Abstract
Primary biliary cirrhosis is characterized by chronic hepatic inflammation and immune mediated apoptosis of bile duct epithelial cells. Delayed macrophage phagocytosis of opsonized apoptotic cells, noted in other autoimmune diseases, may promote inflammation. Recent studies suggest serum anti-CD16 autoantibodies contribute to impaired macrophage phagocytosis by blocking complement receptor 3 (CR3) signaling via CD16. Therefore, serum anti-CD16 levels and the ability of monocyte derived macrophages from individuals with PBC to phagocytosis apoptotic cells were compared to controls. The mean level of anti-CD16 IgM autoantibodies (0.86±0.62 v. 0.35±0.22, respectively, p=0.031) was increased in PBC compared to control sera, and mean PBC phagocytosis of opsonized apoptotic cells was significantly decreased compared to controls (23.9±12.2% v. 43.9±14.4%, respectively, p=0.020). However, PBC phagocytosis of opsonized apoptotic cells was not significantly affected by the presence or absence of autologous serum (20.8±13.5% v. 23.9±12.2%, respectively, p=0.560). PBC phagocytosis of opsonized apoptotic cells inversely correlated with CD16 (and CR3) expression levels on Day 5 after culture in the presence or absence of autologous serum (r=−0.546, p=0.033 and r=−0.519, p=0.042, respectively). Phagocytosis of non-opsonized apoptotic cells did not correlate with CD16 or CR3 expression (p>0.050). In conclusion, PBC macrophage phagocytosis of opsonized apoptotic cells is impaired, irrespective of serum anti-CD16 antibody titers, and may increase hepatic inflammation.
Keywords: macrophage, autoimmunity, opsonization, complement receptor 3, Fc receptor
Introduction
The efficient phagocytosis of apoptotic cells by macrophages is an important regulatory process during inflammation [28]. *. Both macrophage dysfunction and defective opsonization of apoptotic cells have been observed in animal models of autoimmunity and in individuals with autoimmune disease [8, 25]. Individuals with inherited C1q deficiency frequently develop systemic lupus erythematosus [10]. The capacity of macrophages from individuals with systemic lupus erythematosus, who do not have C1q deficiency, to phagocytose apoptotic cells is decreased compared to normal control individuals [13]. Multiple receptors, including complement and Fc receptors for IgG, are involved in macrophage recognition and internalization of apoptotic cells [31]. *. Interestingly, increased titers of autoantibodies against Fc receptors and Fc receptor functional polymorphisms have been reported in autoimmune diseases [4, 18, 23]. A recent study indicated that antibodies against CD16, an Fc receptor for IgG, impair macrophage phagocytosis of complement-opsonized apoptotic cells by disrupting CR3-CD16 interaction [30].
Primary biliary cirrhosis (PBC) is an autoimmune disease characterized by the presence of anti-mitochondrial autoantibodies, high serum IgM levels, and chronic non-suppurative destruction of intra-hepatic biliary epithelial cells [15]. Bile duct destruction is believed due to biliary epithelial cell apoptosis mediated by autoreactive T cells [2, 3, 32]. *. There have been no reports evaluating anti-CD16 autoantibodies in PBC or PBC macrophage phagocytosis of apoptotic cells. However, studies in vivo showing impaired PBC macrophage phagocytosis of opsonized erythrocytes [14, 24] suggest PBC macrophage phagocytosis of apoptotic cells may also be impaired since both involve macrophage complement receptors. Though levels of circulating immune complexes, which may blockade complement and Fc receptors, were increased in PBC, the phagocytosis defect did not correlate with immune complex levels and clearance of micro-aggregated albumin by phagocytes was normal in all patients [14, 24]. *. * PBC patient monocyte derived macrophages demonstrated a similar phagocytosis defect in the presence of autologous serum [22]. To determine the possible role of serum anti-CD16 autoantibodies in impairment of PBC macrophage phagocytosis, the titer of anti-CD16 autoantibodies was examined in PBC sera and the effect of PBC sera on monocyte derived macrophage phagocytosis of apoptotic cells was analyzed.
Materials and Methods
Reagents.
Carboxy-fluorescein succinimidyl ester (CFSE) was obtained from Invitrogen (Eugene, OR). PERCP-conjugated anti-CD14, CD16, and CD11b for flow cytometry were obtained from BD Pharmingen (San Jose, CA) and anti-iC3b was purchased from Quidel (San Diego). Propidium iodide was purchased from Sigma (St. Louis, MO). FBS (lot # SF50602) was obtained from BioWhittaker (Walkersville, MD). All secondary antibodies and isotype control antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). The ECL development kit was purchased from Pierce (Rockford, IL). All other reagents were purchased from Sigma or Fischer unless otherwise specified.
ELISA.
Serum was collected from 10 individuals with PBC and 5 control individuals. Informed consent in writing was obtained from each participant. 96-well plates were coated with soluble CD16 (5 ug/ml) prior to addition of sera (1:20 dilution). IgG or IgM anti-CD16 autoantibodies were detected as previously described [4]. An OD450nm value more than 2 standard deviations above the mean value of the control sera was considered positive with regard to the presence of anti-CD16 autoantibodies. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board.
Preparation of Monocyte Derived Macrophages (MDM).
