Abstract
Nutrient handling by higher organisms is a complex process that is regulated at the transcriptional level. Studies over the past 15 years have highlighted the critical importance of a family of transcriptional regulators termed the Krüppel-like factors (KLFs) in metabolism. Within an organ, distinct KLFs direct networks of metabolic gene targets to achieve specialized functions. This regulation is often orchestrated in concert with recruitment of tissue-specific transcriptional regulators, particularly members of the nuclear receptor family. Upon nutrient entry into the intestine, gut, and liver, KLFs control a range of functions from bile synthesis to intestinal stem cell maintenance to effect nutrient acquisition. Subsequently, coordinated KLF activity across multiple organs distributes nutrients to sites of storage or liberates them for use in response to changes in nutrient status. Finally, in energy-consuming organs like cardiac and skeletal muscle, KLFs tune local metabolic programs to precisely match substrate uptake, flux, and use, particularly via mitochondrial function, with energetic demand; this is achieved in part via circulating mediators, including glucocorticoids and insulin. Here, we summarize current understanding of KLFs in regulation of nutrient absorption, interorgan circulation, and tissue-specific use.
Essential Points
Metabolic homeostasis is tightly regulated at the transcriptional level by a complex network of transcription factors and coregulators
The Krüppel-like factors (KLFs) affect the metabolism of nearly every macronutrient, including carbohydrates, lipids, and amino acids
KLFs are a new family of metabolic regulators that interact with classically studied regulators of metabolism, including nuclear receptors to facilitate metabolic transcription
KLFs orchestrate systemic metabolic homeostasis through transcriptional control of key processes in metabolism, including nutrient uptake, interorgan trafficking, and tissue utilization
Many KLFs are responsive to states of nutrient excess or debt, which allows them to serve as metabolic switches, shifting metabolism between nutrient storage and liberation
Variants of numerous KLFs are implicated in human metabolic disease, making them important targets of investigation
Regulatory maintenance of metabolic homeostasis is critically regulated at the transcriptional level (1). In response to the daily energetic demands, interdependent transcriptional regulators and coregulators transduce upstream nutritional cues to dozens of metabolic gene programs responsible for dynamically balancing energy intake, circulation, storage, and expenditure. Together, these factors subserve cellular function in disparate organs in a cooperative and specific fashion to ultimately (1) generate whole-body responses, (2) restrain extreme metabolic fluctuations, and (3) deliver energetic substrates appropriately in response to changes in physiologic state, such as fasting or exercise. This impressive transcriptional regulatory activity, requisite for mammalian life, can achieve high levels of specificity via multiple mechanisms, including context-dependent recruitment of coactivators or repressors, tissue-restricted expression, and factor-specific cistromes; recent evidence also points to the role of posttranslational modifications in tuning transcription factor activity (2).
Studies over the past several decades have underscored the importance of particular transcriptional gene families in metabolic regulation (3–6). For example, nuclear factors such as the peroxisome proliferator-activated receptors (PPARs), estrogen-related receptors (ERRs), nuclear respiratory factors, CCAAT/element binding proteins (C/EBPs), and the sterol regulatory element binding protein (SREBP) are essential players in metabolic regulation by binding to specific DNA sequence motifs and associating with coregulators in a context-dependent fashion (3–6).
Beyond the canonical ligand-gated nuclear receptors, a new family of metabolic regulators has emerged in recent years: the Krüppel-like factors (KLFs). The KLFs are a family of phylogenetically conserved, broadly expressed transcription factors involved in diverse physiological and pathological processes. This transcription factor family initially was identified by the single Drosophila melanogaster protein Krüppel, and the current 18 members each contain three zinc finger motifs of the C2H2 type, which recognize GC-rich DNA binding sequences and transactivate or repress gene expression. Thousands of KLF response elements are scattered throughout the genome, prohibiting specificity arising from DNA binding motifs. Thus, like the aforementioned ligand-gated nuclear factors, the KLFs use coactivators and corepressors to apply additional regulation to KLF-mediated transcriptional output. These mechanisms, such as posttranslational regulation and descriptions of KLF-associated protein partners, and their roles in wide-ranging KLF biology, are well reviewed elsewhere (7, 8).
Molecular investigations of adipocyte maturation were the first to reveal a link between the KLFs and metabolism (9). Subsequent work has expanded the purview of KLF regulation into nearly every aspect of systemic metabolism. The KLFs are expressed in multiple metabolically active organs, are exquisitely responsive to nutrient status, and exert broad and robust transcriptional control over glucose, lipid, and amino acid nutrient handling. In addition, recent evidence also suggests the KLFs are highly cooperative with other families of metabolic regulators (i.e., the nuclear receptors).
Here, we summarize current understanding of KLFs in the regulation of energy metabolism through control of nutrient intake and acquisition, systemic circulation, and tissue-specific utilization, and discuss how KLFs orchestrate complex physiologic responses such as fasting. First, KLFs located both centrally and peripherally modulate energy intake through feeding behavior. Upon entry into the postprandial state, gut and liver KLFs control a range of functions from bile synthesis to intestinal stem cell maintenance to effect intestinal absorption. Subsequently, KLF activity across multiple organs responds to changes in nutrient status to coordinate distribution of nutrients to sites of storage or liberation for use. Finally, in energy-consuming organs like cardiac and skeletal muscle, KLFs tune local metabolic programs to precisely match energetic demand with substrate uptake, flux, and use; this is achieved in part through circulating mediators, including glucocorticoids and insulin, and through modulation of mitochondrial function. Together, the KLFs orchestrate systemic metabolism through local and multiorgan transcriptional regulation of each macronutrient class.
Energy Intake
Appetite control
The drive to consume is seated in the brain. Eating behavior is sensitive to homeostatic feedback from hormones released peripherally in response to nutrient status; this feedback is an essential mechanism to control food intake and maintain metabolic homeostasis. Two hormones reciprocally signal through the hypothalamus to modulate food intake behavior and are subject to KLF transcriptional control: ghrelin and leptin (Fig. 1a). Originating primarily from specialized cells within the gastrointestinal (GI) tract, ghrelin secretion rises with fasting and falls upon feeding (10). Ghrelin stimulates orexigenic neuropeptide Y and Agouti-related protein (AgRP) neurons to augment appetite (11). Conversely, the adipokine leptin inhibits neuropeptide Y and AgRP neurons while stimulating anorexigenic pro-opiomelanocortin neurons within the arcuate nucleus of the hypothalamus (11).
Figure 1.
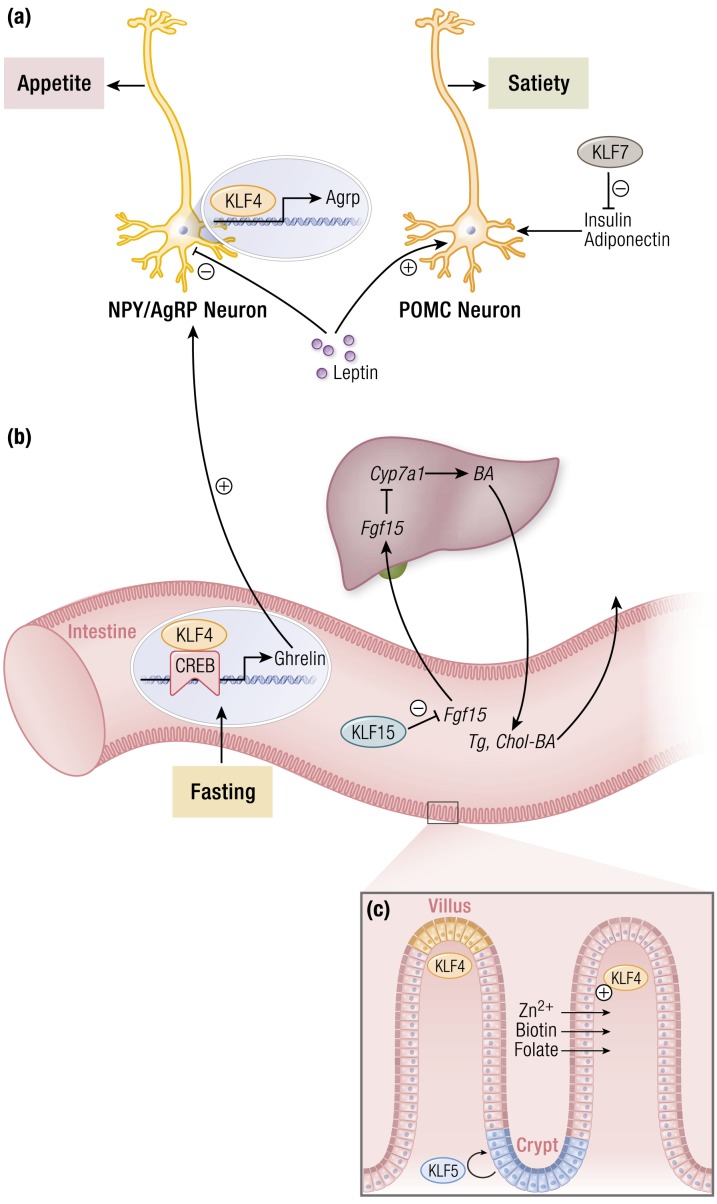
KLF regulation of nutrient acquisition and absorption. (a) Multiple KLFs regulate appetite and feeding behavior centrally and peripherally. Within the hypothalamus, KLF4 expression promotes expression of AgRP to stimulate appetite. Anorexigenic hormones such as leptin inhibit neuronal KLF4 expression, thereby reducing appetite. Peripherally, KLF4 is induced by fasting and cooperates with CREB to augment ghrelin production. This, in turn, propagates orexigenic signaling in the brain. In addition, KLF7 inhibits production of key satiety-mediating factors that would otherwise ultimately signal through POMC neurons to reduce feeding. (b) Intestinal KLF15 promotes hepatic bile acid production through a feedback loop involving inhibition of FGF15 production within the intestines, ultimately relieving FGF15-mediated inhibition of CYP7A1. This reduces intestinal absorption of triglycerides and cholesterol. (c) Intestinal KLFs regulate proper barrier function and absorptive capacity. KLF4 increases specific nutrient transporters to maintain a pool of important cofactors for many metabolic processes. These include zinc, biotin, and folate transporters. Intestinal barrier function is preserved through a coordination of intestinal stem cell self-renewal and eventual differentiation. KLF5 expression in the crypts maintains available populations of intestinal stem cells. Conversely, KLF4 expression is highest at the villi tips and is associated with intestinal cell differentiation. Abbreviations: AgRP, Agouti-related peptide; BA, bile acid; CREB, cAMP response element binding protein; CYP7A1, cytochrome P450 family 7 subfamily A member 1; FGF15, fibroblast growth factor 15; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; TG, triglyceride; chol, cholesterol. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]
KLF4 expression within the GI tract increases in response to fasting and correlates with ghrelin levels (12). Mechanistically, KLF4 associates with the fasting-inducible cAMP response element binding protein (CREB) to directly regulate mRNA transcript levels of ghrelin, and silencing KLF4 greatly reduces ghrelin expression in stimulated human gastric cells (12). Centrally, KLF4 also propagates orexigenic signals while limiting anorexigenic signals like leptin. Within AgRP neurons, KLF4 expression increases during fasting to elevate appetite and promote food intake via direct regulation of AgRP expression in arcuate nucleus neurons independent of Forkhead Box O1 (FoxO1) (13). Leptin suppresses endogenous KLF4 expression through a STAT3-mediated pathway; conversely, forced expression of KLF4 in the arcuate nucleus of rats reduces sensitivity to exogenous leptin (13). Thus, KLF4 seems to favor increased food intake behavior through its activities both in the GI tract and in the hypothalamus. Although many models of diet-induced obesity demonstrate that satiety signals within the hypothalamus are negatively affected by high-fat diet, hypothalamic KLF4 levels do not respond to a high-fat diet regimen—evidence that food intake is a complex behavior subject to layers of control.
