Abstract
Multiple sclerosis (MS) is a disease of the central nervous system characterized by inflammation, demyelination, and neuronal damage. Environmental and genetic factors are associated with the risk of developing MS, but the exact cause still remains unidentified. Epstein-Barr virus (EBV), vitamin D, and smoking are among the most well-established environmental risk factors in MS. Infectious mononucleosis, which is caused by delayed primary EBV infection, increases the risk of developing MS. EBV may also contribute to MS pathogenesis indirectly by activating silent human endogenous retrovirus-W. The emerging B-cell depleting therapies, particularly anti-CD20 agents such as rituximab, ocrelizumab, as well as the fully human ofatumumab, have shown promising clinical and magnetic resonance imaging benefit. One potential effect of these therapies is the depletion of memory B-cells, the primary reservoir site where EBV latency occurs. In addition, EBV potentially interacts with both genetic and other environmental factors to increase susceptibility and disease severity of MS. This review examines the role of EBV in MS pathophysiology and summarizes the recent clinical and radiological findings, with a focus on B-cells and in vivo imaging. Addressing the potential link between EBV and MS allows the better understanding of MS pathogenesis and helps to identify additional disease biomarkers that may be responsive to B-cell depleting intervention.
Keywords: Epstein-Barr virus, multiple sclerosis, meningeal inflammation, magnetic resonance imaging, leptomeningeal contrast enhancement, mononucleosis, human endogeneous retrovirus-W, B-cell
Introduction
Multiple sclerosis (MS) is a disease of the central nervous system characterized by inflammation, blood-brain barrier breakdown, demyelination, lesion formation and axonal damage. The clinical course of relapsing-remitting MS is marked by recurring exacerbations, which are followed by either complete or partial recovery (Reich et al., 2018). Over time, the majority of relapsing-remitting MS (RRMS) patients enter a progressive disease course in which there is gradual worsening of clinical disability with or without superimposed relapses, and eventually become secondary-progressive MS (SPMS) (Lublin, 2014). A small portion of patients diagnosed as primary-progressive MS (PPMS) experience an early and continuous accumulation of physical disability without any superimposed acute relapses. Although MS risk is associated with environmental, neuroimmune, and genetic factors, the exact causative factor for MS is not known. The environmental risk factors most consistently linked MS risk are infection with Epstein-Barr virus (EBV), sun exposure/vitamin D deficiency, and smoking.
EBV or ubiquitous human herpesvirus 4 usually persists asymptomatically in immune-competent and healthy individuals (Figure 1) (Lucas et al., 2011). In the United States, approximately 50% of 6–8 years old children and 90% of 18–19 years old adults exhibit evidence of prior EBV exposure (Balfour et al., 2013). EBV infects B cells and epithelial cells (Lucas et al., 2011). The persistence of EBV in B cells in a latent state is chronic and lifelong.
Figure 1.
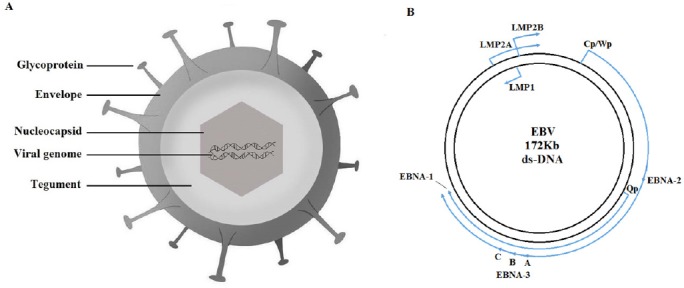
EBV viral structure and genome.
(A) EBV is illustrated in a 2D model. The glycoproteins are embedded on the outer surface of viral envelope. Inside the envelope, the matrix is named tegument. The packaged viral DNA is enclosed within the nucleocapsid. (B) EBV genome shown as a circular, double-stranded DNA. The arrowheads correspond to the direction of latent gene transcription. The outer large arrow represent the transcription of EBNA-2 and EBNA-3 initiated from the Cp or Wp promoter. The inner smaller arrow indicate the transcription of EBNA-1 from the Qp promoter. EBV: Epstein-Barr virus; LMP: latent membrane protein; EBNA: EBV nuclear antigen; VCA: viral capsid antigen; EA: early antigen; Kb: thousand base pair.
Primary EBV infection occurs mainly in children of young age and is generally asymptomatic. However, when EBV infection occurs in adolescent/early adulthood it is more often accompanied by clinically overt symptoms, manifested as infectious mononucleosis (IM) in 30–40% of infected individuals (Sokal et al., 2007). Exposure to EBV can be assessed in the clinical setting using anti-EBV antibody panels and by measuring viral load (Centers for Disease Control and Prevention, 2018). The occurrence of IgG antibodies against EBV early antigen (anti-EA IgG) is a biomarker of active EBV infection (Centers for Disease Control and Prevention, 2018). Anti-EA IgG induction is generally transient: it appears during the acute phase of infections and becomes undetectable 3–6 months after infection. However, anti-EA IgG can persist for years in 20% of the EBV infected patients (Centers for Disease Control and Prevention, 2018). Anti-EBV nuclear antigen-1 (anti-EBNA-1) antibodies appear 2–4 months after infection and persist for the remainder of the host’s life. Similar to anti-EA IgG, seropositivity for anti-EBV Viral Capsid Antigen (anti-VCA) IgM occurs early in EBV infection and disappears after 4–6 months. Anti-VCA IgG appears in the acute phase of EBV infection, peaks at 2–4 weeks, declines slightly and persists for the remainder of the host’s life. Individuals seronegative for VCA are considered susceptible to infectious mononucleosis (Centers for Disease Control and Prevention, 2018).
The initial report of association between infectious mononucleosis occurrence and the subsequent development of MS have been corroborated by multiple meta-analysis (Martyn et al., 1993; Thacker et al., 2006; Handel et al., 2010). Interestingly, the prevalence of both infectious mononucleosis and MS demonstrates a co-localizing and uneven geographical distribution that may be explained by the hygiene hypothesis (Ascherio et al., 2012). According to the hygiene hypothesis, the relative lack of early exposure to common pathogens during childhood in areas of with high levels of sanitation and hygiene may increase the risk for aberrant immune responses and autoimmune diseases if they are exposed to infectious triggers such as EBV later during adolescence and adulthood (Table 1) (Ascherio and Munger, 2007).
Table 1.
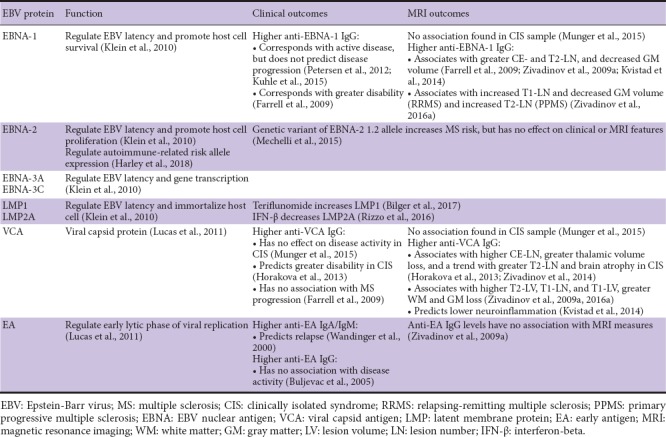
EBV proteins and their effects on MS clinical/MRI outcomes
Memory B-cells play an important role in MS pathogenesis possibly because they are reservoirs for EBV latency and are also antigen-presenting cells, which activate auto-aggressive T-cells against the myelin proteins (Greenfield and Hauser, 2018). Several MS-associated risk alleles responsible for the regulation of B-cell functions, such as antigen presentation and immune-regulation, have been identified (Smets et al., 2018).
In the brain of MS patients, B-cells aggregate within the subarachnoid space into lymphoid-like structures, a process that is guided by the expression of lymphoid-homing chemokines (Franciotta et al., 2008). These lymphoid structures, termed tertiary lymphoid follicles, were isolated from post-mortem MS brains and comprised of EBV-infected B-cells that express genes related to their growth, differentiation, pathogen recognition, and enhanced T-cell activation (Magliozzi et al., 2007; Serafini et al., 2007, 2010; Veroni et al., 2018). Tertiary lymphoid follicles can be potential sites for perpetual and independent lymphocyte activation after the primary autoimmune initiation (Parker Harp et al., 2015). Intrathecal administration of anti-CD20 (a marker for B-cell lineage) in murine experimental autoimmune encephalomyelitis (EAE) model effectively depletes B-cells within the meningeal lymphoid follicles located near MS lesions (Lehmann-Horn et al., 2014). Although most of the disease-modifying therapies currently approved for MS were initially believed to act mainly on T-cells, later investigations showed additional effects on the B-cell population (Baker et al., 2017). The assessment of leptomeningeal contrast enhancement on post-contrast 3D fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) allows the visualization of leptomeningeal inflammation in tertiary lymphoid follicles in vivo, and potentially monitors their associations with disease progression (Absinta et al., 2015). This review evaluates the role of EBV in MS from both clinical and imaging point of view, explores the effects of disease-modifying therapies that might be targeting EBV, and discusses other emerging virus candidates that may be potentially involved in MS pathogenesis. The search strategy is shown in Table 2.
Table 2.
The search strategy of this review

Pathways Involved in MS Pathogenesis
EBV and the two-hit hypothesis of MS
Although EBV seropositivity is frequent in the general population (greater than 90%), the proportion of MS patients with EBV seropositivity is even greater: nearly all MS patients exhibit evidences of past EBV infection (Lucas et al., 2011). Approximately 30–40% of adolescents who contract EBV will develop infectious mononucleosis, and initially present with acute symptoms such as fever and pharyngitis, followed by a feeling of fatigue that can last for several months (Sokal et al., 2007). Occurrence of infectious mononucleosis during the early adulthood has remained as one of the best established risk factors for further MS development (Thacker et al., 2006; Handel et al., 2010). As high as 96% of the published studies that were included in the most up-to-date meta-analysis reported a positive association between infectious mononucleosis and MS (Sheik-Ali, 2017). The mechanism underlying this association remained unexplained, but recent findings suggest that it may be mediated by enhanced blood-brain barrier permeability triggered by the acute primary EBV infection (Ransohoff and Engelhardt, 2012).
In support of this hypothesis, patients with MS also demonstrate elevated intrathecal IgG antibody production against measles virus, rubella virus and varicella zoster virus, a phenomenon called MRZ reaction (Jarius et al., 2017). Paradoxically, intrathecal production of EBV-specific IgG antibodies is significantly lower than IgG antibody production against these listed common viruses (Ruprecht et al., 2018). This paradox has led to the speculation that primary EBV infection initially enhanced blood-brain barrier permeability, allowing pre-existing polyclonal antibody-producing B-cells to enter the central nervous system (CNS), which is eventually detected as a MRZ reaction (Ransohoff and Engelhardt, 2012). In contrast, anti-EBV IgG producing cells were sparse during the initial stage of EBV infection (due to the lack of prior exposure), therefore few will enter CNS and be found intrathecally (Otto et al., 2016; Ruprecht et al., 2018).
The serial events of initial blood-brain barrier disruption and later occurring lymphocyte-based inflammation characterized a “two-hit model” which may explain the link between infectious mononucleosis and the increased MS susceptibility (Ransohoff and Engelhardt, 2012).
B-cells and T-cells
Once B-cells cross the blood-brain barrier and enter CNS, they employ a number of different mechanisms which take part in the development of MS pathology.
