Abstract
Neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s diseases have multifaceted nature because of the different factors contributing to their progression. The complex nature of neurodegenerative diseases has developed a pressing need to design multitarget-directed ligands to address the complementary pathways involved in these diseases. The major enzyme targets for development of therapeutics for Alzheimer’s disease are cholinesterase and β-secretase enzymes. In this review, we discuss recent advances in profiling single target inhibitors based on these enzymes to multitarget-directed ligands as potential therapeutics for this devastating disease. In addition, therapeutics based on iron chelation strategy are discussed as well.
Keywords: multitarget-directed ligands, acetylcholinesterase, β-secretase, Alzheimer's disease, hybridization, neurodegenerative diseases, tacrine, brain permeability
The Challenge of Development of Therapeutics for Multifactorial Conformational Diseases
The World Alzheimer Report 2016 estimated the presence of 46.8 million people worldwide with dementia in 2015 and predicted that the number of patients will be almost tripled by 2050. Therefore, dementia which is associated with neurodegenerative diseases represents one of the major global health crises of the 21st century. The progression of Alzheimer’s (AD) is directly correlated to the deposition of amyloid-β peptides in the human brain as mature fibrils according to the amyloid cascade hypothesis (Karran et al., 2011). Binding of amyloid-β aggregates to neuronal and non-neuronal plasma membranes triggers dysfunction of synapses and neural networks which are correlated with cognitive deficits in AD patients (Mucke and Selkoe, 2012). Multifactorial conditions control amyloid aggregation by modulating the hydrophobic forces in the folding intermediates of amyloid-β peptides, the main contributor to protein misfolding (Figure 1). Consequently, targeting protein aggregation-related neurodegenerative diseases represents a complex challenge in drug discovery and development. Although amyloid fibrils have been considered the main causative of the progression of neurodegenerative diseases, recent research efforts have demonstrated that the neurotoxic effects are mainly attributed to the small soluble oligomeric species. For example, neuronal death in AD animal models was detected in the early amyloid aggregation phase in the presence of the oligomeric species (Sengupta et al., 2016). Amyloid-β oligomers are heterogeneous mixture of polymorphic molecules which significantly hinder investigating their structure and properties resulting in a lack of well-validated biomarkers for clinical trials. Genetic factors are also considered a remarkable factor in the pathogenesis of neurodegenerative diseases. Moreover, the overproduction of reactive oxygen species during oxidative stress has demonstrated to play a key role in the pathophysiology of AD and Parkinson’s disease based on the sensitivity of glial cells and neurons to reactive oxygen species resulting in neuronal damage (Figure 1). On the same line, impaired mitochondrial dynamics (size, movement, shape) and mitochondrial dysfunction have been identified as key factors in the etiology of neurodegeneration (Lin and Beal, 2006). In addition, excessive metal (Cu, Zn, Pb, Fe) accumulation in human brain is a causative factor in the induction of oxidative stress and mitochondrial dysfunction resulting in neurological disorders (Figure 1). Moreover, impaired brain glucose metabolism is postulated to contribute to the progression of AD. In vitro studies demonstrated that polymerization of N-acetylglucosamine can trigger neurotoxicity directly and through microglia activation (Turano et al., 2015). Taking into consideration the multifaceted nature of neurodegenerative diseases, development of multitarget-directed ligands (MTDLs) has evolved as an attractive strategy to target multiple pathways implicated in the progression of neurodegeneration (Hiremathad and Piemontese, 2017).
Figure 1.
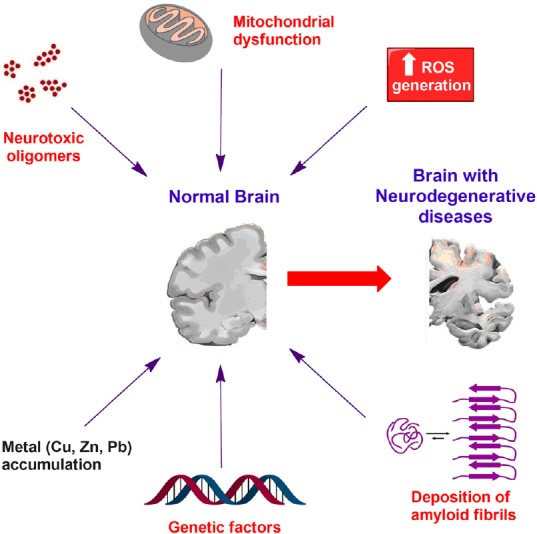
The multifactorial and complex nature of neurodegenerative diseases.
