang XL. Effects of chiral 3-n-butylphthalide on apoptosis induced by transient focal cerebral ischemia in rats. Acta Pharmacol Sin. 2003;24:796–804. [PubMed] [Google Scholar]
- 4.Corpas FJ, Barroso JB. Peroxynitrite (ONOO-) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Ann Bot. 2014;113:87–96. doi: 10.1093/aob/mct260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP, Brownlee M, Bierhaus A, Cooper ME, Forbes JM. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742–752. doi: 10.1681/ASN.2008050514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui LY, Zhu YC, Gao S, Wang JM, Peng B, Ni J, Zhou LX, He J, Ma XQ. Ninety-day administration of dl-3-n-butylphthalide for acute ischemic stroke: a randomized, double-blind trial. Chin Med J (Engl) 2013;126:3405–3410. [PubMed] [Google Scholar]
- 7.Dong GX, Feng YP. Effects of NBP on ATPase and anti-oxidant enzymes activities and lipid peroxidation in transient focal cerebral ischemic rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2002;24:93–97. [PubMed] [Google Scholar]
- 8.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng X, Peng Y, Liu M, Cui L. DL-3-n-butylphthalide extends survival by attenuating glial activation in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology. 2012a;62:1004–1010. doi: 10.1016/j.neuropharm.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Feng XH, Yuan W, Peng Y, Liu MS, Cui LY. Therapeutic effects of dl-3-n-butylphthalide in a transgenic mouse model of amyotrophic lateral sclerosis. Chin Med J (Engl) 2012b;125:1760–1766. [PubMed] [Google Scholar]
- 11.Hopkins N, Cadogan E, Giles S, Bannigan J, McLoughlin P. Type 2 nitric oxide synthase and protein nitration in chronic lung infection. J Pathol. 2003;199:122–129. doi: 10.1002/path.1256. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Liu B, Zuo Y, Liu D, Xie R, Cui W. dl-3-n-butylphthalide suppresses PDGF-BB-stimulated vascular smooth muscle cells proliferation via induction of autophagy. Life Sci. 2016;151:182–188. doi: 10.1016/j.lfs.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, He J, Xiang Y, Wang ZQ, Du G, Wang QS. Changes of perivascular astrocyte foot process in the cerebral cortex of a mouse model of sustained hyperglycemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:3218–3223. [Google Scholar]
- 14.Jia J, Wei C, Liang J, Zhou A, Zuo X, Song H, Wu L, Chen X, Chen S, Zhang J, Wu J, Wang K, Chu L, Peng D, Lv P, Guo H, Niu X, Chen Y, Dong W, Han X, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement. 2016;12:89–99. doi: 10.1016/j.jalz.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 16.Kuo WN, Kreahling JM, Shanbhag VP, Shanbhag PP, Mewar M. Protein nitration. Mol Cell Biochem. 2000;214:121–129. doi: 10.1023/a:1007118300731. [DOI] [PubMed] [Google Scholar]
- 17.Lei H, Zhao CY, Liu DM, Zhang Y, Li L, Wang XL, Peng Y. l-3-n-Butylphthalide attenuates beta-amyloid-induced toxicity in neuroblastoma SH-SY5Y cells through regulating mitochondrion-mediated apoptosis and MAPK signaling. J Asian Nat Prod Res. 2014;16:854–864. doi: 10.1080/10286020.2014.939586. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhang S, Zhang L, Wang R, Wang M. Effects of L-3-n-butylphthalide on cognitive dysfunction and NR2B expression in hippocampus of streptozotocin (STZ)-induced diabetic rats. Cell Biochem Biophys. 2015;71:315–322. doi: 10.1007/s12013-014-0200-5. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Zhang B, Tao Y, Wang Y, Wei H, Zhao J, Huang R, Pei Z. DL-3-n-butylphthalide protects endothelial cells against oxidative/nitrosative stress, mitochondrial damage and subsequent cell death after oxygen glucose deprivation in vitro. Brain Res. 2009;1290:91–101. doi: 10.1016/j.brainres.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Liao SJ, Lin JW, Pei Z, Liu CL, Zeng JS, Huang RX. Enhanced angiogenesis with dl-3n-butylphthalide treatment after focal cerebral ischemia in RHRSP. Brain Res. 2009;1289:69–78. doi: 10.1016/j.brainres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Tewari AK, Zhang L, Green-Church KB, Zweier JL, Chen YR, He G. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: mitochondria as the major target. Biochim Biophys Acta. 2009;1794:476–485. doi: 10.1016/j.bbapap.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda KM, Espey MG, Yamada K, Krishna M, Ludwick N, Kim S, Jourd’heuil D, Grisham MB, Feelisch M, Fukuto JM, Wink DA. Unique oxidative mechanisms for the reactive nitrogen oxide species, nitroxyl anion. J Biol Chem. 2001;276:1720–1727. doi: 10.1074/jbc.M006174200. [DOI] [PubMed] [Google Scholar]