In addition to ghrelin and leptin signaling, there is growing appreciation for other local and circulating mediators of appetite that are under KLF control. KLF7 has a repressive role in the expression of several adipokines, including leptin (14). KLF7 inhibits expression of adiponectin and insulin, both of which suppress appetite centrally (14–16). Multiple KLFs control insulin production and sensitivity. The specific mechanisms behind this are discussed in a later section in this article; however, this regulation is noteworthy in the setting of appetite regulation because the effects of KLF-regulated insulin dynamics likely extend to the hypothalamus, expanding the influence that KLFs have on feeding behavior.
Intestinal absorption
In response to nutrient entry into the duodenum, liver-derived bile acids are secreted into the intestine to facilitate absorption of lipids and fat-soluble vitamins while also serving as a route for excess cholesterol to leave the body. In diseases that affect the liver or biliary tree, such as biliary cholangitis and cirrhosis, suboptimal levels of concentrated bile are released into the small intestine, resulting in impaired lipid absorption and consequent lipid deficiency. Bile acid synthesis, therefore, represents a critical point for transcriptional regulation in metabolic homeostasis. Bile acids act as ligands for nuclear receptors residing in the intestine, such as the farnesoid X receptor (FXR). Activated ileal FXR promotes the expression and secretion of the enterokine fibroblast growth factor 15 (Fgf15) (17) into the circulation, which travels to the liver to repress Cyp7a1, the rate-limiting enzyme in bile acid production (18). KLF15 constitutes a regulatory pathway that controls bile acid pools and lipid and vitamin absorption independent of FXR (Fig. 1b). Ileal KLF15 suppresses expression of Fgf15 to promote bile acid synthesis via Cyp7a1; Klf15−/− mice exhibit reduced tissue bile acid levels simultaneous with deficiencies in triglyceride and cholesterol absorption, which are corrected upon ileectomy (19). In addition, KLF15 expression varies diurnally and, therefore, drives the long-observed rhythmicity of Fgf15 and Cyp7a1 gene expression (19, 20). To date, intestinal KLF15 is, to our knowledge, the only negative transcriptional regulator of bile acid production.
Epithelial barrier function and regenerative potential allow the intestine to be selectively permeable to nutrients while excluding luminal microbes. Intestinal transporters are also essential to ensuring selective uptake of nutrients (Fig. 1c). KLF4 regulates the expression of multiple nutrient transporters, including those for zinc, folate, and biotin absorption (21–23). KLFs are critical in the maintenance of proper intestinal barrier function. For example, KLF4 and KLF5 demonstrate reciprocal effects on intestinal stem cell proliferation and differentiation. Originally termed “gut KLF,” KLF4 expression occurs in a gradient across the intestinal villus, correlating strongly with increasing degree of differentiation, with highest expression in the tips of the villi (24–27). In contrast, KLF5 is enriched in proliferating crypt cells, and loss of intestinal KLF5 expression impairs the intestinal stem cell niche (28–31). Therefore, balanced activity of these two factors throughout the epithelial stem cell life cycle maintains intestinal homeostasis. In addition, KLF5 directly regulates intestinal barrier function. Loss of intestinal KLF5 is associated with barrier breakdown and increased permeability, in part through regulation of desmoglein-2, a desmosomal component (32, 33).
Through the mechanisms described in this section, the KLFs govern eating behavior and subsequent intestinal absorption of nutrients, which, together, affect organismal energy intake. This KLF regulation of energy intake also responds to circulating signals of nutrient status (e.g., to mediate satiety in nutrient replete states). Entry of nutrients into the circulation engages hormonal regulatory mechanisms linked to a new suite of KLF-mediated transcriptional programs in various metabolic organs to handle and distribute energy.
Systemic Nutrient Trafficking
Circulating mediators
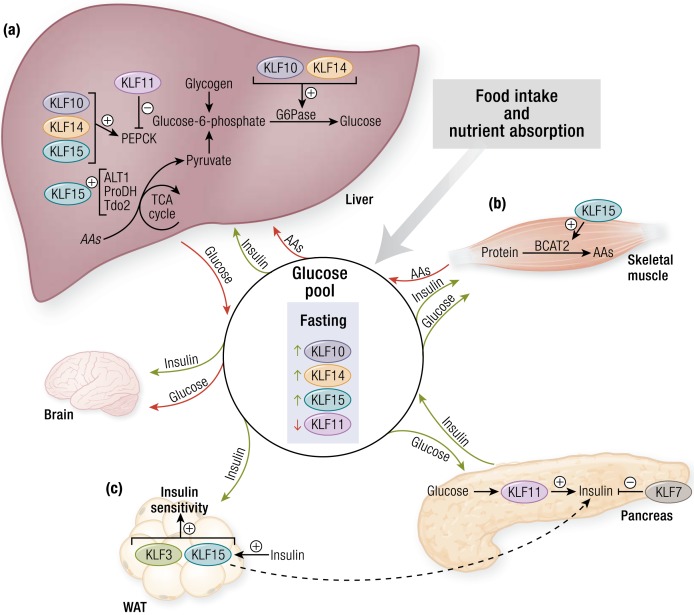
Nutrient trafficking in states of energy debt or surplus is mediated by hormonal signals. Insulin is a powerful anabolic hormone; insulin levels rise in the fed state and stimulate glucose uptake into tissues, glycogen synthesis in the liver and skeletal muscle (lowering blood glucose levels), and triglyceride synthesis in adipose tissue (promoting fat storage). Thus, regulators of insulin production, signaling, and sensitivity greatly influence nutrient handling and energy homeostasis. In human pancreatic β cells, glucose induces expression of KLF11, which directly transactivates the insulin promoter (34–37) (Fig. 2c). This potentiation of insulin transcription depends on recruitment of histone acetyltransferase p300, a commonly recruited coregulator within the KLF family (35, 37). On the other hand, KLF7 may have a suppressive effect; as previously mentioned, KLF7 expressed in insulin-secreting cell lines reduces insulin production (14). KLF15 alters insulin secretion via crosstalk between adipose tissue and the pancreas. Nagare et al. (38) demonstrated that overexpression of adipose tissue KLF15 is protective against diet-induced oxidative stress by suppressing SCD1 in adipose tissue, which results in increased insulin secretion by pancreatic β cells and improved glucose tolerance.
Figure 2.
KLFs control of glucose flux. (a) In fasting, several KLFs (i.e., KLF10, KLF11, KLF14, and KLF15) regulate the expression of key hepatic enzymes involved in amino acid (AA) catabolism (e.g., Alt1, Prodh, Tdo2) and gluconeogenesis (e.g., Pepck, G6Pase). These processes provide a steady source of glucose for glucose-obligate organs such as the brain. (b) KLF15 in skeletal muscle regulates branched-chain AA catabolism. These AAs are shuttled to the liver and used as carbon substrates for gluconeogenesis. (c) In the pancreas, glucose induces KLF11 expression, which leads to increased insulin production. KLF7 opposes the action of KLF11. KLF3 and KLF15 increase insulin sensitivity in WAT, allowing for enhanced glucose uptake. Alt1, alanine aminotransferase 1; BCAT2, branched-chain amino acid transaminase 2; G6Pase, glucose-6-phosphatase; Pepck, phosphoenolpyruvate carboxykinase; Prodh, proline dehydrogenase; Tdo2, tryptophan 2, 3-dioxygenase; TCA, tricarboxylic acid; WAT, white adipose tissue. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]
Beyond regulating insulin production, KLFs also dictate tissue sensitivity to insulin, the most classically described being KLF15. In vitro overexpression of KLF15 in adipocytes and myocytes drives transcription of the insulin-sensitive glucose transporter GLUT4 and facilitates significant glucose uptake into cells (9). This likely occurs via KLF15 cooperation with MEF2A on the Glut4 promoter (9). Conversely, knockdown of KLF15 in adipocytes reduces insulin-mediated glucose uptake and glycolysis (39). Other KLFs known to affect insulin sensitivity include KLF3 and KLF14. Adipose KLF3 binds to and inhibits transcription of Fam132a, which encodes the insulin-sensitizing factor adipolin (40, 41). Global deletion of KLF3 protects mice from diet-induced obesity and metabolic disease, including insulin resistance (40). Interestingly, adipose KLF15 positively regulates adipolin expression, and high-fat diet–induced inflammation represses KLF15 expression, providing an additional link between metabolic inflammation and insulin resistance (41). Whereas KLF3 and KLF15 alter adipose-driven signals affecting insulin sensitivity, KLF14 modifies hepatic insulin signaling. KLF14 is downregulated in multiple cell types of diabetic mice and humans, and its forced overexpression in hepatocytes facilitates insulin-mediated glucose uptake through PI3K/Akt activation (42). In addition, hepatic KLF14 overexpression protects against hyperglycemic- and hyperinsulinemic-stimulated glucose uptake inhibition seen in patients with diabetes. Therefore, KLF14 may represent a dominant positive regulator of insulin signaling within the liver. On the other hand, in mouse models of either global or hepatic deletion of KLF6, animals demonstrate protection from high-fat diet–induced hepatic steatosis and improved insulin sensitivity and glucose tolerance. This may be due to KLF6’s posttranscriptional effects on Pparα and its downstream targets (i.e., Trb3 and Pepck) that regulate insulin signaling and hepatic gluconeogenesis (43).
Glucocorticoids affect systemic glucose handling by promoting hyperglycemia through the induction of hepatic gluconeogenesis and inhibition of skeletal muscle and adipose glucose uptake. Excess glucocorticoid release induces insulin resistance and other manifestations of metabolic syndrome, as seen in Cushing syndrome (44). KLFs are well-known mediators of nuclear receptor signaling, including acting downstream of glucocorticoid receptor (GR) (45). Studies investigating the functional significance of this are largely limited to cell growth and differentiation (46–48). In skeletal muscle, glucocorticoid signaling upregulates KLF15 and improves endurance performance, possibly through upregulating amino acid and lipid metabolic programs (49). In mouse embryonic fibroblasts, KLF15 is a direct target of GR. GR stimulation augments KLF15 expression and contributes to KLF15’s well-documented role in promoting adipogenesis and differentiation (50, 51). Although glucocorticoids and KLFs have independently been shown to control glucose handling, additional work is needed to investigate how these players interact to regulate metabolic homeostasis.
Glucose homeostasis
The body imposes tight constraints on glycemia. Because the brain almost exclusively relies on glucose, plasma glucose levels below ∼3 mM in humans predictably manifest as largely neurologic sequelae. Hyperglycemia occurs most often in the context of diabetes mellitus, and chronically elevated levels of glucose contribute to a host of morbidities, not the least of which are neuropathy and vasculopathy. Thus, robust physiologic mechanisms, especially in the liver, ensure the maintenance of euglycemic conditions. During times of nutrient excess, the liver stores energy in the forms of glycogen and fat. During fasting, the liver buffers glucose levels through glycogenolysis before proceeding to gluconeogenesis, then to ketogenesis while also incorporating substrates released from protein breakdown in skeletal muscle in periods of extended nutrient deprivation.
KLF15 was the first KLF to be linked to metabolism through its regulation of the glucose transporter GLUT4 in adipose and muscle cells (9). Subsequently, KLF10, KLF11, KLF14, and KLF15 were found to have important roles in regulating hepatic gluconeogenesis (Fig. 2a). KLF10, KLF14, and KLF15 in the liver are all induced by fasting and boost expression of gluconeogenic enzymes, whereas KLF11 expression is downregulated in fasting (52–55). Accordingly, studies have shown that the silencing or overexpression of these factors affects systemic glucose dynamics in both physiology and pathology.