ROS: Reactive oxygen species.
Multitarget Therapeutics Based on Cholinesterase and Monoamine Oxidase Inhibitors
Monoamine oxidase (MAO) has a pivotal role in the development of AD through the formation of amyloid plaques and the accumulation of amyloid-β peptides in human brain. In addition, recent studies have demonstrated that MAO-B protein is associated with γ-secretase enzyme and is expressed in high levels not only in astrocytes but in pyramidal neurons of AD brain as well (Schedin-Weiss et al., 2017). Acetylcholinesterase (AChE) enzyme inhibitors are the only Food and Drug Administration (FDA)-approved drugs for AD. Inhibition of AChE is a palliative therapeutic approach for AD based on the dysfunction in basal forebrain cholinergic system in AD patients. Both AChE and MAO inhibitors can improve cognitive functions, memory and alleviate the symptoms associated with AD. Neurodegenerative diseases such as AD have multiple pathways contributing to their pathogenesis. Here comes the MTDL strategy, which provides a more effective way for the treatment of the neurological disorders instead of the classical single drug for a single target strategy. A new homoisoflavonoid derivative linked to a pyridine group proved to have a mixed and balanced inhibitory activity against AChE and MAO-B (Wang et al., 2016). In 2018, a novel propargylamine-modified pyrimidinylthiourea derivative was developed as a potential multitarget agent as it demonstrated a high affinity for the inhibition of both AChE and MAO-B in mouse brain and the ability to alleviate scopolamine-induced cognitive impairment in AD in mice (Xu et al., 2018). Interestingly, the novel pyrimidinylthiourea derivative proved to have good blood-brain barrier permeability, antioxidant, copper chelating properties and good oral bioavailability in pharmacokinetic studies. Glycogen synthase kinase 3β (GSK-3β) is a crucial kinase in AD that contributes to the formation of neurofibrillary tangles in human brain by catalyzing the phosphorylation of serine and threonine residues. Recently, molecular hybridization strategy resulted in new leads as dual AChE/GSK-3β inhibitors in the nanomolar range that alleviated cognitive disorders in animal models (Jiang et al., 2018). In addition, hybridization of different pharmacophore fragments afforded potent antagonists of serotonin 5-HT6 receptors and dual acetyl/butyrylcholinesterase inhibitors that displayed satisfactory blood-brain barrier permeation (Więckowska et al., 2018). Moreover, pyrazolopyrimidinone derivatives were introduced as a novel class that can inhibit butyrylcholinesterase and phosphodiesterase 9 which are involved in different processes of AD progression (Yu et al., 2017). There is a compelling need to further investigate and develop new MTDL strategies as these drugs exhibited significant potential in halting AD or even providing a radical cure for AD.
Multitarget Brain Permeable Iron Chelator Drugs
Altered iron metabolism in human brain is a common event of neurological disorders. Accumulation of iron in the human brain is associated with excessive generation of reactive oxygen species which leads to gradual loss of neurons and diminution in functionality. Moreover, literature reveals the key role of iron in promoting amyloid-β neuro-toxicity in AD by delaying the formation of well-ordered aggregates of amyloid-β (Liu et al., 2011). Thus, cerebral iron homeostasis is identified as a valuable target in designing new therapeutics for aging-related disorders. A multitarget neuroprotective compound M30 was developed with dual iron chelating and MAO-A and -B inhibitory activities (Kupershmidt et al., 2012). The design strategy of M30 embraces molecular hybridization of N-propargyl moiety of rasagiline and 8-hydroxyquinoline scaffold of the iron chelator, VK28. M30 compound displays a wide array of pharmacological activities, including neuro-rescue effects, induction of neuronal differentiation and up-regulation of number of neuroprotective-adaptive mechanisms and pro-survival signaling pathways. In addition, M30 compound exhibits a great impact on the reduction of iron accumulation in the brain, the reduction of the cerebral β-amyloid plaques and showed strong inhibition of both MAO-A and MAO-B activity in aged mice brains. Interestingly, loading of iron chelators on nanoparticles represents a powerful tools in metal chelation therapy for neurological disorders as it exhibits beneficial improvement in blood-brain barrier permeability.
