Keywords: nerve regeneration, chronic cerebral hypoperfusion, vascular dementia, bilateral common carotid artery occlusion, Xiao-Xu-Ming decoction, label-free quantitative proteomics, Morris water maze test, Nano-LC-ESI LTQ-Orbitrap MS/MS technology, neural regeneration
Abstract
Xiao-Xu-Ming decoction has been widely used to treat stroke and sequelae of stroke. We have previously shown that the active fractions of Xiao-Xu-Ming decoction attenuate cerebral ischemic injury. However, the global protein profile and signaling conduction pathways regulated by Xiao-Xu-Ming decoction are still unclear. This study established a two-vessel occlusion rat model by bilateral common carotid artery occlusion. Rats were intragastrically administered 50 or 150 mg/kg Xiao-Xu-Ming decoction for 4 consecutive weeks. Learning and memory abilities were measured with Morris water maze. Motor ability was detected with prehensile test. Coordination ability was examined using the inclined screen test. Neuronal plasticity was observed by immunofluorescent staining. Differentially expressed proteins of rat hippocampus were analyzed by label-free quantitative proteomics. Real time-polymerase chain reaction and western blot assay were used to identify the changes in proteins. Results showed that Xiao-Xu-Ming decoction dramatically alleviated learning and memory deficits, and motor and coordination dysfunction, and increased the expression of microtubule-associated protein 2. Xiao-Xu-Ming decoction extract remarkably decreased 13 upregulated proteins and increased 39 downregulated proteins. The regulated proteins were mainly involved in oxidation reduction process, intracellular signaling cascade process, and protein catabolic process. The signaling pathways were mainly involved in ubiquitin mediated proteolysis and the phosphatidylinositol signaling system. Furthermore, there was an interaction among Rab2a, Ptpn1, Ppm1e, Cdk18, Gorasp2, Eps15, Capza2, Syngap1 and Mt-nd1. Protein analyses confirmed the changes in expression of MT-ND1. The current findings provide new insights into the molecular mechanisms of Xiao-Xu-Ming decoction extract’s effects on chronic cerebral hypoperfusion.
Chinese Library Classification No. R453; R363; R741
Introduction
Vascular dementia is characterized by memory loss, cognitive deficits, and vascular lesions in the brain (Tanaka et al., 2002). Chronic cerebral hypoperfusion has been identified as a risk factor for cognitive decline in vascular dementia (Ihara et al., 2014). Two-vessel occlusion (2-VO), by bilateral common carotid artery occlusion, is a common animal model to investigate the mechanisms of cognitive deficits in chronic cerebral hypoperfusion (Jing et al., 2015a). Substantial evidence has shown that chronic cerebral hypoperfusion may cause cognitive impairment (He et al., 2012; Wang et al., 2012a), but the underlying neurobiological mechanism and the regulation of global protein expression remain poorly understood.
Proteomics plays a very important role in the analysis of proteins and their interactions in a biological system. In addition to the traditional two-dimensional polyacrylamide gel electrophoresis, mass spectrometry has rapidly emerged as a reliable means by which protein expression can be identified and quantified to delineate the change of proteins for biomarker discovery (Ang et al., 2017). In recent years, label-free quantitative proteomics analysis using Nano-LC-ESI LTQ-Orbitrap MS/MS technology has attracted increasing attention because it has some advantages over traditional isotope labeling technology, including that it is faster, simpler and more concise (Tsai et al., 2015). In this study, global protein expression in the hippocampus of rats was analyzed using label-free quantitative proteomics.
Xiao-Xu-Ming decoction (XXM), recorded in “Beiji Qianjin Yao Fang”, written by Sun SimSi-miao in Tang Dynasty (Traditional Chinese Medicine Terminology Committee, 2004), has been used to treat stroke and sequelae of stroke in clinical settings. In our previous studies, the effect of XXM extract against cerebral ischemia was examined by combination high throughput screening with modern pharmacology evaluation technology (Wang et al., 2011, 2012b, 2016). Moreover, we have previously identified the components of XXM (Wang et al., 2014) and determined the pharmacokinetics of some XXM components in rat plasma under normal physiological conditions (Du et al., 2009; Wang et al., 2016). Although our previous studies have demonstrated that XXM exhibits anti-cerebral ischemic effects (Wang et al., 2012a, c), the global protein expression profile and signaling pathways regulated by XXM extract remain unknown. Therefore, in the present investigation, analysis of global protein expression in the hippocampus of 2-VO rats was performed using Nano-LC-ESI LTQ-Orbitrap MS/MS to investigate the molecular events associated with chronic cerebral hypoperfusion and the modulation effects of XXM extract.
Materials and Methods
XXM preparation
The formula of XXM consists of 12 botanical plant drugs including Stephania tetrandra S. Moore (6 g), Ephedra sinica Staph (3 g), Saposhnikovia divaricata (Turcz.) Schischk (6 g), Prunus armeniaca L. Var. Ansu Maxim (9 g), Zingiber officinale Rosc (9 g), Panax ginseng C. A. Mey (3 g), Glycyrrhiza uralensis Fisch (3 g), Aconitum carmichaeli Debx (3 g), Scutellaria baicalensis Georgi (6 g), Cinnamomum cassia Presl (3 g), Ligusticum chuanxiong Hort (3 g), and Paeonia lactiflora Pall (9 g). All crude herbs were purchased from Beijing Tongrentang Co., Ltd. (Beijing, China). The identification and deposition of these medicinal materials, and the preparation of XXM, were completed by Jiangsu Kanion Pharmaceutical Co., Ltd. (China). Briefly, crude herbs were soaked in 95% aqueous ethanol for 2 hours and refluxed by water bath heating for 1 hour. Filtered and mixed suspensions were collected and the concentration was determined. The extraction was extracted with petroleum ether for three times. The 40% ethanol elution fraction and the intermediate layer fraction were incorporated into XXM (Wang et al., 2011).
Animals
Seventy specific-pathogen-free adult male Wistar rats, aged 3 months old, were purchased from the Animal Centre of Beijing Vitra Liver, Beijing, China (license No. SCXK (Jing) 20120001). All rats were housed at 23 ± 2°C and humidity of 55 ± 5% with a regular 12-hour light-dark cycle and allowed free access to water and food. All efforts were made to minimize the suffering and number of animals used according to the Guide for the Care and Use of Laboratory Animals of Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China.
2-VO model establishment
The 2-VO rat model was established by bilateral common carotid artery occlusion according to a previous study (He et al., 2012). In brief, after overnight fasting with free access to tap water, the rats were anesthetized with 10% chloral hydrate. The carotid arteries were separated carefully from the vagus nerve and then doubly ligated via two 5-0 silk sutures. Another group of rats that received the same operation without ligation served as the sham control. Three weeks later, rats that underwent the occlusion were screened using the Morris water maze from day 21 to day 25 to remove those that had an unsuccessful surgery.