Antigen-presentation, one of the major roles of B-cells, involves the processing and presentation of viral antigens for priming cytotoxic T-cells. Healthy B-cells perform destructive processing of self-antigens which results in complete degradation and prevention of autoimmunity (Jakimovski et al., 2017). However, after EBV infection, the B-cells convert into productive processors and cause more overt antigen presentation (Morandi et al., 2017). In vitro studies showed that recombinant human myelin oligodendrocyte glycoprotein (rhMOG) was internalized and cross-presented by EBV-infected B-cells, which were effectively recognized by cytotoxic CD8+ T-cells (Morandi et al., 2017; van Nierop et al., 2017). Furthermore, B-cells derived from relapsing MS patients expressed higher level of CD40 on their surface, suggesting enhanced antigen presentation by B-cells (Mathias et al., 2017). Increased expression of B-cell activation markers in RRMS patients was associated with high level of neurodegeneration, measured as increased number of T1-hypointense (black hole) lesions and lower brain volume (Comabella et al., 2016). Post-mortem brain tissue obtained from a RRMS patient who died of lethal relapse following natalizumab withdrawal revealed high frequency of EBV infected B-cells in the actively demyelinating lesions (Serafini et al., 2017).
Furthermore, memory B-cells were immortalized by EBV and became undetectable by T-cell surveillance (Geginat et al., 2017). EBV-infected memory B-cells expressed lower levels of self-reactive and polyreactive antibodies than their uninfected counterparts (Tracy et al., 2012). Two transcription factors of EBV: EBV-encoded nuclear antigen (EBNA)-3A and EBNA-3C, blocked differentiation of EBV-infected B-cells into terminal plasma cells by interacting with tumor suppressor genes, and thus allowing the virus to escape from T-cell surveillance and maintain long-term latency (Table 1) (Styles et al., 2017).
In animal models, an important MS-related marker is the EBV-induced gene 2 (EBI2), an orphan G-protein coupled receptor on the surface of EBV-infected B-cells that regulates lymphocyte migration (Liu et al., 2011). Apart from its regulatory effect on myelin development, the activation of EBI2 by oxysterols (7-alpha-25-dihydroxycholesterol; 7α-25-OCH) can inhibit the pro-inflammatory cytokine release, thus decreasing consequences such as inflammation, breakdown of blood-brain barrier, and activation of pro-inflammatory microglia (Rutkowska et al., 2017).
Aside from B-cell related pathologies, loss of normal functionality in the effector T-cell population may also underlie MS disease progression. In healthy individuals, EBV infection is kept under control by CD8+ cytotoxic T-cells, which kill off the EBV infected lymphoblastoid cell lines (Khanna and Burrows, 2000). Since specific cytotoxic CD8+ cells are primed to recognize and eliminate infected cells which present latent proteins of EBV, hereafter are referred as latency-specific T-cells. During MS exacerbations, there is an expansion of EBV-specific T-cell population with an increased activity of latency-specific CD8+ T-cells (Latham et al., 2016; Pender et al., 2017). However, as MS progresses, latency-specific CD8+ T-cells demonstrate exhausted phenotype and fail to control the expansion of latently-infected cells. This causes a vicious cycle in which increasing number of infected cells prevents the autoregulatory mechanism and leads to further T-cell exhaustion (Pender et al., 2017). Reoccurring clinical relapses can be associated with inadequate control of EBV reactivations, which leads to increased infection of naïve B-cells and increased viral production (Wandinger et al., 2000; Buljevac et al., 2005). For example, the expression of programmed death-1 (PD1), a receptor associated with CD8+ T-cell activation and cytokine release, was upregulated during the remitting phase of MS (Cencioni et al., 2017).
Genetic susceptibility
In addition to environmental factors such as EBV, the observed familial aggregation of MS suggests that genetic factor is also responsible for the increased susceptibility of individuals to MS (Hemminki et al., 2009). Among all identified genetic loci related to MS, variations within the major histocompatibility complex (MHC) regions exert greatest individual effect on the MS risk (International Multiple Sclerosis Genetics Consortium et al., 2011). Additionally, several studies have suggested that same single nucleotide polymorphisms within the human leukocyte antigen (HLA) gene, as well as non-HLA genes can induce increased anti-EBV antibody response (Zivadinov et al., 2009b; Zhou et al., 2016).
The most implicated HLA-DRB1*1501 allele showed a significant association with the level of anti-EBNA-1 IgG (Rubicz et al., 2013). In a case-control study that enrolled 115 newly diagnosed MS patients and 181 matched controls in Sweden, it was found that the group of individuals who exhibited all three MS risk factors (higher anti-EBNA-1 antibody titer, presence of HLA-DRB1*1501 and absence of HLA-A2) experienced a 16-fold increased susceptibility to MS compared to the reference group comprised of individuals with lower anti-EBNA-1 antibody titer, negative HLA-DRB1*1501 and positive HLA-A2 (Sundqvist et al., 2012). Therefore, induction of anti-EBNA-1 antibody titer and HLA-DRB1*1501 carrier state might have additive effects on the MS risk (Csuka et al., 2013). In that line, enhanced IgG level against latent protein EBNA-1 in MS patients was significantly associated with risk alleles of single nucleotide polymorphism within SOX8, MYB, CARD11, as well as a trend toward significance for HLA-DRB1*1501 (Kreft et al., 2017). A study that followed MS patients for 5 years after their initial demyelinating event found that a significant additive interaction between polymorphism of miR-146a gene and baseline anti-EBNA-1 IgG titers could predict an increased risk of conversion to clinically definite MS and occurrence of relapses (Zhou et al., 2018). Other examples are the HLA-B7 allele and HLA-A2 allele in MS patients, with HLA-B7 positivity being associated with both worse MRI outcomes and a trend towards increased anti-VCA antibody titer, and HLA-A2 positivity associated with lower lesion burden and a trend towards decreased level of the anti-VCA antibody (Table 1) (Zivadinov et al., 2009b). Therefore, MS-related genetic variations may influence individual’s immunocompetence against adverse outcomes associated with EBV infection.
Genetic variants of EBV genome itself, like the EBNA-2 specific 1.2 allele is associated with MS risk, but not with clinical or MRI features (Table 1) (Mechelli et al., 2015). It was recently suggested that EBNA-2 protein forms a complex with RBPJ (recombining binding protein suppressor of hairless, an EBNA-2 cofactor) and human transcription factors to occupy autoimmune-associated genetic risk loci and alter gene expression (Harley et al., 2018). They showed that EBNA-2 protein occupies 44 of the 109 MS risk loci, supporting a potential gene-environment interaction underlying disease pathogenesis (Harley et al., 2018). The EBV-associated gene interaction was also seen in 26 out of 53 risk genes for systemic lupus erythematosus (Harley et al., 2018).
The interaction between environmental viruses and susceptibility genes is additionally supported by a considerable overlap between diseases that are potentially associated with the occurrence of EBV infection (Thorley-Lawson and Gross, 2004). For an example, the increased prevalence of Hodgkin lymphoma in previously diagnosed MS patients is consistent with the environmental and constitutional etiologies (Hjalgrim et al., 2004). This co-occurrence of both diseases had been confirmed by a recent meta-analysis that revealed significant genetic overlap between Hodgkin lymphoma and MS, both of which potentially emerge from the genetic and EBV interaction (Khankhanian et al., 2016). The enhanced expression of HLA-B7 allele has been implicated in Hodgkin lymphoma, as well as in MS patients who presented with worse MRI outcomes and higher anti-VCA antibody titer (Zivadinov et al., 2009b; Huang et al., 2012). Moreover, rheumatoid arthritis, a disease with similar immunological pathophysiology as MS, has been associated with the environmental factors, most commonly with the EBV infection (McInnes and Schett, 2011).
Therefore, dysregulation of host-EBV interaction in MS patients might be enhanced by genetic predispositions for abnormal immune responses towards molecular mimicry-based viruses.
Environmental factors and their relationship with EBV
Other environmental factors such as smoking and vitamin D deficiency can interact with EBV to increase the risk of MS (Horakova et al., 2013; Rolf et al., 2016). The higher incidence of MS observed in regions further from the equator can be explained by their variable exposures to sunlight (Simpson et al., 2011). The major form of circulating vitamin D metabolite, 25-hydroxy vitamin D (25-OH D), requires conversion by the ultraviolet B fraction of sunlight (Munger et al., 2006). Higher vitamin D level is associated with significantly lower risk of MS, possibly through inducing apoptosis of autoreactive B-cells (Rolf et al., 2016). In particular, higher serum vitamin D concentration is associated with reduced occurrence of new contrast enhancing and T2 lesions, lower relapse rate, which suggest a protective role of vitamin D against disease activity (Mowry et al., 2012).
However, the potential interaction between EBV and vitamin D, if any, still remains a matter of debate. Despite lack of association between seasonal variation of infectious mononucleosis and risk for MS, the mean serum 25-OH D level was significantly lower in infectious mononucleosis patients than healthy controls, suggesting a potential interplay between infectious mononucleosis and vitamin D (Maghzi et al., 2016; Downham et al., 2017). When compared to the placebo group, high-dose vitamin D3 treatment for RRMS patients selectively reduced the anti-EBNA-1 antibody level, but not anti-VCA IgG or anti-cytomegalovirus IgG levels (Rolf et al., 2018). This finding corroborated previous identical reports of a positive effect of vitamin D3 supplementation in the ability to reduce anit-EBNA-1 levels, but no effect on other EBV markers (VCA) or other common viruses like cytomegalovirus or varicella zoster virus (Najafipoor et al., 2015; Rosjo et al., 2017). Along the same line, the MS-associated retrovirus (MSRV) DNA copy number is higher in RRMS patients than in healthy controls, an association which is inversely correlated with their serum vitamin D concentration (Mostafa et al., 2017). ZMIZ1, an autoimmune and MS risk gene that is under-expressed in MS patients, can be induced by vitamin D (Fewings et al., 2017). Furthermore, the ZMIZ1 gene has a specific negative relationship with the anti-EBNA-1 levels (Fewings et al., 2017). A study that investigated all three potential risk factors (susceptibility genes, anti-EBV titer, and vitamin D levels) suggested that although anti-EBNA-1 level is not associated with the vitamin D level, its association with HLA-DRB1* 15:01 status still remained significant (Wergeland et al., 2016). Altogether, these findings suggest a complex interaction between EBV, vitamin D, and genetic factors that should be considered in future epidemiological studies.
Smoking is becoming a well-established risk factor for MS development and it is further associated with disease progression measured as increased disability and worse MRI outcomes (Zivadinov et al., 2009a; Wingerchuk, 2012). In MS patients, current smoking status and cumulative tobacco consumption are associated with high levels of EBV-specific antibodies (Nielsen et al., 2007). Smoking enhances the association between high anti-EBNA-1 titer and increased MS risk, however it does not affect the association between HLA-DRB1* 1501 and MS risk (Simon et al., 2010). Smoking induces epigenetic changes such as citrullination, which has been implicated in the conversion of destructive MOG processing to the productive processing by EBV-infected B-cells (Morandi et al., 2017; Olsson et al., 2017). The interaction between smoking and EBV may differ based on the heterogeneity of the study population (Salzer et al., 2014). For instance, a positive interaction between smoking and anti-EBNA-1 antibody titer was only observed in MS subjects older than 26 years old (Salzer et al., 2014). For clinically isolated syndrome (CIS) patients, anti-EBNA-1 antibody and cotinine level (indicator of tobacco use) were not associated with conversion to clinically definite MS or further disease progression (Munger et al., 2015). Other studies suggest that infectious mononucleosis and smoking seem to have independent effects on MS disease outcome (Bjornevik et al., 2017). These findings point towards a complex interaction between smoking and EBV that is indirect and possibly mediated by other variables.
Therefore, the development of MS should not be attributed to any single factor, but to the combined effects of genetic and environmental factors. A case-control study enrolled 282 MS cases and 558 matched controls examined the potential interaction between 5 key MS risk factors (history of infectious mononucleosis, low actinic damage, low serum 25(OH)D concentration, smoking, presence of HLA-DRB1* 1501 genotype), as well as several other risk factors (high anti-EBNA-1 IgG titer, HLA-A2, sun exposure, education level), in relation to the onset of first clinical diagnosis of CNS demyelination (van der Mei et al., 2016). The joint impact of all 5 key risk factors, measured as the summary population attributable fraction, accounted for 63.8% of first clinical diagnosis of demyelination onset. In specific, HLA-DRB1* 1501, smoking, low actinic damage, history of infectious mononucleosis, low 25(OH)D each contributed 45.6%, 40.8%, 30.8%, 24.4% and 15.9%, respectively (van der Mei et al., 2016). The paper also suggested that adding anti-EBNA-1 titer into this multifactorial model would further increase the summary population attributable fraction beyond 63.8%, however this subsample was too small to be included (van der Mei et al., 2016).