Peripheral blood (20cc) was collected from twelve individuals with PBC (histological stage 1 or 2) and age and gender matched healthy controls. Informed consent in writing was obtained from each participant. Mononuclear cells were isolated from peripheral blood using Histopaque-1077 density gradient. Monocyte enrichment was performed by negative selection using Dynal® Monocyte Negative Isolation Kit (Dynal, Oslo, Norway), which uses indirect attachment of magnetic beads to remove T cells, basophils, natural killer cells, B cells, and dendritic cells. The yield and initial viability of the remaining monocyte enriched cells was determined using a hemocytometer and trypan blue exclusion, respectively. 1.5 to 2 million cells were typically obtained with 85+% CD14+ and >95% viable. Cells were cultured in 24-well plastic dishes (200,000 cells/ well) in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with penicillin (50 U/ml), streptomycin (50 μg/ml), L-glutamine (complete RPMI), and either 10% heat-inactivated FBS (HI-FBS) or 10% autologous serum at 37 °C in a humidified 5% CO2 incubator. After 18 h in culture, any non-adherent cells were removed and discarded. On day 1 and day 4 of culture, macrophage colony stimulating factor (M-CSF) (10ng/ml) was added to the culture media. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board.
Preparation of Apoptotic Human Salivary Gland Epithelial Cells (HSG).
The normal human salivary gland epithelial cell line (HSG) was passaged in defined media supplemented with 10% HI-FBS as previously described [26]. HSG loaded with the vital dye CFSE (5 μM for 15 min at 37 C) were irradiated with ultraviolet B light (UV-B) to induce apoptosis as previously described [26]. Induction of apoptosis in HSG by this method has previously proven to be highly reproducible. Additionally, increased salivary gland epithelial cell apoptosis is often noted in PBC [35]. Induction of apoptosis in HSG was confirmed by flow cytometry by annexin V/ propidium iodide staining.
Western Blot Analysis of Opsonization.
16 hours after irradiation, HSG were opsonized at 37°C for 1 h by incubation with 25% autologous or normal human serum. SDS-PAGE, transfer to nitrocellulose, and western blotting using anti-iC3b antibody were performed against lysates of UV-B irradiated, opsonized HSG [26]. Antibody binding was detected using an HRP-conjugated secondary antibody followed by enhanced chemiluminescence. Densitometry was used to compare iC3b staining among the individual lysates while controlling for differences in protein loading by Ponceau staining.
Evaluation of MDM Receptor Expression Level and Viability.
CD14 expression levels of cells isolated by negative selection were measured on Day 0 to assess purity of monocyte preparations. Viability was measured by trypan blue exclusion on Day 0. Only preparations in which the percentage of CD14+ cells exceeded 85% and viability exceeded 90% were analyzed further. MDM cell viability prior to addition of apoptotic HSG was again assessed on Day 5 by flow cytometry. If the % of non-viable cells exceeded 15% further analysis was not performed. CD14, CD11b, and CD16 receptor levels on monocyte derived macrophages (MDM) were measured on Day 5 by staining with receptor-specific, fluorescent-conjugated mAbs prior to addition of apoptotic HSG cells. Isotype control antibodies were employed to detect background staining levels. The relative receptor expression levels were compared by flow cytometry using mean fluorescent intensity values obtained after adjusting for differences in background staining.
Phagocytosis Assays.
Apoptotic HSG cells were added to the wells containing MDM on Day 5 at different ratios for various time periods in pilot studies (not shown). In the final experiments, a 2:1 ratio and a 1 h co-incubation period were used since at higher ratios and longer incubation periods differences in phagocytosis were less pronounced. Non-phagocytosed, apoptotic HSG were removed by extensive washing prior to recovering adherent cells for antibody staining and flow cytometric analysis. The % phagocytosis was calculated as the percentage of CD14+ cells that were also CFSE+. Staining to identify necrotic or apoptotic MDM after addition of apoptotic HSG by flow cytometry was not done, but there were no changes in the forward and side scatter of the cell by flow cytometry suggestive of significant MDM cell death by necrosis or apoptosis.
Statistical Methods.
Student’s t test (two-tailed, unequal variance) was used to compare means for serum anti-CD16 antibody O.D. value, receptor expression levels, and % phagocytosis between individuals with PBC and healthy controls with a p value of ≤ 0.050 considered statistically significant. Spearman rank correlation test was used to analyze correlations between clinical values, serum values and MDM % phagocytosis. An absolute r value greater than 0.500 combined with a p value ≤ 0.050 was considered statistically significant. A negative r value indicates an inverse correlation.
Results
Anti-CD16 serum IgM autoantibody levels are increased among those with PBC.
Sera of five normal controls and ten individuals with non-cirrhotic PBC were screened for IgM and IgG class anti-CD16 autoantibodies using a previously developed ELISA [4]. There was no significant difference between mean normal control and PBC sera anti-CD16 IgG levels (p=0.206) (Table 1). In contrast, the mean anti-CD16 IgM level was significantly higher in PBC sera as opposed to control sera (p=0.031). Concentrations of anti-CD16 IgM autoantibodies 2 fold or more higher than the control mean + 2 SD were detected in 70% of PBC sera. This is consistent with prior studies suggesting the IgM, not IgG, fraction of PBC sera possessed phagocytosis inhibitory factors [22]. Given these results, the effect of autologous PBC serum on monocyte derived macrophage phagocytosis of apoptotic cells was next examined.
Table 1.
Comparison of anti-CD16 Autoantibody Levels in Normal and PBC Sera.
| Antibody Class | Normal (N=5) | PBC (N=10) | p value |
|---|---|---|---|
| IgG (OD450nm) | 0.246±0.050 | 0.332±0.146 | 0.206 |
| IgM (OD450nm) | 0.349±0.224 | 0.859±0.617 | 0.031 |
Values shown are means ± std. dev. in arbitrary units. Student’s t test was used to calculate p values. P values ≤0.050 were considered statistically significant.
