Abstract
Insect societies have evolved a series of disease defenses against pathogens, including social sanitary behavior and individual innate immunity. However, whether sanitary behavior can affect individual innate immunity remains unknown. Here, we exposed the termite Reticulitermes chinensis Snyder to the entomopathogenic fungus Metarhizium anisopliae (Metchnikoff) Sorokin(Ascomycota: Hypocreales), and then measured their allogrooming behavior, conidia load, infection mortality, antifungal activity and immune gene expressions . Our results showed that most of the fungal conidia were fast removed from the cuticles of the grouped termites by intensive allogrooming behavior, resulting in low mortality. The antifungal activity and immune gene expressions (termicin and transferrin) in grouped exposed termites were significantly lower than those in single exposed termite but not significantly different from those in unexposed treatments. These results suggest that allogrooming behavior can fast remove fungal conidia from termite cuticles and then decrease their physiological investment in individual innate immunity.
Keywords: termite, entomopathogenic fungus, survival, antifungal activity, immune gene
In a social context, pathogens can employ social interaction networks to transfer from infectious to susceptible individuals (Schmid-Hempel 1998, Cremer et al. 2018). For example, nesting in pathogen-rich environments, high densities, frequent social contact, and close relatives in some social insects can make disease easier to outbreak when compared with solitary insects (Cremer et al. 2007, Rosengaus et al. 2011, Cremer et al. 2018). To counteract these potential threats, social insects evolve many disease defenses at individual and colony levels (Fefferman et al. 2007, Rosengaus et al. 2011, Parker et al. 2012, Mitaka et al. 2016, Cremer et al. 2018).
Colony-level disease defenses were achieved by cooperation of group members, which can quickly react to the potential threats and benefit the whole colony fitness (Cremer et al. 2007, Rosengaus et al. 2011). For example, allogrooming behavior is one particularly important component of the colony-level sanitary behavior and can externally remove pathogens from cuticles and disinfect the cuticles to inhibit pathogen growth during early-stage infection (Yanagawa and Shimizu 2007, Hamilton and Bulmer 2012, Konrad et al. 2018). When pathogens survive from the external disinfection and invade the body cavity of the hosts, they will cause an internal infection that can induce individual innate immunity, along with an upregulation of immune genes and antifungal activity (Thompson et al. 2003, Bulmer and Crozier 2004, Konrad et al. 2012, Liu et al. 2015). However, the question of whether the decreased infection risk mediated by sanitary behavior affects the internal immune response of individuals remains unexplored.
The subterranean termite Reticulitermes chinensis Snyder is an economically important pest in China (Huang et al. 2013, Yin et al. 2013). The entomopathogenic fungus Metarhizium anisopliae (Metchnikoff) Sorokin(Ascomycota: Hypocreales) has been widely used in biological pest control and is an ideal experimental fungus in which to study social insect immunity (Rosengaus et al. 1998, Liu et al. 2015, Konrad et al. 2018). Here, we used the termite workers and the fungal conidia as a host–pathogen system and then analyzed allogrooming behavior, conidia load, infection mortality, antifungal activity, and immune gene expressions to determine whether sanitary behavior affects individual innate immunity.
Materials and Methods
Experimental Workers
Six colonies of the subterranean termite R. chinensis were collected from Wuhan City in Hubei Province, China. Termite workers were reared under laboratory conditions at a temperature of 25 ± 1°C, 80% relative humidity, and 24-h darkness throughout all the tests. Only healthy workers, which were older than third instar and could normally perform behaviors, were selected for the following different experiments.
Fungal Pathogen
Termites were exposed to the entomopathogenic fungus M. anisopliae (strain IBCCM321.93). The fungus was cultivated on potato dextrose agar (QDRS Biotec, China) for 2–4 weeks, and then it was collected with 0.1% Tween 80 to be made into a conidial suspension stored at 4°C for a maximum of 3–4 weeks. Before each experiment, we measured conidial germination and found that all conidial suspensions had a germination rate of >95%. Exposed termites were inoculated on their abdomen’s surface with a 0.35-μl droplet of the conidial suspension using a pipette (0.1–2.5 μl, Eppendorf). Unexposed termites (controls) were treated with 0.35-μl droplet of conidia-free 0.1% Tween 80 instead of conidia suspension. After being exposed to the conidia or the Tween 80, all treated termites were refrigerated at 4°C for an hour to slow their activity and to precipitate the conidia onto their cuticle before being used in the following experiments (Traniello et al. 2002).