MS Disease Course and EBV Seropositivity
The link between EBV and MS is supported by higher prevalence of EBV infection and by the elevated EBV-specific antibody levels in MS patients when compared to healthy controls (Pender et al., 2014). EBV serostatus is most commonly determined by the presence of antibodies against EBNA-1, VCA, and EA, which are antigens associated with different stages of EBV infection.
The contribution of EBV infection to MS disease progression in early phases of MS is subject to contradictory findings, with some studies suggesting contribution to conversion to clinically definite MS and some not. A country-wide study of the enrolled military personnel demonstrated that among the 10 initially EBV-negative soldiers who later developed MS during their time in the service, they did seroconvert before their disease onset (Levin et al., 2010). A large, multicenter study followed 1047 CIS patients for 4.3 years, and provided valuable results regarding risk factors associated with the conversion to clinically definite MS (Kuhle et al., 2015). Among the many factors that were investigated, they found that only increasing T2 lesion number, earlier age of onset, and oligoclonal bands positivity were strong prognostic markers for conversion to clinically definite MS. Interestingly, in this study, EBV infection, measured by anti-EBNA-1 antibody positivity, was not a marker for disease conversion (Kuhle et al., 2015). A follow-up study from the same CIS sample using a more detailed testing revealed that only 1 out of the 49 originally determined EBV-negative patients was truly seronegative (Dobson et al., 2017). They argued that it was extremely rare for an EBV seronegative individual to develop MS, and suggested that future serological studies should consider testing antibodies across multiple antigens, or using more than one independent antibody screening assays (Dobson et al., 2017). The SET study analyzed anti-EBV antibody levels from 211 CIS patients before initiating interferon-beta (IFN-β) treatment, and obtained clinical and MRI assessments at baseline, 6, 12, and 24 months. They found that anti-EBV VCA antibody level was positively associated with both increased disability and worse MRI outcome over the 2-year course (Horakova et al., 2013). The BENEFIT trial (IFN-β treatment for CIS patients) followed 457 patients for five years and found no association between EBV-specific antibody levels with neither the rate of conversion to clinically definite MS, nor with disease progression measured by accumulation of T2 lesions and development of whole brain atrophy (Munger et al., 2015).
After the MS onset, antibody responses toward EBV antigens correlate with disease activity and disease progression (Christensen, 2006). Recent serological investigations have shown that intermittent EBV reactivation during the active disease course may occur in parallel with exacerbations in RRMS patients. In a sample of 100 MS subjects including RRMS, CIS and PPMS, all individuals showed serological evidence of previous EBV infection, but anti-EBNA-1 IgG titer was the highest in RRMS when compared to PPMS and CIS phenotypes (Farrell et al., 2009). For the RRMS subgroup, the increased serum level of anti-EBNA-1 antibody corresponded with significantly higher number of contrast enhancing lesions and disability accumulation over a five-year period (Farrell et al., 2009). In a group of 19 MS patients who were followed monthly for a year, reinstatement of anti-EA IgM and IgA (markers for active viral replication) and serum EBV DNA positivity were detected in 72.7% of those who experienced acute relapse during the study period, and in 0% of the clinically stable patients Table 1 (Wandinger et al., 2000). EBV reactivation, measured by an increase in the anti-EA IgG level, was seen in 54.5% of patients with exacerbation but in only 12.5% of patients who remained stable during the 1-year period (Wandinger et al., 2000). They suggested that EBV reactivation, indicated by fluctuation of anti-EA IgG level, was associated with clinical disease activity in MS patients (Wandinger et al., 2000). Another study presented with opposite findings: it was shown that anti-EA IgG level remained stable within each of the 73 RRMS patient who were followed for an average of 1.7 years, and there were no association between annual exacerbation rate and anti-EA IgG seropositivity during the follow-up period (Buljevac et al., 2005). They attributed the discrepancy between the two studies to technical differences and the lack of IgA serology (Buljevac et al., 2005).
Within the 100 MS subjects enrolled in the serological study, PPMS patients had significantly lower anti-EBNA-1 IgG titer and higher anti-VCA IgG titer than RRMS patients (Farrell et al., 2009). However, there were no association between changes in EBV antibody titer and disability progression during the 5-year follow-up (Farrell et al., 2009). On the other hand, numbers of MRI studies presented evidences that supported the association between EBV antibody and MS progression, which will be discussed in details later in this review. Therefore, EBV reactivation can be a useful marker for predicting clinical disease activity in RRMS patients, but its significance in monitoring disease progression in progressive MS patients requires future investigation.
EBV and MS: Lessons from MRI Studies
Anti-EBV antibodies and MRI outcomes
The association of EBV and MS has been additionally documented and corroborated with MRI, the most sensitive diagnostic tool for detecting MS development and for monitoring disease progression (Zivadinov et al., 2016a). MRI studies involving CIS or clinically definite MS patients showed that enhanced antibody level against EBV might be associated with increased neuroinflammatory activity. After the first demyelinating event, CIS patients with higher anti-VCA IgG antibody titer demonstrated a greater number of contrast enhancing and T2 lesions, and increased brain atrophy at 2-year follow-up (Horakova et al., 2013). In a longitudinal MRI study that initiated since 1995, the elevation of anti-EBNA-1 IgG level from baseline to the last follow-up was observed in both CIS and RRMS subjects (Farrell et al., 2009). Patients with enhanced anti-EBV antibody response also developed more contrast enhancing lesions, greater T2 lesion volume on MRI, and higher disability score (Farrell et al., 2009). Similar findings were shown in a group of 92 RRMS patients who were treated with IFN-β for 2 years, where greater anti-EBNA-1 antibody level was associated with greater combined unique activity, measured by the sum of the contrast enhancing and new T2 lesions (Kvistad et al., 2014). They also reported an inverse relationship between anti-VCA IgM and the MRI-derived inflammatory activity, which had been attributed to IFN-β treatment response (Kvistad et al., 2014). Moreover, the presence of EBV/human herpes virus 6 DNA within the cerebrospinal fluid (CSF) of MS patients was also associated with increased contrast enhancing lesions (Virtanen et al., 2014). In the analysis of post-mortem MS white matter tissues, both overexpression of pro-inflammatory cytokine interferon-alpha (IFN-α) and EBV-encoded RNA positivity were detected in active areas of white matter lesions (Tzartos et al., 2012). Therefore, EBV infection may have triggered innate immune responses, such as cytokine production which further increased the inflammation within the MS lesions.
In addition to the possible relationship with neuroinflammatory responses discussed above, recent studies have demonstrated that EBV antibody activity could be linked to neurodegeneration in MS patients. Anti-EBV antibody analysis and MRI measurements that were collected over 3 years in a group of RRMS patients revealed that the anti-VCA IgG titer was negatively correlated with grey matter volume (Zivadinov et al., 2009a). This finding was corroborated by a larger cohort study that enrolled more than 1000 CIS, MS (RRMS, SPMS and PPMS), other neurological diseases patients, and matched healthy controls (Zivadinov et al., 2016a). In this study, CIS and MS patients were categorized into highest or lower quartile groups based on their anti-EBV antibody (anti-VCA and anti-EBNA-1) titers (Zivadinov et al., 2016a). The MS patients within the highest anti-VCA quartile displayed significantly increased T2 lesion volume, T1 lesion number and volume, and greater white matter loss than patients in the lower quartile (Zivadinov et al., 2016a). The positive correlation between anti-VCA antibody and cortical atrophy was most prominently observed among RRMS subjects, but not in progressive MS subtypes, possibly due to the small sample size (Zivadinov et al., 2016a). Moreover, the significant difference in MRI outcomes between anti-VCA quartile groups was MS-specific, as it was not observed in the healthy controls or patients with other neurologic diseases (Zivadinov et al., 2016a). On the contrary to the anti-VCA findings, there were no association between anti-EBNA-1 titer and MRI outcomes when comparing between the study groups (Zivadinov et al., 2016a). However, when comparing within the MS subgroups, RRMS patients with highest anti-EBNA-1 quartile showed increased T1 lesion number and decreased grey matter volume than lower quartile group, while PPMS patients with highest EBNA-1 quartile showed increased T2 lesion number than lower quartile group (Zivadinov et al., 2016a). Findings from the CIS population were rather controversial. The BENEFIT trial which followed 468 CIS patients for 2 years reported neither anti-EBNA-1 nor anti-VCA IgG titer affecting MRI outcomes (Munger et al., 2015). The SET study which enrolled 211 CIS patients revealed a trend between highest anti-VCA IgG quartile and decreased percentage brain volume change over 2-year (Horakova et al., 2013). The SET study also showed that CIS patients with the highest anti-VCA IgG quartile demonstrated greater thalamic volume loss, as well as a trend towards greater whole brain and grey matter atrophy (Zivadinov et al., 2014).
The correlation between humoral response to EBV and MRI disease activity supported the involvement of EBV-infected B-cells in grey matter and cortical pathology that can be seen early in the disease. Altogether, these findings reinforced the association between EBV and MS disease progression, and suggests that brain atrophy measurement can be a useful MRI metric in clinical routines (Zivadinov et al., 2016c).
Association of EBV with leptomeningeal inflammation and lymphatic vessels
The discovery of tertiary lymphoid follicles within the meninges of progressive MS patients have elicited new research interests, and several studies reported that these structures might be associated with greater extent of cortical atrophy and higher clinical disability (Magliozzi et al., 2010; Absinta et al., 2015; Makshakov et al., 2017; Zivadinov et al., 2017). Brain tissue analysis of PPMS subjects revealed EBV-infected B-cells expressing EBNA-1 and EBNA-2 that aggregated in both meningeal infiltrations and in cervical lymph nodes, suggesting the involvement of EBV in the progressive disease phenotype (Serafini et al., 2014). The post-mortem tissue analysis from 35 active/inactive MS patients also revealed CD8+ CD57+ T-cells aggregating in these lymphoid structures (Cencioni et al., 2017). Later study corroborated the finding of significantly higher proportion of CD4+/CD8+ T-cells and additionally demonstrated presence of a CD20+/CD138+ population of B-cells in meningeal infiltrates (Veroni et al., 2018). In addition, the expression of IFN-γ, a key regulator for antiviral response, was also enhanced in these meningeal infiltrates (Veroni et al., 2018). Since memory B-cells act as life-long repertoire for latent EBV infection, the aggregation of B-cells and T-cells within tertiary lymphoid follicles may indicate possible site of interaction following virus infection (Jakimovski et al., 2017).
Meningeal infiltrates can be visualized as leptomeningeal contrast enhancement by using the 3D-FLAIR MRI technique (Absinta et al., 2015; Zivadinov et al., 2018b). Leptomeningeal contrast enhancement was previously detected by 3T MRI in 74 of 299 MS patients, and were more prevalent in progressive MS patients than in RRMS patients (Absinta et al., 2015). Interestingly, only one healthy control from this study presented with a focal leptomeningeal contrast enhancement loci (Absinta et al., 2015). They additionally found that the presence of leptomeningeal contrast enhancement was associated with nearby cortical demyelination, and was accompanied by significantly smaller brain volume and white matter volume in MS patients (Absinta et al., 2015). Similarly, leptomeningeal contrast enhancement detected in RRMS patients was associated with reduced thickness of surrounding cortical tissues (Bergsland et al., 2018). A longitudinal study of 50 MS patients (27 RRMS and 23 SPMS) also demonstrated that leptomeningeal contrast enhancement positive patients had significantly greater percentage of total grey matter and cortical volume loss, as well as greater increase in ventricular CSF volume at the 5-year time point than patients without leptomeningeal contrast enhancement (Zivadinov et al., 2017). The association between leptomeningeal contrast enhancement and grey matter atrophy was supported by a similar study, which additionally found that leptomeningeal contrast enhancement correlated with longer disease duration and higher disability (Makshakov et al., 2017). Observation from 7T MRI study revealed that leptomeningeal contrast enhancement occurred in higher frequency in MS patients than prior studies with 3T MRI, which was more closely resembling histological findings (Harrison et al., 2017). In this sample of 29 MS participants (including RRMS, SPMS, and PPMS), at least one focus of LM enhancement was detected in 90% of patients, and these foci were associated with older age and reduced grey matter volume (Harrison et al., 2017). Brain tissue from a mice EAE model showed a higher density of immune cells localized in the meninges, corresponding to the areas of leptomeningeal contrast enhancement (Pol et al., 2017).