Tacrine Derivatives as Multifunctional Compounds for Neurodegenerative Diseases
The current approved therapies for AD are based on improving cholinergic transmission via the inhibition of AChE which only provide fair improvement in memory and cognitive functions. Being a multifaceted neurodegenerative disease, AD needs a comprehensive approach for treatment through multifunctional and multitarget drugs. Tacrine is the earliest centrally acting AChE inhibitor which is considered the prototype lead of this class of drugs. Tacrine was the first clinically effective drug for AD treatment approved by the FDA in 1993. Currently, tacrine is no longer used as a treatment for AD due to serious hepatotoxicity which limited its clinical use. Tacrine initiates reactive oxygen species production and reduces the liver glutathione levels which leads to hepatotoxicity. It was reported that the homo and hetero dimers of tacrine can improve its efficiency, potency and reduce its undesired side effects (Qian et al., 2014). Development of a tacrine derivative with antioxidant properties to counteract the hepatotoxicity of tacrine represents an effective approach in AD treatment. Multi-target tacrine-natural products hybrids have received considerable attention in development of AD therapeutics (Oset-Gasque and Marco-Contelles, 2018). A novel series of multifunctional mercapto-tacrine derivatives was developed that exhibits improvement of synaptic plasticity (long-term potentiation) in hippocampus, neuroprotection and significantly less hepatotoxicity than the parent drug. These novel multifunctional compounds were more efficient in improving cognitive and memory functions in animal models. In addition, the mercapto group in this series played a key role in reducing the hepatotoxicity caused by the free radicals generated by tacrine. A new series of tacrine-propargylamine derivatives was developed for AD treatment which displayed high potency for AChE and butyrylcholinesterase inhibition with significantly lower neurotoxicity and hepatotoxicity compared to tacrine (Mao et al., 2015). Recently, tacrine-hydroxyphenylbenzimidazole hybrids were introduced as multitarget therapeutics that were capable of inhibiting the neurotoxicity induced by Aβ in neuronal cells (Hiremathad et al., 2018). Designing and developing MTDLs by connecting two different classes of compounds into one molecular entity can pave the road towards the discovery of a new revolutionary treatment for neurodegenerative diseases.
Multitarget Therapeutics for Neurodegenerative Diseases Centered on BACE-1
β-Secretase 1 (BACE-1) is an aspartic protease enzyme that catalyzes extracellular cleavage of amyloid precursor protein which produces amyloid-β peptides upon subsequent proteolysis. BACE-1 demonstrated to be a more druggable target for small molecules than amyloid aggregation, thus, BACE-1 inhibitors represent a prominent approach in the discovery of therapeutics for neurodegenerative diseases. Moreover, the identification of crystal structures of BACE-1 bound to various ligands using X-ray crystallography revolutionized structure-based approaches in the development of BACE-1 inhibitors. However, several BACE-1 lead inhibitors failed clinical trials because of dose-limiting side effects and poor in vivo efficacy. Consequently, MTDLs centered on BACE-1 are receiving considerable attention as effective therapeutics for the multifaceted nature of neurological disorders (Prati et al., 2018). Although peptidomimetics represent the early discovered examples of BACE-1 inhibitors, their unfavorable pharmacokinetic properties have shifted research efforts towards development of small molecules non peptide inhibitors. The multi-targeted coumarin derivative (compound 1 in Figure 2) is one of the early examples of potent dual AChE/BACE-1 inhibitors (Piazzi et al., 2008). In addition, introduction of a double bond to the indanone core of FDA-approved donepezil afforded a donepezil-like compound 2 (Figure 2) with dual AChE/BACE-1 inhibitory activity (Costanzo et al., 2016). Recently, we incorporated backbone amide linkers to donepezil analogue 3 (Figure 2) resulting in potent AChE/BACE-1 inhibitory activity in the nanomolar range (Gabr and Abdel-Raziq, 2018). In vitro cytotoxicity screening in neuronal cells and preferable pharmacokinetic profile renders 3 a potential lead for future modifications for development of MTDLs. An emerging therapeutic approach for AD is dual inhibition of BACE-1 and GSK-3β with the aim of obtaining a downstream synergistic effect. Recently, a ligand-based approach afforded triazinone derivatives with favorable pharmacokinetic properties that function as dual BACE-1/GSK-3β inhibitors (Rampa et al., 2017). In addition, a structure-based approach was utilized to design curcumin derivatives with balanced profile against BACE-1 and GSK-3β (Di Martino et al., 2016). Inhibition of NAD(P)H quinone dehydrogenase 1 by the curcumin derivatives is postulated to contribute to their neuroprotective effect by counteracting oxidative stress in neurological disorders (Wu et al., 2013).