Observation of Allogrooming Behavior
Allogrooming behavior is an effective behavioral adaptation to remove conidia from the cuticle of termites. Thus, it is important to determine the changes of this behavioral adaptation between fungus-exposed groups and control groups. For the fungus-exposed groups, two termites were exposed to 5 × 107 conidia/ml conidial suspension. For the control groups, two termites were treated with 0.1% Tween 80. According to the method of Hamilton et al. (2011), a pair of termites was placed in a cell culture dish (3.5 cm in diameter) with a disc of moist filter paper in darkness. Beginning 5 min after cultivation, we video-recorded the events of allogrooming behavior (licking body surface) for a pair of termites by the camera with a nightshot function (HDR-XR550E, SONY) for 30 min. There were nine replicates from three colonies for this experiment.
SEM Observation of Conidia on the Cuticles
Fungus-treated termites were exposed to 5 × 107 conidia/ml suspension. One fungus-treated termite or 20 fungus-treated termites were put in a Petri dish (9.0 cm in diameter) with a disc of moist filter paper. All the petri dishes were kept at 25 ± 1°C and 80% RH in darkness. After 1 or 12 h of M. anisopliae infection, three termites from each treatment were killed with ether fumes and then fixed in 2.5% glutaraldehyde solution at 4 °C. Samples were dehydrated by ethanol method, followed by critical point drying method. The dried samples were fixed on a holder with conductive carbon adhesive tape, followed by coating with platinum. Conidia on the cuticles of the termite samples were observed by using scanning electron microscope (SEM) (JSM-6390, JEOL, Japan). Both unexposed single and unexposed grouped termites after 12-h cultivation were regarded as controls.
Survival
For the mortality assay of exposed single termites, termites were reared individually in Petri dishes (3.5 cm in diameter) with moist filter paper, then each was assigned to one of four treatments: exposed to 0.1% Tween 80 (control), 5 × 103, 5 × 105, and 5 × 107 conidia/ml. Each treatment contained 24 termites from three colonies (eight termites per treatment per colony). Mortality was recorded daily for 7 days and dead termites were removed when recording.
In addition, we compared the infection mortality between single termites and grouped termites under the 5 × 107 conidia/ml treatment. One and twenty termites were exposed and reared in the Petri dishes (9.0 cm in diameter) with moist filter paper for a week. Three colonies were used for this experiment (one exposed termite: 12 termites per colony, 36 termites in total; twenty exposed termites: 20 termites per colony, 60 termites in total). We recorded the number of dead termites every day and the dead termites were removed when recording.
Antifungal Activity
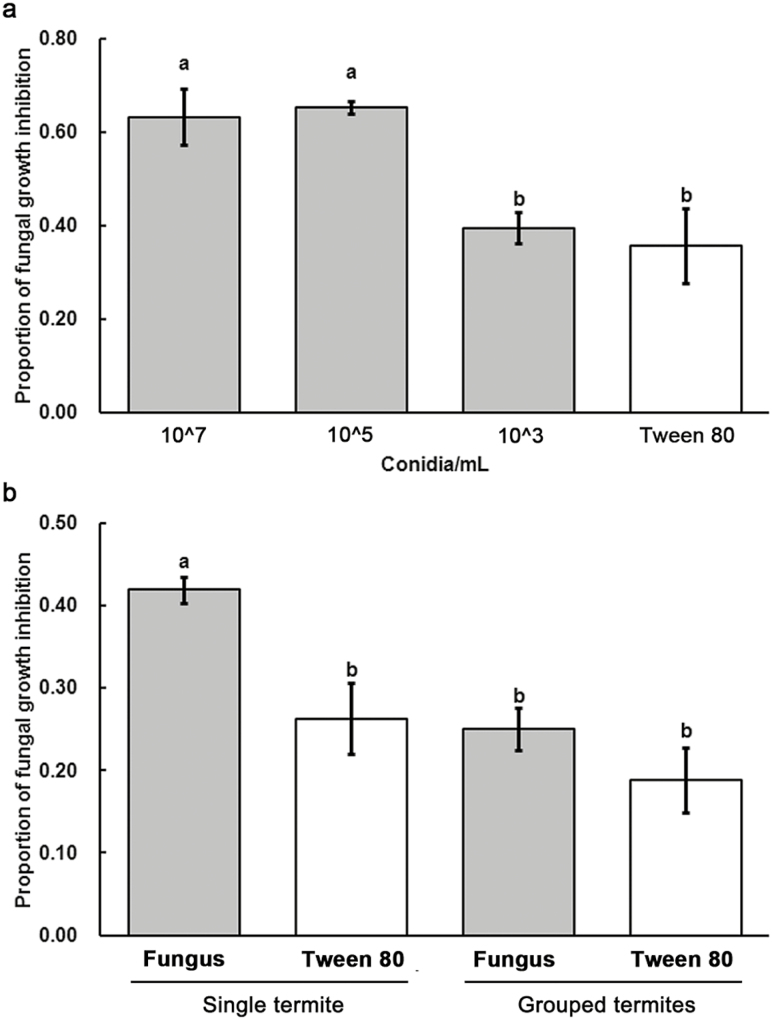
