Keywords: nerve regeneration, induced neurons, plasmid-based, human fibroblasts, nucleofection, Ascl1, miR124, p53, reprogramming, neural regeneration
Abstract
Differentiation of human fibroblasts into functional neurons depends on the introduction of viral-mediated transcription factors, which present risks of viral gene integration and tumorigenicity. In recent years, although some studies have been successful in directly inducing neurons through sustained expression of small molecule compounds, they have only been shown to be effective on mouse-derived cells. Thus, herein we delivered vectors containing Epstein-Barr virus-derived oriP/Epstein-Barr nuclear antigen 1 encoding the neuronal transcription factor, Ascl1, the neuron-specific microRNA, miR124, and a small hairpin directed against p53, into human fibroblasts. Cells were incubated in a neuron-inducing culture medium. Immunofluorescence staining was used to detect Tuj-1, microtubule-associated protein 2, neuron-specific nucleoprotein NeuN and nerve cell adhesion molecules in the induced cells. The proportion of Tuj1-positive cells was up to 36.7% after induction for 11 days. From day 21, these induced neurons showed neuron-specific expression patterns of microtubule-associated protein 2, NeuN and neural cell adhesion molecule. Our approach is a simple, plasmid-based process that enables direct reprogramming of human fibroblasts into neurons, and provides alternative avenues for disease modeling and neurodegenerative medicine.
Chinese Library Classification No. R456; R318; Q2
Introduction
Fibroblasts can be converted, bypassing the pluripotent state, into diverse cell types, such as human induced neurons (Vierbuchen et al., 2010; Pang et al., 2011). Direct reprogramming of neurons has been used to model diseases, and is promising for regenerative medicine (Xu et al., 2015). Most studies on direct conversion of fibroblasts to neurons used doxycycline-regulated lentiviral vectors that introduced specific cell fate-determining transcription factors or microRNAs (Vierbuchen et al., 2010; Caiazzo et al., 2011; Liu et al., 2013; Dell’Anno et al., 2014). However, integration of the viral vectors into the host cell genome can affect expression of endogenous genes and cause insertional mutagenesis (Nienhuis et al., 2006; Okita et al., 2008), which may be problematic for applications of this method.
To overcome the obstacle of viral integration, episomal plasmids can be used to derive non-integrating induced pluripotent stem cells (Kim et al., 2016). In such plasmids, an Epstein-Barr nuclear antigen 1-encoded protein is expressed from a viral promoter after transfection into host cells, and then it recognizes the oriP sequence of the vector and induces plasmid amplification during DNA amplification of the host cell. Episomal vectors can be removed from mammalian cells by simple cell culturing without any additional manipulations (Nanbo et al., 2007; Okita et al., 2013). Here, we report a method for generating functional induced neurons using non-integrating episomal vectors.
Materials and Methods
Plasmid construction
The woodchuck hepatitis post-transcriptional regulatory element was inserted upstream of the polyadenylation signal for efficient gene expression (Niwa et al., 1991). The backbone vectors, pCXLE-hSK and pCXLE-hOCT3/4-shP53-F (Okita et al., 2008), were purchased from Addgene (Plasmid #27078 and Plasmid #27077; Cambridge, MA, USA). The human Ascl1 and microRNA124 (miR124) sequences were amplified by PCR. The pCMV promoter sequence was amplified from pcDNA3.1(-) (Invitrogen, Carlsbad, CA, USA), subcloned into pGEM-T Easy vector (Promega, Madison, WI, USA), and sequenced. The human Ascl1-encoding fragment was digested with EcoRI and subcloned into pCXLE. DNA encoding miR124 and the pCMV promoter (with or without shp53) was digested with BamHI and cloned into pCXLE. The final versions of these combined vectors are shown in Figure 1A.
Figure 1.