Characterization of PBC cohort and monocyte derived macrophage receptor expression.
Healthy control individuals matched for age, race, and gender were studied along with twelve individuals with PBC to compare phagocytosis capacity (Table 2). Individuals with early stage, non-cirrhotic PBC were selected to preclude any effect of cirrhosis on phagocytosis. None had elevated bilirubin levels suggestive of significant cholestasis or malabsorption of fat soluble vitamins. As expected in non-cirrhotic individuals with PBC, the mean Mayo Risk Score, a predictor of time until transplantation, was very low. All individuals were asymptomatic at the time of blood donation. Significantly, the mean serum complement levels (C3, C3d, and C4) were normal in the PBC group, though two PBC subjects had C3 levels slightly above the upper limit of normal.
Table 2.
Demographic and Clinical Data on Control and PBC Individuals for Phagocytosis Assays.
| Controls (N=6) | PBC (N=12) | |
|---|---|---|
| Age (y) | 50.1±8.1 | 56.3±8.4 |
| Gender (F:M) | 5:1 | 11:1 |
| Alkaline phosphatase (U/L) | NA | 180±87 |
| Total bilirubin (mg/dl) | NA | 0.59±0.20 |
| Mayo Risk Score | NA | 4.21±0.25 |
Mean values ± std. dev. are shown. No statistically significant differences were noted using student’s t test (all p>0.050). NA=not applicable.
Rather than examining monocyte phagocytosis, monocytes cultured for 5 days in complete RPMI supplemented with macrophage colony stimulating factor (i.e. monocyte derived macrophages; MDM) were assayed since prior studies demonstrated that PBC MDM phagocytosis correlates with PBC Kupffer cell/macrophage phagocytosis in vivo [1, 14, 22, 24] and minimal CD11b and CD16 expression was observed in freshly isolated monocytes. CD11b and CD16 receptor levels plateau after 3 days in culture (data not shown). The mean MDM receptor levels were slightly higher in the PBC group than in the control group; though, the difference was not statistically significant (all p>0.100) (Table 3). Additionally, culture in HI-FBS versus autologous serum did not significantly affect mean receptor levels on either control or PBC MDM (Table 3) (all p>0.100). Mean serum M-CSF levels were not significantly different between PBC (761±179 pg/ml) and control (673±178 pg/ml) individuals (p=0.382). Thus, supplementation in vitro with exogenous M-CSF did not mask any serum effect due to different macrophage colony stimulating factor levels.
Table 3.
Comparison of receptor expression levels of cultured monocyte-derived macrophages on Day 5.
| Mean Fluorescence Intensity | ||||||
|---|---|---|---|---|---|---|
| Heat-inactivated FBS | Autologous serum | |||||
| CD14 | CD11b | CD16 | CD14 | CD11b | CD16 | |
| Control | 39.7±16.7 | 14.8±2.9 | 23.3±4.2 | 37.8±9.2 | 17.2±4.1 | 25.5±3.4 |
| PBC | 47.8±21.9 | 17.7±4.9 | 24.1±6.8 | 48.3±28.0 | 18.7±7.0 | 27.3±10.5 |
| p value | 0.401 | 0.142 | 0.779 | 0.261 | 0.575 | 0.608 |
Mean fluorescence intensity levels ± std. dev. determined by flow cytometry are provided in arbitrary units. P values shown are for comparison of control and PBC monocyte-derived macrophages (MDM) on Day 5 of culture. Values were compared using student’s t test with p ≤ 0.050 considered statistically significant. P values for comparison of each MDM receptor cultured in heat-inactivated FBS versus autologous serum were all >0.050.
PBC MDM phagocytosis of opsonized apoptotic cells is impaired, but not dependent upon autologous serum.
Apoptosis of the target cells was confirmed morphologically (Fig. 1A) and by annexin V and propidium iodide co-staining (Fig. 1B). A time course study was performed to determine the optimal time point to collect apoptotic target cells without evidence of secondary necrosis. Cell membrane blebbing and apoptotic body formation in more than half of the cells were evident 18h after UV-B irradiation (Fig. 1A, right panel). Annexin V staining detects phosphatydylserine and propidium iodide (PI) staining determines cell permeability. Apoptotic cells are annexin V+/PI−, while necrotic cells are annexin V+/PI+. At 18h after UV-B irradiation, annexin V+/PI− cells typically increased from <5% (Fig. 1B, left panel) to >50% (Fig. 1B, right panel) of target cells with few necrotic cells (V+/PI+) present.
Fig. 1.