For antifungal activity assay (the ability of tissue fluid to inhibit fungal growth) of exposed single termites, termites were exposed to 0.1 % Tween 80, 5 × 103, 5 × 105, and 5 × 107 conidia/ml, respectively. They were isolated from their nestmates and reared in Petri dishes (3.5 cm in diameter) with moist filter paper. There were five replicates (each replicate containing a pool of five termites) from five colonies for this experiment. The antifungal activity was tested after 3 d of fungal infection. The result showed that 5 × 105 conidia/ml was sufficient to upregulate immunity (Fig. 4a) with lower mortality in single termites when compared with the single termites exposed to 5 × 107 conidia/ml (Fig. 3a). Thus, we chose 5 × 105 conidia/ml for the following test.
Fig. 4.
Antifungal activity of the termites among different treatments. (a) The antifungal activity of single termites exposed to 5 × 107, 5 × 105, 5 × 103 conidia/ml, and 0.1% Tween 80, respectively; (b) The antifungal activity of single and grouped termites exposed to 5 × 105 conidia/ml, and single and grouped termites treated by 0.1% Tween 80. Error bars represent mean ± SEM. Different letters over the bars denote significant differences (P < 0.05, Tukey’s HSD).
Fig. 3.
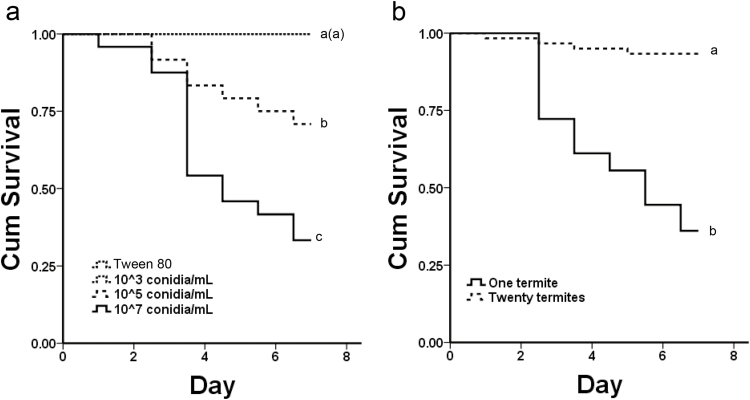
The life span of the termites among different treatments. (a) The life span of the termites exposed to 5 × 107, 5 × 105, 5 × 103 conidia/ml, and 0.1% Tween 80, respectively; (b) the life span of single termites and grouped termites exposed to 5 × 107 conidia/ml. The curves of controls and 5 × 103 conidia/ml treatments largely overlapped and displayed as a single dotted line. Different letters indicated significant differences (P < 0.05, Kaplan-Meier methods).
To measure the antifungal activity of single termites and grouped termites, one and twenty exposed termites were exposed to 5 × 105 conidia/mL, and then reared in Petri dishes (9.0 cm in diameter) with moist filter paper. For the control groups, 1 and 20 unexposed termites were treated with 0.1% Tween 80. There were five replicates (each replicate containing a pool of seven termites) from five colonies for this experiment. The antifungal activity was tested after 3 d of fungal infection. Samples were crushed in 1.5-ml centrifuge tube with liquid nitrogen and then macerated with phosphate buffered saline (PBS) in a proportion of 1 mg of body weight to 5 μl of PBS. About 20 μl supernatants were extracted after centrifugation of the homogenates at 6,000×g for 5 min at 4°C (Thermo Scientific Sorvall Legend Micro 21R). Repeated the protocol and then 10-μl supernatants were extracted and stored at −80°C until the antifungal activity assay; 96-well microplates were used for the antifungal activity assay and each well contained 50-μl potato dextrose (PD), 2-μl blastospores (about 106 spores/ml), and 2-μl supernatants. Additionally, we used 50-μl PD, 2-μl blastospores, 2-μL PBS per well for spores-growth control, 50-μL PD, and 4-μl PBS per well for standards. After about 24-h incubation in constant temperature shaker (200 rpm; 25 ± 1°C), the absorbance of each well was measured by the microplate spectrophotometer (Bio-Rad xMark; 600 nm) (Konrad et al. 2012).
