Abstract
The pathogenesis of glaucoma is still not fully clarified but a growing body of evidence suggests that neuroinflammation and immune response are part of the sequence of pathological events leading to the optic neuropathy. Indeed, inflammation - involving the activation and proliferation of resident glial cells (astrocytes, Muller cells and microglia) and the release of a plethora of anti- and pro-inflammatory cytokines, chemokines and reactive oxygen species - has been reported as common features in clinical and experimental glaucoma. In the insulted retina, as for other neuronal tissues, pathogenic and reparative aspects coexist in the inflammatory process, with extent and persistency affecting the final outcome. In view of this, therapies aimed at modulating the immune and inflammatory responses may represent a promising approach for limiting the optic nerve damage and the loss of retinal ganglion cells associated with glaucoma.
Keywords: glaucoma, retinal ganglion cells, neuroinflammation, neurodegeneration, microglia, oxidative stress, inflammasome, immune response
Glaucoma is a group of chronic optic neuropathies, characterized by the alteration of the optic nerve and the death of retinal ganglion cells and represents a leading cause of irreversible blindness, estimated to affect 76 million in 2020 and 112 million people in 2040 (Tham et al., 2014). Age and elevated intraocular pressure are considered major risk factors for the onset and progression of the disease and, accordingly, all the approved therapies aim to intraocular pressure lowering. However, the high incidence of normal tension glaucoma and the recurrence of patients that continue to lose vision even when intraocular pressure values are successfully controlled, highlight the fact that elevated intraocular pressure is not sufficient nor necessary for developing the disease (Blumberg et al., 2015). Therefore, alternative treatments, beyond and/or independent of intraocular pressure reduction, are required to fulfill the need of a better glaucoma therapy. We have performed a PubMed literature search with the key words: glaucoma and neuroinflammation, glaucoma and microglia activation, glaucoma and inflammasome.
Over the course of the disease, apoptotic retinal ganglion cell death occurs through a complex sequence of pathological events that involve several contributing factors (i.e., modifications of neurotrophin signaling, excitotoxicity, oxidative stress, mitochondrial dysfunction, protein misfolding, hypoxic and ischemic phenomena, autoimmunity and, more recently, autophagy) (Baltmr et al., 2010; Russo et al., 2016).
Although the exact mechanisms by which retinal ganglion cell axons are insulted and finally degenerate are not known, a mounting literature suggests a crucial role for neuroinflammation. In the central nervous system resident astrocytes and microglial cells are responsible for immune surveillance and mediate primary inflammatory responses that protect neural tissue from pathogens and help recover from injury and stress. In the retina and optic nerve three types of resident glial cells (astrocytes, Muller cells and microglia) mediate the inflammatory response. Following glaucoma-related stimuli (i.e., optic nerve transection, ocular hypertension, excitotoxicity) glial cells become reactive and redistribute in the retina and optic nerve, producing mediators, such as cytokines and interleukins, that might affect neuronal survival through both neuroprotective and detrimental effects (Tezel, 2013). Activation of immunocompetent cells has been detected at the optic nerve head and in the retina of animal bearing experimental glaucoma. Indeed, reactive microglia has been identified following optic nerve axotomy, retinal ischemia/reperfusion injury, ocular hypertension and human glaucoma (Russo et al., 2016). Among the factors released by activated microglia, tumor necrosis factor-alpha (TNF-α) and its receptor are important mediators in several models of retinal ganglion cell. A growing body of literature reported a modulation of TNF-α levels in clinical glaucoma suggesting that a relation exists between this cytokine and the progression of the disease (Tong et al., 2017). Interestingly, it has been recently reported in a mouse model of corneal chemical injury that TNF-α inhibition, prevents monocyte infiltration and microglia activation and reduce susceptibility to develop secondary glaucoma and retinal ganglion cell death (Paschalis et al., 2018). Interleukin-1β (IL-1β) is also a key microglia-derived factors for glaucoma progression acting by stimulating the production of matrix mellanoproteinase-9, nitric oxide and reactive oxygen species. Another pro-inflammatory cytokine released by microglia and astrocytes, interleukine-6 (IL-6) is an important component of the pressure-induced retinal response and it exerts neuroprotective effects on retinal ganglion cells challenged by pressure (Sappington et al., 2006). Besides being involved in the release of cytokines and chemokines, activated astrocytes and Muller cells up-regulate structural proteins such as glial fibrillary acidic protein; the biological role of this response has not been clarified though it seems to be an essential part of gliosis (Russo et al., 2016).