KLF15 coordinates with peroxisome proliferator–activated receptor-γ coactivator 1α (PGC-1α) and directly acts on the promoter of phosphoenolpyruvate carboxykinase (Pepck), an enzyme that catalyzes an irreversible step in gluconeogenesis, to initiate Pepck gene transcription (53). Liver-specific KLF15 depletion results in reduced expression of PEPCK and glucose-6-phosphatase (G6Pase). Interestingly, KLF15 also regulates a number of enzymes involved in amino acid catabolism [e.g., alanine aminotransferase 1 (Alt1), proline dehydrogenase (Prodh), and tryptophan 2,3-dioxygenase (Tdo2)] (56, 57). Tellingly, mice with systemic KLF15 deficiency demonstrate impaired skeletal muscle amino acid catabolism (discussed later in this article) and, consequently, these animals are unable to mobilize and use gluconeogenic substrates once glucose and glycogen stores are depleted, thus leading to severe hypoglycemia after an overnight fast (57). KLF15, therefore, not only controls hepatic gluconeogenic enzymes directly but also influences the supply of gluconeogenic carbon substrates from skeletal muscle and their use in the liver. KLF15’s role in gluconeogenesis is important in mediating the therapeutic effects of metformin, a medication used for the treatment of type 2 diabetes. Administration of metformin to either cultured hepatocytes or mice results in reduced KLF15 expression and reduced glucose production. Upon restoration of KLF15 expression, these effects on glucose output are attenuated (56).
Importantly, KLF15’s effects on blood glucose levels occur in a circadian fashion, allowing organism behavior (e.g., feeding and sleeping) to be synchronized with nutrient handling throughout the day/night cycle. Klf15 is subject to CLOCK/BMAL1 control and its rhythmicity is abolished in mice with mutations of core clock genes (e.g., Per1/2) (58). Circadian glucose homeostasis is critically dependent on rhythmic KLF15 expression and through it, coordination of diurnal amino acid supply and use in the liver as gluconeogenic substrates (58). In hepatocytes as well, KLF15 expression oscillates on a 24-hour cycle, suggestive of its role in conferring rhythmicity to hepatic glucose metabolism. A second hepatic KLF, KLF10, also possessed a robust circadian pattern that is abolished in clock-deficient Bmal1 knockout mice, and luciferase promoter assays have demonstrated that the Klf10 promoter is a target of the CLOCK-BMAL1 heterodimer (59). In the liver, transcriptome data from Klf10−/− mice reveal enrichment of 158 genes involved in lipid and carbohydrate metabolism, the majority of which are clock controlled. Compared with wild-type mice, male Klf10−/− mice have hyperglycemia, both postprandially and during fasting, and this phenotype is accompanied by a time-of-day dependent upregulation of Pepck. Studies also show that KLF10 directly suppresses Pepck promoter activity (59). Disruption of circadian rhythmicity in humans such as that seen in shift work is well documented to be associated with metabolic pathologies such as type 2 diabetes mellitus T2DM) (60, 61). Given this, the role of KLF15 and KLF10 oscillations in regulating human metabolic disease is a potentially important area of investigation.
Overexpression of either KLF10 or KLF14 in primary hepatocytes increases cellular glucose output as a result of these transcription factors directly binding and activating the promoter of Pgc-1α (54, 55). Pgc-1a is an important transcription factor that drives the expression of a number of key gluconeogenic enzyme genes, such as Pck1, which encodes PEPCK, and G6pc, which encodes G6Pase (62). In vivo evidence demonstrates that forced expression of hepatic KLF10 and KLF14 in C57BL/6J mice leads to increased plasma glucose levels and glucose intolerance. Interestingly, hepatic knockdown of these KLFs in a diabetic mouse model results in normalization of blood glucose levels and improvement in glucose tolerance (54, 55).
Hepatic KLF11 acts antiparallel to KLF10, KLF14, and KLF15 (52). In the fasted state, PGC-1α and PEPCK are elevated, whereas KLF11 expression is low. KLF11 acts on the Pepck promoter to suppress transcription. Concordantly, hepatic silencing of KLF11 leads to disruptions in glucose homeostasis; whereas, adenovirus-mediated overexpression of hepatic KLF11 alleviates hyperglycemia and glucose intolerance in db/db mice (52).
Lipid flux and use
Efficient long-term energy storage falls primarily within the purview of white adipose tissue (WAT), a major regulator of lipid partitioning in the body. When caloric intake outstrips the rate of expenditure, excess energy is stored via either increased commitment of stem cells to the adipocyte lineage or hypertrophy of mature white adipocytes. The activation of lipogenic programs and the uptake of lipids from the circulation trap nearly 100% of postprandial fatty acids liberated by lipoprotein lipase (63). Endocrine functions are also ascribed to WAT via the release of secreted proteins, including leptin, adiponectin, resistin, and others, as previously discussed (64). These processes are sensitive to adipogenic stimuli such as insulin or glucocorticoids, and they are subject to control by master transcriptional regulators such as PPARγ and the C/EBPs (65).
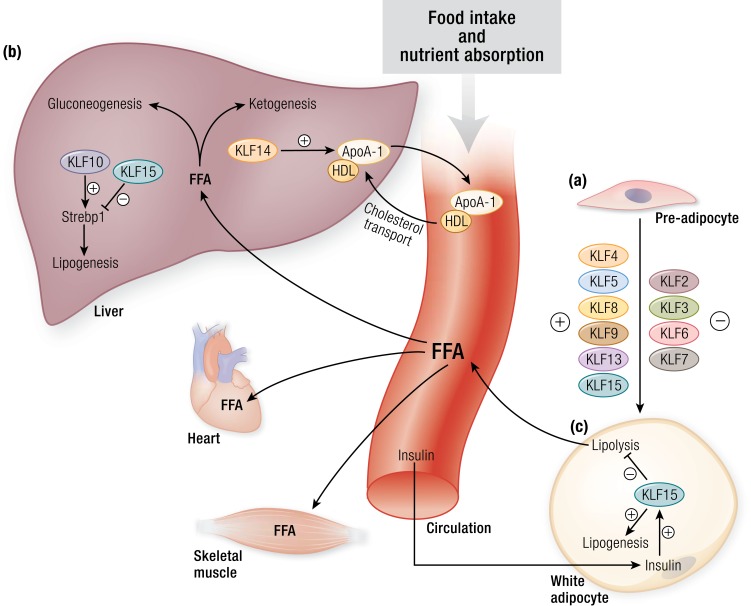
Several KLF family members are positive and negative regulators of adipocyte development (Fig. 3a), as reviewed elsewhere (66). Their effects depend on timing and degree of expression during differentiation, which complicates the interpretation of studies that inactivate the KLFs throughout the process of white adipocyte differentiation. Early studies reported that KLF4 levels are elevated early during differentiation of 3T3-L1 cells, and KLF4 is required for not only DLK2-mediated transcriptional regulation of adipogenesis but for the full adipogenic program (67, 68). However, in studies using cells differentiated from primary progenitors, KLF4 was required for A2bAR signaling–mediated inhibition of adipogenesis (69). More recently, a report using conditional KLF4-KO mice suggested that KLF4 may be dispensable for adipogenesis in vivo. KLF8 is required for 3T3-L1 differentiation and acts by driving C/EBPα and PPARγ activity (70). Similarly, KLF13 activates PPARγ and promotes porcine adipocyte differentiation, and two separate reports show KLF9 is important in the early and middle phases of 3T3-L1 differentiation through its action on the promoters of C/EBPβ and PPARγ, respectively (71–73). C/EBPβ and Δ induce KLF5 during adipogenesis and together activate PPARγ and Krox20 (an early adipogenic factor), a process that may require GSK3β activity (74, 75). Evidence of KLF5’s role as a positive regulator of adipocyte development comes from studies with Klf5+/− mice, which demonstrate impaired WAT development and smaller WAT mass; additionally, isolated embryonic fibroblasts from these animals exhibit poor differentiation (74). Recent transcriptomic analysis of adipogenesis using human stromal cells identified KLF15 as highly upregulated during differentiation (76), consistent with earlier reports that KLF15 in 3T3-L1 adipocytes is required for PPARγ2 expression and normal differentiation (51). KLF15 modulation of adipogenesis is sensitive to several upstream signals, including glucocorticoids, interleukin-17, and circadian regulation (50, 77, 78).
Figure 3.
KLFs in adipogenesis, lipid partitioning, and lipid uptake. (a) KLF4, KLF5, KLF8, KLF9, KLF13, and KLF15 act as positive regulators of adipocyte development, whereas, KLF2, KLF3, KLF6, and KLF7 act as negative regulators. Several of these KLFs interact with nuclear factors such as the PPARs and C/EBPs and coregulators such as CtBP to control adipogenesis. Inactivation of KLFs result in impaired WAT differentiation and development. (b) KLFs play an important role in hepatic lipid metabolism. KLF14 is a transcriptional regulator of Apoa1 and consequently affects systemic HDL levels and cholesterol efflux. Hepatic KLF10 and KLF15 regulate transcription of Srebp1. These transcription factors affect several genes involved in lipogenesis and in gluconeogenesis. (c) In the fed state, insulin induces WAT KLF15 expression, which consequently drives lipogenesis and inhibits lipolysis. Conversely, loss of WAT KLF15 leads to increased lipolysis, thus contributing to increased FFAs in the circulation. These FFAs are then taken up and used by different organs such as the heart and skeletal muscle. ApoA1, apolipoprotein A1; CtBP, C-terminal binding protein 1; FFA, free fatty acid; HDL, high-density lipoprotein; Srebp1, sterol regulatory element-binding protein 1. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]
Several KLFs are negative regulators of adipogenesis. KLF2 was the first family member identified as a negative regulator of adipogenesis by virtue of its ability to inhibit PPARγ in 3T3-L1 cells (79). Consistent with this observation, mouse embryonic fibroblasts from Klf2−/− embryos were shown to differentiate early in the adipogenic process (80). Interestingly, in the context of adipogenesis, Klf2 is subject to posttranscriptional regulation via Stau1-mediated mRNA decay, suggesting an additional level of complexity in the regulation of adipogenesis (81). KLF3 decreases during 3T3-L1 differentiation, and forced overexpression of KLF3 in 3T3-L1 cells inhibits differentiation (82). Mechanistically, KLF3 may recruit CtBP corepressors and together, repress the C/EBPα promoter; therefore, with diminishing KLF3 levels, C/EBPα is de-repressed in adipocytes. Although initially paradoxical, the finding that Klf3−/− mice have smaller fat pads than control cohorts may be rationalized by early and inappropriate activation of C/EBPα before its normal rise during differentiation (82). Similar to overexpression of KLF3, overexpression of KLF7 in 3T3-L1 adipocytes and chicken preadipocytes also inhibits adipogenesis. On the other hand, KLF6 inhibits 3T3-L1 differentiation through the recruitment of HDAC3 to repress Dlk1 (83).
Classically recognized for their thermogenic capabilities, brown adipose tissue as well as “inducible brown adipocytes” or “beige/brite adipocytes,” are emerging as important contributors of systemic metabolism because they are able to convert excess nutrients into heat. In response to cold, brown adipose tissue upregulates expression of uncoupling protein 1 (UCP1), a unique protein that uncouples the electron transport chain to produce heat; WAT can activate specific gene programs to induce “browning” to produce beige/brite adipocytes with thermogenic capabilities. KLFs affect brown adipocyte differentiation and browning of WAT. KLF11 and KLF15 are highly expressed in brown adipocytes and directly regulate the expression of UCP1 during brown adipocyte differentiation (84). Furthermore, KLF11 associates with PPARγ superenhancers to activate “browning” genes to induce beige adipocytes in the context of rosiglitazone stimulation (85, 86). Whereas KLF11 is essential for promoting brown adipocyte differentiation, KLF16 overexpression inhibits PPARγ in brown preadipocytes and consequently reduces differentiation (87). KLF control of the size of the brown and beige adipocytes pool ultimately affects the efficiency with which the body burns excess energy and maintains nutrient homeostasis.