Expression of Immune Genes
To determine immune-gene expressions of single termites and grouped termites, 1 termite and 20 exposed termites were exposed to 5 × 105 conidia/ml and then reared in Petri dishes (9.0 cm in diameter) with moist filter paper. For the control groups, 1 termite and 20 unexposed termites were treated with 0.1% Tween 80. There were three replicates (each replicate containing a pool of seven termites) from three colonies for this experiment. The immune gene expressions were tested after 5 d of fungal infection. Samples were crushed in 1.5-ml centrifuge tube with liquid nitrogen, using sterilized disposable tissue grinding pestles. Total RNA was extracted from the samples using RNAiso Plus according to manufacturer protocol (TaKaRa, Dalian, China). The purity and concentration of the extracted RNA were determined by Thermo NANO DROP 2000 Spectrophotometer. Approximately, 1-μg RNA was treated by PrimeScriptTM RT Reagent Kit with gDNA Eraser (Perfect Real Time; TakaRa). The cDNA products were diluted in a proportion of 1 μl of cDNA to 20 μl of deionized water as templates for the real-time polymerase chain reaction (PCR) of termicin (Bulmer and Crozier 2004), transferrin (Thompson et al. 2003), and β-actin (reference gene) (Hamilton and Bulmer 2012). The quantitative reaction was performed on My IQ 2 Two color Real-Time PCR Detection System (Bio-Rad). Reaction mixtures, containing 10 μl of SYBR Premix Ex Taq II (TaKaRa), 7.2 μl of deionized water, 0.4 μl of forward primer (10 μM), 0.4 μl of reserve primer (10 μM), and 2 μl of template cDNA, were performed as the following reaction condition: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 58°C for 30 s.
The primers used for real-time PCR are listed in Table 1. The expressions of groups exposed to fungus were calibrated by those of groups exposed to 0.1% Tween 80. The relative gene expressions were normalized by the method of 2-ΔΔ Ct (Livak and Schmittgen 2001). The real-time PCR was performed on three biological replicates, each containing three technical replicates (Liu et al. 2015).
Table 1.
Primers used for real-time RT-PCR in this study
| Gene name | Orientation | (5′→3′) Primer sequence |
|---|---|---|
| β-actin | Forward | ctcaggtgatggtgtctc |
| Reverse | caggtagtcggtcaagtc | |
| termicin | Forward | tcgtctttctggtcgtcgtagtg |
| Reverse | cagtggtgatagagatgata | |
| transferrin | Forward | atgcacggcttgcctgttat |
| Reverse | tgtgtgcgctctgtgagca |
Statistical Analysis
All of the data were analyzed with IBM SPSS (Statistical Package for the Social Sciences) Statistics 19.0 software. Behavioral observations were analyzed with paired t-test. Survival was analyzed with a Cox proportional regression for assessing variables affecting survival and then was analyzed with Kaplan–Meier methods for life span under different conidial concentrations and termite quantities. For antifungal activity and immune-gene expression, data analysis was performed with one-way ANOVAs using Tukey’s HSD test for significant differences.
Results
Observation of Allogrooming Behavior
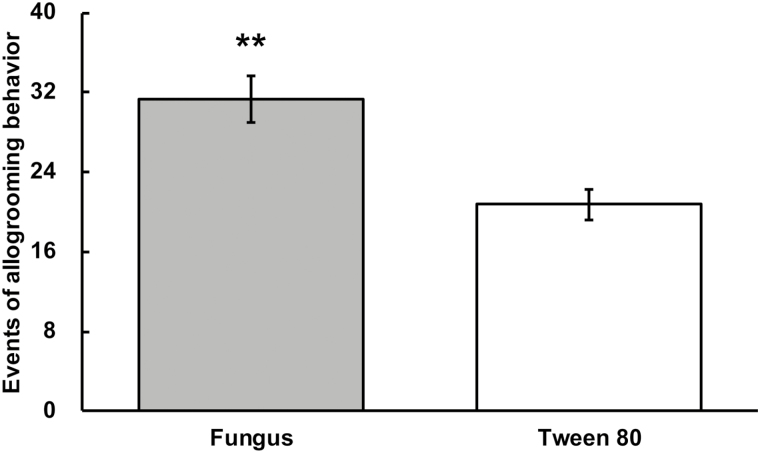
Allogrooming behavior occurred significantly more frequently in the fungus-treated groups than in the controls (Fig. 1; T = 3.777; df= 8; P = 0.005).