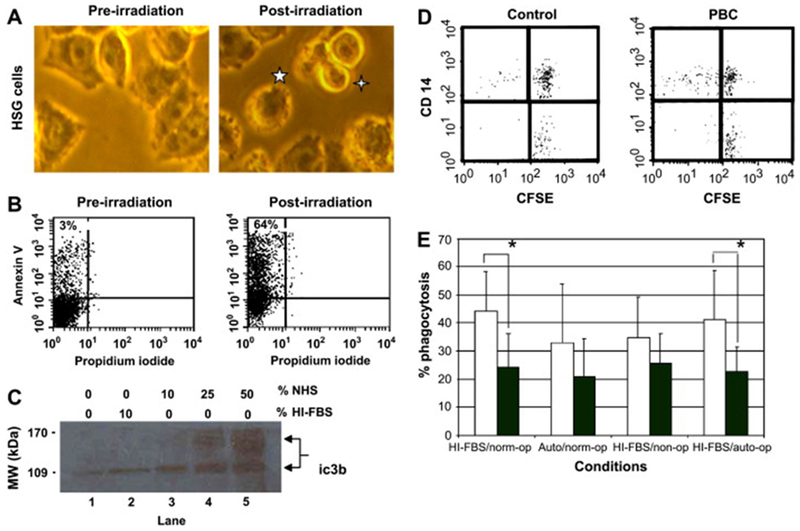
Monocyte derived macrophage (MDM) phagocytosis of apoptotic HSG under different conditions. (A & B) Evaluation of target cell apoptosis. Microscopic assessment of apoptosis of target cells before (A, left panel) and 18h after (A, right panel) UV-B irradiation. A typical apoptotic cell with membrane blebbing (5-pointed star) and an apoptotic body (4-pointed star) are denoted in the right panel (20× objective). Flow cytometric assessment of external phosphatydylserine staining of non-permeabilized target cells by PE-conjugated Annexin V before (B, left panel) and 18h after (B, right panel) irradiation of target cells with UV-B light. Apoptosis induction was confirmed by detection of an increased percentage of annexin V+/PI− (apoptotic) target cells after UV-B irradiation. This analysis was repeated prior to each phagocytosis experiment. Representative images are shown. (C) Evaluation of opsonization efficiency. Immunoblot detection of iC3b attachment to UV-B irradiated target cells after incubation with varying concentrations of normal human serum (NHS) and HI-FBS for 1h at 37C. Student’s t test was used to compare iC3b bands by densitometry. P values ≤ 0.050 were considered significant. No significant increase in iC3b attachment was noted after incubation with 10% HI-FBS (lane 2) compared to no serum (lane 1). Incubation in 25% normal human serum significantly increased iC3b attachment to UV-B irradiated HSG (lane 4). The experiment was repeated three times and a representative image is shown. (D) Two-color flow cytometry was used to evaluate phagocytosis by MDM (CD14+) of apoptotic HSG cells (CFSE+). Dual stained cells (upper right quadrant) indicate MDM that have phagocytosed an apoptotic cell. MDM that have not phagocytosed an apoptotic cell are represented in the upper left quadrant. Non-apoptotic HSG and non-phagocytosed apoptotic HSG are depicted in the lower right quadrant. Representative control (left panel) and PBC (right panel) flow cytometry images are shown. (E) Mean % phagocytosis ± std. dev. of apoptotic cells by control (white) and PBC (black) MDM after culture in media supplemented with HI-FBS or autologous serum (Auto). Apoptotic target cells were opsonized with normal serum (norm-op), no serum (non-op), or autologous serum (auto-op) prior to culture with monocyte derived macrophages. Control MDM % phagocytosis was significantly greater than PBC MDM % phagocytosis with respect to phagocytosis of opsonized apoptotic cells after MDM culture in HI-FBS. The presence of autologous serum did not significantly alter PBC MDM phagocytosis. A * indicates a statistically significant difference by student’s t test (p ≤ 0.050). Duplicate wells were used for each condition.
Western blot analysis of iC3b binding was performed to determine the concentration of serum needed to efficiently opsonize the apoptotic cells (Fig. 1C). By densitometry, opsonization by iC3b was statistically significantly increased (p<0.050) using 25% normal human serum (90±3.5 units, lane 4) compared to no serum (33.8±5.9 units, lane 1) or 10% HI-FBS (42±2.5 units, lane 2). Using 50% normal human serum (92±6.5 units, lane 5) did not further significantly increase opsonization compared to 25% serum. In phagocytosis assays, phagocytosis of apoptotic cells incubated in 10% HI-FBS (non-op) were compared to those incubated in 25% normal human serum (norm-op) or 25% autologous serum (auto-op).
Phagocytosis of opsonized, apoptotic target cells (CFSE+) by control versus PBC CD14+ MDM was compared by flow cytometry (e.g. Fig. 1D, left and right panels, respectively). The percentage of CD14+ cells that were also CFSE+ (i.e. % phagocytosis) was calculated for MDM cultured in HI-FBS or autologous serum and then exposed to either non-opsonized or opsonized apoptotic cells (Fig. 1E). Mean PBC MDM % phagocytosis (Fig. 1E, black bars) of apoptotic cells was consistently decreased compared to controls (Fig. 1E, white bars), though the decrease was only statistically significant for cells cultured in HI-FBS and exposed to opsonized apoptotic cells (Fig. 1E, HI-FBS/norm-op and HI-FBS/auto-op). Unexpectedly, mean PBC MDM % phagocytosis was not inhibited by culture in autologous serum compared to HI-FBS (20.8±13.5% v. 23.9±12.2%, respectively, p=0.560). Two individuals with previously demonstrated high serum titers of anti-CD16 IgM autoantibody titers did have a large decrease in % phagocytosis in the presence of autologous serum. However, serum anti-CD16 IgM autoantibody titers did not statistically significantly correlate with % phagocytosis (r=−0.400, p=0.390). As suspected PBC macrophages had impaired phagocytosis of opsonized apoptotic cells, but impairment was not dependent upon incubation with autologous serum.
To determine the importance of apoptotic cell opsonization in the observed impaired phagocytosis, non-opsonized apoptotic cells were added to MDM cultured in HI-FBS. A trend towards decreased phagocytosis by control MDM was noted when non-opsonized apoptotic cells were substituted for opsonized apoptotic cells (34.8±14.5% v. 43.9±14.3%, respectively, p=0.298) In contrast, lack of opsonization of apoptotic cells did not decrease PBC MDM phagocytosis (25.6±10.6% v. 23.9±12.2%, respectively, p=0.719). PBC MDM phagocytosis of non-opsonized apoptotic cells was lower than control MDM, but the difference was not statistically significant (p=0.210). Defective PBC MDM phagocytosis was only statistically significant for phagocytosis of opsonized apoptotic cells.