Several KLFs influence lipid metabolism through control of hepatic lipoprotein synthesis and lipogenesis (Fig. 3b). As a direct transcriptional regulator of Apoa1, KLF14 controls high-density lipoprotein cholesterol levels in circulation and, therefore, cholesterol efflux to the liver (88). Interestingly, this role in lipoprotein assembly may be ancient; control of lipoprotein levels in murine models is mirrored in KLF regulation of lipoproteins in Caenorhabditis elegans. Klf3 mutations in C. elegans result in decreased expression of dsc-4 and the five vit genes, which are C. elegans orthologs of mammalian microsomal triglyceride transfer protein and apolipoprotein B, both of which are critical for lipoprotein assembly and secretion (89, 90). KLF10 exerts direct control over lipid metabolism in a circadian fashion in the liver; as mentioned, the Klf10 promoter contains an enhancer-box response element and therefore acquires the CLOCK-BMAL1 heterodimer, resulting in rhythmic expression of a majority of KLF10 regulated genes involved in lipid metabolism (as well as gluconeogenesis), such as Srebp1c (59). The Klf10 promoter also contains several carbohydrate response elements, one of which is used by carbohydrate response element–binding protein to directly couple glucose levels in the liver with hepatic lipogenesis (91).
Independent of adipocyte number, KLF15 also regulates lipogenesis and lipolysis. KLF15, in physical association with the lipogenesis regulator LXR/RXR, regulates transcript levels of Srebf1 to exert control over the hepatic lipogenic program and influence triglyceride levels in the circulation. Upon fasting, RIP140 forms a repressive complex with LXR/RXR and KLF15, thus reducing Srebp-1c expression and subsequent lipogenic enzyme expression. While in the postprandial state, hepatic KLF15 levels decrease, and the coactivator SRC-1 replaces RIP140 to drive SREBP-1c promoter activation (92). Also in the postprandial state, insulin induces WAT KLF15, which, in turn, suppresses lipolysis and enhances lipogenesis (Fig. 3c). Adipose tissue–specific KLF15 knockout mice exhibit decreased adiposity, lower levels of leptin, elevated levels of adiponectin, enhanced insulin sensitivity, improved endurance-exercise capacity, and enhanced liver ketogenesis (39). Indeed, both systemic KLF15-deficient and adipose tissue–specific KLF15-deficient mice are protected from diet-induced obesity (39, 93).
Amino acid metabolism and nitrogen disposal
Amino acids serve as an energy shuttle in the body: Carbon skeletons from amino acids are used by major metabolic pathways such as gluconeogenesis, ketogenesis, and fatty acid synthesis. Owing to mammals’ inability to fix nitrogen, they detoxify the body of nitrogen byproducts of amino acid metabolism by converting ammonia to urea. Of the KLFs, KLF15, in particular, has been heavily implicated in the control of amino acid metabolism and subsequent nitrogen detoxification and disposal (94). In addition, KLF15 plays a key role in controlling the circadian rhythmicity of these processes, allowing for the coupling between behavior (e.g., sleeping and eating) and nutrient handling by the body.
In the fasted state, skeletal muscle releases alanine and other amino acids into the circulation for uptake and use by the liver for gluconeogenesis. This process of transferring and repurposing nutrients is critical to preserving systemic euglycemia and the continued function of obligate glucose users such as the brain. Systemic KLF15-deficient mice demonstrate derangements in serum amino acid concentrations: Specifically, proline, tyrosine, leucine, and valine levels are decreased, and glutamine levels are increased (57). Gray et al. (57) found that Klf15−/− mice have significantly reduced expression of several amino acid degradation enzymes in the liver and skeletal muscle: Alt1, Prodh, Tdo2, branched-chain aminotransferase 2 (Bcat2), and 4-hydroxyphenylpyruvic acid dioxygenase (Hpd) (Fig. 2b). Consequently, compared with control animals, these KLF15-deficient mice experience severe hypoglycemia in the contexts of overnight fasts and prolonged high-protein diets (57).
In humans, nitrogen homeostasis exhibits 24-hour periodicity. Consequently, plasma urea concentration and several amino acids [e.g., alanine and the branched-chain amino acids (BCAA)] exhibit circadian rhythmicity (58). Jeyaraj et al. (58) have demonstrated that the expression of amino acid metabolism and nitrogen detoxification genes exhibit diurnal rhythmicity orchestrated by KLF15 in mice. The diurnal rhythmicity of Alt and Bcat2 expression is disrupted in Klf15−/− mice; these animals have decreased alanine and glutamine concentrations and a buildup of BCAA concentrations. In addition, systemic KLF15 deficiency results in reduced ornithine transcarbamylase activity, increased urine ammonia levels, and cognitive dysfunction resulting from hyperammonemia, clearly indicating KLF15’s important role in nitrogen homeostasis (58).
In the myocardium, cardiac KLF15 has a role in rhythm, imparting diurnal oscillations to transcripts of genes involved in BCAA catabolism. KLF15 underlies temporal partitioning of cardiac functions, such that during the resting phase, remodeling and repair processes dominate, whereas during the active phase, there is an enrichment of catabolic pathways (95, 96).
It is thought that dysregulation of KLF15-mediated amino acid metabolism plays an important role in the progression of muscle pathologies (e.g., heart failure and Duchenne muscular dystrophy) (49, 97, 98). Several studies have shown that these diseases are associated with loss of KLF15, consequent downregulation of BCAA catabolism enzymes, and eventual reactive oxygen species’ release secondary to toxic buildup of BCAAs (49, 99). Elucidating these pathways may serve as a basis for developing therapies against striated muscle pathology; indeed, KLF15 overexpression in skeletal muscle or BCAA supplementation in murine spinal muscular atrophy models (Taiwanese Smn−/−, SMN2, and Smn2B/−) extended lifespan and improved markers of muscle atrophy (100).
Thus, energetic substrate distribution is highly dynamic and dependent on coordinated KLF activity across multiple tissues. On the one hand, KLFs in liver and WAT regulate bidirectional movement of nutrients during fasting or fed states. On the other hand, KLFs in striated muscle often control gene programs that promote unidirectional uptake of substrates for oxidation. This may be mediated by circulating factors like insulin or glucocorticoids, but is certain to include more (e.g., adipokines).
Energy Consumption/Substrate Oxidation
Hormonal signals and the delivery of fatty acid substrates to organs of high metabolic demand use metabolic programs that uptake these lipids, transport them into mitochondria, and eventually oxidize them as sources of energy. Skeletal and cardiac muscle account for ∼40% of the body’s energy expenditure at rest but nearly 100% during aerobic exercise. Within these tissues, several KLFs are critical regulators of lipid flux and oxidation (101, 102). Indeed, because cardiac and skeletal muscles are highly metabolically plastic and preferentially consume fat sources of energy, KLF activity couples muscle substrate preference with energy supply.
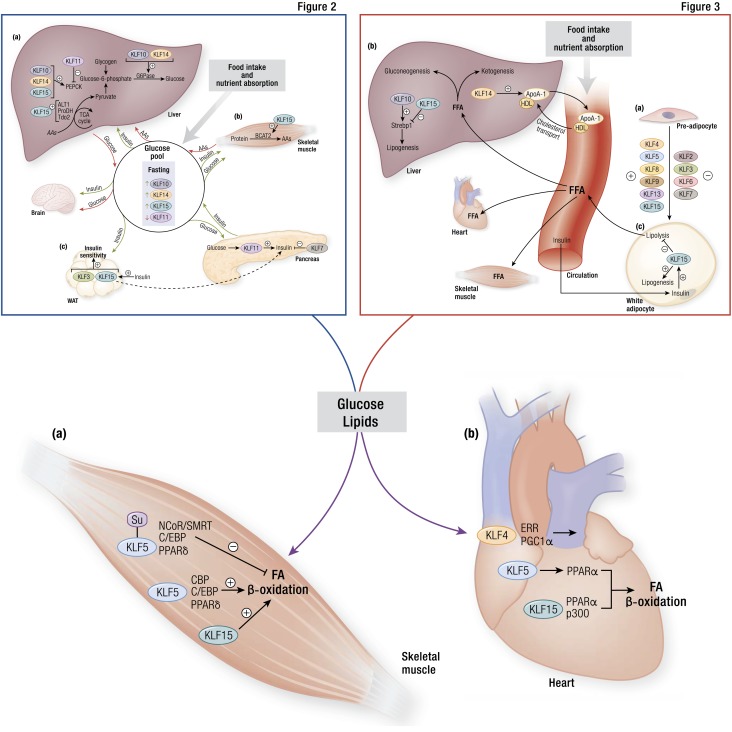
As first observed by Oishi et al. (103), Klf5 is induced in the soleus muscle of mice fed a high-fat diet, and Klf5+/− mice demonstrated enhanced oxygen consumption but similar respiratory exchange ratios compared with wild-type mice, presumably due to Klf5 regulation of CPT1b, ACADM, ACOX1, UCP2, and UCP3 in skeletal muscle. These target genes are shared by the ligand-inducible and lipid metabolism regulator PPARΔ (104), such that transcriptional activity of fatty acid metabolism genes in skeletal muscle is mediated by a multimember protein complex including KLF5, PPARΔ, as well as C/EBP, NCoR, and SMRT, wherein association is at least partly determined by SUMOylation (103). Our group has identified KLF15 as an essential regulator of fat use in skeletal muscle in the context of exercise and fasting. Acute exercise induces KLF15 in muscle and KLF15 is more highly expressed in slow-twitch soleus muscle than in fast-twitch vastus lateralis, a phenomenon consistent with muscle fiber–type switching in exercise (105). Klf15−/− mice experience poor exercise tolerance and decreased mitochondrial oxidation coincident with lowered expression of genes involved in fatty acid transport and oxidation, despite compensatory increases in Ppargc1a and Esrrg (105). Importantly, these mice exhibit an increased respiratory exchange ratio as well as elevated plasma fatty acid concentrations after exercise, suggesting that in the absence of KLF15, these mice shift substrate preference in skeletal muscle toward carbohydrates and away from lipid oxidation (105). Furthermore, KLF15 is responsive to fasting and controls expression of other fasting-inducible genes (106). KLF activity in skeletal muscle, therefore, shapes intracellular lipid use pathways to respond to nutrient availability and composition (Fig. 4a).
Figure 4.
KLFs in substrate oxidation within metabolically active organs. (a) Within skeletal muscle, posttranslational modification of KLF5 serves as a switch for fatty acid (FA) oxidation. When SUMOylated, KLF5 associates with NCoR/SMRT, C/EBP, and PPARδ to inhibit β-oxidation. Conversely, when KLF5 is not SUMOylated, it associates with CBP, C/EBP, and PPARδ to promote FA breakdown. Similarly, skeletal muscle KLF15 favors FA oxidation, although the precise mechanisms remain unclear. (b) Multiple KLFs participate in cardiac muscle FA oxidation. Through its cooperation with ERR and PGC1α, KLF4 promotes mitochondrial health and function, including FA oxidation. KLF5 promotes PPARα transcription, whereas KLF15 cooperates with PPARα and p300 to foster oxidation. CBP, CREB-binding protein; PGC1α, PPAR γ coactivator 1-α; NCoR/SMRT, nuclear receptor corepressor/silencing mediator of retinoid and thyroid hormone receptor; Su, SUMOylated. [© 2019 Illustration Presentation ENDOCRINE SOCIETY]
“Genome-wide studies have identified important human KLF genetic variation associated with a multitude of metabolic pathologies.”