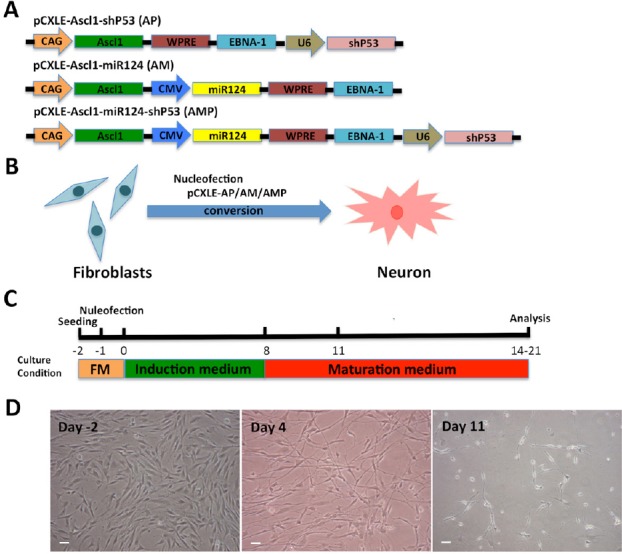
Direct conversion of human fibroblasts into neurons by nucleofection of non-viral vectors.
(A) The three combinations of episomal vectors: pCXLE-Ascl1-shP53 (AP), pCXLE-Ascl1-miR124 (AM) and pCXLE-Ascl1-miR124-shP53 (AMP). (B, C) The protocol of human foreskin fibroblast cell conversion into neurons. FM: Full medium. (D) Cell morphology during fibroblast reprogramming in the AMP group. Day 2: fibroblast cell morphology; Day 4: the cells gradually changed their morphological characteristics; Day 11: the shape of the cells exhibited neuron-like morphology. Scale bars: 50 μm.
Cell cultures and plasmid transfection
Human foreskin fibroblast cells were purchased from ATCC Global Bioresource Center (Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 10% penicillin and 10% streptomycin. Cultures were grown under 5% CO2 at 37°C and passaged every 2–3 days. One million human foreskin fibroblast cells in Human Nucleofector Stem Cell Kit 1 buffer (Lonza, Allendale, NJ, USA) were nucleofected with 5 μg of pCXLE using Nucleofection Program A23, in accordance with the manufacturer’s protocol. Then, cells were cultured in fibroblast medium for 24 hours. One day after nucleofection, the medium was replaced with the induction medium, DMEM:Nutrient Mixture F-12, with N2 supplement, B27 supplement (both from Invitrogen), 20 μM vitamin C (Sigma, St. Louis, MO, USA), 3 μM CHIR99021 (Stemgent, Beltsville, MD, USA), 1 μM purmorphamine (Selleckchem, Houston, TX, USA), 20 μM brain-derived neurotrophic factor, 20 μM glial-derived neurotrophic factor, 20 μM nerve growth factor (all from Invitrogen) and 10 μM Y27632 (Stemgent, Beltsville, MD, USA). Eight days after induction, the medium was replaced with maturation medium, which was induction medium without CHIR99021 and purmorphamine.
Immunofluorescence staining
Cells were cultured in 12-well plates. On days 11, 21 and 29 after induction, cells were fixed in 4% paraformaldehyde (Sigma) for 20 minutes at room temperature, permeabilized with 0.1% Triton X-100 for 15 minutes at room temperature, blocked in 3% bovine serum albumin for 30 minutes at room temperature, and then incubated overnight with primary antibodies at 4°C. The primary antibodies used (all from Millipore, Temecula, CA, USA; all diluted 1:1000; mouse anti-human) were: class III β-tubulin (Tuj-1), microtubule-associated protein 2 (Map2), neuron-specific nuclear protein (NeuN) and neural cell adhesion molecule (NCAM). The following day, cells were incubated with goat anti-mouse AlexaFluor 488 or 594-labeled secondary antibodies (1:1000; Invitrogen) for 1 hour at 37°C. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:5000; ZhongShan Goldenbridge Biotechnology Co., Ltd., Beijing, China) for 5 minutes at room temperature. Morphology was examined using a Leica TCS SP5 X confocal laser scanning microscope (Wetzlar, Hesse, Germany). Experiments were repeated five times. At least 10 immunofluorescence staining pictures were taken per experiment, and all the Tuj-1-positive cells were counted. The reprogramming efficiency was expressed as Tuj-1-positive cells/total cells.