Opsonization of apoptotic cells with iC3b using PBC sera is normal.
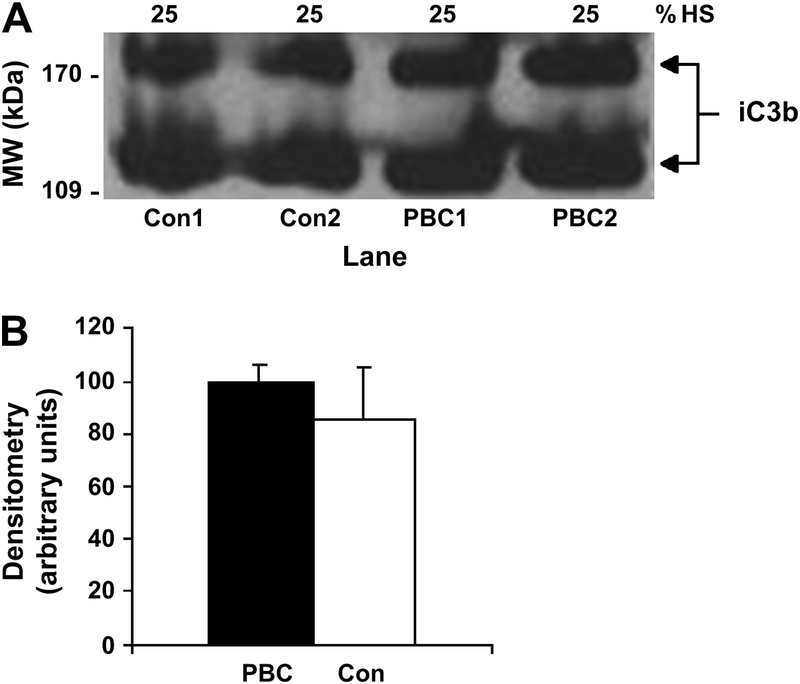
Impaired opsonization of apoptotic cells may also contribute to impaired macrophage phagocytosis of apoptotic cells in vivo. Opsonization of yeast, though not E. coli, by PBC serum has been reported to be abnormal in 23% of individuals with PBC [37]. However, using autologous serum as opposed to normal human serum did not affect mean % phagocytosis by control MDM (41.1±17.6% v. 43.9±14.3%, p=0.77, respectively) or PBC MDM (23.0±8.5% v. 23.9±12.1%, p=0.82, respectively) (Fig. 1E). Opsonization of apoptotic cells with iC3b appeared equivalent by western blot analysis after incubation of apoptotic cells with either PBC or control serum (Fig. 2A). By densitometry, the mean intensity of the iC3b bands was not statistically significantly different between cells opsonized with PBC or control serum (Fig. 2B). Taken together, these results suggest an intrinsic PBC MDM defect, not serum anti-CD16 autoantibodies or aberrant complement activation, may be primarily responsible for the observed impairment in phagocytosis of apoptotic cells.
Fig. 2.
Immunoblot detection of iC3b attachment. Apoptotic HSG cells were incubated with media supplemented with 25% human serum (HS) from individuals with PBC or controls for 1h at 37C. After washing, cells were lysed and immunoblotted with anti-iC3b antibody followed by ECL development. (A) A representative immunoblot image is shown. (B) The mean densitometry of the iC3b bands demonstrated no statistically significant difference between iC3b attachment to apoptotic cells following incubation with PBC or control (Con) serum (p > 0.050). Student’s t test was used for the comparison.
PBC MDM receptor levels and serum bilirubin inversely correlated with phagocytosis of opsonized apoptotic cells.
The mean fluorescent intensities of CD14, CD16, and CD11b staining of Day 5 PBC MDM were measured using specific mAb and examined for correlations with % phagocytosis of apoptotic HSG cells (Table 4). Regardless of culture in HI-FBS or autologous serum, PBC MDM CD16 staining on Day 5 statistically significantly correlated inversely with phagocytosis of apoptotic cells opsonized with normal human serum (r=−0.519, p=0.042 and r=−0.547, p=0.033, respectively). PBC MDM CD11b staining also correlated inversely with phagocytosis of opsonized apoptotic cells (r=−0.541, p=0.035; r=−0.512, p=0.044, respectively). Levels of CD11b and CD16 staining positively correlated with each other, not CD14 staining, on MDM cultured in autologous serum (r=0.576, p=0.025). These findings may reflect coordinated interaction between CD16 and CR3 (CD11b) during phagocytosis of apoptotic cells. In contrast, CD14 staining significantly correlated inversely with phagocytosis of non-opsonized apoptotic cells (r=−0.547, p=0.033) and autologous serum opsonized cells (r=−0.578, p=0.026).
Table 4.