XXM extract intervention
After screening the 2-VO model, the successful 2-VO rats were randomly divided into different groups (10 rats per group): 2-VO group treated with saline solution (1 mL/100 g), and 2-VO rats treated with XXM 50 or 150 mg/kg. The sham rats were treated with saline (1 mL/100 g). Daily oral administration of XXM or vehicle lasted for 4 weeks from day 26 to day 52 after surgery.
Morris water maze test for memory
Learning and memory testing (10 rats per group) was performed from day 53 to day 57 after surgery with the Morris water maze according to previous methods with modification (Jing et al., 2015b). A circular black tank (diameter 2 m, height 60 cm) was filled with water up to 30 cm (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College). A black escape platform was used as a rescue island for the rats. Video tracking software was used to record and analyze swimming time, distance and speed of the rats. For the water navigation task, rats first received swimming training on day 1. For the following four consecutive days, rats received a learning trial to escape the water by finding an invisible platform 1.5 cm under water. On day 6, rats received a probe trial in which the platform was removed within 60 seconds of the last learning trial. The escape latency in the learning trial and the travel path in the probe trial were monitored and analyzed by a video camera linked to an animal behavioral recording system.
Prehensile test for motor balance ability
Motor ability (10 rats per group) was performed with the prehensile test (Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College) on day 53 after surgery to quantitatively evaluate the motor function of the 2-VO model rats (Seo et al., 2010). Briefly, the forepaws of a rat were placed on a steel rope, 70 cm long and 5 mm in diameter. The time spent on the rope was recorded. Rats were scored according to the following criteria: animal fell within 10 seconds from start on the rope, 0 points; paws hung on the rope for 1 minute, 1 point; tried to climb up the nylon rope, 2 points; at least one hind paw hanging on the line, 3 points; limbs and tail around the rope, 4 points; attempted to escape at the end of the horizontal part, 5 points.
Detection of coordination ability using the inclined screen test
Motor ability (10 rats per group) was evaluated using an inclined screen test according to previous methods with modification (Yonemori et al., 1998; Yan et al., 2013). In brief, rats were placed on a board (25 cm × 15 cm) with an 85° angle, and the maintenance time of the animal on the inclined plate was recorded. Animals were scored according to the following criteria: time on the board was 3 seconds or less, 0 points; 4–10 seconds, 1 point; 11–20 seconds, 2 points; 21–30 seconds, 3 points; and more than 30 seconds, 4 points. The test was ended at 2 minutes.
Neuronal plasticity detection by immunofluorescent staining with microtubule-associated protein 2 (MAP2) antibody
After behavioral testing, three rats from each group were anesthetized with 10% chloral hydrate and perfused with 4% paraformaldehyde for immunohistochemistry. The brain tissues were fixed, dehydrated, embedded, and sliced (He et al., 2012). Immunohistochemistry was performed with an anti-MAP2 antibody (mouse monoclonal antibody; Abcam, Cambridge, UK). Briefly, sections were boiled to retrieve antigen, and nonspecific binding was blocked with 5% normal goat serum in phosphate-buffered saline at room temperature for 2 hours. Sections were then incubated with MAP2 antibody (1:200). After washing, the sections were incubated with Cy3-conjugated secondary antibody (goat to mouse monoclonal antibody; 1:1000; Abcam) at 37°C for 2 hours. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI) for 10 minutes at room temperature. Sections were observed and photographed under a fluorescence microscope (BX41; Olympus, Tokyo, Japan).
Hippocampal tissue preparation for label-free proteomics
Hippocampal tissue was lysed with tissue lysis solution containing a protease inhibitor cocktail on ice for 1 hour, and centrifuged at 12,000 × g for 20 minutes. The supernatant was extracted and protein concentration was determined by bicinchoninic acid assay. Protein (200 μg) for each sample was digested according to the filter-assisted sample preparation procedure (Xu et al., 2017). Briefly, the detergent, DTT and other low-molecular-weight components were removed using 100 μL UA buffer (8 M Urea, 100 mM Tris-HCl, pH 8.5) by repeated ultrafiltration (Millipore Corporation, Billerica, MA, USA), facilitated by centrifugation at 14,000 × g for 15 minutes. Then, 100 μL of 0.1 M iodoacetamide in urea-acetate buffer was added to block reduced cysteine residues, and the samples were incubated for 30 minutes in darkness at room temperature. The filter was washed with 100 μL urea-acetate buffer three times and then with 100 μL 50 mM NH4HCO3 twice. Finally, the protein suspension was digested with trypsin overnight at 37°C. The resulting peptides were collected as a filtrate. Then, the samples were speed-vacuum dried and re-suspended in 0.1% formic acid prior to liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) analyses.
LC-MS/MS analysis
Analysis of samples was performed on a nano-LC-ESI-LTQ-Orbitrap MS/MS system (Thermo Fisher Scientific, Waltham, MA, USA) in Health Science Center, Peking University, Beijing, China. The samples were online-purified using Acclaim PepMap100 C18 cartridge (C18-A1, 100 μm I.D.×20 mm, 5 μm). The purified samples were then separated using Acclaim PepMap100 C18 capillary column (C18-A2, 75 μm I.D.×100 mm, 3 μm). The separation of the digests was achieved at 300 nL/min flow rate, using the gradient elution of 0.1% formic acid with a concentration of between 2% and 40% acetonitrile for 60 minutes. Nano spray Flex ion source voltage was maintained at 2.2 kV; capillary temperature was set to 250°C; rail operation used data dependent mode of hydrazine; scanning range was m/z 350–2000; and the resolution was set to 60,000 (m/z 400). After the completion of the full scan, LTQ was used to meet the MS/MS conditions at the intensity of the top 15 ions by collision induced ionization (collision induced dissociation, CID) for further analysis. The LC-MS/MS was repeated three times in each group to ensure the accuracy of the experiment, and the results of the mass spectra of the three samples were combined as the mass spectra of the samples.
Protein identification and quantitation
The LC-MS/MS data were analyzed using MaxQuant software (http://www.coxdocs.org/doku.php?id=maxquant:start), where the quantitation of proteins was based on ion intensity and spectral count (Luber et al., 2010). In the MaxQuant analysis for protein identification, MS/MS data were submitted to the Uniprot human protein database (https://www.uniprot.org) using the Andromeda search engine. The statistical analysis was also carried out in the MaxQuant software. P-values with the means of the two groups were calculated. Fold of change FDR of 1.2 fold (up or down) and P-value of 0.05 were used as a joint threshold to define biologically significant differences in protein expression. The evaluation of protein-protein interactions was analyzed using the STRING knowledge database (https://string-db.org/cgi/input.pl).
Validation of proteomic results by real time-polymerase chain reaction (RT-PCR) assay
To confirm changes in protein expression, RT-PCR was performed. Total RNA of hippocampal tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed using an RT-PCR kit (Invitrogen). The primer of MT-ND1 was: F 5′-TCC TAA CAC TCC TAA TCC CAA T-3′, R 5′-GCC TTT GCG TAG TTG TAT GTA G-3′. The GAPDH gene was used as an endogenous control for normalization. The primer of GAPDH was: F 5′-GCT CTC TGC TCC TCC CTG TTC TA-3′, R 5′-TGG TAA CCA GGC GTC CGA TA-3′. Relative quantification was performed using the SYBR Green RT-PCR reagent (Invitrogen).