Although the presence of leptomeningeal contrast enhancement is not a MS-specific sign and has been detected in other non-MS inflammatory neurologic conditions including human T-lymphotropic virus-associated myelopathy, Susac’s syndrome, neuromyelitis optica spectrum disorder, and human immunodeficiency virus (HIV)-infected individuals, these structures seem to be involved in aberrant CNS-specific autoimmune processes (Absinta et al., 2017b).
A consensus MRI guideline for measuring leptomeningeal contrast enhancement has yet to be established, but recent studies have attempted to improve this imaging technique for translation into clinical routine (Zivadinov et al., 2018b). The sensitivity and specificity of three methods used for MR imaging of leptomeningeal contrast enhancement in MS patients showed that subtraction images of pre/postcontrast 3D-FLAIR improve the most accurate diagnosis and significantly reduce imaging time for detecting leptomeningeal contrast enhancement (Zivadinov et al., 2018b). The study of leptomeningeal contrast enhancement is especially important for understanding the mechanism underlying the observed positive correlation between EBV antibody titer and cortical atrophy in longitudinal studies. With further development of a standardized protocol, leptomeningeal contrast enhancement can potentially be useful for detecting EBV-related brain pathology in clinical routine examination and contribute to identification of new therapeutic targets.
The discovery of tertiary lymphoid follicles within meninges of MS brain containing large proportion of EBV-activated B-cells and T-cells might suggest an alternative route of entry for lymphocytes into the CNS region. Self-antigen presentation by EBV-immortalized B-cells may be important for the reactivation of T-cells occurring in the subarachnoid space (Louveau et al., 2016). Evidence from EAE studies suggested that T-cells caused the development of meningeal infiltrates and local invasion of the parenchyma, events that were tightly associated with disease onset (Kivisakk et al., 2009). This is supported by evidence from another EAE study which showed that at 10 days-post induction, higher gadolinium signal intensity was detected in superior sagittal sinus (major draining vein for the anterior cerebral cortex) of EAE mice brain than in healthy mice brain (Pol et al., 2017). The enhanced signal intensity was also associated with higher density of immune cells (Pol et al., 2017). It was proposed that under pathological conditions, antigen-specific T-cells leave the peripheral blood stream and enter into the CSF at choroid plexus sites, a place ideal for their re-stimulation by local myeloid antigen presenting cells (Ransohoff and Engelhardt, 2012). These T-cells then release cytokines that increase the permeability of blood-brain barrier to other peripheral lymphocytes, thus enabling them to enter the brain parenchyma and cause further damage (Ransohoff and Engelhardt, 2012).
Newer imaging technology has enabled the identification of conventional lymphatic vessels that carry fluid and immune cells from meninges/CSF to deep cervical lymph nodes (Louveau et al., 2015). Increasing evidence now suggests that bidirectional trafficking of B-cells can occur between the CNS and cervical lymph nodes via the meningeal lymphatic vessels. The meninges and tertiary lymphoid follicles are important sites for lymphocyte affinity maturation, and their functions as entry points for autoreactive T-cells to the CNS. The described rudimentary lymphatic vessel have been additionally shown in vivo in higher level primates and in humans (Absinta et al., 2017a).
Human Endogenous Retrovirus W
In addition to EBV, other viral agents such as human endogenous retroviruses are thought to be involved in MS onset (Tao et al., 2017). An initial large-scale, comparative cohort study suggested that HIV was associated with a lower risk for MS, possibly either due to the immune-suppressive state induced by the virus itself, or by the effects of HIV-associated antiretroviral therapies (Gold et al., 2015). This study followed up 21,207 HIV-positive patients and 5 million controls between the years of 1999 to 2011, and showed that the rate of developing MS in people with HIV was relative lower than people without (Gold et al., 2015). One such therapeutic target implicated in both HIV and MS is the envelope protein of human endogenous retrovirus W (HERV-W), or MSRV/syncytin-1. Multiple studies were able to isolate MSRV from cells of MS patients, such as in leptomeningeal cells, monocytes and EBV-transformed B-cells (Dolei and Perron, 2009). Enhanced MSRV expression was found in MS brains when compared to other neurological disorders (Antony et al., 2004).
A potential pathological cascade triggered by syncytin-1 induction is the release of soluble factors by astrocytes, such as inducible nitric oxide synthase and other redox reactants, which might lead to oligodendrocyte damage and cell death (Antony et al., 2004). In vivo mice model also supported the role of syncytin-1 in demyelination and impaired neurological functions, both which can be rescued by antioxidant ferulic acid (Antony et al., 2004). Furthermore, another study had shown that HERV-W obtruded remyelination by inhibiting differentiation of oligodendrocyte precursor cells (OPCs) (Kremer et al., 2013). This was achieved by the env protein activating TLR4 on OPCs, which induced nitric oxide synthase levels and increased production of pro-inflammatory cytokines (Kremer et al., 2013). All 75 MS brain samples acquired from two separate studies were positive for MSRV-env protein, with specifically high expression of MSRV in macrophages and microglia near active lesion sites, and lower expression in normal-appearing white matter, while no MSRV expression was detected in healthy controls (van Horssen et al., 2016). HERV-W activity (measured by MSRV-type transcript expression in peripheral blood mononuclear cells, PBMC) was higher in patients with infectious mononucleosis, and in healthy controls with high anti-EBV antibodies (Mameli et al., 2013). These findings suggest that silent MSRV may be activated by environmental triggers, such as an EBV infection, to directly contribute to MS development (Kury et al., 2018). Future studies are required for a better understanding of the role of MSRV and its interaction with EBV in the MS pathogenesis.
MS Treatments and EBV
Standard disease-modifying therapies in MS
The clinical effect of disease-modifying therapies may interfere with the rate and extent of the persistent EBV infection. IFN-β, a commonly used disease-modifying therapy for RRMS, has potent antiviral activity. Interestingly, EBV and other viruses have evolved molecular mechanisms to evade the antiviral effects of Type 1 interferons such as IFN-β. However, IFN-β treatment results in apoptosis of CD27+ memory B-cells, which are reservoir for EBV infection and decrease the expression of EBV gene LMP2A (Rizzo et al., 2016). IFN-β alternatively increased the expression of apoptotic markers such as Annexin-V and active caspase-3 and induced specific depletion of memory B-cells (Jakimovski et al., 2018). Similar study followed 6 patients who remained active despite IFN-β treatment and found significantly increased anti-EBNA-1 antibody level at one-year mark (Petersen et al., 2012). An MRI study showed that the positive correlation between anti-EBNA-1 antibody titer and contrast enhancing lesions, T2-lesion number became insignificant after initiating IFN-β treatment (Kvistad et al., 2014). The lack of significant association between EBV and CIS-clinically definite MS disease conversion reported in the SET and BENEFIT trials may also be related to clinical benefit of IFN-β (Horakova et al., 2013) (Munger et al., 2015).
The therapeutic effects of natalizumab may also involve the regulation of humoral responses against EBV. The expression of miR-155, a microRNA involved in pro-inflammatory cytokine expression, was induced in un-treated MS patients compared to healthy controls (Mameli et al., 2016). This higher miR155 expression was positively correlated with anti-EBNA-1 antibody level in MS samples, and it was down-regulated by 6 months of natalizumab treatment (Mameli et al., 2016). Another group assessed the EBV-specific CD8+ T-cell response among 14 MS patients after 8–16 months of natalizumab treatment, and they reported that the frequency of EBV lytic antigen-specific CD8+ T-cells was significantly higher in untreated clinically active patients than in treated inactive patients (Angelini et al., 2013). Another study presented negative findings, by showing that a group of 20 MS patients who were followed for 21 months during natalizumab treatment experienced no significant differences in serum anti-EBNA-1 IgG levels (Castellazzi et al., 2015). This was similar to another study that reported RRMS patients treated with either IFN-β or natalizumab for 12 months experienced no significant changes in their anti-EBNA-1 antibody titer, demonstrating no treatment effect on humoral EBV response (Raffel et al., 2014). Future studies are needed to verify the actual effects of IFN-β and natalizumab on anti-EBV antibodies.
Other disease-modifying therapies have also been shown to exert therapeutic effects on B-cell subpopulations (Baker et al., 2017). Glatiramer acetate-treated patients demonstrated altered B-cell compositions and restored cytokine production by CD19+ B-cells (Ireland et al., 2014). Additionally, the treatment group exhibited lower CD19+ B-cell count and diminished CD27+ B-cells when compared to healthy controls and matched untreated patients (Ireland et al., 2014). Four to six months of dimethyl fumarate treatment in RRMS patients also significantly reduced the total number of CD27+ memory B-cells (Lundy et al., 2016).
B-cell depletion therapies
Since EBV establishes long-term latency within the memory B-cells, elimination of infected B-cells may improve the control of EBV infection. Rituximab, ocrelizumab, and ofatumumab are anti-CD20 mAbs that induce marked depletion of CD20-expressing lymphocytes (Greenfield and Hauser, 2018). In a murine EAE model, intrathecal injection of anti-CD20 efficiently depleted B-cells within the tertiary lymphoid follicles near CNS lesions (Lehmann-Horn et al., 2014). This finding supported the potential of B-cell depleting therapy and the utility of leptomeningeal contrast enhancement in the drug efficacy monitoring.
When compared to the complete and long-lasting peripheral B-cell depletion, the depletion of CSF B-cells by intrathecal rituximab treatment was incomplete and only transient (Bhargava et al., 2018). This finding was consistent with findings from the RIVITALISE trial in which SPMS patients treated with intrathecal rituximab demonstrated incomplete depletion of CNS harbored B-cells and ultimately lack of efficacy in clinical outcome measures (Komori et al., 2016). The differences between ocrelizumab and rituximab in their ability to deplete CD20-expressing B-cells might be the result of the variable extents in triggering complement-dependent and antibody-dependent cellular cytotoxicity (Jakimovski et al., 2017).
In contrast to B-cell depletion therapies, the augmentation of memory B-cells worsens symptoms in MS. Atacicept depletes mature B-cells but upregulates interleukin-15, which in turn can lead to increased production of memory B-cells and induction of relapse rates in MS patients (Ma et al., 2014). Although the long-term risks associated with anti-B-cell therapies have not been fully characterized, these evidence underscore the potential of B-cells as a therapeutic target in MS.
The effect of disease-modifying therapies on leptomeningeal contrast enhancement still remains poorly understood. However, because these lymphoid follicles contain CD20+ B-cells, it is reasonable to expect B-cell depleting therapies to be effective in reducing the prevalence of leptomeningeal contrast enhancement (Jakimovski et al., 2017; Zurawski et al., 2017). The limited data suggest that intrathecal administration of rituximab did not disrupt tertiary lymphoid follicles in progressive MS patients (Bhargava et al., 2018). At 24-month post-treatment, there was no change in the number of leptomeningeal contrast enhancement despite the reduction of peripheral blood B-cell (Bhargava et al., 2018).