Figure 2.
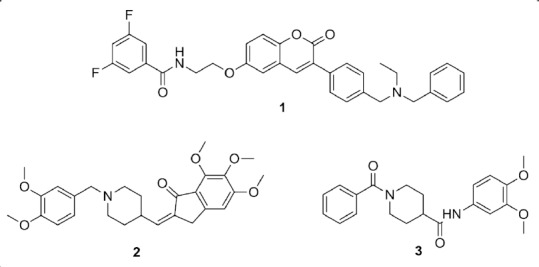
Chemical structures of compounds 1–3.
Conclusions and Future Perspective
Tremendous research efforts are directed towards development of small molecules targeting protein misfolding-related diseases. The majority of discovered small molecules as therapeutics for amyloid aggregation-related diseases shared failure in clinical trials owing to their poor in vivo efficacy and unfavorable pharmacokinetic profiles. However, the positive topline results of Tafamidis from phase 3 in transthyretin cardiomyopathy patients announced by Pfizer in 2018 holds promise for the future of small molecules as potential therapeutics for protein misfolding diseases. MTDLs represent effective tools in facing the unfathomable complexity of neurological disorder. The superior therapeutic profiles of MTDLs to single-target small molecules are attributed to the ability of MTDLs to target multiple major pathological cascades of neurodegenerative diseases. In this context, we are currently optimizing lead 3 as a potential AD therapeutic through dual AChE/BACE-1 inhibition in combination with a third target. Tailoring the identified lead compounds to iron-chelating ability and antioxidant activity is anticipated to result in favorable neuroprotective profile.
Additional file: Open peer review report 1 (146.2KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Paulina Carriba, Cardiff University, UK.
P-Reviewer: Carriba P; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Costanzo P, Cariati L, Desiderio D, Sgammato R, Lamberti A, Arcone R, Salerno R, Nardi M, Masullo M, Oliverio M. Design, synthesis, and evaluation of donepezil-like compounds as AChE and BACE-1 inhibitors. ACS Med Chem Lett. 2016;7:470–475. doi: 10.1021/acsmedchemlett.5b00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Martino RM, De Simone A, Andrisano V, Bisignano P, Bisi A, Gobbi S, Rampa A, Fato R, Bergamini C, Perez DI, Martinez A, Bottegoni G, Cavalli A, Belluti F. Versatility of the curcumin scaffold: discovery of potent and balanced dual BACE-1 and GSK-3beta inhibitors. J Med Chem. 2016;59:531–544. doi: 10.1021/acs.jmedchem.5b00894. [DOI] [PubMed] [Google Scholar]
- 3.Gabr MT, Abdel-Raziq MS. Design and synthesis of donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg Chem. 2018;80:245–252. doi: 10.1016/j.bioorg.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Hiremathad A, Piemontese L. Heterocyclic compounds as key structures for the interaction with old and new targets in Alzheimer’s disease therapy. Neural Regen Res. 2017;12:1256–1261. doi: 10.4103/1673-5374.213541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiremathad A, Keri RS, Esteves AR, Cardoso SM, Chaves S, Santos MA. Novel tacrine-hydroxyphenylbenzimidazole hybrids as potential multitarget drug candidates for Alzheimer’s disease. Eur J Med Chem. 2018;148:255–267. doi: 10.1016/j.ejmech.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Jiang XY, Chen TK, Zhou JT, He SY, Yang HY, Chen Y, Qu W, Feng F, Sun HP. Dual GSK-3beta/AChE inhibitors as a new strategy for multitargeting anti-Alzheimer’s disease drug discovery. ACS Med Chem Lett. 2018;9:171–176. doi: 10.1021/acsmedchemlett.7b00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 8.Kupershmidt L, Amit T, Bar-Am O, Youdim MB, Weinreb O. Neuroprotection by the multitarget iron chelator M30 on age-related alterations in mice. Mech Ageing Dev. 2012;133:267–274. doi: 10.1016/j.mad.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 10.Liu B, Moloney A, Meehan S, Morris K, Thomas SE, Serpell LC, Hider R, Marciniak SJ, Lomas DA, Crowther DC. Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J Biol Chem. 2011;286:4248–4256. doi: 10.1074/jbc.M110.158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao F, Li J, Wei H, Huang L, Li X. Tacrine-propargylamine derivatives with improved acetylcholinesterase inhibitory activity and lower hepatotoxicity as a potential lead compound for the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2015;30:995–1001. doi: 10.3109/14756366.2014.1003212. [DOI] [PubMed] [Google Scholar]