Validation of proteomic results by western blot assay
Hippocampal tissues were stored at −80°C until analysis. The hippocampal tissues were lysed with radioimmune precipitation assay buffer in an ice bath for 30 minutes, and centrifuged at 12,000 r/min for 10 minutes. The supernatant was collected. The samples were mixed with loading buffer and heated at 95°C for 10 minutes to denature proteins. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 5% bovine serum albumin in Tris-buffered saline solution containing Tween for 1 hour and then incubated with anti-MT-ND1 (rabbit, 1:1000; Abcam) and anti-β-actin antibody (rabbit, 1:2000; Abcam) at 4°C overnight. After being washed, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (goat, 1:5000; Abcam) for 2 hours at room temperature followed by washing, and proteins were detected using an enhanced chemiluminescence system. The signal densities on the blots were normalized using an internal control (optical density detected protein/optical density internal control).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.02 (GraphPad Software Inc., San Diego, CA, USA). Results were expressed as the mean ± SD. The data were analyzed using one-way analysis of variance followed by paired t-test (for comparing two value sets). P < 0.05 was considered statistically significant.
Results
XXM extract reduces 2-VO-induced learning and memory deficits in rats
Learning and memory ability was assessed using the Morris water test. During learning trials, as shown in Figure 1A, 2-VO model rats exhibited significantly longer escape latency compared with that of the sham rats from day 3 to day 5 (day 3, P < 0.05; day 4, P < 0.01; day 5, P < 0.01). XXM extract treatment shortened the escape latency in 2-VO rats. On day 4, XXM extract 150 mg/kg significantly decreased the latency compared with that of the 2-VO group (P < 0.05). On day 5, XXM extract 50 and 150 mg/kg both significantly decreased the latency compared with that of the 2-VO group (both P < 0.01). In the probe trial test on the last day, as shown in Figure 1B, XXM extract 50 and 150 mg/kg both significantly decreased the latency compared with that of the 2-VO group (both P < 0.01). As shown in Figure 1C, the number of crossings over the platform area in rats with XXM extract 150 mg/kg treatment was significantly increased compared with that of 2-VO rats (P < 0.01). There were no significant differences in the speed of swimming among the groups (P > 0.05; Figure 1D).
Figure 1.
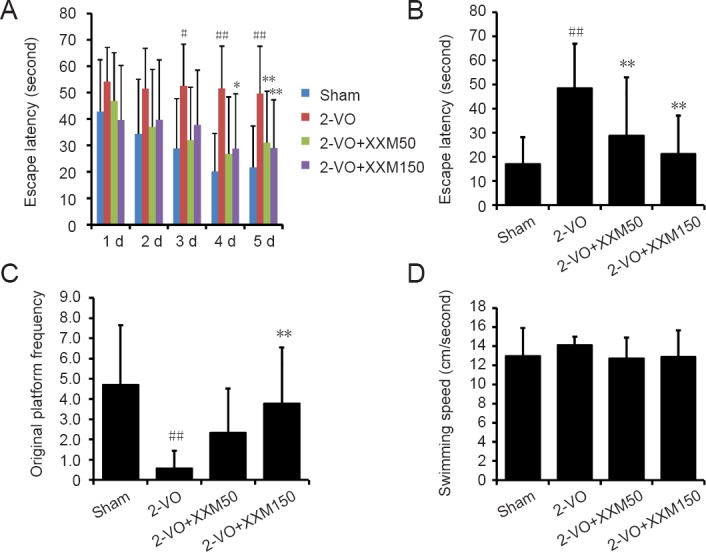
Changes in memory behavior using Morris water test in 2-VO rats after XXM extract intervention.
(A) Escape latencies in the place navigation test. (B) Escape latency during the probe test. (C) Number of crossings over the place where the platform had been hidden during the probe test. (D) Swimming speed during the probe test. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by t-test). #P < 0.05, ##P < 0.01, vs. sham group; *P < 0.05, **P < 0.01, vs. 2-VO group. 2-VO: Two-vessel occlusion; XXM: Xiao-Xu-Ming decoction; D: day.
XXM extract reduces 2-VO-induced motor balance and coordination ability deficits in rats
Changes in prehensile traction ability were tested by prehensile ability test and oblique board test. In the prehensile ability test, as shown in Figure 2A, the prehensile ability score in 2-VO rats was significantly decreased compared with that of sham rats (P < 0.05). XXM extract 150 mg/kg treatment significantly alleviated 2-VO-induced motor balance and coordination ability deficits in rats (P < 0.01; Figure 2A). In addition, in the oblique board test, the score was significantly decreased in 2-VO rats compared with sham rats (P < 0.05), and XXM extract 150 mg/kg treatment significantly alleviated 2-VO-induced motor ability deficits (P < 0.01; Figure 2B).
Figure 2.
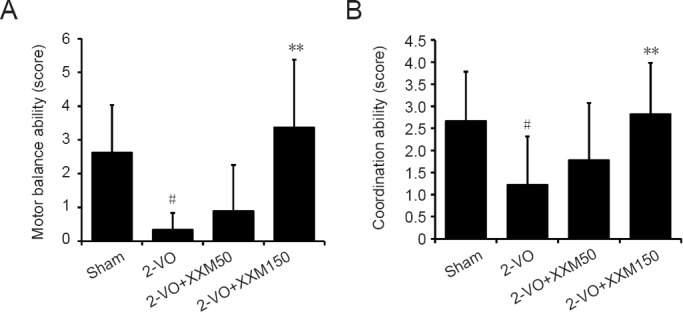
Changes in motor ability using prehensile test and inclined oblique screen test in 2-VO rats after XXM extract intervention.
(A) Motor balance ability by prehensile test; (B) Coordination ability by inclined oblique screen test. Data are expressed as the mean ± SD (n = 10; one-way analysis of variance followed by t-test). #P < 0.05, vs. sham group; **P < 0.01, vs. 2-VO group. 2-VO: Two-vessel occlusion; XXM: Xiao-Xu-Ming decoction.
XXM extract alleviates 2-VO-induced neuronal plasticity injury in the hippocampal CA1 region in rats
Representative photomicrographs of the MAP2 staining results for each group are shown in Figure 3. In the sham group, the neurons in the CA1 region were normal and did not show any cell damage. However, the MAP2 protein of surviving neurons in the 2-VO group was markedly decreased compared with that in the sham rats. Obvious recovery of neuron loss was observed in rats treated with XXM extract 150 mg/kg.
Figure 3.