Statistical analysis
All values are expressed as the mean ± SEM. Differences between two groups were compared with unpaired t-test using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Direct conversion of human fibroblasts into neurons using an integration-free episomal system
The direct conversion of fibroblasts into neurons is relatively fast when cells are infected with lentiviruses expressing transcription factors such as Ascl1, miRNA124 and p53 shRNA (Cheng et al., 2009; Vierbuchen et al., 2010; Pang et al., 2011; Jiang et al., 2015). However, as explained in the Introduction, this approach is problematic. Here, we attempted to reprogram fibroblasts into neurons by combining Ascl1, miRNA124 and p53 shRNA, using an integration-free episomal system. The combined episomal vectors pCXLE-Ascl1-shP53 (AP), pCXLE-Ascl1-miR124 (AM) and pCXLE-Ascl1-miR124-shP53 (AMP) were transfected into human foreskin fibroblasts by nucleofection (Figure 1A and B). Human foreskin fibroblasts were cultured in induction medium after nucleofection, and the culture medium was replaced by maturation medium on day 8 after induction. The induced neurons were maintained in culture in the maturation medium for 2 to 3 weeks for further analysis (Figure 1C). Neuron-like cells started to emerge on day 4 after plasmid nucleofection. Figure 1D shows the cell morphology during the fibroblast reprogramming.
Induced cells express early- and late-stage neuronal markers
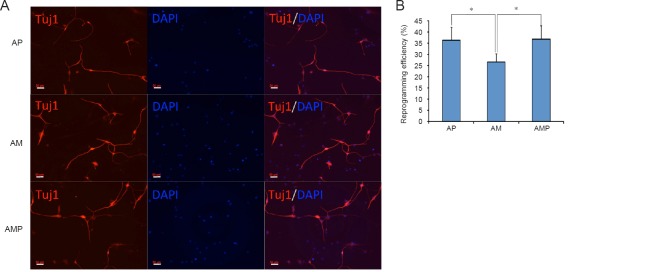
To determine whether these neuron-like cells had characteristics of early-stage neurons, we assessed the expression of neuronal markers. As Figure 2A shows, the neuron-specific marker, Tuj-1, was expressed in these cells 11 days after induction. The reprogramming efficiency of the AP group (Ascl1 + shp53) and the AMP group (Ascl1 + miR124 + shp53) was higher than that of the AM group (Ascl1 + miR124) (P < 0.05; Figure 2B). We further investigated whether these converted neurons became mature neurons. Immunofluorescence staining demonstrated the expression of the neuron-specific markers, Map2 and NeuN, in the converted cells on day 21 (Figure 3A). Furthermore, the marker, NCAM, was detected on day 29 (Figure 3B). There was no significant difference in the expression levels of the mature neuronal markers among the three groups (P > 0.05). However, the morphology of neurons from the AMP group was better than that of neurons from the AP group, which may be caused by the neurogenesis-promoting role of miR124.
Figure 2.
Immunofluorescence staining of early-stage conversion neurons.
(A) Tuj-1 was expressed in these cells on day 11 after induction. The fluorescent indicator is AlexaFluor 488 (red). Scale bars: 50 μm. (B) The reprogramming efficiency. The reprogramming efficiency is expressed as Tuj-1-positive cells/total cells. Data are expressed as the mean ± SEM (unpaired t-test). Five independent experiments were carried out, each with three replicates. *P < 0.05. AP: Ascl1 + shP53; AM: Ascl1 + miR124; AMP: Ascl1 + miR124 + shP53. Tuj-1: Class III b-tubulin; DAPI: 4′,6-diamidino-2-phenylindole.
Figure 3.