Fig. 1.
Events of allogrooming behavior. A pair of termites was exposed to fungal conidia (gray) or 0.1% Tween 80 (White). Error bars represent mean ± SEM. Asterisks denote significant differences (**P < 0.01, paired t-test).
SEM Observation of Conidia on the Cuticles
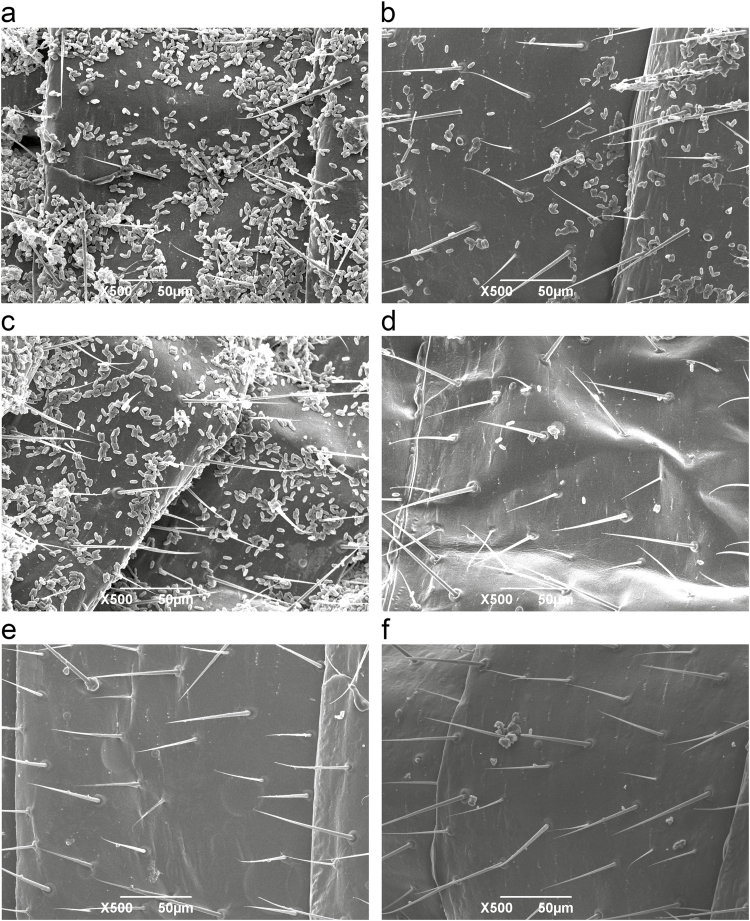
Compared with the conidia on the cuticle of single exposed termites (Fig. 2a and c), most of the conidia on the cuticles of grouped exposed termites were removed by intensive allogrooming behavior after 1- and 12-h infection (Fig. 2b and d). After 12-h cultivation, there were no conidia in unexposed single and grouped termites (Fig. 2e and f).
Fig. 2.
The fungal conidia on termite cuticles. Conidia on the cuticle of single exposed termites after 1 h (a) and 12 h (c) infection. Conidia on the cuticles of grouped exposed termites after 1 h (b) and 12 h (d) infection. After 12 h cultivation, unexposed single (e) and grouped (f) termites were regarded as controls.
Survival
The exposed termites show concentration-dependent susceptibility to M. anisopliae. For the survival of individuals under different conidial concentrations, the colony had the negligible effect on termite survival (Wald = 0.001; df = 1; P = 0.970), whereas the conidial concentrations had the significant effect (Wald = 20.605; df = 1; P < 0.001). The survival under the 5 × 107 conidia/ml treatment was significantly lower than under the other treatments (Fig. 3a; 107 vs 105: χ2 = 6.559, P = 0.01; 107 vs 103: χ2 = 24.269, P < 0.001; 107 vs Tween 80: χ2 = 24.269, P < 0.001). The survival under the 105 conidia/ml treatment was significantly lower than under the 5 × 103 conidia/ml and 0.1% Tween 80 treatments (Fig. 3a; 105 vs 103: χ2 = 8.054, P = 0.005; 105 vs Tween 80: χ2 = 8.054, P = 0.005).