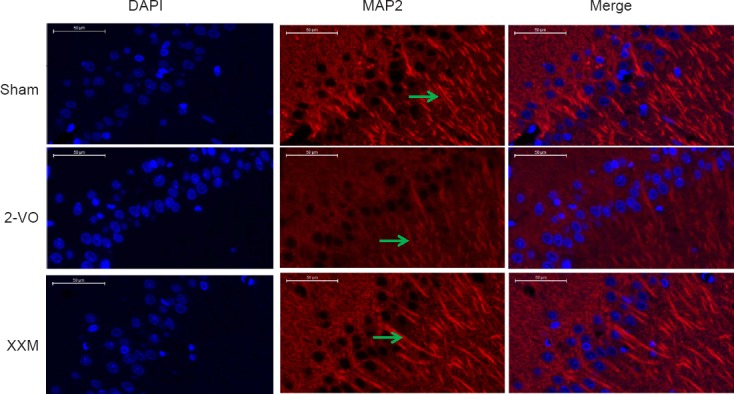
MAP2 immunohistochemistry staining in hippocampal CA1 region of 2-VO rats after XXM extract treatment.
MAP2 protein of surviving neurons in the 2-VO group was markedly decreased compared with the sham rats. Obvious recovery of neuron loss was observed in rats treated with XXM 150 mg/kg. Arrows point to the MAP2-immunoreative cells. Scale bars: 50 μm. Original magnification: 100×. 2-VO: Two-vessel occlusion; XXM: Xiao-Xu-Ming decoction; MAP2: microtubule-associated protein 2; DAPI: 4′,6-diamidino-2-phenylindole.
Differentially expressed protein analysis in the hippocampus
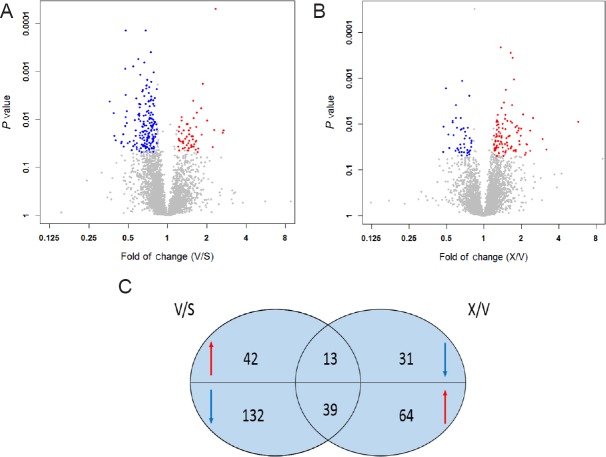
Data matrices of protein intensities were obtained by preprocessing LC-MS/MS based on ion intensity and spectral count. In the MaxQuant analysis, compared with sham group, 55 proteins were upregulated and 171 proteins were downregulated in the 2-VO group (Figure 4A). Compared with the 2-VO group, 102 proteins were upregulated and 44 proteins were downregulated in the XXM-treated group (Figure 4B). Furthermore, comprehensive analysis found that 52 proteins were regulated in 2-VO rats after treatment with XXM extract. Of these 52 proteins, XXM extract significantly decreased 13 2-VO-induced upregulated proteins and increased 39 2-VO-induced downregulated proteins (Figure 5).
Figure 4.
Volcano plot displaying different proteins in 2-VO rat hippocampus with XXM extract treatment.
Scatter plots of fold change (x-axis) against log P-value (y-axis) of all quantified proteins. Up- and downregulated proteins are colored red and blue, respectively. (A) Volcano plot displaying difference in proteins between 2-VO group and sham group. (B) Volcano plot displaying difference in proteins between XXM treatment group and 2-VO group. (C) Comprehensive analysis of differentially expressed proteins following treatment with XXM extract in 2-VO rats. S: Sham group; V: 2-VO group; X: XXM group. 2-VO: Two-vessel occlusion; XXM: Xiao-Xu-Ming decoction.
Figure 5.
Differentially expressed protein profiling using a label-free quantitative proteomic approach.
Gene names are listed on the right, and animal groups are indicated on bottom. The color key indicates the expression level (red indicates upregulation; green indicates downregulation).
Categorization of differentially expressed proteins based on gene ontological and signaling pathway enrichment analysis
To further understand the protein changes, 52 differentially expressed proteins were analyzed in terms of ontology categories and signaling pathways. Gene ontology (GO) categories included “Biological Process (BP)”, “Molecular Function (MF)”, and “Cellular Component (CC)” based on their annotation in GO domain. The BP analysis showed that XXM extract-regulated proteins were involved mainly in oxidation reduction process, intracellular signaling cascade process, and protein catabolic processes (Figure 6A). The MF analysis showed that XXM extract-regulated proteins were involved mainly in ion binding proteins, cation binding proteins, and metal ion binding proteins (Figure 6B). The CC analysis showed that XXM extract-regulated proteins were associated mainly with cell fraction proteins, mitochondrial proteins, and cytosol proteins (Figure 6C). The signaling pathways regulated by XXM extract were involved mainly in ubiquitin-mediated proteolysis and the phosphatidylinositol signaling system (Figure 6D).
Figure 6.
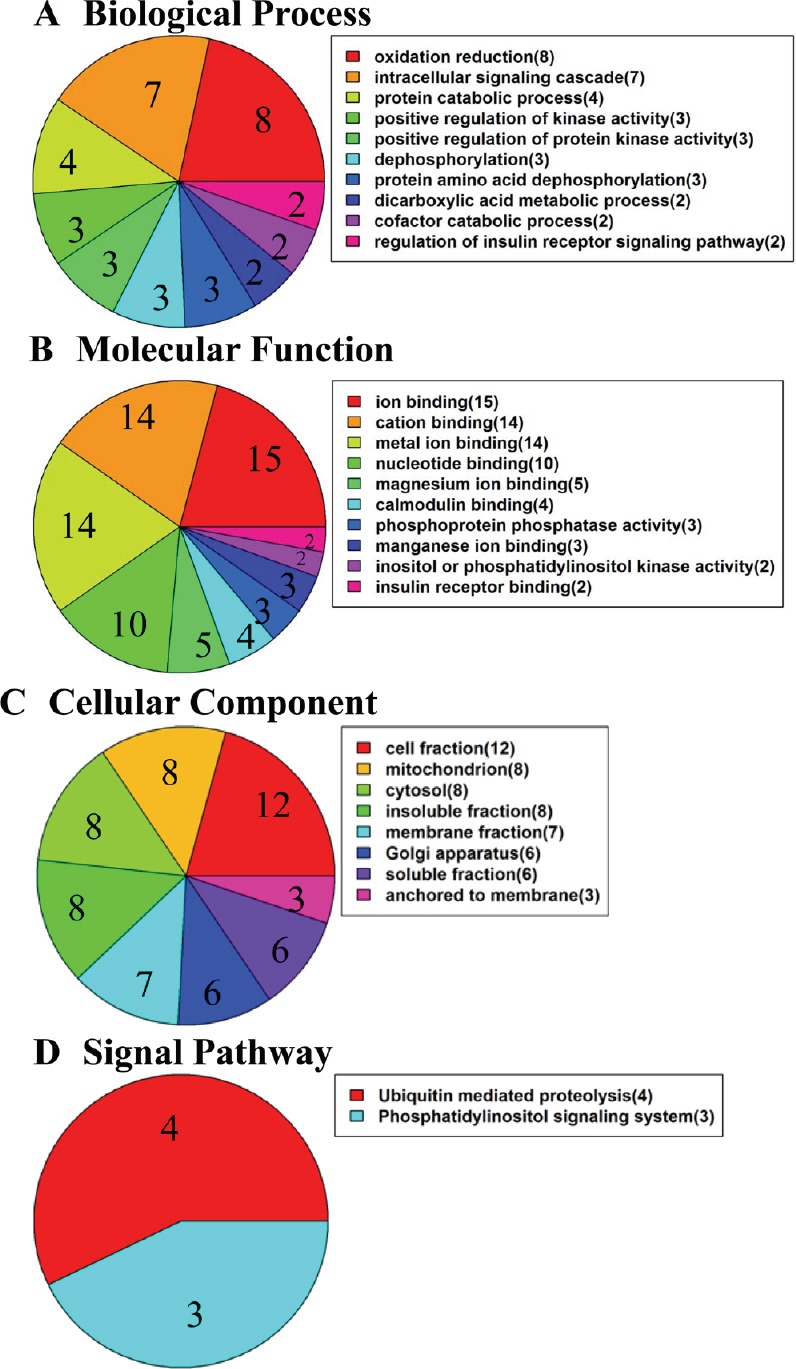
Gene ontology analyses of differentially regulated proteins.