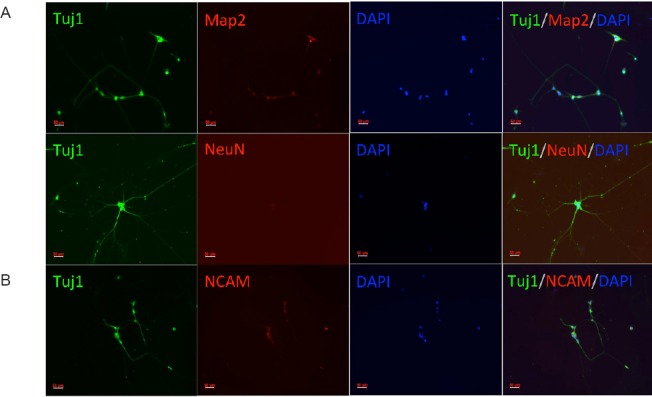
Immunofluorescence staining of late-stage neurons in the AMP group.
(A) Expression of neuron-specific Map2 and NeuN in the converted cells was detected by day 21 in the AMP group. (B) NCAM, another neuron-specific marker (Fiszbein et al., 2015) was detected in the converted cells on day 29. AMP: Ascl1 + miR124 + shP53; Tuj-1: Class III b-tubulin; Map2: microtubule-associated protein 2; NeuN: neuron-specific nuclear protein; NCAM: neural cell adhesion molecule; DAPI: 4′,6-diamidino-2-phenylindole. Scale bars: 50 μm.
Discussion
A big problem in neurodegenerative medicine is obtaining functional neurons for therapeutic treatment. Many studies have reported direct conversion of somatic cells to functional neurons (Vierbuchen et al., 2010; Caiazzo et al., 2011; Pang et al., 2011; Pfisterer et al., 2011; Son et al., 2011; Karow et al., 2012; Zhang et al., 2013). Direct lineage reprogramming is a promising fast approach for manipulating cell fate that avoids the risk of teratoma associated with pluripotent stem cells. However, most of these studies used viral vectors, with the associated risk of genomic modifications and positional mutagenesis with the insertion of transgenes (Nienhuis et al., 2006; Okita et al., 2008; Lombardo et al., 2011). Recent developments led to plasmid-based protocols (Capetian et al., 2016). Here, we used episomal vector combinations including three factors (Ascl1, miR124 and shP53) to induce reprogramming and obtain induced neurons without viral integration.
Ascl1 plays an important role in differentiation and maturation of neurons in vivo and in vitro (Chanda et al., 2014; Liu et al., 2015), and introduction of Ascl1 as a single reprogramming factor is enough to generate neuronal cells. MiR124, which is only expressed in neurons, promotes neurogenesis in vitro and in vivo, and may regulate the activity of postmitotic neurons (Krichevsky et al., 2006; Cheng et al., 2009; Åkerblom et al., 2012). p53 inhibition using direct viral infection or transient transfection enhanced the efficiency of induced pluripotent stem cell formation from fibroblasts by reducing apoptosis following the initial transgene induction (Hong et al., 2009; Kawamura et al., 2009). The suppression of p53 has recently been demonstrated to enhance the efficiency of the transcription factor-mediated conversion of human fibroblasts into neurons (Jiang et al., 2015). The present study used Ascl1, miR124 and p53 shRNA to successfully direct conversion of human fibroblasts into neurons. We also found that shp53 enhanced the reprogramming efficiency.
Our approach is a simple plasmid-based process that enables direct reprogramming of human fibroblasts into neurons, and provides alternative avenues for disease modeling and neurodegenerative medicine. However, our study has some limitations. We did not examine the electrophysiological properties of these neurons because of laboratory conditions. Furthermore, we only tested a few transcription factors and additional transcription factors should be examined in the system, as they may further enhance the reprogramming efficiency.
Additional file: Open peer review report 1 (53.8KB, pdf) .
Footnotes
Conflicts of interest: The authors have declared that no competing interests exist.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81471126 (to XZC) and 81771216 (to XZC); the Natural Science Foundation of Zhejiang Province of China, No. LY17H090005 (to JLP); a grant from the Medical Science and Technology Plan Project of Zhejiang Province of China, No. 2016KYB119 (to JLP). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hao Chen, Shanghai 6th People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, China.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81471126 (to XZC) and 81771216 (to XZC); the Natural Science Foundation of Zhejiang Province of China, No. LY17H090005 (to JLP); a grant from the Medical Science and Technology Plan Project of Zhejiang Province of China, No. 2016KYB119 (to JLP).
