Abstract
Neuroendocrine tumors (NETs) are relatively rare and highly heterogeneous neoplasms. Despite this, recent studies from North America and Central Europe have suggested an increase in incidence. In Latin America, NET data are scarce and scattered with only a few studies reporting registries. Our goal was to establish a NET registry in Chile. Here, we report the establishment and our first 166 NET patients. We observed a slight preponderance of males, a median age at diagnosis of 53 years and a median overall survival of 110 months. As anticipated, most tumors were gastroenteropancreatic (GEP). Survival analyses demonstrated that non-GEP or stage IV tumors presented significantly lower overall survival (OS). Similarly, patients with surgery classified as R0 had better OS compared to R1, R2, or no surgery. Furthermore, patients with elevated chromogranin A (CgA) or high Ki67 showed a trend to poorer OS; however, these differences did not reach statistical significance (log-rank test p = 0.07). To the best of our knowledge, this is the first report of a NET registry in Chile. Median OS in our registry (110 months) is in line with other registries from Argentina and Spain. Other variables including age at diagnosis and gender were similar to previous studies; however, our data indicate a high proportion of small-bowel NETs compared to other cohorts, reflecting the need for NET regional registries. Indeed, these registries may explain regional discrepancies in incidence and distribution, adding to our knowledge on this seemingly rare, highly heterogeneous disease.
Electronic supplementary material
The online version of this article (10.1007/s12672-018-0354-5) contains supplementary material, which is available to authorized users.
Keywords: Neuroendocrine tumors, Cancer registry, Chromogranin A, Overall survival
Introduction
Neuroendocrine tumors (NETs) are a group of highly heterogeneous neoplasms that arise from cells of the diffuse neuroendocrine system that extend throughout the body. Initially described over a century ago by Oberndorfer [1], NETs are characterized by the secretion of biologically active peptides or neuropeptides that give rise to a variety of clinical syndromes, including the carcinoid syndrome. Despite this, in certain cases, NETs can remain clinically silent and undiagnosed until advanced stage disease. Anatomically, NETs are frequently located in the gastrointestinal tract; however, they can also originate in other organs such as the pancreas, lungs, adrenal glands, and the thyroid. Indeed, the vast majority of NET cases (75%) originate in the gastroenteropancreatic (GEP) system [2] and these cases are collectively referred to as GEP-NETs.
Still regarded as relatively rare tumors, several studies have previously reported NET incidence rates across North American [3, 4], Western European [5–8], and Asian [9, 10] countries. Interestingly, studies in North America and the United Kingdom (UK) indicate a significant increase in the incidence of NETs over the last decades. Data from the Surveillance, Epidemiology, and End Results (SEER) program registry in the USA involved > 35,000 patients and compared the NET age-adjusted incidence over the 1973–2004 period. This study found a significant increase in NET incidence from 1.9 to 5.25 per 100,000 per year during this period [3]. Similarly, a study in Canada that involved > 5000 patients reported an increase in incidence from 2.48 to 5.86 per 100,000 per year over the period 1994–2009 [4]. Furthermore, a study in the UK that identified > 10,000 cases of gastrointestinal NETs during the 1971–2007 period also found an increase from 0.27 to 1.32 per 100,000 in men and from 0.35 to 1.33 per 100,000 in women [6]. Although this increase can be attributed to an improvement in diagnostic techniques and a rise in NET/cancer awareness, a definitive explanation for this phenomenon is still pending, as is the discrepancy in incidence between the UK and North America. These regional differences further highlight the need to establish and maintain regional and country-based tumor registries.