(A) Biological Process-based analysis. (B) Molecular Function-based analysis. (C) Cellular Component-based analysis. (D) Signaling pathway analysis.
Protein-protein interactions
Protein-protein interactions were analyzed using the STRING knowledge database. The analysis indicated close connectivity proteins including Rab2a, Ptpn1, Ppm1e, Cdk18, Gorasp2, Eps15, Capza2, Syngap1, and Mt-nd1 (Figure 7). Remarkably, proteome profiles revealed several types of proteins that participate in the physiological process of chronic cerebral ischemia.
Figure 7.
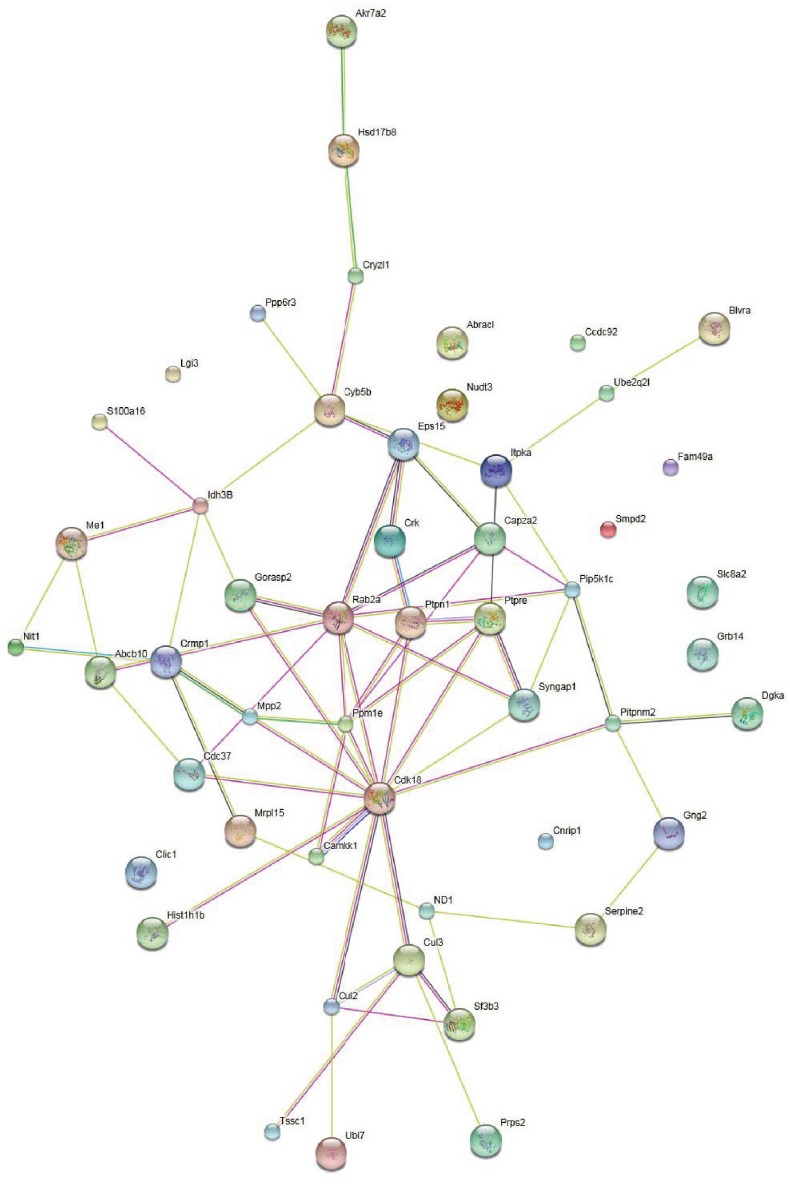
Interactions of the differentially expressed proteins through network analysis using String database.
Network nodes represent proteins (each node represents all the proteins produced by a single, protein-coding gene locus). Edges represent protein-protein associations. Colored nodes: query proteins and first shell of interactions. White nodes: second shell of interactions. Empty nodes: proteins of unknown three-dimensional structure. Filled nodes: Some three-dimensional structure is known or predicted.
Validation of proteomic results
MT-ND1, identified as differentially expressed regulated by XXM extract in 2-VO rats, was validated using quantitative RT-PCR and western blot assay. At the mRNA level, compared with the sham group, the mRNA expression of Mt-nd1 was significantly decreased in the 2-VO group (P < 0.01), and XXM extract treatment increased the mRNA level of Mt-nd1 (P < 0.05; Figure 8A). The protein expression of MT-ND1 was significantly downregulated in the 2-VO group compared with the sham group (P < 0.01; Figure 8B), which is consistent with the proteomic screen results. XXM extract treatment upregulated the protein expression of MT-ND1 (P < 0.05) compared with 2-VO rats, which is also consistent with the proteomic screen results.
Figure 8.
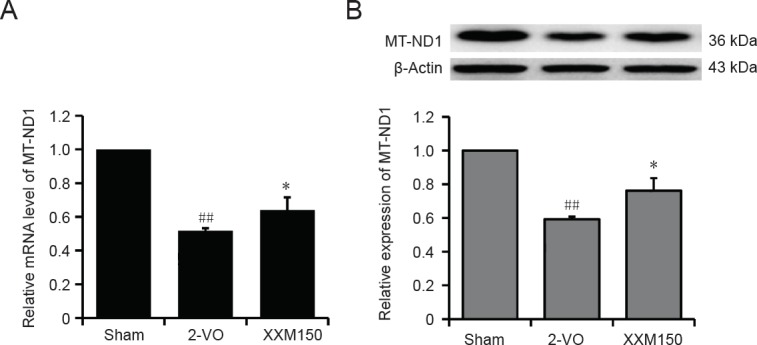
Identification of differentially expressed proteins MT-ND1 in hippocampal tissue of 2-VO rats.