Therapies with unique mechanisms of action
Teriflunomide stands out as a unique disease-modifying therapies with less destructive effects on the host immune system. This compound inhibits de novo pyrimidine synthesis pathway and suppresses proliferation of EBV-transformed lymphocytes without affecting cell viability (Bar-Or et al., 2014). Studies have shown that treating lymphoblastoid cell line with teriflunomide enhanced expression of the LMP1 and p53 genes, suggesting altered gene expression in latent EBV-infected B-cells, as well as reduced viral replication and increased apoptosis in infected B-cells (Table 1) (Bilger et al., 2017). Both phase 3 teriflunomide trials (TOWER and TESMO) showed that RRMS patients undergoing high-dosage treatment had significant reductions in annualized relapse rate and accumulated disability compared to the placebo group (Wolinsky et al., 2013; Confavreux et al., 2014). This therapy was also beneficial based on MRI outcomes, demonstrating reduction of total lesion volume and cortical atrophy (Wolinsky et al., 2013; Radue et al., 2017). The baseline differences in brain volume and diffusion tensor imaging measurements between RRMS and matched-healthy controls diminished at 12-month after patients initiated teriflunomide treatment (Zivadinov et al., 2018a). By comparing anti-EBV antibody titers at baseline and 12-month follow-up, Zivadinov et al. (2016b) found that MS patients with the highest decrease in anti-VCA IgG developed less grey matter and cortical atrophy, and those with the highest decrease in anti-EBNA-1 IgG developed less hippocampal atrophy. While the majority of disease-modifying therapies that are currently available for MS treatment exert their effects through inducing active depletion of the overall lymphocyte population, teriflunomide may possess a unique mechanism of action which limits the involvement of autoreactive T-cells and EBV-infected B-cells in neuroinflammation.
Investigations of CD8+ T-cells have led to the development of a potential therapeutic target. Specific lytic CD8+ T-cells against the EBV antigen BMLF1 can eliminate replicating EBV-activated B-cells after being transferred into EBV-infected mice model (Antsiferova et al., 2014). The expansion of EBV-specific T-cells by in vitro stimulation with AdE1-LMPpoly was used to treat a 42-year-old SPMS patient with CD8+ T-cell deficiency (Pender et al., 2014). Following the treatment, the level of EBV-specific CD8+ T-cells increased, the CE lesions and intrathecal IgG production decreased and the patient reported sustained improvement of physical symptoms (Pender et al., 2014).
Retroviral treatments and EBV
Interestingly, anti-retroviral therapies have shown considerable benefits for treating MS patients (Drosu et al., 2018). In the previously discussed comparative cohort HIV study, the lower MS rate among HIV-positive subjects suggested possible protective effects exerted by antiretroviral medications against MS development (Gold et al., 2015). This was further supported by a recent case report of a 25-year-old female student who recovered from a MS exacerbation and remained clinically and MRI stable after being treated with Combivir (Drosu et al., 2018). An initial study reported enhanced MSRV-env transcript level following infectious mononucleosis, suggesting MSRV as a mediator between EBV and MS (Mameli et al., 2013). As mentioned previously, the env protein also interrupts OPC differentiation by inducing inducible nitric oxide synthase and pro-inflammatory cytokine release (Kremer et al., 2013). OPC cultures that were incubated with anti-MSRV-env antibody GNbAC1 before env stimulation showed significantly reduced inducible nitric oxide synthase transcript level and restored myelin expression compared to OPC cultures that were incubated with buffer (Kremer et al., 2015). A phase 2 trial of GNbAC1 showed reduced MSRV-env and MSRV-pol expressions in all 10 RRMS patients, and 9 out of 10 treated patients remained MRI stable at the 6-month time point (Derfuss et al., 2015). Despite the favorable safety profile of the drug, the interpretation of the efficacy data from this phase 2a trial was limited and warranted a subsequent phase 2b or phase 3 trial with larger cohort and longer duration period (Curtin et al., 2016).
Traditional disease-modifying therapies are also associated with changes in MSRV-env expression (Mameli et al., 2015). For an example, 6 months of IFN-β treatment decreases MSRV viremia and the immune response against MSRV-env, whereas a 1-year treatment with natalizumab strongly reduces the HERV-W/MSRV/Syncytin-1 expression in RRMS patients (Arru et al., 2014; Mameli et al., 2015). These findings suggest that measuring MSRV level in MS patients under therapy can be important for monitoring disease progression and treatment efficacy (Arru et al., 2014).
Potential EBV vaccine
Although EBV infection is ubiquitous and often persists asymptomatically among immunocompetent individuals, a primary immunological response manifested as infectious mononucleosis has been implicated as the trigger of multiple disorders. Accumulating evidence have supported the strong association between infectious mononucleosis and MS, thus leading to the speculation of early prevention of infectious mononucleosis as an intervention for reducing MS risk (Cohen, 2015).
Currently there is no licensed-EBV vaccine, and the development of such vaccine has faced a few challenges. Since the majority of EBV-infected individuals will remain asymptomatic, additional MS susceptibility markers need to be identified in order to recognize the most suitable population for EBV vaccination. Additionally, the specific EBV strain that is associated with MS disease pathology also has to be clarified. The modestly effective vaccination could postpone the EBV infection from childhood to adolescence, a period when one is most sensitive to environmental influences, and thus increase the risk for the negative outcomes associated with EBV immune response (Olsson et al., 2017). Therefore, the vaccine needs to be delicately designed before any large scale studies are administered.
Despite many difficulties associated creating an EBV vaccine, valuable results have strengthened the rationale for its development. The closest attempt was made with gp350, an EBV surface protein that mediates the attachment of the virus to B-cells. An earlier Phase II clinical trial of gp350-based infectious mononucleosis vaccine showed that the vaccinated group was 4.8 times less likely to develop infectious mononucleosis than the placebo group (Sokal et al., 2007). However, this vaccine did not affect the rate of initial EBV infection (Sokal et al., 2007). A prospective study followed university freshmen before and after EBV seroconversion and recorded the occurrence of infectious mononucleosis (Grimm et al., 2016). They showed that gp350 level was inversely correlated with the severity of infectious mononucleosis, supporting the protective role of gp350 against aberrant immunological response towards EBV. Interestingly, the seroprevalence of EBV declined between the year of 2006 and 2012, implicating larger number of EBV naïve adolescents and greater susceptibility for potential infectious mononucleosis occurrence in future generations.
Conclusion
The conclusion drawn from the studies that are discussed in this review seems to align with a “fertile field hypothesis” which describes a heightened immunological state triggered by the initial viral infection. This would ultimately decrease the threshold for activating auto-aggressive T-cells in the presence of later events. In order to accurately describe the role of viruses in MS, there is an urgent need to establish standardized virus screening protocols. Nevertheless, recent B-cells research have become increasingly important for understanding the MS pathophysiology and may assist in addressing the potential link between EBV and MS tertiary lymphoid follicles comprising mainly of memory B-cells can be assessed by in vivo MRI imaging and might be useful in monitoring disease activity.
Additional file: Open peer review reports 1 (120.3KB, pdf) and 2 (117.9KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
P-Reviewers: Akaishi T, Lin W; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
Open peer reviewers: Tetsuya Akaishi, Tohoku University, Japan; Wensheng Lin, University of Minnesota Twin Cities, USA.
References
- 1.Absinta M, Ha SK, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017a;6:e29738. doi: 10.7554/eLife.29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Absinta M, Vuolo L, Rao A, Nair G, Sati P, Cortese IC, Ohayon J, Fenton K, Reyes-Mantilla MI, Maric D, Calabresi PA, Butman JA, Pardo CA, Reich DS. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85:18–28. doi: 10.1212/WNL.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Absinta M, Cortese IC, Vuolo L, Nair G, de Alwis MP, Ohayon J, Meani A, Martinelli V, Scotti R, Falini A, Smith BR, Nath A, Jacobson S, Filippi M, Reich DS. Leptomeningeal gadolinium enhancement across the spectrum of chronic neuroinflammatory diseases. Neurology. 2017b;88:1439–1444. doi: 10.1212/WNL.0000000000003820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelini DF, Serafini B, Piras E, Severa M, Coccia EM, Rosicarelli B, Ruggieri S, Gasperini C, Buttari F, Centonze D, Mechelli R, Salvetti M, Borsellino G, Aloisi F, Battistini L. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013;9:e1003220. doi: 10.1371/journal.ppat.1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antony JM, van Marle G, Opii W, Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K, Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat Neurosci. 2004;7:1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- 6.Antsiferova O, Muller A, Ramer PC, Chijioke O, Chatterjee B, Raykova A, Planas R, Sospedra M, Shumilov A, Tsai MH, Delecluse HJ, Munz C. Adoptive transfer of EBV specific CD8+ T cell clones can transiently control EBV infection in humanized mice. PLoS Pathog. 2014;10:e1004333. doi: 10.1371/journal.ppat.1004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arru G, Leoni S, Pugliatti M, Mei A, Serra C, Delogu LG, Manetti R, Dolei A, Sotgiu S, Mameli G. Natalizumab inhibits the expression of human endogenous retroviruses of the W family in multiple sclerosis patients: a longitudinal cohort study. Mult Scler. 2014;20:174–182. doi: 10.1177/1352458513494957. [DOI] [PubMed] [Google Scholar]
- 8.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker D, Marta M, Pryce G, Giovannoni G, Schmierer K. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine. 2017;16:41–50. doi: 10.1016/j.ebiom.2017.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balfour HH, Jr, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age-specific prevalence of Epstein-Barr virus infection among individuals aged 6-19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208:1286–1293. doi: 10.1093/infdis/jit321. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Or A, Pachner A, Menguy-Vacheron F, Kaplan J, Wiendl H. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74:659–674. doi: 10.1007/s40265-014-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergsland N, Ramasamy D, Hojnacki D, Weinstock-Guttman B, Zivadinov R. Leptomeningeal inflammation is related to cortical thinning in relapsing-remitting multiple sclerosis. Neurology. 2018;90:S6.008. doi: 10.3174/ajnr.A6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhargava P, Wicken C, Smith M, Cortese I, Reich D, Calabresi P, Mowry E. Phase 1 trial of intrathecal rituximab in progressive MS patients with evidence of leptomeningeal contrast enhancement. Neurology. 2018;90:3–393. doi: 10.1016/j.msard.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilger A, Plowshay J, Ma S, Nawandar D, Barlow EA, Romero-Masters JC, Bristol JA, Li Z, Tsai MH, Delecluse HJ, Kenney SC. Leflunomide/teriflunomide inhibit Epstein-Barr virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget. 2017;8:44266–44280. doi: 10.18632/oncotarget.17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornevik K, Riise T, Bostrom I, Casetta I, Cortese M, Granieri E, Holmoy T, Kampman MT, Landtblom AM, Magalhaes S, Pugliatti M, Wolfson C, Myhr KM. Negative interaction between smoking and EBV in the risk of multiple sclerosis: The EnvIMS study. Mult Scler. 2017;23:1018–1024. doi: 10.1177/1352458516671028. [DOI] [PubMed] [Google Scholar]