For the survival between single termite and grouped termites, the termite quantity (Wald = 21.913; df = 1; P < 0.001) rather than colony (Wald = 0.090; df = 1; P = 0.764) had significantly impact on termite survival. The survival of grouped exposed termites was significantly higher than that of single exposed termites (Fig. 3b; χ2 = 38.498, P < 0.001).
Antifungal Activity
After 3 d of cultivation, significant differences were detected in antifungal activity of single termite under different conidial concentrations (Fig. 4a; F = 8.598, df = 3, 16; P = 0.001). Antifungal activity of single termite exposed to 5 × 107 and 5 × 105 conidia/ml was significantly higher than that of single termite exposed to 5 × 103 conidia/ml (Fig. 4a; P < 0.05) and 0.1% Tween 80 (Fig. 4a; P < 0.05). Antifungal activity of single termite exposed to 5 × 103 conidia/ml was not significantly higher than that of single termites exposed to 0.1% Tween 80 (Fig. 4a; P > 0.05).
After 3 d of cultivation, termite quantity had a significant effect on antifungal activity of individuals under 5 × 105 conidia/ml (Fig. 4b; F = 8.598, df = 3, 16; P = 0.001). Antifungal activity of single exposed termite was significantly higher than that of single unexposed termite, and grouped exposed and unexposed termites (Fig. 4b; P < 0.05). There were no significant differences in antifungal activity among single unexposed termite, and grouped exposed and unexposed termites (Fig. 4b; P > 0.05).
Expression of Immune Genes
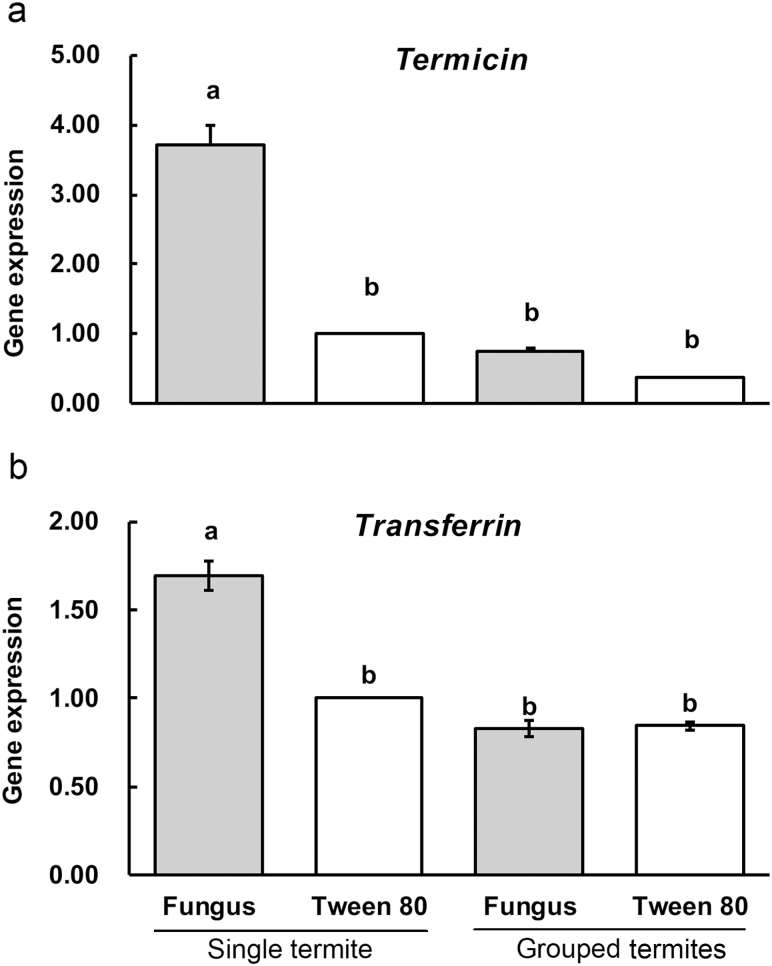
After 5 d of cultivation, the expression of the immune gene termicin in single exposed termite significantly higher than that in single unexposed termite, and grouped exposed and unexposed termites (Fig. 5a; F = 115.992; df = 3, 8; P < 0.001). Also, the expression of the immune gene transferrin in single exposed termite was significantly upregulated when compared with the other three treatments (Fig. 5b; F = 67.517; df = 3, 8; P < 0.001). However, the two immune genes exhibited no significant differences among single unexposed termite, and grouped exposed and unexposed termites (P > 0.05).
Fig. 5.