Inflammasomes is a group of cytosolic multiprotein complexes and sensors that regulate the activation of caspase-1 and coordinates inflammation in response to infectious microbes and stress factors (Zanon-Moreno et al., 2018). Following its activation, caspase-1 induces the maturation of cytokines such as IL-1 beta and interleukin-18 contributing to the progression of the inflammatory process. Different types of inflammasomes have been identified, including the ones controlled by nod like receptor proteins (NLRP1, NLRP2, NLRP3) that operate in innate immunity. In particular NLRP1 and NLRP3 play a crucial role in the pathogenesis of glaucoma. Indeed, a study conducted in human glaucomatous eye reported an increase of NLRP3 inflammasome, caspase-1 and caspase-8 levels. Furthermore, NLRP3 as well as NLRP1 inflammasomes are present in mice and rat models of acute glaucoma (Yerramothu et al., 2018).
Based on these observations, biological therapies currently used for autoimmune diseases (i.e., multiple sclerosis, rheumatoid arthritis, psoriatic arthritis and spondyloarthropathies) could be suitable to modulate inflammation and immune response in glaucoma. These therapeutics include: IL-1 receptor inhibitors (anakinra), anti-IL-6 monoclonal antibody (clazakizumab), TNF-α blockers (monoclonal antibodies: infliximab, golimumab and adalimumab), non-TNF-α blockers (fusion protein: abatacept; monoclonal antibodies: tocilizumab and rituximab) (Zanon-Moreno et al., 2018).
Cytokine signaling and inflammasome are linked to the “redox - sensitive” master transcriptional regulator nuclear factor-kappa B (NF-κB). Indeed, activation of the NF-κB pathway leads to increased IL-1 expression promoting the secondary inflammatory cytokines cascade in microglia (i.e., TNF-α) and astrocytes (i.e., IL-6). Proteomic analysis of glaucomatous retinas from humans and rats showed upregulation of kinases involved in the activation of NF-κB pathway, such as: RIPK (receptor-interacting proteine kinase), NIK (NF-κB inducing kinase) and IκK (I kappa B kinase) (Tezel, 2013). Accordingly, tempol, a superoxide dismutase mimetic and free radical scavenger, reduced activation of NF-κB limiting the neuroinflammatory process and preventing ocular hypertension-induced axon and retinal ganglion cell loss (Yang et al., 2016).
Oxidative stress is critical for the initiation and dysregulation of immune activity during glaucomatous degeneration. The oxidative process and the production of reactive oxygen species regulate immune response by stimulating the antigen presenting ability of glial cells and increasing the generation of advanced glycation end products which modulate cellular function through the binding of specific receptors (i.e., RAGE, receptor for advanced glycation end product). advanced glycation end product-binding receptors, which are mostly found on monocytes, pericytes, endothelial cells, macrophages, microglia and astrocytes, induce the release of profibrotic cytokines, such as transforming growth factor-beta and proinflammatory cytokines, such as TNF-α and IL-6 (Tezel, 2013).
In view of the role played by oxidative stress in the developing of inflammatory response, antioxidant strategies represent a possible approach for limiting the inflammatory component of glaucoma. Several compounds, often of natural origin, are endowed with antioxidant actvities and have been shown to be neuroprotective in experimental models of glaucoma. Coenzyme Q10, an important component of the mitochondrial electron transport chain, is a potent antioxidant and our and other groups reported the ability of coenzyme Q10 to prevent retinal ganglion cells loss in in vivo and in vitro model of glaucoma. Moreover, topical coenzyme Q10 has been shown to positively affect retinal function in patients with primary open-angle glaucoma (Parisi et al., 2014). Alpha-lipoic acid and superoxide dismutase, two essential antioxidants, as well as Ginkgo biloba leaf extract and Lycium barbarum aqueous extract, two traditional Chinese medicines, show neuroprotective effects in patients with normal tension glaucoma and in an ocular hypertension model of glaucoma, respectively (Song et al., 2015).
Glial cells, in addition to innate immune activities, through the release of cytokines and chemokines play important roles in the adaptive immune response in glaucoma and, expressing major histocompatibility complex molecules, function as resident antigen-presenting cells (Tezel, 2013).