- 23.Negi G, Kumar A, Sharma SS. Concurrent targeting of nitrosative stress-PARP pathway corrects functional, behavioral and biochemical deficits in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;391:102–106. doi: 10.1016/j.bbrc.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Peng Y, Zeng X, Feng Y, Wang X. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol. 2004;43:876–881. doi: 10.1097/00005344-200406000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Gallardo RV, Noriega-Cisneros R, Esquivel-Gutierrez E, Calderon-Cortes E, Cortes-Rojo C, Manzo-Avalos S, Campos-Garcia J, Salgado-Garciglia R, Montoya-Perez R, Boldogh I, Saavedra-Molina A. Effects of diabetes on oxidative and nitrosative stress in kidney mitochondria from aged rats. J Bioenerg Biomembr. 2014;46:511–518. doi: 10.1007/s10863-014-9594-4. [DOI] [PubMed] [Google Scholar]
- 26.Schonfeld P, Wojtczak L. Brown adipose tissue mitochondria oxidizing fatty acids generate high levels of reactive oxygen species irrespective of the uncoupling protein-1 activity state. Biochim Biophys Acta. 2012;1817:410–418. doi: 10.1016/j.bbabio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Sefi M, Fetoui H, Soudani N, Chtourou Y, Makni M, Zeghal N. Artemisia campestris leaf extract alleviates early diabetic nephropathy in rats by inhibiting protein oxidation and nitric oxide end products. Pathol Res Pract. 2012;208:157–162. doi: 10.1016/j.prp.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Singh R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Stavniichuk R, Shevalye H, Lupachyk S, Obrosov A, Groves JT, Obrosova IG, Yorek MA. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014;30:669–678. doi: 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 31.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, Lindvall O. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke. 2007;38:3032–3039. doi: 10.1161/STROKEAHA.107.488445. [DOI] [PubMed] [Google Scholar]
- 32.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–634. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 33.Wang CY, Wang ZY, Xie JW, Wang T, Wang X, Xu Y, Cai JH. Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer’s disease mouse model. Neurobiol Aging. 2016a;38:32–46. doi: 10.1016/j.neurobiolaging.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 34.Wang F, Ma J, Han F, Guo X, Meng L, Sun Y, Jin C, Duan H, Li H, Peng Y. DL-3-n-butylphthalide delays the onset and progression of diabetic cataract by inhibiting oxidative stress in rat diabetic model. Sci Rep. 2016b;6:19396. doi: 10.1038/srep19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Chopp M, Zhang ZG. PDE5 inhibitors promote recovery of peripheral neuropathy in diabetic mice. Neural Regen Res. 2017;12:218–219. doi: 10.4103/1673-5374.200804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteman M, Ketsawatsakul U, Halliwell B. A reassessment of the peroxynitrite scavenging activity of uric acid. Ann N Y Acad Sci. 2002;962:242–259. doi: 10.1111/j.1749-6632.2002.tb04072.x. [DOI] [PubMed] [Google Scholar]
- 37.Xu ZZ, Wang M, Wang YJ, Niu HX, Li XY, Zhou WD, Zhu Y, Long HB. Effect of nitrotyrosine on renal expressions of NF-kappaB, MCP-1 and TGF-beta1 in rats with diabetic nephropathy. Nan Fang Yi Ke Da Xue Xue Bao. 2013;33:346–350. [PubMed] [Google Scholar]
- 38.Yang X, Yao W, Shi H, Liu H, Li Y, Gao Y, Liu R, Xu L. Paeoniflorin protects Schwann cells against high glucose induced oxidative injury by activating Nrf2/ARE pathway and inhibiting apoptosis. J Ethnopharmacol. 2016;185:361–369. doi: 10.1016/j.jep.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Yin T, Hou R, Liu S, Lau WB, Wang H, Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–361. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Puchowicz MA, LaManna JC, Xu K. Protective effect of Dl-3-n-butylphthalide on recovery from cardiac arrest and resuscitation in rats. Adv Exp Med Biol. 2016a;923:31–36. doi: 10.1007/978-3-319-38810-6_4. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XG, Zhang YQ, Cheng QP, Cao Y, Sun JM, Lv XF. The impact of insulin pump therapy to oxidative stress in patients with diabetic nephropathy. Eur J Med Res. 2018;23:7. doi: 10.1186/s40001-018-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Huang LJ, Shi S, Xu SF, Wang XL, Peng Y. L-3-n-butylphthalide rescues hippocampal synaptic failure and attenuates neuropathology in aged APP/PS1 mouse model of Alzheimer’s disease. CNS Neurosci Ther. 2016b;22:979–987. doi: 10.1111/cns.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao W, Luo C, Wang J, Gong J, Li B, Gong Y, Wang J, Wang H. 3-N-butylphthalide improves neuronal morphology after chronic cerebral ischemia. Neural Regen Res. 2014;9:719–726. doi: 10.4103/1673-5374.131576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Zhang Y, Yang C. Protective effect of 3-n-butylphthalide against hypertensive nephropathy in spontaneously hypertensive rats. Mol Med Rep. 2015;11:1448–1454. doi: 10.3892/mmr.2014.2791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.