NET incidence and/or prevalence in Latin American countries remain largely unreported, and clinical literature is extremely scarce [11, 12]. An observational study in Argentina documented a total of 532 NET cases that included 461 GEP-NETs and 71 bronchial NETs [12]. This study demonstrated that 26.9% of GEP-NETs were located at the small bowel, followed by pancreas (25.2%), colon–rectum (12.4%), appendix (7.6%), gastric (6.9%), esophagus (2.8%), and duodenum (2.0%), with a further 16.3% reported as unknown origin. A NET registry from Brazil has documented baseline information on the first 1000 patients and classified the majority of tumors as thoracic (71.6%), followed by GEP-NETs (20.2%), head and neck (1.5%), skin (0.9%), genitourinary tract (0.6%), adrenal (0.4%), biliary (0.3%), prostate (0.3%), esophagus (0.1%), breast (0.2%), kidney (0.1%), ovary (0.2%), and 3.6% classified as unknown origin [11].These results again demonstrate the population-specific incidence of the NET classified malignancies.
Herein, we deliver the first multicentric Chilean registry of NET patients. Of the 166 patients currently incorporated into this registry, we report that 115 are GEP-NETs and 51 classified as non-GEP-NETs. Interestingly, in contrast to previously reported finding from other NET registries, the Chilean registry data shows a high proportion of small-bowel tumors (46%) and stage IV metastatic disease at diagnosis (62%). Patient characteristics (age, sex, etc.) and survival rates were similar to those reported in other regions.
Materials and Methods
The First Chilean NET Patient Registry: Participating Institutions
A team of specialists that included medical oncologists, endocrinologists, gastroenterologists, nurses, and molecular biologists generated this registry. The registry was funded by Novartis, Chile. Novartis had no access to patient raw data or any participation in the establishment of the database, data acquisition, or analysis. This study was designed as an observational, multicenter, prospective, and retrospective registry, and approved by the institutional review board and ethics committee in all participating institutions, in accordance with the Declaration of Helsinki, Good Clinical Practices, and Chilean regulations. Participating institutions with ethics approval included the following: Hospital Base de Valdivia, Hospital de Punta Arenas, Hospital Dr. Sotero del Rio, Hospital Base de Osorno, Hospital Regional de Concepcion, and Hospital Clinico de la Universidad de Chile. Written informed consent was obtained from all participating patients.
Chromogranin A, Intraplatelet Serotonin, and 5-Hydroxy-Indoleacetic Acid
Chromogranin A (CgA) levels were obtained from plasma samples using an ELISA kit from EURODIAGNOSTICA. Similarly, intraplatelet (I-P) serotonin levels were obtained from plasma by high-performance liquid chromatography (HPLC). 5-Hydroxy-indoleacetic acid (5-HIAA) was also obtained by HPLC but using 24-h collected urine samples.
Data Acquisition
The Chilean registry involved the development of an online database of NET patients. Collected patient data were entered into a virtual platform at www.clinicaldata.cl. Patients were enrolled starting in July 2015 through July 2017. Data were also retrospectively collected from medical records at participating institutions. The database consisted of 86 validated entries that included basic information, demographics, onset symptoms, tumor characteristics, diagnostic procedures, treatment regime (if any), and clinical outcome. All physicians received prior training and were responsible for entering data into the registry. To assess the quality of the data entered in www.clinicaldata.cl, a trained monitor for the study periodically visited each participating center to review the relevant patient medical records.
Inclusion and Exclusion Criteria
The registry included adult individuals (> 18 years old), diagnosed with histologically confirmed NETs and at least 3 months of follow-up with access to clinical information. Exclusion criteria consisted of patients with missing or incomplete information, absence of clinical follow-up data, or those unable or unwilling to sign written informed consent.
Statistical Analysis
Continuous variables entered in the registry were expressed as mean plus/minus standard deviation or by median values and range (minimum and maximum) values according to their distribution (normal vs. not normal). Categorical variables were expressed as percentages (%). Statistical comparisons among groups were performed by Student’s t test when data were normally distributed; otherwise, the Mann–Whitney U test was performed. The distribution of continuous variables for > 2 groups was analyzed by the ANOVA or Kruskal–Wallis test, depending on data normality. The differences in categorical variables were tested by Fisher’s exact test. Survival curves were calculated using the Kaplan–Meier estimate method and different variables were compared by the log-rank test. All statistical analyses were performed using GraphPad Prism 7 or R statistical software. All analyses were two-tailed and significance was set at p ≤ 0.05.