P-Reviewer: Chen H; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Bell M, Raye W, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Åkerblom M, Sachdeva R, Barde I, Verp S, Gentner B, Trono D, Jakobsson J. MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci. 2012;32:8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 3.Capetian P, Azmitia L, Pauly MG, Krajka V, Stengel F, Bernhardi EM, Klett M, Meier B, Seibler P, Stanslowsky N, Moser A, Knopp A, Gillessen-Kaesbach G, Nikkhah G, Wegner F, Dobrossy M, Klein C. Plasmid-based generation of induced neural stem cells from adult human fibroblasts. Front Cell Neurosci. 2016;10:245. doi: 10.3389/fncel.2016.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chanda S, Ang CE, Davila J, Pak C, Mall M, Lee QY, Ahlenius H, Jung SW, Sudhof TC, Wernig M. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Reports. 2014;3:282–296. doi: 10.1016/j.stemcr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dell’Anno MT, Caiazzo M, Leo D, Dvoretskova E, Medrihan L, Colasante G, Giannelli S, Theka I, Russo G, Mus L, Pezzoli G, Gainetdinov RR, Benfenati F, Taverna S, Dityatev A, Broccoli V. Remote control of induced dopaminergic neurons in parkinsonian rats. J Clin Invest. 2014;124:3215–3229. doi: 10.1172/JCI74664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiszbein A, Schor IE, Kornblihtt AR. Chapter 11 - Fundamentals of NCAM expression, function, and regulation of alternative splicing in neuronal differentiation. Neural Surf Antigens. 2015;112:131–140. [Google Scholar]
- 8.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H, Xu Z, Zhong P, Ren Y, Liang G, Schilling HA, Hu Z, Zhang Y, Wang X, Chen S, Yan Z, Feng J. Cell cycle and p53 gate the direct conversion of human fibroblasts to dopaminergic neurons. Nat Commun. 2015;6:10100. doi: 10.1038/ncomms10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karow M, Sánchez R, Schichor C, Masserdotti G, Ortega F, Heinrich C, Gascón S, Khan MA, Lie DC, Dellavalle A, Cossu G, Goldbrunner R, Götz M, Berninger B. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11:471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Izpisúa Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460:1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SM, Kim JW, Kwak TH, Park SW, Kim KP, Park H, Lim KT, Kang K, Kim J, Yang JH, Han H, Lee I, Hyun JK, Bae YM, Scholer HR, Lee HT, Han DW. Generation of integration-free induced neural stem cells from mouse fibroblasts. J Biol Chem. 2016;291:14199–14212. doi: 10.1074/jbc.M115.713578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Miao Q, Yuan J, Han S, Zhang P, Li S, Rao Z, Zhao W, Ye Q, Geng J, Zhang X, Cheng L. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardo A, Cesana D, Genovese P, Di Stefano B, Provasi E, Colombo DF, Neri M, Magnani Z, Cantore A, Lo Riso P, Damo M, Pello OM, Holmes MC, Gregory PD, Gritti A, Broccoli V, Bonini C, Naldini L. Site-specific integration and tailoring of cassette design for sustainable gene transfer. Nat Methods. 2011;8:861–869. doi: 10.1038/nmeth.1674. [DOI] [PubMed] [Google Scholar]
- 17.Nanbo A, Sugden A, Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26:4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nienhuis AW, Dunbar CE, Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 20.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 21.Okita K, Yamakawa T, Matsumura Y, Sato Y, Amano N, Watanabe A, Goshima N, Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells. 2013;31:458–466. doi: 10.1002/stem.1293. [DOI] [PubMed] [Google Scholar]
- 22.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Du Y, Deng H. Direct lineage reprogramming: strategies, mechanisms, and applications. Cell Stem Cell. 2015;16:119–134. doi: 10.1016/j.stem.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.