PBC Monocyte Derived Macrophage (MDM) Receptor and Serum Biochemical Levels Correlate with Phagocytose of Apoptotic Cells.
| % Phagocytosis | CD11b | CD16 | CD14 | T bili | Chol ratio | |
|---|---|---|---|---|---|---|
| HI-FBS | r value | −0.541 | −0.519 | 0.000 | −0.509 | 0.102 |
| norm-op | p value | 0.035 | 0.042 | 1.000 | 0.046 | 0.376 |
| Auto | r value | −0.512 | −0.547 | −0.332 | −0.306 | 0.109 |
| norm-op | p value | 0.044 | 0.033 | 0.146 | 0.167 | 0.668 |
| HI-FBS | r value | 0.318 | −0.133 | −0.547 | −0.224 | 0.571 |
| non-op | p value | 0.157 | 0.340 | 0.033 | 0.242 | 0.026 |
| HI-FBS | r value | −0.014 | −0.297 | −0.578 | −0.250 | 0.472 |
| auto-op | p value | 0.883 | 0.174 | 0.025 | 0.217 | 0.061 |
Spearman analysis was used to assess correlations between MDM phagocytosis and MDM receptor expression levels as well as serum biochemical values. An r value ≥/0.500/ was considered a strong correlation and statistically significant if the p value was ≤ 0.050. A (−) sign indicates an inverse correlation. % phagocytosis refers to the percentage of CD14+ MDM that phagocytosed an apoptotic cell under the indicated conditions. Cells were cultured in media supplemented with HI-FBS or autologous serum (Auto) and then exposed to apoptotic cells opsonized with normal human serum (norm-op), autologous serum (auto-op), or HI-FBS (non-op). T bill is total serum bilirubin and ratio is serum total cholesterol divided by HDL cholesterol levels.
With respect to phagocytosis of opsonized apoptotic cells and laboratory values, the only other statistically significant correlation was an inverse relationship with serum total bilirubin values (r=−0.509, p=0.046), which were all well within the normal range (0.2 to 1.3 mg/dl). Serum total bilirubin levels also correlated with PBC MDM CD11b (r=0.513, p=0.044) and CD16 (r=0.555, p=0.030) expression levels after culture in HI-FBS. MRS and age did not correlate with PBC MDM phagocytosis or CD16 and CD11b receptor levels (all p>0.050), suggesting disease severity is not responsible for the above correlations with total bilirubin levels. The serum laboratory value that correlated statistically significantly with PBC MDM phagocytosis of non-opsonized apoptotic cells was the total/HDL cholesterol ratio (r=0.571, p=0.026). The correlation between the total/HDL cholesterol ratio and PBC MDM phagocytosis of autologous serum opsonized apoptotic cells was narrowly statistically non-significant (0.472, p=0.061). PBC serum lipid concentrations may influence macrophage phagocytosis of apoptotic cells.
Lastly, vitamin D3 regulates macrophage phagocytosis and immune system function [34]. Seven of twelve individuals with PBC had borderline low serum 25-hydroxyvitamin D3 levels between 20 and 30 ng/ml despite taking 400 IU of cholecalciferol daily. However, serum 25-hydroxyvitamin D3 concentrations did not correlate with MDM phagocytosis of apoptotic cells (all p>0.050). Serum 25-hydroxyvitamin D3 levels did strongly correlate inversely with serum alkaline phosphatase (r=−0.712, p=0.005), IgM (r=−0.565, p=0.028), and C3 (r=−0.782, p=0.001) levels. Thus, a low serum vitamin D3 concentration in PBC correlated with increased concentrations of these markers of hepatic inflammation even in those with early PBC.
Discussion
This study demonstrates that serum titers of anti-CD16 IgM autoantibodies are increased and macrophage phagocytosis of opsonized apoptotic cells is decreased in PBC. The phagocytosis dysfunction is consistent with prior studies demonstrating impaired PBC patient clearance of opsonized aged, erythrocytes in vivo and in vitro *[1, 14, 20, 22, 24]. As in those studies, macrophage dysfunction was present in those with early stage disease *, suggesting these findings are not secondary to cirrhosis *. Impaired macrophage phagocytosis of apoptotic cells appears to be a common feature of individuals with PBC and may enhance pro-inflammatory responses within the liver.
The cause of this impairment in macrophage phagocytosis of apoptotic cells in PBC is not certain. There were no differences in cell viability between PBC samples either at Day 0 or Day 5 to suggest that autophagy [19] resulted in pre-activation of MDM from certain individuals. In a few cases the presence of autologous serum, with high titers of anti-CD16 IgM autoantibodies, strongly inhibited phagocytosis. This may have been due to high titers of anti-CD16 IgM autoantibodies and not non-specific immune complex blockade of CD16 since serum complement levels were not reduced. However, impaired PBC MDM phagocytosis of apoptotic cells was serum-independent in most cases, though one cannot rule out a persistent effect of autologous serum factors even after prolonged culture in the absence of autologous serum. The serum-independent nature of the defect in most cases favors the presence of an intrinsic functional defect in phagocytosis by PBC macrophages of apoptotic cells. *.
Macrophage phagocytosis of apoptotic cells is a complex process which may be impaired due to a variety of intrinsic defects [29, 31]. These intrinsic defects can be subdivided between defects in apoptotic cell recognition and defects in internalization of bound apoptotic cells. In our study, the inverse correlation between PBC MDM % phagocytosis and PBC MDM CD16 (and CR3) levels prior to addition of apoptotic cells suggests the increased presence of an autoimmunity-related CD16 polymorphism amongst those with PBC. The prevalence of the V158 allele of the Fc gamma receptor IIIA gene, which encodes CD16 on macrophages and NK cells, is increased in other autoimmune diseases [7]. This CD16 polymorphism is associated with higher macrophage CD16 expression, lower affinity binding and impaired macrophage clearance of opsonized target cells [12, 17]. Interestingly this CD16 polymorphism is also associated with a reduced risk of coronary artery disease [9]. Macrophage internalization of apoptotic cells is most strongly triggered by CD16 and CR3, which act cooperatively to mediate internalization [27, 31]. Thus, the observed correlation between high CD16 expression levels and poor PBC macrophage phagocytosis of apoptotic cells may be due to an increased frequency of the Fc gamma RIIIA-V158 polymorphism among those with PBC.