Much like the skeletal muscle KLFs, several cardiac KLFs broadly control lipid flux and oxidation in response to physiologic state, thereby enabling the enormous metabolic flexibility exhibited by the heart (Fig. 4b). Cardiomyocyte Klf15 is fasting inducible and requisite for cardiac adaptation to fasting (107). It is also elevated during postnatal maturation as the murine fetal heart transitions toward adulthood and dramatically increases its reliance on oxidative metabolism (108). Just as in skeletal muscle, higher plasma levels of fatty acids and triglycerides in the Klf15−/− mouse exist in stark contrast to the reduced levels of intramyocardial fatty acids and triglycerides, and mitochondria isolated from Klf15−/− mice oxidize long-chain fatty acids poorly (108). In this context, KLF15 associates with PPARα as well as coactivators such as p300 to cooperatively control lipid gene expression and transduce incoming physiologic signals to cellular metabolism (109). Recent work by Drosatos et al. (110) points to KLF5 as another regulator of fatty acid oxidation through its control of PPARα expression, suggesting that KLF control of cardiomyocyte fat metabolism occurs not only in cooperation with nuclear receptors but also at a level upstream.
In the heart, KLF control of oxidative metabolism extends to broad regulation of mitochondrial biogenesis, dynamics, and degradation. Deletion of cardiac KLF4 in mice reveals altered expression of mitochondrial genes, disordered mitochondrial morphology (e.g., fragmentation, reduced volume density, reduced mitochondrial genomic DNA), and impaired mitochondrial respiration (111). Specifically, both small and giant mitochondria accumulate in KLF4 knockout mouse hearts, suggestive of a defect in mitochondrial fission and fusion processes as well as autophagic clearance (111). These changes ultimately reduce cardiac resiliency in the face of high metabolic requirement, for example, during postnatal cardiac maturation, with normal aging, and in response to pressure overload (111, 112). KLF4 associates with PGC-1α and ERRα to form a tripartite molecular complex and cooperatively regulate transcription of common gene targets, notably those involved in mitochondrial biogenesis and autophagy; indeed, KLF4 is required for ERR/PGC-1α gene activation (111). Independent of ERR/PGC-1α, KLF4 also regulates several mitochondrial encoded genes (111).
Dysregulation of KLFs in Human Disease
Obesity and metabolic disease remain pervasive health concerns, increasing risk of multiple leading causes of mortality, including cardiovascular disease and cancer. Despite this, therapeutic options targeting these processes remain only partially effective at preventing or slowing progression of disease. Given the widespread reach of KLFs in physiological metabolic control, it is not surprising that genome-wide studies have identified important human KLF genetic variation associated with a multitude of metabolic pathologies. Variants in KLF14, KLF11, KLF7, and KLF15 are associated with diabetes, and KLF6, KLF7, KLF9, KLF13, and KLF15 are associated with increased body mass index (BMI). In addition, single nucleotide polymorphism (SNP) variants in KLF2 and KLF6 are associated with NAFLD, whereas KLF14 variation is also correlated with dyslipidemia and coronary artery disease (CAD). We focus here primarily on evidence of KLF involvement from human studies.
KLFs in diseases of glucose homeostasis
Numerous studies have implicated KLFs in human metabolic diseases of glucose intolerance and insulin resistance such as T2DM and maturity onset of diabetes in the young (MODY). Several different groups have recently identified SNPs in KLF14 that are associated with T2DM susceptibility (113–117). Although most disease-associated SNPs are cis-acting gain- or loss-of-function mutations, Small et al. (118) showed that SNPs within the KLF14 loci are also associated with trans-mediated changes of expression in other genes important in dictating metabolic parameters, such as insulin resistance. Remarkably, one gene that was identified as being under trans regulation of KLF14 in metabolic disease is KLF13, an example of intrafamily regulation not uncommon to the KLFs. Interestingly, in a recent follow-up study, the authors observed that the cis and trans effects of this KLF14 mutation disproportionately skew toward female mice (119). After ruling out sex hormone–based dimorphism, the authors attributed this phenomenon to females requiring higher basal levels of KLF14, as evidenced by elevated expression in adipose tissue of females compared with males. In addition to mutational events affecting KLF14 function, Bacos et al. (120) showed that epigenetic alterations in KLF14 might also contribute to metabolic disease. In pancreatic islets and blood, KLF14 methylation increases with age and is associated with altered insulin secretion commonly seen in the aging pancreas (120). These studies demonstrate that loss of KLF14 function through mutational or epigenetic means is highly associated with metabolic disease.
Variants of KLF11 are also associated with diabetes pathogenesis in humans. SNPs in KLF11 were first identified in the context of families with early-onset diabetes, hence its characterization as the causative mutation in MODY7 (35, 36). Also, one variant has been identified within the insulin promoter that prohibits KLF11 binding, leading to neonatal diabetes mellitus (34). Mechanistic investigation has elucidated widespread effects of KLF11 in glucose homeostasis. In pancreatic β cells, KLF11 is induced by glucose and augments insulin production (36). The Gln62Arg variant of KLF11 is a gain-of-function mutation that permits KLF11 binding of the corepressor mSin3A, eventually repressing insulin transcription (36). Conversely, the Ala347Ser variant of KLF11 inhibits the ability of the transcriptional regulatory domain 3 (TRD3) to bind to guanine nucleotide-binding protein β2 (Gβ2), thus diminishing glucose-stimulated insulin secretion (121). In addition to regulating insulin production, KLF11 interacts with p300 and promotes transcription of pancreatic-duodenal homeobox-1 (Pdx-1), a master regulator of pancreas development and function (34, 35). Interestingly, mutations in Pdx-1 are causative for MODY4 (122), suggesting there is a regulatory network of MODY genes in which KLF11 participates.
Although less well characterized, other KLFs have also been identified in genome-wide association and expression studies to play a role in diabetes including KLF7 and KLF15 (123–125). For example, the KLF15 rs9838915 SNP is associated with diabetic cardiomyopathy and was a predictor of hospitalization for heart failure (126). Considering the pervasive role of KLF15 in multiple facets of metabolism, it remains one of the most promising regulators to target in human metabolic disease and, as such, requires prompt and thorough exploration.
KLFs in obesity
Although there is considerable overlap between the pathogenesis of diabetes and obesity, several KLF variants are only associated with increased body mass and adiposity. For example, a link between BMI and KLF9 SNP exists in East Asian populations; however, it is not associated with increased risk of diabetes (127). Interestingly, the rs11142387 SNP of KLF9 demonstrates gene-gene interactions with an SNP at the myostatin (MSTN) locus, offering possible mechanistic insight that needs further exploration (127). Additional SNPs in KLF6 and KLF7 have been identified that are associated with obesity (128, 129). In addition to KLF SNP variants contributing to metabolic disease, alterations in KLF gene dosage within cells, such as that seen in copy number variations (CNVs), epigenetic silencing, and transcriptional regulation, have a demonstrated link to human obesity. In children with elevated BMI, KLF13 is hypermethylated in blood and metabolic cells (e.g., preadipocytes, pancreatic islets) (130). Interestingly, in their study, Koh et al. (130) also identified KLF13 SNPs in these patients, associated with hypermethylation of CpG sites, suggesting that polymorphisms in KLF13 may also dictate epigenetic changes that drive disease. In a recently published article exploring CNVs as a potential mechanism of disease in isolated cases of early-onset obesity, KLF15 CNV was observed to be associated with higher BMI (131). Unsurprisingly, adipose mRNA expression of KLF15 was also lower in these obese patients. Given the evidence that pathologic expression of multiple KLFs is capable of contributing to obesity, correction of KLF expression by genetic, epigenetic, or transcriptional means represents an exciting potential strategy in combating obesity.
KLFs in dyslipidemia
Abnormal concentrations of plasma lipids and carrier proteins are important risk factors for cardiovascular disease as well as for nonalcoholic fatty liver disease (NAFLD), both of which are associated with development of metabolic syndrome. Because KLFs are important effectors of lipid handling, it is unsurprising that KLF variants are seen in human dyslipidemia. One particular SNP at the KLF14 locus is associated with decreased high-density lipoprotein cholesterol and an elevated risk for CAD (119, 132). Similar to trends seen with glucose dynamics (119), there are also sex-specific differences in lipid profiles between males and females with the rs4731702 KLF14 SNP, further demonstrating the predilection of this variant to more robustly affect female patients (119, 132).
NAFLD is a consequence of increased hepatic triglyceride accumulation, eventually leading to inflammatory damage and fibrosis. Although KLF variants contributing to dyslipidemia may also increase risk for NAFLD development, there is a paucity of literature to confirm this. Instead, studies suggest that SNPs in KLFs may dictate the severity of progression in patients with NAFLD. Indeed, two reports identify a loss-of-function KLF6 SNP, which is linked to advanced NAFLD (43, 133, 134), and another two have identified a loss-of-function SNP in KLF6 that decreases risk of fibrotic progression in patients with NAFLD (135, 136). Interestingly, one splice variant of KLF6 shown to be protective against NAFLD progression, KLF6-IVS1-27A, also protects against other metabolic predictors of NAFLD severity, such as insulin resistance by restricting KLF6’s activation of glucokinase transcription (134). In addition to KLF6, KLF2 has also been linked to mouse models of NAFLD. KLF2 expression promotes NAFLD by positively regulating hepatocyte expression of CD36, a cell surface receptor responsible for fatty acid uptake into the liver (137). Currently, however, no human correlates have been found implicating KLF2 variants with amelioration of NAFLD.
Dyslipidemia is also highly correlated with the progression of atherosclerosis . The KLFs are heavily implicated in the pathogenesis of atherosclerosis, predominantly through their involvement in the vascular inflammatory response to oxidized low-density lipoprotein (138). As previously discussed, SNPs in KLF14 are associated with CAD, a manifestation of atherosclerosis (132). Despite the large body of evidence in mice and cell culture implicating KLFs in the formation of atherosclerosis, there is a relative dearth of evidence describing patients with vascular disease who have KLF mutations. Additional investigation is necessary to fully understand the importance of KLFs in human atherosclerotic disease.