- 17.Buljevac D, van Doornum GJ, Flach HZ, Groen J, Osterhaus AD, Hop W, van Doorn PA, van der Meche FG, Hintzen RQ. Epstein-Barr virus and disease activity in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76:1377–1381. doi: 10.1136/jnnp.2004.048504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellazzi M, Delbue S, Elia F, Gastaldi M, Franciotta D, Rizzo R, Bellini T, Bergamaschi R, Granieri E, Fainardi E. Epstein-Barr virus specific antibody response in multiple sclerosis patients during 21 months of natalizumab treatment. Dis Markers. 2015;2015:901312. doi: 10.1155/2015/901312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cencioni MT, Magliozzi R, Nicholas R, Ali R, Malik O, Reynolds R, Borsellino G, Battistini L, Muraro PA. Programmed death 1 is highly expressed on CD8(+) CD57(+) T cells in patients with stable multiple sclerosis and inhibits their cytotoxic response to Epstein-Barr virus. Immunology. 2017;152:660–676. doi: 10.1111/imm.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Epstein-Barr Virus and Infectious Mononucleosis. USA: Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 21.Christensen T. The role of EBV in MS pathogenesis. Int MS J. 2006;13:52–57. [PubMed] [Google Scholar]
- 22.Cohen JI. Epstein-barr virus vaccines. Clin Transl Immunology. 2015;4:e32. doi: 10.1038/cti.2014.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comabella M, Canto E, Nurtdinov R, Rio J, Villar LM, Picon C, Castillo J, Fissolo N, Aymerich X, Auger C, Rovira A, Montalban X. MRI phenotypes with high neurodegeneration are associated with peripheral blood B-cell changes. Hum Mol Genet. 2016;25:308–316. doi: 10.1093/hmg/ddv473. [DOI] [PubMed] [Google Scholar]
- 24.Confavreux C, O’Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, Wolinsky JS, Bagulho T, Delhay JL, Dukovic D, Truffinet P, Kappos L, Group TT. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 25.Csuka D, Simon D, Hobor R, Uray K, Prohaszka Z, Banlaki Z, Jani PK, Szilagyi A, Hudecz F, Rajczy K, Beke G, Boros Major A, Tordai A, Illes Z, Berki T, Czirjak L, Fust G. Serum concentration of immunoglobulin G-type antibodies against the whole Epstein-Barr nuclear antigen 1 and its aa35-58 or aa398-404 fragments in the sera of patients with systemic lupus erythematosus and multiple sclerosis. Clin Exp Immunol. 2013;171:255–262. doi: 10.1111/cei.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curtin F, Porchet H, Glanzman R, Schneble HM, Vidal V, Audoli-Inthavong ML, Lambert E, Hartung HP. A placebo randomized controlled study to test the efficacy and safety of GNbAC1, a monoclonal antibody for the treatment of multiple sclerosis - Rationale and design. Mult Scler Relat Disord. 2016;9:95–100. doi: 10.1016/j.msard.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Derfuss T, Curtin F, Guebelin C, Bridel C, Rasenack M, Matthey A, Du Pasquier R, Schluep M, Desmeules J, Lang AB, Perron H, Faucard R, Porchet H, Hartung HP, Kappos L, Lalive PH. A phase IIa randomised clinical study of GNbAC1, a humanised monoclonal antibody against the envelope protein of multiple sclerosis-associated endogenous retrovirus in multiple sclerosis patients. Mult Scler. 2015;21:885–893. doi: 10.1177/1352458514554052. [DOI] [PubMed] [Google Scholar]
- 28.Dobson R, Kuhle J, Middeldorp J, Giovannoni G. Epstein-Barr-negative MS: a true phenomenon? Neurol Neuroimmunol Neuroinflamm. 2017;4:e318. doi: 10.1212/NXI.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dolei A, Perron H. The multiple sclerosis-associated retrovirus and its HERV-W endogenous family: a biological interface between virology, genetics, and immunology in human physiology and disease. J Neurovirol. 2009;15:4–13. doi: 10.1080/13550280802448451. [DOI] [PubMed] [Google Scholar]
- 30.Downham C, Visser E, Vickers M, Counsell C. Season of infectious mononucleosis as a risk factor for multiple sclerosis: A UK primary care case-control study. Mult Scler Relat Disord. 2017;17:103–106. doi: 10.1016/j.msard.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Drosu NC, Edelman ER, Housman DE. Could antiretrovirals be treating EBV in MS? A case report. Mult Scler Relat Disord. 2018;22:19–21. doi: 10.1016/j.msard.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrell RA, Antony D, Wall GR, Clark DA, Fisniku L, Swanton J, Khaleeli Z, Schmierer K, Miller DH, Giovannoni G. Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology. 2009;73:32–38. doi: 10.1212/WNL.0b013e3181aa29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fewings NL, Gatt PN, McKay FC, Parnell GP, Schibeci SD, Edwards J, Basuki MA, Goldinger A, Fabis-Pedrini MJ, Kermode AG, Manrique CP, McCauley JL, Nickles D, Baranzini SE, Burke T, Vucic S, Stewart GJ, Booth DR. The autoimmune risk gene ZMIZ1 is a vitamin D responsive marker of a molecular phenotype of multiple sclerosis. J Autoimmun. 2017;78:57–69. doi: 10.1016/j.jaut.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. 2008;7:852–858. doi: 10.1016/S1474-4422(08)70192-3. [DOI] [PubMed] [Google Scholar]
- 35.Geginat J, Paroni M, Pagani M, Galimberti D, De Francesco R, Scarpini E, Abrignani S. The enigmatic role of viruses in multiple sclerosis: molecular mimicry or disturbed immune surveillance? Trends Immunol. 2017;38:498–512. doi: 10.1016/j.it.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gold J, Goldacre R, Maruszak H, Giovannoni G, Yeates D, Goldacre M. HIV and lower risk of multiple sclerosis: beginning to unravel a mystery using a record-linked database study. J Neurol Neurosurg Psychiatry. 2015;86:9–12. doi: 10.1136/jnnp-2014-307932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenfield AL, Hauser SL. B-cell therapy for multiple sclerosis: entering an era. Ann Neurol. 2018;83:13–26. doi: 10.1002/ana.25119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm JM, Schmeling DO, Dunmire SK, Knight JA, Mullan BD, Ed JA, Brundage RC, Hogquist KA, Balfour HH., Jr Prospective studies of infectious mononucleosis in university students. Clin Transl Immunology. 2016;5:e94. doi: 10.1038/cti.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handel AE, Williamson AJ, Disanto G, Handunnetthi L, Giovannoni G, Ramagopalan SV. An updated meta-analysis of risk of multiple sclerosis following infectious mononucleosis. PLoS One. 2010;5:e12496. doi: 10.1371/journal.pone.0012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harley JB, Chen X, Pujato M, Miller D, Maddox A, Forney C, Magnusen AF, Lynch A, Chetal K, Yukawa M, Barski A, Salomonis N, Kaufman KM, Kottyan LC, Weirauch MT. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat Genet. 2018;50:699–707. doi: 10.1038/s41588-018-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison DM, Wang KY, Fiol J, Naunton K, Royal W, 3rd, Hua J, Izbudak I. Leptomeningeal enhancement at 7T in multiple sclerosis: frequency, morphology, and relationship to cortical volume. J Neuroimaging. 2017;27:461–468. doi: 10.1111/jon.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemminki K, Li X, Sundquist J, Hillert J, Sundquist K. Risk for multiple sclerosis in relatives and spouses of patients diagnosed with autoimmune and related conditions. Neurogenetics. 2009;10:5–11. doi: 10.1007/s10048-008-0156-y. [DOI] [PubMed] [Google Scholar]
- 43.Hjalgrim H, Rasmussen S, Rostgaard K, Nielsen NM, Koch-Henriksen N, Munksgaard L, Storm HH, Melbye M. Familial clustering of Hodgkin lymphoma and multiple sclerosis. J Natl Cancer Inst. 2004;96:780–784. doi: 10.1093/jnci/djh135. [DOI] [PubMed] [Google Scholar]
- 44.Horakova D, Zivadinov R, Weinstock-Guttman B, Havrdova E, Qu J, Tamano-Blanco M, Badgett D, Tyblova M, Bergsland N, Hussein S, Willis L, Krasensky J, Vaneckova M, Seidl Z, Lelkova P, Dwyer MG, Zhang M, Yu H, Duan X, Kalincik T, et al. Environmental factors associated with disease progression after the first demyelinating event: results from the multi-center SET study. PLoS One. 2013;8:e53996. doi: 10.1371/journal.pone.0053996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, Kushekhar K, Nolte I, Kooistra W, Visser L, Bouwman I, Kouprie N, Veenstra R, van Imhoff G, Olver B, Houlston RS, Poppema S, Diepstra A, Hepkema B, van den Berg A. HLA associations in classical hodgkin lymphoma: EBV status matters. PLoS One. 2012;7:e39986. doi: 10.1371/journal.pone.0039986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, et al. International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ireland SJ, Guzman AA, O’Brien DE, Hughes S, Greenberg B, Flores A, Graves D, Remington G, Frohman EM, Davis LS, Monson NL. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurol. 2014;71:1421–1428. doi: 10.1001/jamaneurol.2014.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jakimovski D, Kolb C, Ramanathan M, Zivadinov R, Weinstock-Guttman B. Interferon beta for multiple sclerosis. Cold Spring Harb Perspect Med. 2018 doi: 10.1101/cshperspect.a032003. doi: 10.1101/cshperspect.a032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jakimovski D, Weinstock-Guttman B, Ramanathan M, Kolb C, Hojnacki D, Minagar A, Zivadinov R. Ocrelizumab: a B-cell depleting therapy for multiple sclerosis. Expert Opin Biol Ther. 2017;17:1163–1172. doi: 10.1080/14712598.2017.1347632. [DOI] [PubMed] [Google Scholar]
- 50.Jarius S, Eichhorn P, Franciotta D, Petereit HF, Akman-Demir G, Wick M, Wildemann B. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol. 2017;264:453–466. doi: 10.1007/s00415-016-8360-4. [DOI] [PubMed] [Google Scholar]
- 51.Khankhanian P, Cozen W, Himmelstein DS, Madireddy L, Din L, van den Berg A, Matsushita T, Glaser SL, More JM, Smedby KE, Baranzini SE, Mack TM, Lizee A, de Sanjose S, Gourraud PA, Nieters A, Hauser SL, Cocco P, Maynadie M, Foretova L, et al. Meta-analysis of genome-wide association studies reveals genetic overlap between Hodgkin lymphoma and multiple sclerosis. Int J Epidemiol. 2016;45:728–740. doi: 10.1093/ije/dyv364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khanna R, Burrows SR. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu Rev Microbiol. 2000;54:19–48. doi: 10.1146/annurev.micro.54.1.19. [DOI] [PubMed] [Google Scholar]
- 53.Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ. Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol. 2009;65:457–469. doi: 10.1002/ana.21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein G, Klein E, Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem Biophys Res Commun. 2010;396:67–73. doi: 10.1016/j.bbrc.2010.02.146. [DOI] [PubMed] [Google Scholar]
- 55.Komori M, Lin YC, Cortese I, Blake A, Ohayon J, Cherup J, Maric D, Kosa P, Wu T, Bielekova B. Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol. 2016;3:166–179. doi: 10.1002/acn3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kreft KL, Van Nierop GP, Scherbeijn SMJ, Janssen M, Verjans G, Hintzen RQ. Elevated EBNA-1 IgG in MS is associated with genetic MS risk variants. Neurol Neuroimmunol Neuroinflamm. 2017;4:e406. doi: 10.1212/NXI.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kremer D, Forster M, Schichel T, Gottle P, Hartung HP, Perron H, Kury P. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation blockade. Mult Scler. 2015;21:1200–1203. doi: 10.1177/1352458514560926. [DOI] [PubMed] [Google Scholar]