Genetic polymorphisms not directly related to recognition of opsonized apoptotic cells may also affect PBC macrophage phagocytosis. *. For example, the vitamin D receptor BsmI polymorphism that is more frequent in PBC [6, 36] is known to impair macrophage phagocytosis [33]. Whether this impairment includes phagocytosis of iC3b opsonized targets is unknown. The positive correlation between serum total cholesterol/HDL cholesterol ratio and macrophage phagocytosis of apoptotic cells is intriguing since * PBC carriers of the epsilon4 Apo E allele have increased total cholesterol/HDL cholesterol ratios and more severe disease sooner after diagnosis [5]. The functional effect of the epsilon4 Apo E allele on macrophage phagocytosis of apoptotic cells is unknown, but Apo E−/− mice have decreased macrophage phagocytosis of apoptotic cells and a systemic pro-inflammatory state [11]. Other genetic polymorphisms associated PBC involving toll-like receptor 9 and mannose binding lectin may also directly or indirectly affect macrophage phagocytosis of apoptotic cells [16, 21].
In conclusion, macrophage phagocytosis of apoptotic cells is significantly impaired in PBC. Though anti-CD16 autoantibodies were present at significantly higher levels in PBC sera, autologous sera did not affect PBC macrophage phagocytosis of apoptotic cells in most individuals. Regardless of the presence or absence of autologous serum, PBC macrophage CD16 expression levels correlated inversely with phagocytosis efficiency. In other autoimmune diseases, CD16 genetic polymorphisms as well as polymorphisms in other genes associated with decreased macrophage phagocytosis are increased in frequency. Further studies are needed to examine the prevalence of these polymorphisms in those with PBC.
Acknowledgements
This study was supported by grants from the NIH (DK59653) (JAO), New York City Speaker’s Fund (JAO) and the Hirschl/Weill-Caulier Trust (JAO).
Abbreviations:
- PBC
primary biliary cirrhosis
- HSG
human salivary gland epithelial cells
- UV-B
ultraviolet B light
- HI-FBS
heat-inactivated fetal bovine serum
- MDM
monocyte derived macrophage
- MRS
Mayo Risk Score
- CFSE
carboxyfluoroscein succinimidyl ester
- NHS
normal human serum
- HDL
high density lipoprotein
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Al-Aghbar MN, Neuberger J, Williams R, Eddleston AL. Mononuclear cell complement receptor blockade in primary biliary cirrhosis. Gut 1985;26:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, Huebert RC, van de Water J, LaRusso NF, Gershwin ME, Gores GJ, Odin JA. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun 2006;27:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun 2006;27:50–53. [DOI] [PubMed] [Google Scholar]
- [4].Boros P, Odin JA, Chen J, Unkeless JC. Specificity and class distribution of Fc gamma R-specific autoantibodies in patients with autoimmune disease. J Immunol 1994;152:302–306. [PubMed] [Google Scholar]
- [5].Corpechot C, Benlian P, Barbu V, Chazouilleres O, Poupon RE, Poupon R. Apolipoprotein E polymorphism, a marker of disease severity in primary biliary cirrhosis? J Hepatol 2001;35:324–328. [DOI] [PubMed] [Google Scholar]
- [6].Fan LY, Zhong RQ, Tu XQ, Zhu Y, Gong CL, Zhou L, Zhao ZX, Feltens R, Pfeiffer T. [Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune liver diseases on Chinese]. Zhonghua Yi Xue Za Zhi 2003;83:1852–1855. [PubMed] [Google Scholar]
- [7].Fossati G, Bucknall RC, Edwards SW. Fcgamma receptors in autoimmune diseases. Eur J Clin Invest 2001;31:821–831. [DOI] [PubMed] [Google Scholar]
- [8].Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, Kern P, Herrmann M. Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 2007;28:114–121. [DOI] [PubMed] [Google Scholar]
- [9].Gavasso S, Nygard O, Pedersen ER, Aarseth JH, Bleie O, Myhr KM, Vedeler CA. Fcgamma receptor IIIA polymorphism as a risk-factor for coronary artery disease. Atherosclerosis 2005;180:277–282. [DOI] [PubMed] [Google Scholar]
- [10].Ghebrehiwet B, Peerschke EI. Role of C1q and C1q receptors in the pathogenesis of systemic lupus erythematosus. Curr Dir Autoimmun 2004;7:87–97. [DOI] [PubMed] [Google Scholar]
- [11].Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol 2004;173:6366–6375. [DOI] [PubMed] [Google Scholar]
- [12].Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, et al. Increased natural killer cell expression of CD16, and augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIA-158 V/V and V/F polymorphism. Blood 2007;110(7):2561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum 1998;41:1241–1250. [DOI] [PubMed] [Google Scholar]
- [14].Jaffe CJ, Vierling JM, Jones EA, Lawley TJ, Frank MM. Receptor specific clearance by the reticuloendothelial system in chronic liver diseases. Demonstration of defective C3b-specific clearance in primary biliary cirrhosis. J Clin Invest 1978;62:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med 2005;353:1261–1273. [DOI] [PubMed] [Google Scholar]
- [16].Kikuchi K, Lian ZX, Kimura Y, Selmi C, Yang GX, Gordon SC, Invernizzi P, Podda M, Coppel RL, Ansari AA, Ikehara S, Miyakawa H, Gershwin ME. Genetic polymorphisms of toll-like receptor 9 influence the immune response to CpG and contribute to hyper-IgM in primary biliary cirrhosis. J Autoimmun 2005;24:347–352. [DOI] [PubMed] [Google Scholar]