Expressions of the two immune genes among different treatments. Single and grouped termites were treated by 5 × 105 conidia/ml or 0.1% Tween 80. Error bars represent mean ± SEM. Different letters over the bars denote significant differences (P < 0.05, Tukey’s HSD).
Discussion
Sanitary behaviors can effectively reduce the risk of disease for pathogen-exposed individuals (Yanagawa and Shimizu 2007, Konrad et al. 2018). When insects somehow contact the pathogenic fungus, the fungal conidia may transfer to their cuticles, and hence, the insects become pathogen-exposed individuals. During early-stage infection, the conidia those attach to the insect cuticles loosely can be cleared by sanitary behaviors among insects (Rosengaus et al. 1998, Calleri et al 2006, Yanagawa and Shimizu 2007, Cremer et al. 2007, Konrad et al. 2018). However, when the conidia germinate and fully attach to the cuticles, it will be very difficult to remove them. If the pathogens cause disease in exposed individuals, innate immunity will be activated to kill pathogens or inhibit pathogen growth in vivo (Hamilton et al. 2011, Konrad et al. 2012, Zeng et al. 2016, Liu et al. 2015) . In this study, we found that grouped termites immediately increased allogrooming behavior when they were exposed to the pathogen, resulting in apparently decreased pathogen loads. Since termites exhibited concentration-dependent susceptibility to the pathogens, the decreased pathogen loads had less impact on infection mortality of termites under group conditions. Therefore, allogrooming behavior among termite nestmates during early infection was an effective mechanism to reduce the risk of disease and improve the survival of group members.
However, when pathogens successfully invade the body cavities of termites, individual innate immunity will work (Thompson et al. 2003, Bulmer and Crozier 2004). Termites exposed to pathogens exhibit the upregulation of antifungal activity and expression of immune proteins to kill pathogens or inhibit pathogen growth (Bulmer and Crozier, 2004, Rosengaus et al. 2007; Zeng et al. 2016, Liu et al. 2015). Among these immune proteins, termicin is a cysteine-rich antimicrobial peptide, exhibiting strong killing activity against fungal pathogens (Bulmer and Crozier 2004). Transferrin may protect free iron ions from being utilized by fungus so as to inhibit fungal growth in termite hemocoel (Thompson et al. 2003). However, grouped fungus-exposed termites in this study did not upregulate their antifungal activities and expression of the immune genes termicin and transferrin, which might be caused by social sanitary behaviors. Since allogrooming behavior is an important component of sanitary cares to disinfect insect cuticles and clear pathogens (Yanagawa and Shimizu 2007, Hamilton and Bulmer 2012, Konrad et al. 2018), it may be difficult for the pathogens to succeed in invading into the termite hemocoel. Hence, the host immune system in vivo is unnecessary to be activated for recognizing the pathogens, upregulating immune proteins, and increasing antifungal activity. Therefore, we inferred that allogrooming behavior decreased physiological investments in individual innate immunity by preventing pathogens from invading hosts.
In social insects, allogrooming can not only remove pathogens from the cuticle to reduce the risk of disease (Cremer et al. 2007, Yanagawa and Shimizu 2007) but also may result in the transfer sublethal doses of pathogens to naïve nestmates to immunize them against pathogens (Traniello et al. 2002, Konrad et al. 2012, Liu et al. 2015). This kind of social immunization can decrease susceptibility of social insects to the same pathogens (Traniello et al. 2002, Konrad et al. 2012, Liu et al. 2015). However, recent work has shown that low-level infected individuals by social immunization are more susceptible to a different pathogen, so the immunized social insects reduced the allogrooming frequency toward the individuals exposed to a different pathogen to avoid superinfections (Konrad et al. 2018). Thus, social insects always choose the optimum defensive strategies to resist infections of different pathogens (Cremer et al. 2007). In the future, more studies on the immune system in social insects need be carried out to deeply understand the evolution of social immunity (Cremer et al. 2018).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31572322), and the Fundamental Research Funds for the Central Universities (2662016PY062).