Conclusion
Evidence over the past two decades has positioned the KLFs as essential regulators of metabolism by virtue of their own actions and coordination with canonical nuclear receptor signaling. Alone or in concert with coregulators, KLFs affect a broad spectrum of metabolic functions in nearly every major organ and in response to diverse physiologic needs. KLF transcriptional output directs nutrient intake, systemic trafficking (i.e., glucose flux, lipid partitioning, amino acid catabolism), and oxidation for energy. The physiologic importance of the KLFs is reflected in genome-wide association studies and other large-scale studies that routinely identify KLF genetic variants associated with metabolic disease. Many aspects of KLF regulation of metabolism remain to be elucidated. The profound impact of this complex family of metabolic regulators on human health and disease is only now becoming clear, and it is expected that future investigations will assign new metabolic roles to the KLFs.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health (NIH) Grants R01DK111478, R35HL135789, and R01HL086548 (to M.K.J.); T32GM007250 (to P.N.H., L.F., and D.R.S.), F30AG054237 (to P.N.H.), T32HL134622 (to L.F.), and F30HL139014 (to D.R.S.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- AgRP
Agouti-related protein
- BCAA
branched-chain amino acid
- BMI
body mass index
- C/EBP
CCAAT/element binding protein
- CAD
coronary artery disease
- CNV
copy number variation
- ERR
estrogen-related receptor
- Fgf15
fibroblast growth factor
- FXR
farnesoid X receptor
- GI
gastrointestinal
- GR
glucocorticoid receptor
- KLF
Krüppel-like factor
- MODY
maturity onset of diabetes in the young
- NAFLD
nonalcoholic fatty liver disease
- Pepck
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome proliferator–activated receptor-γ coactivator 1α
- PPAR
peroxisome proliferator-activated receptor
- SNP
single nucleotide polymorphism
- SREBP
sterol regulatory element binding protein
- T2DM
type 2 diabetes mellitus
- WAT
white adipose tissue
References
- 1. Desvergne B, Michalik L, Wahli W. Transcriptional regulation of metabolism. Physiol Rev. 2006;86(2):465–514. [DOI] [PubMed] [Google Scholar]
- 2. Lempradl A, Pospisilik JA, Penninger JM. Exploring the emerging complexity in transcriptional regulation of energy homeostasis. Nat Rev Genet. 2015;16(11):665–681. [DOI] [PubMed] [Google Scholar]
- 3. Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the Big Bang. Cell. 2014;157(1):255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oñate SA, Tsai SY, Tsai MJ, O’Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270(5240):1354–1357. [DOI] [PubMed] [Google Scholar]
- 5. Debevec D, Christian M, Morganstein D, Seth A, Herzog B, Parker M, White R. Receptor interacting protein 140 regulates expression of uncoupling protein 1 in adipocytes through specific peroxisome proliferator activated receptor isoforms and estrogen-related receptor alpha. Mol Endocrinol. 2007;21(7):1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77(1):289–312. [DOI] [PubMed] [Google Scholar]
- 7. Oishi Y, Manabe I. Krüppel-like factors in metabolic homeostasis and cardiometabolic disease. Front Cardiovasc Med. 2018;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev. 2010;90(4):1337–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gray S, Feinberg MW, Hull S, Kuo CT, Watanabe M, Sen-Banerjee S, DePina A, Haspel R, Jain MK. The Krüppel-like factor KLF15 regulates the insulin-sensitive glucose transporter GLUT4. J Biol Chem. 2002;277(37):34322–34328. [DOI] [PubMed] [Google Scholar]
- 10. Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281(5):1220–1225. [DOI] [PubMed] [Google Scholar]
- 11. Coll AP, Farooqi IS, O’Rahilly S. The hormonal control of food intake. Cell. 2007;129(2):251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee HJ, Kang YM, Moon CS, Joe MK, Lim JH, Suh YH, Song J, Jung MH. KLF4 positively regulates human ghrelin expression. Biochem J. 2009;420(3):403–411. [DOI] [PubMed] [Google Scholar]
- 13. Imbernon M, Sanchez-Rebordelo E, Gallego R, Gandara M, Lear P, Lopez M, Dieguez C, Nogueiras R. Hypothalamic KLF4 mediates leptin’s effects on food intake via AgRP. Mol Metab. 2014;3(4):441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawamura Y, Tanaka Y, Kawamori R, Maeda S. Overexpression of Kruppel-like factor 7 regulates adipocytokine gene expressions in human adipocytes and inhibits glucose-induced insulin secretion in pancreatic beta-cell line. Mol Endocrinol. 2006;20(4):844–856. [DOI] [PubMed] [Google Scholar]
- 15. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. [DOI] [PubMed] [Google Scholar]
- 16. Coope A, Milanski M, Araújo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett. 2008;582(10):1471–1476. [DOI] [PubMed] [Google Scholar]
- 17. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–225. [DOI] [PubMed] [Google Scholar]
- 18. Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50(10):1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han S, Zhang R, Jain R, Shi H, Zhang L, Zhou G, Sangwung P, Tugal D, Atkins GB, Prosdocimo DA, Lu Y, Han X, Tso P, Liao X, Epstein JA, Jain MK. Circadian control of bile acid synthesis by a KLF15-Fgf15 axis [published correction appears in Nat Commun. 2015;6:8270]. Nat Commun. 2015;6(1):7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reidling JC, Said HM. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: confirmation of promoter activity in vivo. Am J Physiol Cell Physiol. 2007;292(4):C1305–C1312. [DOI] [PubMed] [Google Scholar]
- 22. Liuzzi JP, Guo L, Chang SM, Cousins RJ. Krüppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2009;296(3):G517–G523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furumiya M, Inoue K, Ohta K, Hayashi Y, Yuasa H. Transcriptional regulation of PCFT by KLF4, HNF4α, CDX2 and C/EBPα: implication in its site-specific expression in the small intestine. Biochem Biophys Res Commun. 2013;431(2):158–163. [DOI] [PubMed] [Google Scholar]
- 24. Flandez M, Guilmeau S, Blache P, Augenlicht LH. KLF4 regulation in intestinal epithelial cell maturation. Exp Cell Res. 2009;314(20):3712–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuruvilla JG, Kim CK, Ghaleb AM, Bialkowska AB, Kuo CJ, Yang VW. Krüppel-like factor 4 modulates development of BMI1(+) intestinal stem cell-derived lineage following γ-radiation-induced gut injury in mice. Stem Cell Reports. 2016;6(6):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu T, Chen X, Zhang W, Li J, Xu R, Wang TC, Ai W, Liu C. Krüppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS One. 2012;7(2):e32492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem. 1996;271(33):20009–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conkright MD, Wani MA, Anderson KP, Lingrel JB. A gene encoding an intestinal-enriched member of the Krüppel-like factor family expressed in intestinal epithelial cells. Nucleic Acids Res. 1999;27(5):1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nandan MO, Ghaleb AM, Liu Y, Bialkowska AB, McConnell BB, Shroyer KR, Robine S, Yang VW. Inducible intestine-specific deletion of Krüppel-like factor 5 is characterized by a regenerative response in adult mouse colon. Dev Biol. 2014;387(2):191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nandan MO, Ghaleb AM, Bialkowska AB, Yang VW. Krüppel-like factor 5 is essential for proliferation and survival of mouse intestinal epithelial stem cells. Stem Cell Res (Amst). 2015;14(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuruvilla JG, Ghaleb AM, Bialkowska AB, Nandan MO, Yang VW. Role of Krüppel-like factor 5 in the maintenance of the stem cell niche in the intestinal crypt. Stem Cell Transl Investig. 2015;2(2):e839. [PMC free article] [PubMed] [Google Scholar]
- 32. McConnell BB, Kim SS, Yu K, Ghaleb AM, Takeda N, Manabe I, Nusrat A, Nagai R, Yang VW. Kruppel-like factor 5 is important for maintenance of crypt architecture and barrier function in mouse intestine. Gastroenterology. 2011;141(4):1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Y, Chidgey M, Yang VW, Bialkowska AB. Krüppel-like factor 5 is essential for maintenance of barrier function in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2017;313(5):G478–G491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonnefond A, Lomberk G, Buttar N, Busiah K, Vaillant E, Lobbens S, Yengo L, Dechaume A, Mignot B, Simon A, Scharfmann R, Neve B, Tanyolaç S, Hodoglugil U, Pattou F, Cavé H, Iovanna J, Stein R, Polak M, Vaxillaire M, Froguel P, Urrutia R. Disruption of a novel Kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.-331 INS mutation found in neonatal diabetes mellitus. J Biol Chem. 2011;286(32):28414–28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez-Zapico ME, van Velkinburgh JC, Gutiérrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem. 2009;284(52):36482–36490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clément K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci USA. 2005;102(13):4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perakakis N, Danassi D, Alt M, Tsaroucha E, Mehana AE, Rimmer N, Laubner K, Wang H, Wollheim CB, Seufert J, Päth G. Human Krüppel-like factor 11 differentially regulates human insulin promoter activity in β-cells and non-β-cells via p300 and PDX1 through the regulatory sites A3 and CACCC box. Mol Cell Endocrinol. 2012;363(1-2):20–26. [DOI] [PubMed] [Google Scholar]
- 38. Nagare T, Sakaue H, Matsumoto M, Cao Y, Inagaki K, Sakai M, Takashima Y, Nakamura K, Mori T, Okada Y, Matsuki Y, Watanabe E, Ikeda K, Taguchi R, Kamimura N, Ohta S, Hiramatsu R, Kasuga M. Overexpression of KLF15 transcription factor in adipocytes of mice results in down-regulation of SCD1 protein expression in adipocytes and consequent enhancement of glucose-induced insulin secretion. J Biol Chem. 2011;286(43):37458–37469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Matoba K, Lu Y, Zhang R, Chen ER, Sangwung P, Wang B, Prosdocimo DA, Jain MK. Adipose KLF15 controls lipid handling to adapt to nutrient availability. Cell Reports. 2017;21(11):3129–3140. [DOI] [PubMed] [Google Scholar]
- 40. Bell-Anderson KS, Funnell AP, Williams H, Mat Jusoh H, Scully T, Lim WF, Burdach JG, Mak KS, Knights AJ, Hoy AJ, Nicholas HR, Sainsbury A, Turner N, Pearson RC, Crossley M. Loss of Krüppel-like factor 3 (KLF3/BKLF) leads to upregulation of the insulin-sensitizing factor adipolin (FAM132A/CTRP12/C1qdc2). Diabetes. 2013;62(8):2728–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Enomoto T, Ohashi K, Shibata R, Higuchi A, Maruyama S, Izumiya Y, Walsh K, Murohara T, Ouchi N. Adipolin/C1qdc2/CTRP12 protein functions as an adipokine that improves glucose metabolism. J Biol Chem. 2011;286(40):34552–34558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang M, Ren Y, Lin Z, Tang C, Jia Y, Lai Y, Zhou T, Wu S, Liu H, Yang G, Li L. Krüppel-like factor 14 increases insulin sensitivity through activation of PI3K/Akt signal pathway. Cell Signal. 2015;27(11):2201–2208. [DOI] [PubMed] [Google Scholar]
- 43. Bechmann LP, Vetter D, Ishida J, Hannivoort RA, Lang UE, Kocabayoglu P, Fiel MI, Muñoz U, Patman GL, Ge F, Yakar S, Li X, Agius L, Lee YM, Zhang W, Hui KY, Televantou D, Schwartz GJ, LeRoith D, Berk PD, Nagai R, Suzuki T, Reeves HL, Friedman SL. Post-transcriptional activation of PPAR alpha by KLF6 in hepatic steatosis. J Hepatol. 2013;58(5):1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chanson P, Salenave S.. Metabolic syndrome in Cushing's syndrome. Neuroendocrinology. 2010;92(suppl 1):96–101. [DOI] [PubMed] [Google Scholar]