Results
Patient Population
This registry enrolled a total of 166 eligible NET patients. The median age at diagnosis was 53 years (range 23–85) and male patients accounted for 54.2% (n = 90) of the entries. The majority, 115 out of 166 tumors (64%) were registered as GEP-NETs in line with previous reports. Within GEP tumors, the most frequent primary tumor site was the small bowel (46%, n = 53). Tumors were predominantly metastatic at diagnosis, classified as stage IV (62%). The majority of patients also had an ECOG performance status 0–1 (92%) and their histology classified as low-grade, well-differentiated (56%). In 92 patients, Ki67 was assessed by immunohistochemistry with 43% being classified as intermediate grade/moderately differentiated with a Ki67 level in the 3–20% range. Basic information, clinico-pathological characteristics, and demographics of patients are summarized in Table 1.
Table 1.
Demographic and biological characteristics of registered Chilean NET patients. * Thoracic NET cases includes broncho/pulmonary and thymic tumors; ** unknown site NETs include breast (not primary) and other with uncertain origin
| All patients | GEP | Pancreatic | Gastric | Small bowel | Colorectal | Appendicular | Hepatobiliary | Thoracic* | Unknown** | |
|---|---|---|---|---|---|---|---|---|---|---|
| Median age/years (range) | 53 (22–85) | 52 (25–81) | 48 (25–78) | 58 (29–81) | 55 (27–77) | 52.5 (25–70) | 42 (28–63) | 60 (49–71) | 46 (22–73) | 61 (33–75) |
| Median OS/months | 110 | 168 | ND | 31 | 168 | 83 | ND | ND | ND | 68 |
| 5-year OS rate (%) | 71.5 | 78.5 | 75.1 | ND | 86.5 | 68.8 | ND | ND | 70 | 50 |
| Gender n (%) | 166 (100) | 115 (100) | 35 (100) | 9 (100) | 53 (100) | 12 (100) | 4 (100) | 2 (100) | 11 (100) | 9 (100) |
| Male | 90 (54) | 64 (56) | 21 (60) | 4 (44.4) | 32 (60) | 5 (41.7) | 1 (25) | 1 (50) | 7 (63.6) | 3 (33.3) |
| Female | 76 (46) | 51 (44) | 14 (40) | 5 (55.6) | 21 (40) | 7 (58.3) | 3 (75) | 1 (50) | 4 (36.4) | 6 (66.7) |
| Tumor stage n (%) | 145 (100) | 102 (100) | 30 (100) | 7 (100) | 51 (100) | 9 (100) | 3 (100) | 2 (100) | 11 (100) | 8 (100) |
| Stage I | 17 (12) | 14 (14) | 4 (13.3) | 4 (57.1) | 3 (5.9) | 1 (11.1) | 2 (66.7) | 0 (0) | 3 (27.3) | 0 |
| Stage II | 17 (12) | 8 (8) | 4 (13.3) | 0 | 3 (5.9) | 0 | 1 (33.3) | 0 (0) | 2 (18.2) | 3 (37.5) |
| Stage III | 21 (14) | 16 (15) | 5 (16.7) | 0 | 8 (15.7) | 2 (22.2) | 0 | 1 (50) | 1 (9.1) | 0 |