- 58.Kremer D, Schichel T, Forster M, Tzekova N, Bernard C, van der Valk P, van Horssen J, Hartung HP, Perron H, Kury P. Human endogenous retrovirus type W envelope protein inhibits oligodendroglial precursor cell differentiation. Ann Neurol. 2013;74:721–732. doi: 10.1002/ana.23970. [DOI] [PubMed] [Google Scholar]
- 59.Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, Bestwick JP, Meier UC, Marta M, Dalla Costa G, Runia T, Evdoshenko E, Lazareva N, Thouvenot E, Iaffaldano P, Direnzo V, Khademi M, Piehl F, Comabella M, Sombekke M, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult Scler. 2015;21:1013–1024. doi: 10.1177/1352458514568827. [DOI] [PubMed] [Google Scholar]
- 60.Kury P, Nath A, Creange A, Dolei A, Marche P, Gold J, Giovannoni G, Hartung HP, Perron H. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24:379–394. doi: 10.1016/j.molmed.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kvistad S, Myhr KM, Holmoy T, Bakke S, Beiske AG, Bjerve KS, Hovdal H, Loken-Amsrud KI, Lilleas F, Midgard R, Njolstad G, Pedersen T, Benth JS, Wergeland S, Torkildsen O. Antibodies to Epstein-Barr virus and MRI disease activity in multiple sclerosis. Mult Scler. 2014;20:1833–1840. doi: 10.1177/1352458514533843. [DOI] [PubMed] [Google Scholar]
- 62.Latham LB, Lee MJ, Lincoln JA, Ji N, Forsthuber TG, Lindsey JW. Antivirus immune activity in multiple sclerosis correlates with MRI activity. Acta Neurol Scand. 2016;133:17–24. doi: 10.1111/ane.12417. [DOI] [PubMed] [Google Scholar]
- 63.Lehmann-Horn K, Kinzel S, Feldmann L, Radelfahr F, Hemmer B, Traffehn S, Bernard CC, Stadelmann C, Bruck W, Weber MS. Intrathecal anti-CD20 efficiently depletes meningeal B cells in CNS autoimmunity. Ann Clin Transl Neurol. 2014;1:490–496. doi: 10.1002/acn3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levin LI, Munger KL, O’Reilly EJ, Falk KI, Ascherio A. Primary infection with the Epstein-Barr virus and risk of multiple sclerosis. Ann Neurol. 2010;67:824–830. doi: 10.1002/ana.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Yang XV, Wu J, Kuei C, Mani NS, Zhang L, Yu J, Sutton SW, Qin N, Banie H, Karlsson L, Sun S, Lovenberg TW. Oxysterols direct B-cell migration through EBI2. Nature. 2011;475:519–523. doi: 10.1038/nature10226. [DOI] [PubMed] [Google Scholar]
- 66.Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron. 2016;91:957–973. doi: 10.1016/j.neuron.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lublin FD. New multiple sclerosis phenotypic classification. Eur Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 69.Lucas RM, Hughes AM, Lay ML, Ponsonby AL, Dwyer DE, Taylor BV, Pender MP. Epstein-Barr virus and multiple sclerosis. J Neurol Neurosurg Psychiatry. 2011;82:1142–1148. doi: 10.1136/jnnp-2011-300174. [DOI] [PubMed] [Google Scholar]
- 70.Lundy SK, Wu Q, Wang Q, Dowling CA, Taitano SH, Mao G, Mao-Draayer Y. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurol Neuroimmunol Neuroinflamm. 2016;3:e211. doi: 10.1212/NXI.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma N, Xiao H, Marrero B, Xing C, Wang X, Zheng M, Han G, Chen G, Hou C, Shen B, Li Y, Wang R, Jiang Z. Combination of TACI-IgG and anti-IL-15 treats murine lupus by reducing mature and memory B cells. Cell Immunol. 2014;289:140–144. doi: 10.1016/j.cellimm.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 72.Maghzi H, Ataei B, Khorvash F, Yaran M, Maghzi AH. Association between acute infectious mononucleosis and vitamin D deficiency. Viral Immunol. 2016;29:398–400. doi: 10.1089/vim.2016.0038. [DOI] [PubMed] [Google Scholar]
- 73.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 74.Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 75.Makshakov G, Magonov E, Totolyan N, Nazarov V, Lapin S, Mazing A, Verbitskaya E, Trofimova T, Krasnov V, Shumilina M, Skoromets A, Evdoshenko E. Leptomeningeal contrast enhancement is associated with disability progression and grey matter atrophy in multiple sclerosis. Neurol Res Int. 2017;2017:8652463. doi: 10.1155/2017/8652463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mameli G, Cossu D, Cocco E, Frau J, Marrosu MG, Niegowska M, Sechi LA. Epitopes of HERV-Wenv induce antigen-specific humoral immunity in multiple sclerosis patients. J Neuroimmunol. 2015;280:66–68. doi: 10.1016/j.jneuroim.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Mameli G, Arru G, Caggiu E, Niegowska M, Leoni S, Madeddu G, Babudieri S, Sechi GP, Sechi LA. Natalizumab therapy modulates miR-155, miR-26a and proinflammatory cytokine expression in MS patients. PLoS One. 2016;11:e0157153. doi: 10.1371/journal.pone.0157153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG, Maida I, Babudieri S, Serra C, Manetti R, Mura MS, Dolei A. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: the missing link with multiple sclerosis? PLoS One. 2013;8:e78474. doi: 10.1371/journal.pone.0078474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martyn CN, Cruddas M, Compston DA. Symptomatic Epstein-Barr virus infection and multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:167–168. doi: 10.1136/jnnp.56.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mathias A, Perriard G, Canales M, Soneson C, Delorenzi M, Schluep M, Du Pasquier RA. Increased ex vivo antigen presentation profile of B cells in multiple sclerosis. Mult Scler. 2017;23:802–809. doi: 10.1177/1352458516664210. [DOI] [PubMed] [Google Scholar]
- 81.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 82.Mechelli R, Manzari C, Policano C, Annese A, Picardi E, Umeton R, Fornasiero A, D’Erchia AM, Buscarinu MC, Agliardi C, Annibali V, Serafini B, Rosicarelli B, Romano S, Angelini DF, Ricigliano VA, Buttari F, Battistini L, Centonze D, Guerini FR, et al. Epstein-Barr virus genetic variants are associated with multiple sclerosis. Neurology. 2015;84:1362–1368. doi: 10.1212/WNL.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morandi E, Jagessar SA, t Hart BA, Gran B. EBV infection empowers human B cells for autoimmunity: role of autophagy and relevance to multiple sclerosis. J Immunol. 2017;199:435–448. doi: 10.4049/jimmunol.1700178. [DOI] [PubMed] [Google Scholar]
- 84.Mostafa A, Jalilvand S, Shoja Z, Nejati A, Shahmahmoodi S, Sahraian MA, Marashi SM. Multiple sclerosis-associated retrovirus, Epstein-Barr virus, and vitamin D status in patients with relapsing remitting multiple sclerosis. J Med Virol. 2017;89:1309–1313. doi: 10.1002/jmv.24774. [DOI] [PubMed] [Google Scholar]
- 85.Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, Gourraud PA, Brenneman D, Owen MC, Qualley P, Bucci M, Hauser SL, Pelletier D. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72:234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 87.Munger KL, Fitzgerald KC, Freedman MS, Hartung HP, Miller DH, Montalban X, Edan G, Barkhof F, Suarez G, Radue EW, Sandbrink R, Kappos L, Pohl C, Ascherio A. No association of multiple sclerosis activity and progression with EBV or tobacco use in BENEFIT. Neurology. 2015;85:1694–1701. doi: 10.1212/WNL.0000000000002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Najafipoor A, Roghanian R, Zarkesh-Esfahani SH, Bouzari M, Etemadifar M. The beneficial effects of vitamin D3 on reducing antibody titers against Epstein-Barr virus in multiple sclerosis patients. Cell Immunol. 2015;294:9–12. doi: 10.1016/j.cellimm.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 89.Nielsen TR, Pedersen M, Rostgaard K, Frisch M, Hjalgrim H. Correlations between Epstein-Barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Mult Scler. 2007;13:420–423. doi: 10.1177/1352458506071470. [DOI] [PubMed] [Google Scholar]
- 90.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 91.Otto C, Hofmann J, Ruprecht K. Antibody producing B lineage cells invade the central nervous system predominantly at the time of and triggered by acute Epstein-Barr virus infection: A hypothesis on the origin of intrathecal immunoglobulin synthesis in multiple sclerosis. Med Hypotheses. 2016;91:109–113. doi: 10.1016/j.mehy.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 92.Parker Harp CR, Archambault AS, Sim J, Ferris ST, Mikesell RJ, Koni PA, Shimoda M, Linington C, Russell JH, Wu GF. B cell antigen presentation is sufficient to drive neuroinflammation in an animal model of multiple sclerosis. J Immunol. 2015;194:5077–5084. doi: 10.4049/jimmunol.1402236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pender MP, Csurhes PA, Burrows JM, Burrows SR. Defective T-cell control of Epstein-Barr virus infection in multiple sclerosis. Clin Transl Immunology. 2017;6:e126. doi: 10.1038/cti.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pender MP, Csurhes PA, Smith C, Beagley L, Hooper KD, Raj M, Coulthard A, Burrows SR, Khanna R. Epstein-Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis. Mult Scler. 2014;20:1541–1544. doi: 10.1177/1352458514521888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Petersen T, Moller-Larsen A, Ellermann-Eriksen S, Thiel S, Christensen T. Effects of interferon-beta therapy on elements in the antiviral immune response towards the human herpesviruses EBV, HSV, and VZV, and to the human endogenous retroviruses HERV-H and HERV-W in multiple sclerosis. J Neuroimmunol. 2012;249:105–108. doi: 10.1016/j.jneuroim.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Pol S, Schweser F, Bertolino N, Preda M, Svienson M, Sudyn M, Babek N, Siebert D, Zivadinov R. Serial MRI of leptomeningeal inflammation in rodent experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. Neurology. 2017;88:P1. doi: 10.1016/j.expneurol.2019.01.013. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Radue EW, Sprenger T, Gaetano L, Mueller-Lenke N, Cavalier S, Thangavelu K, Panzara MA, Donaldson JE, Woodward FM, Wuerfel J, Wolinsky JS, Kappos L. Teriflunomide slows BVL in relapsing MS: A reanalysis of the TEMSO MRI data set using SIENA. Neurol Neuroimmunol Neuroinflamm. 2017;4:e390. doi: 10.1212/NXI.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Raffel J, Dobson R, Gafson A, Mattoscio M, Muraro P, Giovannoni G. Multiple sclerosis therapy and Epstein-Barr virus antibody titres. Mult Scler Relat Disord. 2014;3:372–374. doi: 10.1016/j.msard.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 99.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 100.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rizzo F, Giacomini E, Mechelli R, Buscarinu MC, Salvetti M, Severa M, Coccia EM. Interferon-beta therapy specifically reduces pathogenic memory B cells in multiple sclerosis patients by inducing a FAS-mediated apoptosis. Immunol Cell Biol. 2016;94:886–894. doi: 10.1038/icb.2016.55. [DOI] [PubMed] [Google Scholar]
- 102.Rolf L, Muris AH, Hupperts R, Damoiseaux J. Illuminating vitamin D effects on B cells--the multiple sclerosis perspective. Immunology. 2016;147:275–284. doi: 10.1111/imm.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rolf L, Muris AH, Mathias A, Du Pasquier R, Koneczny I, Disanto G, Kuhle J, Ramagopalan S, Damoiseaux J, Smolders J, Hupperts R. Exploring the effect of vitamin D3 supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult Scler. 2018;24:1280–1287. doi: 10.1177/1352458517722646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosjo E, Lossius A, Abdelmagid N, Lindstrom JC, Kampman MT, Jorgensen L, Sundstrom P, Olsson T, Steffensen LH, Torkildsen O, Holmoy T. Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein-Barr virus in relapsing-remitting multiple sclerosis. Mult Scler. 2017;23:395–402. doi: 10.1177/1352458516654310. [DOI] [PubMed] [Google Scholar]