Involvement of the immune system in glaucoma includes also the modulation toward both an increase or decrease, of serum autoantibodies titres in a variety of retina and optic nerve proteins. Indeed, upregulation of antibodies against heat shock protein 60, heat shock protein 70, alpha-fodrin or myelin basic protein as well as downregulation of antibodies directed to γ-synuclein, glial fibrillary acidic protein or vimentin were reported in the sera of glaucoma patients (Tezel, 2013; Von Thun Und Hohenstein-Blaul et al., 2017). The pathological role of the detected autoantibodies has not been yet clarified, but it is known that up-regulated autoantibodies can be auto-aggressive and lead to pathogenic conditions. Therefore, targeting autoimmunity might represent another possible approach for glaucoma therapy. However, it must be also considered that the downregulation of some autoantibodies in glaucoma patients could lead to a loss of protective autoimmunity. Furthermore, autoantibody could be interpreted as biomarkers and be useful in glaucoma diagnosis offering the chance of slowing down the development of glaucoma by early diagnosis and treatment (Von Thun Und Hohenstein-Blaul et al., 2017).
Recent studies suggest the repurposing of antibiotics with anti-inflammatory properties for glaucoma treatment. Minocycline, a second-generation tetracycline with antimicrobial and anti-inflammatory activities, has been reported to afford neuroprotection in experimental models of glaucoma, such as intraocular pressure elevation and optic nerve transection (Levkovitch-Verbin et al., 2014). The neuroprotection afforded by minocycline appears to be mediated at least by two mechanisms: attenuation of innate and adaptive immunity, in particular preventing the activation of microglia, and blockade of apoptotic cascade (Russo et al., 2016).
Azithromycin is a widely used semi-synthetic macrolide antibiotic endowed with anti-inflammatory and immunomodulatory properties. We have recently reported the first evidence of the neuroprotective effect of azithromycin in an experimental model of acute glaucoma induced by transient elevation of intraocular pressure; azithromycin-mediated neuroprotective effects were ascribed to the ability of the drug of modifying the inflammatory state of the retina (Varano et al., 2017). Similarly, the neuroprotective effect of azithromycin has been previously reported in mice subjected to ischemic stroke; in this model azithromycin reduced brain injury by decreasing the infiltration of inflammatory, myeloid, cells in the ischemic hemisphere and promoting the macrophage transition towards the M2 phenotype (a phenotype that stimulates angiogenesis, tissue remodeling and repair) (Amantea et al., 2016). These data suggest that systemic treatment with azithromycin might exert peripheral immunomodulatry effects that, in turn, affect neuronal survival in central areas. Since azithromycin is characterized by a documented ability to reach high tissue concentration after oral administration and has a favorable safety profile, it could be identified as a promising candidate for treating ocular disorders associated with retinal ganglion cell degeneration.
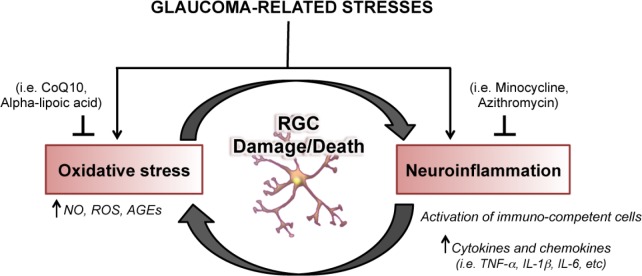
In conclusion, a growing body of evidence supports the notion that neuroinflammation and neurodegeneration coexist in several central and peripheral pathologies and have often a reciprocal causative role. Furthermore, pathogenic and reparative properties have been attributed to the inflammatory and immune response triggered by neuronal insults (Soto and Howell, 2014). Accordingly, over the course of the optic neuropathy, activation of the immune system may act as a double-edge sword having both harmful or beneficial effects on retinal ganglion cell survival. Therefore, developing new therapeutic approaches aimed at modulating, rather than suppressing, the neuroinflammation and the activation of immune system might hold great potential for the treatment of glaucoma though this implies a careful choice of the druggable targets and the consideration of a flexible therapy that must be redesigned depending on the disease state (Figure 1).
Figure 1.
Neuroinflammation and oxidative stress lead to retinal ganglion cell (RGC) neurodegeneration.
CoQ10: Coenzyme Q10; NO: nitric oxide; ROS: reactive oxygen species; AGEs: advanced glycation end products; TNF-α: tumor necrosis factor-alpha; IL-1β: interleukin-1β; IL-6: interleukin-6.