- 45. Knoedler JR, Denver RJ. Krüppel-like factors are effectors of nuclear receptor signaling. Gen Comp Endocrinol. 2014;203:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cruz-Topete D, He B, Xu X, Cidlowski JA. Krüppel-like factor 13 is a major mediator of glucocorticoid receptor signaling in cardiomyocytes and protects these cells from DNA damage and death. J Biol Chem. 2016;291(37):19374–19386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bagamasbad P, Ziera T, Borden SA, Bonett RM, Rozeboom AM, Seasholtz A, Denver RJ. Molecular basis for glucocorticoid induction of the Kruppel-like factor 9 gene in hippocampal neurons. Endocrinology. 2012;153(11):5334–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, Stockmeier CA, Meyer JH, Urrutia R, Miczek KA, Austin MC, Wang J, Paul IA, Woolverton WL, Seo S, Sittman DB, Ou XM. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287(29):24195–24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Morrison-Nozik A, Anand P, Zhu H, Duan Q, Sabeh M, Prosdocimo DA, Lemieux ME, Nordsborg N, Russell AP, MacRae CA, Gerber AN, Jain MK, Haldar SM. Glucocorticoids enhance muscle endurance and ameliorate Duchenne muscular dystrophy through a defined metabolic program. Proc Natl Acad Sci USA. 2015;112(49):E6780–E6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Asada M, Rauch A, Shimizu H, Maruyama H, Miyaki S, Shibamori M, Kawasome H, Ishiyama H, Tuckermann J, Asahara H. DNA binding-dependent glucocorticoid receptor activity promotes adipogenesis via Krüppel-like factor 15 gene expression. Lab Invest. 2011;91(2):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M. Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem. 2005;280(13):12867–12875. [DOI] [PubMed] [Google Scholar]
- 52. Zhang H, Chen Q, Jiao T, Cui A, Sun X, Fang W, Xie L, Liu Y, Fang F, Chang Y. Involvement of KLF11 in hepatic glucose metabolism in mice via suppressing of PEPCK-C expression. PLoS One. 2014;9(2):e89552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Teshigawara K, Ogawa W, Mori T, Matsuki Y, Watanabe E, Hiramatsu R, Inoue H, Miyake K, Sakaue H, Kasuga M. Role of Krüppel-like factor 15 in PEPCK gene expression in the liver. Biochem Biophys Res Commun. 2005;327(3):920–926. [DOI] [PubMed] [Google Scholar]
- 54. Wang M, Tang L, Liu D, Ying QL, Ye S. The transcription factor Gbx2 induces expression of Kruppel-like factor 4 to maintain and induce naïve pluripotency of embryonic stem cells. J Biol Chem. 2017;292(41):17121–17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang X, Chen Q, Sun L, Zhang H, Yao L, Cui X, Gao Y, Fang F, Chang Y. KLF10 transcription factor regulates hepatic glucose metabolism in mice. Diabetologia. 2017;60(12):2443–2452. [DOI] [PubMed] [Google Scholar]
- 56. Takashima M, Ogawa W, Hayashi K, Inoue H, Kinoshita S, Okamoto Y, Sakaue H, Wataoka Y, Emi A, Senga Y, Matsuki Y, Watanabe E, Hiramatsu R, Kasuga M. Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes. 2010;59(7):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gray S, Wang B, Orihuela Y, Hong EG, Fisch S, Haldar S, Cline GW, Kim JK, Peroni OD, Kahn BB, Jain MK. Regulation of gluconeogenesis by Krüppel-like factor 15. Cell Metab. 2007;5(4):305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeyaraj D, Scheer FA, Ripperger JA, Haldar SM, Lu Y, Prosdocimo DA, Eapen SJ, Eapen BL, Cui Y, Mahabeleshwar GH, Lee HG, Smith MA, Casadesus G, Mintz EM, Sun H, Wang Y, Ramsey KM, Bass J, Shea SA, Albrecht U, Jain MK. Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 2012;15(3):311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Guillaumond F, Gréchez-Cassiau A, Subramaniam M, Brangolo S, Peteri-Brünback B, Staels B, Fiévet C, Spelsberg TC, Delaunay F, Teboul M. Kruppel-like factor KLF10 is a link between the circadian clock and metabolism in liver. Mol Cell Biol. 2010;30(12):3059–3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26(5):423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26(5):926–941. [DOI] [PubMed] [Google Scholar]
- 62. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. [DOI] [PubMed] [Google Scholar]
- 63. Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes. 2002;51(9):2684–2690. [DOI] [PubMed] [Google Scholar]
- 64. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. [DOI] [PubMed] [Google Scholar]
- 65. Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol. 2010;323(1):20–34. [DOI] [PubMed] [Google Scholar]
- 66. Pollak NM, Hoffman M, Goldberg IJ, Drosatos K. Krüppel-like factors: crippling and un-crippling metabolic pathways. JACC Basic Transl Sci. 2018;3(1):132–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Birsoy K, Chen Z, Friedman J. Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 2008;7(4):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rivero S, Díaz-Guerra MJ, Monsalve EM, Laborda J, García-Ramírez JJ. DLK2 is a transcriptional target of KLF4 in the early stages of adipogenesis. J Mol Biol. 2012;417(1-2):36–50. [DOI] [PubMed] [Google Scholar]
- 69. Eisenstein A, Carroll SH, Johnston-Cox H, Farb M, Gokce N, Ravid K. An adenosine receptor-Krüppel-like factor 4 protein axis inhibits adipogenesis. J Biol Chem. 2014;289(30):21071–21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee H, Kim HJ, Lee YJ, Lee MY, Choi H, Lee H, Kim JW. Krüppel-like factor KLF8 plays a critical role in adipocyte differentiation. PLoS One. 2012;7(12):e52474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kimura H, Fujimori K. Activation of early phase of adipogenesis through Krüppel-like factor KLF9-mediated, enhanced expression of CCAAT/enhancer-binding protein β in 3T3-L1 cells. Gene. 2014;534(2):169–176. [DOI] [PubMed] [Google Scholar]
- 72. Pei H, Yao Y, Yang Y, Liao K, Wu JR. Krüppel-like factor KLF9 regulates PPARγ transactivation at the middle stage of adipogenesis. Cell Death Differ. 2011;18(2):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jiang S, Wei H, Song T, Yang Y, Zhang F, Zhou Y, Peng J, Jiang S. KLF13 promotes porcine adipocyte differentiation through PPARγ activation. Cell Biosci. 2015;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1(1):27–39. [DOI] [PubMed] [Google Scholar]
- 75. Cervantes-Camacho C, Beltrán-Langarica A, Ochoa-Uribe AK, Marsch-Moreno M, Ayala-Sumuano JT, Velez-delValle C, Kuri-Harcuch W. The transient expression of Klf4 and Klf5 during adipogenesis depends on GSK3β activity. Adipocyte. 2015;4(4):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ambele MA, Dessels C, Durandt C, Pepper MS. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res (Amst). 2016;16(3):725–734. [DOI] [PubMed] [Google Scholar]
- 77. Aggarwal A, Costa MJ, Rivero-Gutiérrez B, Ji L, Morgan SL, Feldman BJ. The circadian clock regulates adipogenesis by a Per3 crosstalk pathway to Klf15. Cell Reports. 2017;21(9):2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ahmed M, Gaffen SL. IL-17 inhibits adipogenesis in part via C/EBPα, PPARγ and Krüppel-like factors. Cytokine. 2013;61(3):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J Biol Chem. 2003;278(4):2581–2584. [DOI] [PubMed] [Google Scholar]
- 80. Wu J, Srinivasan SV, Neumann JC, Lingrel JB. The KLF2 transcription factor does not affect the formation of preadipocytes but inhibits their differentiation into adipocytes. Biochemistry. 2005;44(33):11098–11105. [DOI] [PubMed] [Google Scholar]
- 81. Cho H, Kim KM, Han S, Choe J, Park SG, Choi SS, Kim YK. Staufen1-mediated mRNA decay functions in adipogenesis. Mol Cell. 2012;46(4):495–506. [DOI] [PubMed] [Google Scholar]
- 82. Sue N, Jack BH, Eaton SA, Pearson RC, Funnell AP, Turner J, Czolij R, Denyer G, Bao S, Molero-Navajas JC, Perkins A, Fujiwara Y, Orkin SH, Bell-Anderson K, Crossley M. Targeted disruption of the basic Krüppel-like factor gene (Klf3) reveals a role in adipogenesis. Mol Cell Biol. 2008;28(12):3967–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Li D, Yea S, Li S, Chen Z, Narla G, Banck M, Laborda J, Tan S, Friedman JM, Friedman SL, Walsh MJ. Krüppel-like factor-6 promotes preadipocyte differentiation through histone deacetylase 3-dependent repression of DLK1. J Biol Chem. 2005;280(29):26941–26952. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto K, Sakaguchi M, Medina RJ, Niida A, Sakaguchi Y, Miyazaki M, Kataoka K, Huh NH. Transcriptional regulation of a brown adipocyte-specific gene, UCP1, by KLF11 and KLF15. Biochem Biophys Res Commun. 2010;400(1):175–180. [DOI] [PubMed] [Google Scholar]
- 85. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Loft A, Forss I, Siersbæk MS, Schmidt SF, Larsen AS, Madsen JG, Pisani DF, Nielsen R, Aagaard MM, Mathison A, Neville MJ, Urrutia R, Karpe F, Amri EZ, Mandrup S. Browning of human adipocytes requires KLF11 and reprogramming of PPARγ superenhancers. Genes Dev. 2015;29(1):7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jang MK, Lee S, Jung MH. RNA-Seq analysis reveals a negative role of KLF16 in adipogenesis. PLoS One. 2016;11(9):e0162238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Guo Y, Fan Y, Zhang J, Lomberk GA, Zhou Z, Sun L, Mathison AJ, Garcia-Barrio MT, Zhang J, Zeng L, Li L, Pennathur S, Willer CJ, Rader DJ, Urrutia R, Chen YE. Perhexiline activates KLF14 and reduces atherosclerosis by modulating ApoA-I production. J Clin Invest. 2015;125(10):3819–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang J, Bakheet R, Parhar RS, Huang CH, Hussain MM, Pan X, Siddiqui SS, Hashmi S. Regulation of fat storage and reproduction by Krüppel-like transcription factor KLF3 and fat-associated genes in Caenorhabditis elegans. J Mol Biol. 2011;411(3):537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang J, Hashmi S, Cheema F, Al-Nasser N, Bakheet R, Parhar RS, Al-Mohanna F, Gaugler R, Hussain MM, Hashmi S. Regulation of lipoprotein assembly, secretion and fatty acid β-oxidation by Krüppel-like transcription factor, klf-3. J Mol Biol. 2013;425(15):2641–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Iizuka K, Takeda J, Horikawa Y. Krüppel-like factor-10 is directly regulated by carbohydrate response element-binding protein in rat primary hepatocytes. Biochem Biophys Res Commun. 2011;412(4):638–643. [DOI] [PubMed] [Google Scholar]
- 92. Takeuchi Y, Yahagi N, Aita Y, Murayama Y, Sawada Y, Piao X, Toya N, Oya Y, Shikama A, Takarada A, Masuda Y, Nishi M, Kubota M, Izumida Y, Yamamoto T, Sekiya M, Matsuzaka T, Nakagawa Y, Urayama O, Kawakami Y, Iizuka Y, Gotoda T, Itaka K, Kataoka K, Nagai R, Kadowaki T, Yamada N, Lu Y, Jain MK, Shimano H. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting. Cell Reports. 2016;16(9):2373–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jung DY, Chalasani U, Pan N, Friedline RH, Prosdocimo DA, Nam M, Azuma Y, Maganti R, Yu K, Velagapudi A, O’Sullivan-Murphy B, Sartoretto JL, Jain MK, Cooper MP, Urano F, Kim JK, Gray S. KLF15 is a molecular link between endoplasmic reticulum stress and insulin resistance. PLoS One. 2013;8(10):e77851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fan L, Hsieh PN, Sweet DR, Jain MK. Krüppel-like factor 15: Regulator of BCAA metabolism and circadian protein rhythmicity. Pharmacol Res. 2018;130:123–126. [DOI] [PubMed] [Google Scholar]
- 95. Zhang L, Prosdocimo DA, Bai X, Fu C, Zhang R, Campbell F, Liao X, Coller J, Jain MK. KLF15 establishes the landscape of diurnal expression in the heart. Cell Reports. 2015;13(11):2368–2375. [DOI] [PubMed] [Google Scholar]
- 96. Hsieh PN, Zhang L, Jain MK. Coordination of cardiac rhythmic output and circadian metabolic regulation in the heart. Cell Mol Life Sci. 2018;75(3):403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Haldar SM, Lu Y, Jeyaraj D, Kawanami D, Cui Y, Eapen SJ, Hao C, Li Y, Doughman YQ, Watanabe M, Shimizu K, Kuivaniemi H, Sadoshima J, Margulies KB, Cappola TP, Jain MK. Klf15 deficiency is a molecular link between heart failure and aortic aneurysm formation. Sci Transl Med. 2010;2(26):26ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto YM, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy [published correction appears in Proc Natl Acad Sci USA. 2007;104(34):13851]. Proc Natl Acad Sci USA. 2007;104(17):7074–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, Jeyaraj D, Youn JY, Ren S, Liu Y, Rau CD, Shah S, Ilkayeva O, Gui WJ, William NS, Wynn RM, Newgard CB, Cai H, Xiao X, Chuang DT, Schulze PC, Lynch C, Jain MK, Wang Y.. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure.Circulation. 2016;133(21):2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Walter LM, Deguise MO, Meijboom KE, Betts CA, Ahlskog N, van Westering TLE, Hazell G, McFall E, Kordala A, Hammond SM, Abendroth F, Murray LM, Shorrock HK, Prosdocimo DA, Haldar SM, Jain MK, Gillingwater TH, Claus P, Kothary R, Wood MJA, Bowerman M. Interventions targeting glucocorticoid-Krüppel-like factor 15-branched-chain amino acid signaling improve disease phenotypes in spinal muscular atrophy mice. EBioMedicine. 2018;31:226–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275(2 Pt 1):E249–E258. [DOI] [PubMed] [Google Scholar]