- 105.Rubicz R, Yolken R, Drigalenko E, Carless MA, Dyer TD, Bauman L, Melton PE, Kent JW, Jr, Harley JB, Curran JE, Johnson MP, Cole SA, Almasy L, Moses EK, Dhurandhar NV, Kraig E, Blangero J, Leach CT, Goring HH. A genome-wide integrative genomic study localizes genetic factors influencing antibodies against Epstein-Barr virus nuclear antigen 1 (EBNA-1) PLoS Genet. 2013;9:e1003147. doi: 10.1371/journal.pgen.1003147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ruprecht K, Wildemann B, Jarius S. Low intrathecal antibody production despite high seroprevalence of Epstein-Barr virus in multiple sclerosis: a review of the literature. J Neurol. 2018;265:239–252. doi: 10.1007/s00415-017-8656-z. [DOI] [PubMed] [Google Scholar]
- 107.Rutkowska A, Sailer AW, Dev KK. EBI2 receptor regulates myelin development and inhibits LPC-induced demyelination. J Neuroinflammation. 2017;14:250. doi: 10.1186/s12974-017-1025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Salzer J, Stenlund H, Sundstrom P. The interaction between smoking and Epstein-Barr virus as multiple sclerosis risk factors may depend on age. Mult Scler. 2014;20:747–750. doi: 10.1177/1352458513507820. [DOI] [PubMed] [Google Scholar]
- 109.Serafini B, Rosicarelli B, Aloisi F, Stigliano E. Epstein-barr virus in the central nervous system and cervical lymph node of a patient with primary progressive multiple sclerosis. J Neuropathol Exp Neurol. 2014;73:729–731. doi: 10.1097/NEN.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 110.Serafini B, Scorsi E, Rosicarelli B, Rigau V, Thouvenot E, Aloisi F. Massive intracerebral Epstein-Barr virus reactivation in lethal multiple sclerosis relapse after natalizumab withdrawal. J Neuroimmunol. 2017;307:14–17. doi: 10.1016/j.jneuroim.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 111.Serafini B, Severa M, Columba-Cabezas S, Rosicarelli B, Veroni C, Chiappetta G, Magliozzi R, Reynolds R, Coccia EM, Aloisi F. Epstein-Barr virus latent infection and BAFF expression in B cells in the multiple sclerosis brain: implications for viral persistence and intrathecal B-cell activation. J Neuropathol Exp Neurol. 2010;69:677–693. doi: 10.1097/NEN.0b013e3181e332ec. [DOI] [PubMed] [Google Scholar]
- 112.Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, Andreoni L, Trivedi P, Salvetti M, Faggioni A, Aloisi F. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–2912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sheik-Ali S. Infectious mononucleosis and multiple sclerosis - Updated review on associated risk. Mult Scler Relat Disord. 2017;14:56–59. doi: 10.1016/j.msard.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 114.Simon KC, van der Mei IA, Munger KL, Ponsonby A, Dickinson J, Dwyer T, Sundstrom P, Ascherio A. Combined effects of smoking, anti-EBNA antibodies, and HLA-DRB1* 1501 on multiple sclerosis risk. Neurology. 2010;74:1365–1371. doi: 10.1212/WNL.0b013e3181dad57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simpson S, Jr, Blizzard L, Otahal P, Van der Mei I, Taylor B. Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol Neurosurg Psychiatry. 2011;82:1132–1141. doi: 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 116.Smets I, Fiddes B, Garcia-Perez JE, He D, Mallants K, Liao W, Dooley J, Wang G, Humblet-Baron S, Dubois B, Compston A, Jones J, Coles A, Liston A, Ban M, Goris A, Sawcer S. Multiple sclerosis risk variants alter expression of co-stimulatory genes in B cells. Brain. 2018 doi: 10.1093/brain/awx372. doi: 10.1093/brain/awx372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sokal EM, Hoppenbrouwers K, Vandermeulen C, Moutschen M, Leonard P, Moreels A, Haumont M, Bollen A, Smets F, Denis M. Recombinant gp350 vaccine for infectious mononucleosis: a phase 2, randomized, double-blind, placebo-controlled trial to evaluate the safety, immunogenicity, and efficacy of an Epstein-Barr virus vaccine in healthy young adults. J Infect Dis. 2007;196:1749–1753. doi: 10.1086/523813. [DOI] [PubMed] [Google Scholar]
- 118.Styles CT, Bazot Q, Parker GA, White RE, Paschos K, Allday MJ. EBV epigenetically suppresses the B cell-to-plasma cell differentiation pathway while establishing long-term latency. PLoS Biol. 2017;15:e2001992. doi: 10.1371/journal.pbio.2001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sundqvist E, Sundstrom P, Linden M, Hedstrom AK, Aloisi F, Hillert J, Kockum I, Alfredsson L, Olsson T. Epstein-Barr virus and multiple sclerosis: interaction with HLA. Genes Immun. 2012;13:14–20. doi: 10.1038/gene.2011.42. [DOI] [PubMed] [Google Scholar]
- 120.Tao C, Simpson S, Jr, Taylor BV, van der Mei I. Association between human herpesvirus & human endogenous retrovirus and MS onset & progression. J Neurol Sci. 2017;372:239–249. doi: 10.1016/j.jns.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 121.Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- 122.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 123.Tracy SI, Kakalacheva K, Lunemann JD, Luzuriaga K, Middeldorp J, Thorley-Lawson DA. Persistence of Epstein-Barr virus in self-reactive memory B cells. J Virol. 2012;86:12330–12340. doi: 10.1128/JVI.01699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tzartos JS, Khan G, Vossenkamper A, Cruz-Sadaba M, Lonardi S, Sefia E, Meager A, Elia A, Middeldorp JM, Clemens M, Farrell PJ, Giovannoni G, Meier UC. Association of innate immune activation with latent Epstein-Barr virus in active MS lesions. Neurology. 2012;78:15–23. doi: 10.1212/WNL.0b013e31823ed057. [DOI] [PubMed] [Google Scholar]
- 125.van der Mei I, Lucas RM, Taylor BV, Valery PC, Dwyer T, Kilpatrick TJ, Pender MP, Williams D, Chapman C, Otahal P, Ponsonby AL. Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Mult Scler. 2016;22:461–469. doi: 10.1177/1352458515594040. [DOI] [PubMed] [Google Scholar]
- 126.van Horssen J, van der Pol S, Nijland P, Amor S, Perron H. Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis. Mult Scler Relat Disord. 2016;8:11–18. doi: 10.1016/j.msard.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 127.van Nierop GP, van Luijn MM, Michels SS, Melief MJ, Janssen M, Langerak AW, Ouwendijk WJD, Hintzen RQ, Verjans G. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 2017;134:383–401. doi: 10.1007/s00401-017-1744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Veroni C, Serafini B, Rosicarelli B, Fagnani C, Aloisi F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J Neuroinflammation. 2018;15:18. doi: 10.1186/s12974-017-1049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Virtanen JO, Wohler J, Fenton K, Reich DS, Jacobson S. Oligoclonal bands in multiple sclerosis reactive against two herpesviruses and association with magnetic resonance imaging findings. Mult Scler. 2014;20:27–34. doi: 10.1177/1352458513490545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, Wessel K, Kirchner H, Hennig H. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–184. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- 131.Wergeland S, Myhr KM, Loken-Amsrud KI, Beiske AG, Bjerve KS, Hovdal H, Midgard R, Kvistad SS, Holmoy T, Riise T, Torkildsen O. Vitamin D, HLA-DRB1 and Epstein-Barr virus antibody levels in a prospective cohort of multiple sclerosis patients. Eur J Neurol. 2016;23:1064–1070. doi: 10.1111/ene.12986. [DOI] [PubMed] [Google Scholar]
- 132.Wingerchuk DM. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord. 2012;5:13–22. doi: 10.1177/1756285611425694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wolinsky JS, Narayana PA, Nelson F, Datta S, O’Connor P, Confavreux C, Comi G, Kappos L, Olsson TP, Truffinet P, Wang L, Miller A, Freedman MS. Teriflunomide Multiple Sclerosis Oral (TEMSO) Trial Group (2013) Magnetic resonance imaging outcomes from a phase III trial of teriflunomide. Mult Scler. 19:1310–1319. doi: 10.1177/1352458513475723. [DOI] [PubMed] [Google Scholar]
- 134.Zhou Y, Chen M, Simpson S, Jr, Lucas RM, Charlesworth JC, Blackburn N, van der Mei I, Ponsonby AL, Ausimmune Aig, Taylor BV. Common genetic variation within miR-146a predicts disease onset and relapse in multiple sclerosis. Neurol Sci. 2018;39:297–304. doi: 10.1007/s10072-017-3177-1. [DOI] [PubMed] [Google Scholar]
- 135.Zhou Y, Zhu G, Charlesworth JC, Simpson S, Jr, Rubicz R, Goring HH, Patsopoulos NA, Laverty C, Wu F, Henders A, Ellis JJ, van der Mei I, Montgomery GW, Blangero J, Curran JE, Johnson MP, Martin NG, Nyholt DR, Taylor BV, consortium AN. Genetic loci for Epstein-Barr virus nuclear antigen-1 are associated with risk of multiple sclerosis. Mult Scler. 2016;22:1655–1664. doi: 10.1177/1352458515626598. [DOI] [PubMed] [Google Scholar]
- 136.Zivadinov R, Bergsland N, Hagemeier J, Carl E, Kolb H, Hojnacki D, Weinstock-Guttman B. Effect of teriflunomide on gray and white matter brain pathology in multiple sclerosis using volumetric and diffusion-tensor imaging MRI measures. J Neurol Sci. 2018a;388:175–181. doi: 10.1016/j.jns.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 137.Zivadinov R, Cerza N, Hagemeier J, Carl E, Badgett D, Ramasamy DP, Weinstock-Guttman B, Ramanathan M. Humoral response to EBV is associated with cortical atrophy and lesion burden in patients with MS. Neurol Neuroimmunol Neuroinflamm. 2016a;3:e190. doi: 10.1212/NXI.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zivadinov R, Ramasamy DP, Hagemeier J, Kolb C, Bergsland N, Schweser F, Dwyer MG, Weinstock-Guttman B, Hojnacki D. Evaluation of leptomeningeal contrast enhancement using pre-and postcontrast subtraction 3D-FLAIR Imaging in multiple sclerosis. AJNR Am J Neuroradiol. 2018b;39:642–647. doi: 10.3174/ajnr.A5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zivadinov R, Kolb C, Modi N, Carl J, Hagemeier J, Bergsland N, Ramasamy D, Durfee J, Weinstock-Guttman B. Effect of Teriflunomide (Aubagio®) on gray matter pathology in multiple sclerosis. Neurology. 2016;86:P3–062. [Google Scholar]
- 140.Zivadinov R, Zorzon M, Weinstock-Guttman B, Serafin M, Bosco A, Bratina A, Maggiore C, Grop A, Tommasi MA, Srinivasaraghavan B, Ramanathan M. Epstein-Barr virus is associated with grey matter atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009a;80:620–625. doi: 10.1136/jnnp.2008.154906. [DOI] [PubMed] [Google Scholar]
- 141.Zivadinov R, Jakimovski D, Gandhi S, Ahmed R, Dwyer MG, Horakova D, Weinstock-Guttman B, Benedict RR, Vaneckova M, Barnett M, Bergsland N. Clinical relevance of brain atrophy assessment in multiple sclerosis. Implications for its use in a clinical routine. Expert Rev Neurother. 2016c;16:777–793. doi: 10.1080/14737175.2016.1181543. [DOI] [PubMed] [Google Scholar]
- 142.Zivadinov R, Ramasamy DP, Vaneckova M, Gandhi S, Chandra A, Hagemeier J, Bergsland N, Polak P, Benedict RH, Hojnacki D, Weinstock-Guttman B. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: A retrospective, pilot, observational longitudinal study. Mult Scler. 2017;23:1336–1345. doi: 10.1177/1352458516678083. [DOI] [PubMed] [Google Scholar]
- 143.Zivadinov R, Weinstock-Guttman B, Zorzon M, Uxa L, Serafin M, Bosco A, Bratina A, Maggiore C, Grop A, Tommasi MA, Srinivasaraghavan B, Ramanathan M. Gene-environment interactions between HLA B7/A2, EBV antibodies are associated with MRI injury in multiple sclerosis. J Neuroimmunol. 2009b;209:123–130. doi: 10.1016/j.jneuroim.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 144.Zivadinov R, Chin J, Horakova D, Bergsland N, Weinstock-Guttman B, Tamano-Blanco M, Badgett D, Hagemeier J, Tyblova M, Carl E, Krasensky J, Vaneckova M, Seidl Z, Dwyer MG, Havrdova E, Ramanathan M. Humoral responses to herpesviruses are associated with neurodegeneration after a demyelinating event: results from the multi-center set study. J Neuroimmunol. 2014;273:58–64. doi: 10.1016/j.jneuroim.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 145.Zurawski J, Lassmann H, Bakshi R. Use of magnetic resonance imaging to visualize leptomeningeal inflammation in patients with multiple sclerosis: a review. JAMA Neurol. 2017;74:100–109. doi: 10.1001/jamaneurol.2016.4237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