Additional file: Open peer review report 1 (113.6KB, pdf) .
Acknowledgments:
We apologize to those authors whose relevant work was not cited due to space constraints.
Footnotes
Financial support: None.
Conflicts of interest: None declared.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Eleftherios Paschalis Ilios, Harvard Medical School, USA.
P-Reviewer: Ilios EP; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Amantea D, Certo M, Petrelli F, Tassorelli C, Micieli G, Corasaniti MT, Puccetti P, Fallarino F, Bagetta G. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Exp Neurol 275 Pt. 2016;1:116–125. doi: 10.1016/j.expneurol.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–566. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg D, Skaat A, Liebmann JM. Emerging risk factors for glaucoma onset and progression. Prog Brain Res. 2015;221:81–101. doi: 10.1016/bs.pbr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Levkovitch-Verbin H, Waserzoog Y, Vander S, Makarovsky D, Piven I. Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma. Graefes Arch Clin Exp Ophthalmol. 2014;252:761–772. doi: 10.1007/s00417-014-2588-4. [DOI] [PubMed] [Google Scholar]
- 5.Parisi V, Centofanti M, Gandolfi S, Marangoni D, Rossetti L, Tanga L, Tardini M, Traina S, Ungaro N, Vetrugno M, Falsini B. Effects of coenzyme Q10 in conjunction with vitamin E on retinal-evoked and cortical-evoked responses in patients with open-angle glaucoma. J Glaucoma. 2014;23:391–404. doi: 10.1097/IJG.0b013e318279b836. [DOI] [PubMed] [Google Scholar]
- 6.Paschalis EI, Lei F, Zhou C, Kapoulea V, Thanos A, Dana R, Vavvas DG, Chodosh J, Dohlman CH. The role of microglia and peripheral monocytes in retinal damage after corneal chemical injury. Am J Pathol. 2018;188:1580–1596. doi: 10.1016/j.ajpath.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G, Morrone LA. Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. Eur J Pharmacol. 2016;787:134–142. doi: 10.1016/j.ejphar.2016.03.064. [DOI] [PubMed] [Google Scholar]
- 8.Sappington RM, Chan M, Calkins DJ. Interleukin-6 protects retinal ganglion cells from pressure-induced death. Invest Ophthalmol Vis Sci. 2006;47:2932–2942. doi: 10.1167/iovs.05-1407. [DOI] [PubMed] [Google Scholar]
- 9.Song W, Huang P, Zhang C. Neuroprotective therapies for glaucoma. Drug Des Devel Ther. 2015;9:1469–1479. doi: 10.2147/DDDT.S80594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soto I, Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. 2014;4:a017269. doi: 10.1101/cshperspect.a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezel G. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol. 2013;13:23–31. doi: 10.1016/j.coph.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Tong Y, Zhou YL, Zheng Y, Biswal M, Zhao PQ, Wang ZY. Analyzing cytokines as biomarkers to evaluate severity of glaucoma. Int J Ophthalmol. 2017;10:925–930. doi: 10.18240/ijo.2017.06.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varano GP, Parisi V, Adornetto A, Cavaliere F, Amantea D, Nucci C, Corasaniti MT, Morrone LA, Bagetta G, Russo R. Post-ischemic treatment with azithromycin protects ganglion cells against retinal ischemia/reperfusion injury in the rat. Mol Vis. 2017;23:911–921. [PMC free article] [PubMed] [Google Scholar]
- 15.Von Thun Und Hohenstein-Blaul N, Kunst S, Pfeiffer N, Grus FH. Biomarkers for glaucoma: from the lab to the clinic. Eye (Lond) 2017;31:225–231. doi: 10.1038/eye.2016.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Hondur G, Tezel G. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2016;57:2344–2354. doi: 10.1167/iovs.16-19153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yerramothu P, Vijay AK, Willcox MDP. Inflammasomes the eye and anti-inflammasome therapy. Eye (Lond) 2018;32:491–505. doi: 10.1038/eye.2017.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanon-Moreno V, Raga-Cervera J, Garcia-Medina JJ, Benitez-Del-Castillo J, Vinuesa-Silva I, Torregrosa S, Pinazo-Duran MD. New horizons for the treatment of glaucoma. I: Neuroinflammation and inflammasomes. Arch Soc Esp Oftalmol. 2018;93:e7–9. doi: 10.1016/j.oftal.2017.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.