- 102. Müller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3(2):113–122. [DOI] [PubMed] [Google Scholar]
- 103. Oishi Y, Manabe I, Tobe K, Ohsugi M, Kubota T, Fujiu K, Maemura K, Kubota N, Kadowaki T, Nagai R. SUMOylation of Krüppel-like transcription factor 5 acts as a molecular switch in transcriptional programs of lipid metabolism involving PPAR-delta. Nat Med. 2008;14(6):656–666. [DOI] [PubMed] [Google Scholar]
- 104. Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. [DOI] [PubMed] [Google Scholar]
- 105. Haldar SM, Jeyaraj D, Anand P, Zhu H, Lu Y, Prosdocimo DA, Eapen B, Kawanami D, Okutsu M, Brotto L, Fujioka H, Kerner J, Rosca MG, McGuinness OP, Snow RJ, Russell AP, Gerber AN, Bai X, Yan Z, Nosek TM, Brotto M, Hoppel CL, Jain MK. Kruppel-like factor 15 regulates skeletal muscle lipid flux and exercise adaptation. Proc Natl Acad Sci USA. 2012;109(17):6739–6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yamamoto J, Ikeda Y, Iguchi H, Fujino T, Tanaka T, Asaba H, Iwasaki S, Ioka RX, Kaneko IW, Magoori K, Takahashi S, Mori T, Sakaue H, Kodama T, Yanagisawa M, Yamamoto TT, Ito S, Sakai J. A Kruppel-like factor KLF15 contributes fasting-induced transcriptional activation of mitochondrial acetyl-CoA synthetase gene AceCS2. J Biol Chem. 2004;279(17):16954–16962. [DOI] [PubMed] [Google Scholar]
- 107. Sugi K, Hsieh PN, Ilkayeva O, Shelkay S, Moroney B, Baadh P, Haynes B, Pophal M, Fan L, Newgard CB, Prosdocimo DA, Jain MK. Kruppel-like factor 15 is required for the cardiac adaptive response to fasting. PLoS One. 2018;13(2):e0192376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Prosdocimo DA, Anand P, Liao X, Zhu H, Shelkay S, Artero-Calderon P, Zhang L, Kirsh J, Moore D, Rosca MG, Vazquez E, Kerner J, Akat KM, Williams Z, Zhao J, Fujioka H, Tuschl T, Bai X, Schulze PC, Hoppel CL, Jain MK, Haldar SM. Kruppel-like factor 15 is a critical regulator of cardiac lipid metabolism. J Biol Chem. 2014;289(9):5914–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Prosdocimo DA, John JE, Zhang L, Efraim ES, Zhang R, Liao X, Jain MK. KLF15 and PPARα cooperate to regulate cardiomyocyte lipid gene expression and oxidation. PPAR Res. 2015;2015:201625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Drosatos K, Pollak NM, Pol CJ, Ntziachristos P, Willecke F, Valenti MC, Trent CM, Hu Y, Guo S, Aifantis I, Goldberg IJ. Cardiac myocyte KLF5 regulates PPARα expression and cardiac function. Circ Res. 2016;118(2):241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Liao X, Zhang R, Lu Y, Prosdocimo DA, Sangwung P, Zhang L, Zhou G, Anand P, Lai L, Leone TC, Fujioka H, Ye F, Rosca MG, Hoppel CL, Schulze PC, Abel ED, Stamler JS, Kelly DP, Jain MK. Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis. J Clin Invest. 2015;125(9):3461–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hsieh PN, Sweet DR, Fan L, Jain MK. Aging and the Krüppel-like factors. Trends Cell Mol Biol. 2017;12:1–15. [PMC free article] [PubMed] [Google Scholar]
- 113. Long J, Edwards T, Signorello LB, Cai Q, Zheng W, Shu XO, Blot WJ. Evaluation of genome-wide association study-identified type 2 diabetes loci in African Americans. Am J Epidemiol. 2012;176(11):995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nielsen T, Sparsø T, Grarup N, Jørgensen T, Pisinger C, Witte DR, Hansen T, Pedersen O; Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium . Type 2 diabetes risk allele near CENTD2 is associated with decreased glucose-stimulated insulin release. Diabetologia. 2011;54(5):1052–1056. [DOI] [PubMed] [Google Scholar]
- 115. Ohshige T, Iwata M, Omori S, Tanaka Y, Hirose H, Kaku K, Maegawa H, Watada H, Kashiwagi A, Kawamori R, Tobe K, Kadowaki T, Nakamura Y, Maeda S. Association of new loci identified in European genome-wide association studies with susceptibility to type 2 diabetes in the Japanese. PLoS One. 2011;6(10):e26911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Rees SD, Hydrie MZ, Shera AS, Kumar S, O’Hare JP, Barnett AH, Basit A, Kelly MA. Replication of 13 genome-wide association (GWA)-validated risk variants for type 2 diabetes in Pakistani populations. Diabetologia. 2011;54(6):1368–1374. [DOI] [PubMed] [Google Scholar]
- 117. Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Bengtsson Boström K, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney AS, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PR, Jørgensen T, Kao WH, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JR, Petersen AK, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CN, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann HE, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI; MAGIC Investigators; GIANT Consortium . Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42(7):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Small KS, Hedman AK, Grundberg E, Nica AC, Thorleifsson G, Kong A, Thorsteindottir U, Shin SY, Richards HB, Soranzo N, Ahmadi KR, Lindgren CM, Stefansson K, Dermitzakis ET, Deloukas P, Spector TD, McCarthy MI; GIANT Consortium; MAGIC Investigators; DIAGRAM Consortium; MuTHER Consortium . Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes [published correction appears in Nat Genet. 2011;43;1040]. Nat Genet. 2011;43(6):561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Small KS, Todorčević M, Civelek M, El-Sayed Moustafa JS, Wang X, Simon MM, Fernandez-Tajes J, Mahajan A, Horikoshi M, Hugill A, Glastonbury CA, Quaye L, Neville MJ, Sethi S, Yon M, Pan C, Che N, Viñuela A, Tsai PC, Nag A, Buil A, Thorleifsson G, Raghavan A, Ding Q, Morris AP, Bell JT, Thorsteinsdottir U, Stefansson K, Laakso M, Dahlman I, Arner P, Gloyn AL, Musunuru K, Lusis AJ, Cox RD, Karpe F, McCarthy MI. Regulatory variants at KLF14 influence type 2 diabetes risk via a female-specific effect on adipocyte size and body composition [published correction appears in Nat Genet. 2018;50:1342] Nat Genet. 2018;50(4):572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bacos K, Gillberg L, Volkov P, Olsson AH, Hansen T, Pedersen O, Gjesing AP, Eiberg H, Tuomi T, Almgren P, Groop L, Eliasson L, Vaag A, Dayeh T, Ling C. Blood-based biomarkers of age-associated epigenetic changes in human islets associate with insulin secretion and diabetes. Nat Commun. 2016;7:11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lomberk G, Grzenda A, Mathison A, Escande C, Zhang JS, Calvo E, Miller LJ, Iovanna J, Chini EN, Fernandez-Zapico ME, Urrutia R. Krüppel-like factor 11 regulates the expression of metabolic genes via an evolutionarily conserved protein interaction domain functionally disrupted in maturity onset diabetes of the young. J Biol Chem. 2013;288(24):17745–17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sachdeva MM, Claiborn KC, Khoo C, Yang J, Groff DN, Mirmira RG, Stoffers DA. Pdx1 (MODY4) regulates pancreatic beta cell susceptibility to ER stress. Proc Natl Acad Sci USA. 2009;106(45):19090–19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kanazawa A, Kawamura Y, Sekine A, Iida A, Tsunoda T, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kawai K, Iiizumi T, Fujioka T, Imanishi M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Single nucleotide polymorphisms in the gene encoding Krüppel-like factor 7 are associated with type 2 diabetes. Diabetologia. 2005;48(7):1315–1322. [DOI] [PubMed] [Google Scholar]
- 124. Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, Das SK. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes. 2011;60(3):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kulyté A, Ehrlund A, Arner P, Dahlman I. Global transcriptome profiling identifies KLF15 and SLC25A10 as modifiers of adipocytes insulin sensitivity in obese women. PLoS One. 2017;12(6):e0178485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Patel SK, Wai B, Lang CC, Levin D, Palmer CNA, Parry HM, Velkoska E, Harrap SB, Srivastava PM, Burrell LM. Genetic variation in Kruppel like factor 15 is associated with left ventricular hypertrophy in patients with type 2 diabetes: discovery and replication cohorts. EBioMedicine. 2017;18:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Okada Y, Kubo M, Ohmiya H, Takahashi A, Kumasaka N, Hosono N, Maeda S, Wen W, Dorajoo R, Go MJ, Zheng W, Kato N, Wu JY, Lu Q, Tsunoda T, Yamamoto K, Nakamura Y, Kamatani N, Tanaka T; GIANT consortium . Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet. 2012;44(3):302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Zobel DP, Andreasen CH, Burgdorf KS, Andersson EA, Sandbaek A, Lauritzen T, Borch-Johnsen K, Jørgensen T, Maeda S, Nakamura Y, Eiberg H, Pedersen O, Hansen T. Variation in the gene encoding Krüppel-like factor 7 influences body fat: studies of 14 818 Danes [published correction appears in Eur J Endocrinol. 2010;162(1):191] Eur J Endocrinol. 2009;160(4):603–609. [DOI] [PubMed] [Google Scholar]
- 129. Salinas YD, Wang L, DeWan AT. Multiethnic genome-wide association study identifies ethnic-specific associations with body mass index in Hispanics and African Americans. BMC Genet. 2016;17(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Koh IU, Lee HJ, Hwang JY, Choi NH, Lee S. Obesity-related CpG methylation (cg07814318) of Kruppel-like factor-13 (KLF13) gene with childhood obesity and its cis-methylation quantitative loci. Sci Rep. 2017;7(May):45368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pettersson M, Viljakainen H, Loid P, Mustila T, Pekkinen M, Armenio M, Andersson-Assarsson JC, Mäkitie O, Lindstrand A. Copy number variants are enriched in individuals with early-onset obesity and highlight novel pathogenic pathways. J Clin Endocrinol Metab. 2017;102(8):3029–3039. [DOI] [PubMed] [Google Scholar]
- 132. Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci USA. 1998;95(16):9500–9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bechmann LP, Gastaldelli A, Vetter D, Patman GL, Pascoe L, Hannivoort RA, Lee UE, Fiel I, Muñoz U, Ciociaro D, Lee YM, Buzzigoli E, Miele L, Hui KY, Bugianesi E, Burt AD, Day CP, Mari A, Agius L, Walker M, Friedman SL, Reeves HL. Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology. 2012;55(4):1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Miele L, Beale G, Patman G, Nobili V, Leathart J, Grieco A, Abate M, Friedman SL, Narla G, Bugianesi E, Day CP, Reeves HL. The Kruppel-like factor 6 genotype is associated with fibrosis in nonalcoholic fatty liver disease. Gastroenterology. 2008;135(1):282–291.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Nobili V, Donati B, Panera N, Vongsakulyanon A, Alisi A, Dallapiccola B, Valenti L. A 4-polymorphism risk score predicts steatohepatitis in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2014;58(5):632–636. [DOI] [PubMed] [Google Scholar]
- 137. Chen JL, Lu XJ, Zou KL, Ye K. Krüppel-like factor 2 promotes liver steatosis through upregulation of CD36. J Lipid Res. 2014;55(1):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sweet DR, Fan L, Hsieh PN, Jain MK. Krüppel-like factors in vascular inflammation: mechanistic insights and therapeutic potential. Front Cardiovasc Med. 2018;5(February):6. [DOI] [PMC free article] [PubMed] [Google Scholar]