- [17].Kumpel BM, De Haas M, Koene HR, Van De Winkel JG, Goodrick MJ. Clearance of red cells by monoclonal IgG3 anti-D in vivo is affected by the VF polymorphism of Fcgamma RIIIa (CD16). Clin Exp Immunol 2003;132:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lamour A, Baron D, Soubrane C, Cartron J, Khayat D, Adler Y, Le Goff P, Youinou P. Anti-Fc gamma receptor III autoantibody is associated with soluble receptor in rheumatoid arthritis serum and synovial fluid. J Autoimmun 1995;8:249–265. [DOI] [PubMed] [Google Scholar]
- [19].Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, Mackay IR, Gershwin ME. Autophagy: Highlighting a novel player in the autoimmunity scenario. J Autoimmun 2007;29:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Loof L, Hakansson L, Nyberg A, Venge P. Defective C3b receptor-mediated phagocytosis of neutrophils in patients with primary biliary cirrhosis. Scand J Gastroenterol 1987;22:1169–1174. [DOI] [PubMed] [Google Scholar]
- [21].Matsushita M, Miyakawa H, Tanaka A, Hijikata M, Kikuchi K, Fujikawa H, Arai J, Sainokami S, Hino K, Terai I, Mishiro S, Gershwin ME. Single nucleotide polymorphisms of the mannose-binding lectin are associated with susceptibility to primary biliary cirrhosis. J Autoimmun 2001;17:251–257. [DOI] [PubMed] [Google Scholar]
- [22].Minuk GY, Vergalla J, Hanson RG, Hoofnagle JH, Frank MM, Jones EA. Anticomplement receptor activity in the serum of patients with primary biliary cirrhosis. Gut 1986;27:324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morgan AW, Griffiths B, Ponchel F, Montague BM, Ali M, Gardner PP, Gooi HC, Situnayake RD, Markham AF, Emery P, Isaacs JD. Fcgamma receptor type IIIA is associated with rheumatoid arthritis in two distinct ethnic groups. Arthritis Rheum 2000;43:2328–2334. [DOI] [PubMed] [Google Scholar]
- [24].Nilsson Ekdahl K, Loof L, Ahrenstedt O, Nilsson UR, Nilsson B. Defective elimination of C3b/iC3b-coated autologous erythrocytes in patients with primary biliary cirrhosis, alcoholic cirrhosis, and ulcerative colitis. J Lab Clin Med 1997;130:285–292. [DOI] [PubMed] [Google Scholar]
- [25].O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun 2006;26:104–115. [DOI] [PubMed] [Google Scholar]
- [26].Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest 2001;108:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ohkuro M, Ogura-Masaki M, Kobayashi K, Sakai M, Takahashi K, Nagasawa S. Effect of iC3b binding to immune complexes upon the phagocytic response of human neutrophils: synergistic functions between Fc gamma R and CR3. FEBS Lett 1995;373:189–192. [DOI] [PubMed] [Google Scholar]
- [28].Peng Y, Martin DA, Kenkel J, Zhang K, Ogden CA, Elkon KB. Innate and adaptive immune response to apoptotic cells. J Autoimmun 2007; September 19; [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pittoni V, Valesini G. The clearance of apoptotic cells: implications for autoimmunity. Autoimmun Rev 2002;1:154–161. [DOI] [PubMed] [Google Scholar]
- [30].Preynat-Seauve O, Villiers CL, Jourdan G, Richard MJ, Plumas J, Favier A, Marche PN, Favrot MC. An interaction between CD16 and CR3 enhances iC3b binding to CR3 but is lost during differentiation of monocytes into dendritic cells. Eur J Immunol 2004;34:147–155. [DOI] [PubMed] [Google Scholar]
- [31].Roos A, Xu W, Castellano G, Nauta AJ, Garred P, Daha MR, van Kooten C. Mini-review: A pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol 2004;34:921–929. [DOI] [PubMed] [Google Scholar]
- [32].Salunga TL, Cui ZG, Shimoda S, Zheng HC, Nomoto K, Kondo T, Takano Y, Selmi C, Alpini G, Gershwin ME, Tsuneyama K. Oxidative stress-induced apoptosis of bile duct cells in primary biliary cirrhosis. J Autoimmun 2007;29:78–86. [DOI] [PubMed] [Google Scholar]
- [33].Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol 2004;24:523–532. [DOI] [PubMed] [Google Scholar]
- [34].Spittler A, Willheim M, Leutmezer F, Ohler R, Krugluger W, Reissner C, Lucas T, Brodowicz T, Roth E, Boltz-Nitulescu G. Effects of 1 alpha,25-dihydroxyvitamin D3 and cytokines on the expression of MHC antigens, complement receptors and other antigens on human blood monocytes and U937 cells: role in cell differentiation, activation and phagocytosis. Immunology 1997;90:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tsuneyama K, Van De Water J, Yamazaki K, Suzuki K, Sato S, Takeda Y, Ruebner B, Yost BA, Nakanuma Y, Coppel RL, Gershwin ME. Primary biliary cirrhosis an epithelitis: evidence of abnormal salivary gland immunohistochemistry. Autoimmunity 1997;26:23–31. [DOI] [PubMed] [Google Scholar]
- [36].Vogel A, Strassburg CP, Manns MP. Genetic association of vitamin D receptor polymorphisms with primary biliary cirrhosis and autoimmune hepatitis. Hepatology 2002;35:126–131. [DOI] [PubMed] [Google Scholar]
- [37].Wyke RJ, Rajkovic IA, Williams R. Impaired opsonization by serum from patients with chronic liver disease. Clin Exp Immunol 1983;51:91–98. [PMC free article] [PubMed] [Google Scholar]