References Cited
- Bulmer M. S., and Crozier R. H.. 2004. Duplication and diversifying selection among termite antifungal peptides. Mol. Biol. Evol. 21: 2256–2264. [DOI] [PubMed] [Google Scholar]
- Calleri D. V. II, McGrail Reid E., Rosengaus R. B., Vargo E. L., and Traniello J. F.. 2006. Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc. Biol. Sci. 273: 2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer S., Armitage S. A., and Schmid-Hempel P.. 2007. Social immunity. Curr. Biol. 17: R693–R702. [DOI] [PubMed] [Google Scholar]
- Cremer S., Pull C. D., and Fürst M. A.. 2018. Social immunity: emergence and evolution of colony-level disease protection. Annu. Rev. Entomol. 63: 105–123. [DOI] [PubMed] [Google Scholar]
- Fefferman N. H., Traniello J. F. A., Rosengaus R. B., and Calleri D. V.. 2007. Evolution of disease prevention and resistance in social insects: modeling the survival consequences of immunity, hygienic behavior and colony organization. Behav. Ecol. Sociobiol. 61: 565–577. [Google Scholar]
- Hamilton C., and Bulmer M. S.. 2012. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev. Comp. Immunol. 36: 372–377. [DOI] [PubMed] [Google Scholar]
- Hamilton C., Lejeune B. T., and Rosengaus R. B.. 2011. Trophallaxis and prophylaxis: social immunity in the carpenter ant Camponotus pennsylvanicus. Biol. Lett. 7: 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q. Y., Li G. H., Husseneder C., and Lei C. L.. 2013. Genetic analysis of population structure and reproductive mode of the termite Reticulitermes chinensis Snyder. Plos One. 8: e69070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad M., Vyleta M. L., Theis F. J., Stock M., Tragust S., Klatt M., Drescher V., Marr C., Ugelvig L. V., and Cremer S.. 2012. Social transfer of pathogenic fungus promotes active immunisation in ant colonies. Plos Biol. 10: e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad M., Pull C. D., Metzler S., Seif K., Naderlinger E., Grasse A. V., and Cremer S.. 2018. Ants avoid superinfections by performing risk-adjusted sanitary care. Proc. Natl. Acad. Sci. USA. 115: 2782–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li G., Sun P., Lei C., and Huang Q.. 2015. Experimental verification and molecular basis of active immunization against fungal pathogens in termites. Sci. Rep. 5: 15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., and Schmittgen T. D.. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆ Ct Method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mitaka Y., Kobayashi K., and Matsuura K.. 2016. Caste-, sex-, and age-dependent expression of immune-related genes in a Japanese subterranean termite, Reticulitermes speratus. Plos One. 12: e0175417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Guarna M. M., Melathopoulos A. P., Moon K. M., White R., Huxter E., Pernal S. F., and Foster L. J.. 2012. Correlation of proteome-wide changes with social immunity behaviors provides insight into resistance to the parasitic mite, Varroa destructor, in the honey bee (Apis mellifera). Genome Biol. 13: R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengaus R. B., Maxmen A. M., Coates L. E., and Traniello J. F. A.. 1998. Disease resistance: a benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav. Ecol. Sociobi. 44: 125–134. [Google Scholar]
- Rosengaus R. B., Cornelisse T., Guschanski K., and Traniello J. F.. 2007. Inducible immune proteins in the dampwood termite Zootermopsis angusticollis. Naturwissenschaften. 94: 25–33. [DOI] [PubMed] [Google Scholar]
- Rosengaus R. B., Traniello J. F. A., and Bulmer M. S.. 2011. Ecology, behavior and evolution of disease resistance in termites, pp 165–191 In Bignell D. E., Roisin Y., and Lo N. (eds.), In biology of termites: a modern synthesis. Springer, Dordrecht, the Netherlands. [Google Scholar]
- Schmid-Hempel P. 1998. Parasites in social insects. Princeton University Press, Princeton, NJ. [Google Scholar]
- Thompson G. J., Crozier Y. C., and Crozier R. H.. 2003. Isolation and characterization of a termite transferrin gene up-regulated on infection. Insect Mol. Biol. 12: 1–7. [DOI] [PubMed] [Google Scholar]
- Traniello J. F., Rosengaus R. B., and Savoie K.. 2002. The development of immunity in a social insect: evidence for the group facilitation of disease resistance. Proc. Natl. Acad. Sci. U. S. A. 99: 6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa A., and Shimizua S.. 2007. Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. Biocontrol. 52: 75–85. [Google Scholar]
- Yin L. F., Su X. H., Wang K., and Xing L. X.. 2013. Termites species and damage to urban trees in Shanxi and Gansu provinces. Plant Protect. 39: 151–155. [Google Scholar]
- Zeng Y., Hu X. P., and Suh S. J.. 2016. Characterization of antibacterial activities of eastern subterranean termite, Reticulitermes flavipes, against human pathogens. Plos One. 11: e0162249. [DOI] [PMC free article] [PubMed] [Google Scholar]