(A) Verification of the differentially expressed proteins by quantitative real time-polymerase chain reaction. Results are expressed as the ratio to the sham group. (B) Verification of the differentially expressed proteins using western blot assay. The results are expressed as the ratio of relative protein expression (optical density ratio of target protein/β-actin) to the sham group. Data are expressed as the mean ± SD (n = 3; one-way analysis of variance followed by t-test). ##P < 0.01, vs. sham group; *P < 0.05, vs. 2-VO group. 2-VO: Two-vessel occlusion; XXM: Xiao-Xu-Ming decoction.
Discussion
Label-free quantitative proteomics is an important quantitative mass spectrometry method developed in recent years. By comparing the signal intensity of peptide segments in different samples, the corresponding protein is quantified, which is mainly used for differential protein screening in different groups. The technology does not require expensive isotopic labels as internal standards to improve the detection efficiency of low abundance proteins. However, different response signals and the difficulty of internal peptide synthesis remain as limitations for the absolute quantification of protein (Keilhauer et al., 2015).
XXM is a well-known traditional herbal prescription for treatment of stroke or stroke sequela. In recent years, a growing number of studies have demonstrated that XXM extract protects against cerebral ischemic injury, which may be mediated by preserving mitochondrial integrity, reducing apoptosis, and alleviating blood-brain barrier disruption (Lan et al., 2013, 2014). Our previous studies (Wang et al., 2012a, c) have also demonstrated that XXM can attenuate the impairment of learning and memory, dysfunction of cerebrovascular reactivity and mitochondrial function induced by chronic cerebral ischemic in rats.
Vascular dementia is a common type of dementia in aged people, and is caused by problems in the supply of blood to the brain, typically by a series of minor strokes (Battistin and Cagnin, 2010; Zhang, 2016; Zhu et al., 2018). Vascular dementia patients suffer from forgetfulness, depression and anxiety, disorientation, and loss of executive functions (Venkat et al., 2015). The insufficient blood supply to the brain leads to worsening cognitive decline that occurs step by step (Sandu et al., 2015). Restorative strategies after stroke are focused on the remodeling of cerebral endothelial cells, neurons, neural precursor cells and glial cells (Hermann et al., 2015; Chen et al., 2018). Therefore, understanding progressive loss of functional neuronal networks becomes essential to develop efficient drugs to prevent cognitive decline in the elderly (Popa-Wagner et al., 2015). Our previous study has demonstrated that 2-VO leads to neuronal loss and neuroinflammation in the cortex and hippocampus, and causes impairments of learning and memory and dysfunction of motor ability in rats. In this study, our results showed that 2-VO induced dysfunction of learning and memory ability, and motor balance and coordination deficits in rats. XXM extract improved these 2-VO-induced deficits in rats. These results suggest that XXM extract improves the cognitive and motor symptoms in the stroke rat model, which is consistent with the classical prescription of XXM for stroke treatment.
Cerebral ischemia leads to delayed neuronal death in the hippocampal CA1 region (Danielisová et al., 2009). The cognitive deficits caused by ischemia have been shown to be strongly correlated with neuronal plasticity in the hippocampal CA1 region. MAP2, a cytoskeletal phosphor protein, is mainly associated with microtubules and neuronal plasticity (Liu et al., 2005). Therefore, in this study, neuroprotection was evaluated by MAP2-labeled immunofluorescence. The results showed that the MAP2 protein of surviving neurons in the 2-VO group was markedly decreased compared with that in the sham rats. Obvious recovery of neuronal loss was observed in rats treated with XXM extract.
Chronic cerebral hypoperfusion causes learning and memory impairments and increases the risk of Alzheimer’s disease and vascular dementia through several biologically plausible pathways, yet the mechanisms underlying the disease process and differentiated protein profile remain unclear. The hippocampus is essential for memory and is particularly vulnerable to ischemia-reperfusion (Sugawara et al., 2002; Buzsáki and Moser, 2013). Chronic cerebral ischemia often leads to delayed neuronal death and cognitive deficits, which have been shown to be strongly correlated with neuronal plasticity in the hippocampal CA1 region (Zuloaga et al., 2015). Thus, in this study, the global protein expression profile in the hippocampus of rats was investigated using label-free quantitative proteomics to explore the molecular events associated with chronic cerebral hypoperfusion and the modulation of XXM extract. Proteomics is a technique that allows for the examination of all expressed proteins under different conditions, making it an ideal tool for gathering information on both the level of individual proteins and their interplay in complexes, network modules and signaling pathways associated with specific biological functions (Walther and Mann, 2010). High-throughput experimental platforms are usually used in proteomics studies. LC-MS/MS can measure the abundance of peptides in given biological conditions by determining thousands of proteins from biological samples simultaneously (Aebersold and Mann, 2003; Altelaar et al., 2013). Label-free quantitative proteomics is growing rapidly because it is more reliable, versatile and cost-effective than labelled quantitation (Zhong et al., 2016). The untargeted proteomic analysis of the hippocampus to identify candidate proteins was performed with statistically significant differences after XXM extract treatment in 2-VO rats. The results showed that 52 proteins were differentially expressed following treatment with XXM extract in 2-VO rats. The GO analysis indicated that differentially expressed proteins in XXM extract-regulated proteins were associated with diverse molecular functions and biological processes. The Biological Processes included mainly oxidation reduction process, intracellular signaling cascade process, and protein catabolic processes. These results were in accordance with our previous investigation, which demonstrated that XXM extract protects brain mitochondrial homeostasis and improves mitochondrial function in rats with chronic cerebral ischemia (Wang et al., 2012c). The Molecular Functions of the proteins included mainly ion binding proteins, cation binding proteins, and metal ion binding proteins. The Cellular Components included mainly cell fraction proteins, mitochondria proteins, and cytosol proteins. The signaling pathways were involved mainly in ubiquitin mediated proteolysis and the phosphatidylinositol signaling system. To evaluate the protein-protein interactions, the STRING knowledge database was analyzed, and close connectivity proteins were identified including Rab2a, Ptpn1, Ppm1e, Cdk18, Gorasp2, Eps15, Capza2 and Syngap1, and Mt-nd1. Remarkably, proteome profiles revealed several types of proteins that participated in the physiological process of chronic cerebral ischemia. The results from this study demonstrate the power of combining untargeted and targeted quantitation methods for a comprehensive proteomic analysis to evaluate changes in protein levels.