- 12.Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oset-Gasque MJ, Marco-Contelles J. Tacrine-natural-product hybrids for Alzheimer's disease therapy. Curr Med Chem. 2018 doi: 10.2174/0929867325666180403151725. doi: 10.2174/0929867325666180403151725. [DOI] [PubMed] [Google Scholar]
- 14.Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F, Recanatini M, Andrisano V, Rampa A. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg Med Chem Lett. 2008;18:423–426. doi: 10.1016/j.bmcl.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 15.Prati F, Bottegoni G, Bolognesi ML, Cavalli A. BACE-1 inhibitors: from recent single-target molecules to multitarget compounds for Alzheimer’s disease. J Med Chem. 2018;61:619–637. doi: 10.1021/acs.jmedchem.7b00393. [DOI] [PubMed] [Google Scholar]
- 16.Qian S, He L, Mak M, Han Y, Ho CY, Zuo Z. Synthesis, biological activity, and biopharmaceutical characterization of tacrine dimers as acetylcholinesterase inhibitors. Int J Pharm. 2014;477:442–453. doi: 10.1016/j.ijpharm.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 17.Rampa A, Gobbi S, Concetta Di Martino RM, Belluti F, Bisi A. Dual BACE-1/GSK-3beta inhibitors to combat Alzheimer’s disease: a focused review. Curr Top Med Chem. 2017;17:3361–3369. doi: 10.2174/1568026618666180112161406. [DOI] [PubMed] [Google Scholar]
- 18.Schedin-Weiss S, Inoue M, Hromadkova L, Teranishi Y, Yamamoto NG, Wiehager B, Bogdanovic N, Winblad B, Sandebring-Matton A, Frykman S, Tjernberg LO. Monoamine oxidase B is elevated in Alzheimer disease neurons, is associated with gamma-secretase and regulates neuronal amyloid beta-peptide levels. Alzheimers Res Ther. 2017;9:57. doi: 10.1186/s13195-017-0279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sengupta U, Nilson AN, Kayed R. The role of amyloid-beta oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turano E, Busetto G, Marconi S, Guzzo F, Farinazzo A, Commisso M, Bistaffa E, Angiari S, Musumeci S, Sotgiu S, Bonetti B. Neurotoxicity and synaptic plasticity impairment of N-acetylglucosamine polymers: implications for Alzheimer’s disease. Neurobiol Aging. 2015;36:1780–1791. doi: 10.1016/j.neurobiolaging.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Sun Y, Guo Y, Wang Z, Huang L, Li X. Dual functional cholinesterase and MAO inhibitors for the treatment of Alzheimer’s disease: synthesis, pharmacological analysis and molecular modeling of homoisoflavonoid derivatives. J Enzyme Inhib Med Chem. 2016;31:389–397. doi: 10.3109/14756366.2015.1024675. [DOI] [PubMed] [Google Scholar]
- 22.Więckowska A, Wichur T, Godyń J, Bucki A, Marcinkowska M, Siwek A, Więckowski K, Zaręba P, Knez D, Głuch-Lutwin M, Kazek G, Latacz G, Mika K, Kołaczkowski M, Korabecny J, Soukup O, Benkova M, Kieć-Kononowicz K, Gobec S, Malawska B. Novel multitarget-directed ligands aiming at symptoms and causes of Alzheimer’s disease. ACS Chem Neurosci. 2018;9:1195–1214. doi: 10.1021/acschemneuro.8b00024. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Li Q, Wang X, Yu S, Li L, Wu X, Chen Y, Zhao J, Zhao Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS One. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu YX, Wang H, Li XK, Dong SN, Liu WW, Gong Q, Wang TD, Tang Y, Zhu J, Li J, Zhang HY, Mao F. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;143:33–47. doi: 10.1016/j.ejmech.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Yu YF, Huang YD, Zhang C, Wu XN, Zhou Q, Wu D, Wu Y, Luo HB. Discovery of novel pyrazolopyrimidinone derivatives as phosphodiesterase 9A inhibitors capable of inhibiting butyrylcholinesterase for treatment of alzheimer’s disease. ACS Chem Neurosci. 2017;8:2522–2534. doi: 10.1021/acschemneuro.7b00268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