Mitochondria are the main source of energy to sustain cellular metabolism and integrity. The decrease in oxygen supply during ischemia impairs energy production by these organelles. Mitochondrial membrane respiratory chain NADH dehydrogenase (Complex I) is the largest of the five multi-subunit complexes constituting the human oxidative phosphorylation system to transfer electrons from NADH to the respiratory chain (Gorman et al., 2015). Hippocampal mitochondrial dysfunction has been considered to play a major role in the pathogenesis of vascular dementia. Complex I is made up of 44 different subunits, including one subunit that is found twice, for a total of 45 subunits (Lim et al., 2016). Seven of these subunits (ND1, ND2, ND3, ND4, ND4L, ND5, and ND6) are encoded by mitochondrial DNA and are found within the hydrophobic membrane arm where they form the core proton translocation machinery of the complex. MT-ND1, the core subunit of Complex I, plays an important structural role, as it is believed to form part of the ubiquinone binding pocket at the interface between the membrane and matrix arms, which is believed to belong to the minimal assembly required for catalysis (Efremov et al., 2010). In the present study, the quantitative proteomics results showed that XXM treatment upregulated MT-ND1 expression in 2-VO rats. Consistent with this finding, the RT-PCR and western blot assays also revealed that MT-ND1 expression levels were upregulated after XXM extract treatment in 2-VO rats. Our previous investigation has found that XXM might have therapeutic potential for the treatment of dementia caused by chronic cerebral hypoperfusion because of its protective effect on brain mitochondrial homeostasis and function. It is concluded that the mitochondrial protection of XXM extract could be associated with the upregulated protein expression of MT-ND1 in chronic cerebral hypoperfusion.
In summary, the present study indicated that XXM extract could protect against 2-VO-induced chronic cerebral injury in rats by modulating multiple proteins, which could suggest potential targets for the development of treatments for vascular dementia.
Additional file: Open peer review reports 1 (61.8KB, pdf) , 2 (61.8KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing interests.
Financial support: This study was supported in part by the National Natural Science Foundation of China, No. 81473383 (to YHW); the Significant New-Drugs Creation of Science and Technology Major Projects in China, No. 2018ZX09711001-003-019 (to YHW); the Innovation Fund for Graduate of Beijing Union Medical College of China, No. 2017-1007-02 (to XC). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Care and Use Committee at Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Chao Jiang, Weapon Industry Health Research Institute, China; Aurel Popa-Wagner, University Medicine Rostock, Germany.
Funding: This study was supported in part by the National Natural Science Foundation of China, No. 81473383 (to YHW); the Significant New-Drugs Creation of Science and Technology Major Projects in China, No. 2018ZX09711001-003-019 (to YHW); the Innovation Fund for Graduate of Beijing Union Medical College of China, No. 2017-1007-02 (to XC).
P-Reviewers: Jiang C, Popa-Wagner A; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: MoCollum L, Norman C, Qiu Y, Song LP; T-Editor: Liu XL
References
- 1.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 2.Altelaar AF, Munoz J, Heck AJ. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet. 2013;14:35–48. doi: 10.1038/nrg3356. [DOI] [PubMed] [Google Scholar]
- 3.Ang CS, Baker MS, Nice EC. Mass spectrometry-based analysis for the discovery and validation of potential colorectal cancer stool biomarkers. Methods Enzymol. 2017;586:247–274. doi: 10.1016/bs.mie.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Battistin L, Cagnin A. Vascular cognitive disorder. A biological and clinical overview. Neurochem Res. 2010;35:1933–1938. doi: 10.1007/s11064-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 5.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen DP, Hou SH, Guo Y, Hu ZZ, Hu XH, Chen YG, Chen MS. Human placenta-derived mesenchymal stem cell transplantation provides neuroprotection against cerebral infarction in rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2018;22:65–69. [Google Scholar]
- 7.Danielisová V, Gottlieb M, Némethová M, Kravčuková P, Domoráková I, Mechírová E, Burda J. Bradykinin postconditioning protects pyramidal ca1 neurons against delayed neuronal death in rat hippocampus. Cell Mol Neurobiol. 2009;29:871–878. doi: 10.1007/s10571-009-9369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du K, Wu C, Ding C, Zhao S, Qin H, Zhang J. Simultaneous LC–MS Analysis and of wogonin and oroxylin a in rat plasma, and pharmacokinetic studies after administration of the active fraction from Xiao-Xu-Ming Decoction. Chromatographia. 2009;69:1259. [Google Scholar]
- 9.Efremov RG, Baradaran R, Sazanov LA. The architecture of respiratory complex I. Nature. 2010;465:441–445. doi: 10.1038/nature09066. [DOI] [PubMed] [Google Scholar]
- 10.Gorman GS, Blakely EL, Hornig-Do HT, Tuppen HA, Greaves LC, He L, Baker A, Falkous G, Newman J, Trenell MI, Lecky B, Petty RK, Turnbull DM, McFarland R, Taylor RW. Novel MTND1 mutations cause isolated exercise intolerance, complex I deficiency and increased assembly factor expression. Clin Sci (Lond) 2015;128:895–904. doi: 10.1042/CS20140705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He XL, Wang YH, Bi MG, Du GH. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur J Pharmacol. 2012;680:41–48. doi: 10.1016/j.ejphar.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Hermann DM, Buga AM, Popa-Wagner A. Neurovascular remodeling in the aged ischemic brain. J Neural Transm (Vienna) 2015;122(Suppl 1):S25–33. doi: 10.1007/s00702-013-1148-0. [DOI] [PubMed] [Google Scholar]
- 13.Ihara M, Taguchi A, Maki T, Washida K, Tomimoto H. A mouse model of chronic cerebral hypoperfusion characterizing features of vascular cognitive impairment. Methods Mol Biol. 2014;1135:95–102. doi: 10.1007/978-1-4939-0320-7_8. [DOI] [PubMed] [Google Scholar]
- 14.Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, Li W, Ruan Y, Huang L. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015a;35:1249–1259. doi: 10.1038/jcbfm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jing Z, Shi C, Zhu L, Xiang Y, Chen P, Xiong Z, Li W, Ruan Y, Huang La. Chronic cerebral hypoperfusion induces vascular plasticity and hemodynamics but also neuronal degeneration and cognitive impairment. J Cereb Blood Flow Metab. 2015b;35:1249–1259. doi: 10.1038/jcbfm.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keilhauer EC, Hein MY, Mann M. Accurate protein complex retrieval by affinity enrichment mass spectrometry (AE-MS) rather than affinity purification mass spectrometry (AP-MS) Mol Cell Proteomics. 2015;14:120–135. doi: 10.1074/mcp.M114.041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan R, Xiang J, Wang GH, Li WW, Zhang W, Xu LL, Cai DF. Xiao-Xu-Ming Decoction protects against blood-brain barrier disruption and neurological injury induced by cerebral ischemia and reperfusion in rats. Evid Based Complement Alternat Med. 2013;2013:629782. doi: 10.1155/2013/629782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan R, Zhang Y, Xiang J, Zhang W, Wang GH, Li WW, Xu LL, Cai DF. Xiao-Xu-Ming decoction preserves mitochondrial integrity and reduces apoptosis after focal cerebral ischemia and reperfusion via the mitochondrial p53 pathway. J Ethnopharmacol. 2014;151:307–316. doi: 10.1016/j.jep.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 19.Lim SC, Hroudová J, Van Bergen NJ, Lopez Sanchez MI, Trounce IA, McKenzie M. Loss of mitochondrial DNA-encoded protein ND1 results in disruption of complex I biogenesis during early stages of assembly. FASEB J. 2016;30:2236–2248. doi: 10.1096/fj.201500137R. [DOI] [PubMed] [Google Scholar]
- 20.Liu HX, Zhang JJ, Zheng P, Zhang Y. Altered expression of MAP-2, GAP-43, and synaptophysin in the hippocampus of rats with chronic cerebral hypoperfusion correlates with cognitive impairment. Brain Res Mol Brain Res. 2005;139:169–177. doi: 10.1016/j.molbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, Akira S, Wiegand M, Hochrein H, O’Keeffe M, Mann M. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32:279–289. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Popa-Wagner A, Buga AM, Popescu B, Muresanu D. Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J Neural Transm (Vienna) 2015;122(Suppl 1):S47–54. doi: 10.1007/s00702-013-1129-3. [DOI] [PubMed] [Google Scholar]
- 23.Sandu RE, Buga AM, Uzoni A, Petcu EB, Popa-Wagner A. Neuroinflammation and comorbidities are frequently ignored factors in CNS pathology. Neural Regen Res. 2015;10:1349–1355. doi: 10.4103/1673-5374.165208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo HG, Kim DY, Park HW, Lee SU, Park SH. Early motor balance and coordination training increased synaptophysin in subcortical regions of the ischemic rat brain. J Korean Med Sci. 2010;25:1638–1645. doi: 10.3346/jkms.2010.25.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugawara Y, Kikuchi T, Ueda T, Nishizaki M, Nakata S, Mochizuki T, Ikezoe J. Usefulness of brain SPECT to evaluate brain tolerance and hemodynamic changes during temporary balloon occlusion test and after permanent carotid occlusion. J Nucl Med. 2002;43:1616–1623. [PubMed] [Google Scholar]
- 26.Tanaka K, Wada-Tanaka N, Miyazaki I, Nomura M, Ogawa N. Chronic cerebral hypoperfusion induces striatal alterations due to the transient increase of NO production and the depression of glutathione content. Neurochem Res. 2002;27:331–336. doi: 10.1023/a:1014967414468. [DOI] [PubMed] [Google Scholar]
- 27.Traditional Chinese Medicine Terminology Committee. Basic Terms of Traditional Chinese Medicine. Beijing: Science Press; 2005. [Google Scholar]
- 28.Tsai TH, Song E, Zhu R, Di Poto C, Wang M, Luo Y, Varghese RS, Tadesse MG, Ziada DH, Desai CS, Shetty K, Mechref Y, Ressom HW. LC-MS/MS-based serum proteomics for identification of candidate biomarkers for hepatocellular carcinoma. Proteomics. 2015;15:2369–2381. doi: 10.1002/pmic.201400364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108. doi: 10.1016/j.expneurol.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walther TC, Mann M. Mass spectrometry-based proteomics in cell biology. J Cell Biol. 2010;190:491–500. doi: 10.1083/jcb.201004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Jia Z, Wang Z, Hu T, Qin H, Du G, Wu C, Zhang J. Pharmacokinetics of 21 active components in focal cerebral ischemic rats after oral administration of the active fraction of Xiao-Xu-Ming decoction. J Pharm Biomed Anal. 2016;122:110–117. doi: 10.1016/j.jpba.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 32.Wang CH, Wu CS, Qin HL, Zhang JL. Rapid discovery and identification of 68 compounds in the active fraction from Xiao-Xu-Ming decoction (XXMD) by HPLC-HRMS and MTSF technique. Chin Chem Lett. 2014;25:1648–1652. [Google Scholar]
- 33.Wang Y, Qin H, He X, Du G. Activity evaluation of components and preparation of effective components group of xiaoxuming decoction for anti-cerebral ischemic. Zhongguo Zhong Yao Za Zhi. 2011;36:2140–2144. [PubMed] [Google Scholar]
- 34.Wang YH, He XL, Yang HG, Qin HL, Du GH. Effects of the active components of Chinese herbal medicine Xiaoxuming Decoction on memory behavior and brain injury in rats with chronic cerebral ischemia. Zhong Xi Yi Jie He Xue Bao. 2012a;10:91–99. doi: 10.3736/jcim20120114. [DOI] [PubMed] [Google Scholar]
- 35.Wang YH, Hem XL, Yang HG, Qin HL, Du GH. Effects of the effective components group of Xiaoxuming Decoction on MCAO rats. Zhongguo Yaoxue Zazhi. 2012b;47:194–198. [Google Scholar]
- 36.Wang YH, He XL, Li XX, Qin HL, Du GH. Effects of the effective component group of Chinese herbal medicine Xiaoxuming Decoction on brain mitochondria in rats with chronic cerebral ischemia. Zhong Xi Yi Jie He Xue Bao. 2012c;10:569–576. doi: 10.3736/jcim20120513. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, Li Z, Wang L, Chen F, Chi Z, Gu M, Li S, Wu D, Miao J, Zhang Y, Hao L, Fan Y. Label-free quantitative proteomics reveals differentially expressed proteins in high risk childhood acute lymphoblastic leukemia. J Proteomics. 2017;150:1–8. doi: 10.1016/j.jprot.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Yan C, Zhang J, Wang S, Xue G, Hou Y. Neuroprotective effects of rutaecarpine on cerebral ischemia reperfusion injury. Neural Regen Res. 2013;8:2030–2038. doi: 10.3969/j.issn.1673-5374.2013.22.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yonemori F, Yamaguchi T, Yamada H, Tamura A. Evaluation of a motor deficit after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1998;18:1099–1106. doi: 10.1097/00004647-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Zhang XA. Cortical mechanism underlying aerobic exercise promoting cognitive function of vascular dementia rats:study protocol for a randomized controlled trial. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:6943–6949. [Google Scholar]
- 41.Zhong L, Zhou J, Chen X, Lou Y, Liu D, Zou X, Yang B, Yin Y, Pan Y. Quantitative proteomics study of the neuroprotective effects of B12 on hydrogen peroxide-induced apoptosis in SH-SY5Y cells. Sci Rep. 2016;6:22635. doi: 10.1038/srep22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu JD, Wang JJ, Zhang XH, Yu Y, Kang ZS. Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen Res. 2018;13:664–672. doi: 10.4103/1673-5374.230292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuloaga KL, Zhang W, Yeiser LA, Stewart B, Kukino A, Nie X, Roese NE, Grafe MR, Pike MM, Raber J, Alkayed NJ. Neurobehavioral and imaging correlates of hippocampal atrophy in a mouse model of vascular cognitive impairment. Transl Stroke Res. 2015;6:390–398. doi: 10.1007/s12975-015-0412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.