| Stage IV | 90 (62) | 64 (63) | 17 (56.7) | 3 (42.9) | 37 (72.5) | 6 (66.7) | 0 | 1 (50) | 5 (45.5) | 5 (62.5) |
| ECOG n (%) | 129 (100) | 82 (100) | 25 (100) | 6 (100) | 40 (100) | 7 (100) | 3 (100) | 2 (100) | 10 (100) | 8 (100) |
| 0 | 49 (38) | 32 (39) | 10 (40) | 2 (33.3) | 16 (40) | 1 (14.3) | 3 (100) | 1 (50) | 4 (40) | 1 (12.5) |
| 1 | 69 (54) | 43 (52) | 13 (52) | 3 (50) | 20 (50) | 6 (85.7) | 0 | 1 (50) | 6 (60) | 6 (75) |
| 2 | 7 (5) | 6 (8) | 2 (8) | 1 (16.7) | 3 (7.5) | 0 | 0 | 0 | 0 | 1 (12.5) |
| 3 | 4 (3) | 1 (1) | 0 | 0 | 1 (2.5) | 0 | 0 | 0 | 0 | 0 |
| Tumor grade n (%) | 159 (100) | 111 (100) | 34 (100) | 8 (100) | 51 (100) | 12 (100) | 4 (100) | 2 (100) | 10 (100) | 8 (100) |
| 1 | 90 (56) | 67 (60) | 21 (61.8) | 2 (25) | 36 (70.6) | 6 (50) | 2 (50) | 0 | 5 (50) | 2 (25) |
| 2 | 34 (22) | 31 (28) | 10 (29.4) | 3 (37.5) | 14 (27.4) | 4 (33.3) | 0 | 0 | 0 | 0 |
| 3 | 35 (22) | 13 (12) | 3 (8.8) | 3 (37.5) | 1 (2) | 2 (16.7) | 2 (50) | 2 (100) | 5 (50) | 6 (75) |
| Ki-67 n (%) | 92 (100) | 71 (100) | 19 (100) | 8 (100) | 29 (100) | 9 (100) | 4 (100) | 2 (100) | 8 (100) | 4 (100) |
| 0–2% | 27 (29) | 25 (35) | 6 (31.6) | 4 (50) | 13 (44.8) | 3 (33.3) | 3 (75) | 0 | 2 (25) | 0 |
| 3–20% | 39 (43) | 33 (47) | 9 (47.4) | 4 (50) | 15 (51.7) | 5 (55.6) | 0 | 0 | 2 (25) | 1 (25) |
| > 20% | 26 (28) | 13 (18) | 4 (21) | 0 | 1 (3.5) | 1 (1.1) | 1 (25) | 2 (100) | 4 (50) | 3 (75) |
| CgA n (%) | 57 (100) | 52 (100) | 21 (100) | 3 (100) | 23 (100) | 3 (100) | 0 | 2 (100) | 3 (100) | 1 (100) |
| < 100 ng/mL | 27 (47) | 23 (44) | 8 (38.1) | 1 (33.3) | 12 (52.2) | 2 (66.7) | 0 | 0 | 2 (66.7) | 1 (100) |
| > 100 ng/mL | 30 (53) | 29 (56) | 13 (61.9) | 2 (66.7) | 11 (47.8) | 1 (33.3) | 0 | 2 (100) | 1 (33.3) | 0 |
| I-P serotonin n (%) | 45 (100) | 43 (100) | 8 (100) | 1 (100) | 30 (100) | 4 (100) | 0 | 0 | 0 | 0 |
| 400–800 ng/109 plat. | 12 (27) | 10 (23) | 2 (25) | 0 | 6 (20) | 2 (50) | 0 | 0 | 0 | 0 |
| > 800 ng/109 plat. | 33 (73) | 33 (77) | 6 (75) | 1 (100) | 24 (80) | 2 (50) | 0 | 0 | 0 | 0 |
| 5-HIAA n (%) | 52 (100) | 49 (100) | 11 (100) | 0 | 33 (100) | 4 (100) | 0 | 0 | 0 | 0 |
| < 100 μmol/h | 21 (40) | 18 (37) | 7 (63.6) | 0 | 8 (24.2) | 2 (50) | 0 | 0 | 0 | 0 |
| > 100 μmol/h | 31 (60) | 31 (63) | 4 (36.4) | 0 | 25 (75.8) | 2 (50) | 0 | 0 | 0 | 0 |
NET Biomarkers and Quality of Surgery
Chromogranin A (CgA), intraplatelet (I-P) serotonin, and 5-hydroxy-indoleacetic acid (5-HIAA) were measured as diagnostic biomarkers in subsets of patients. First, CgA levels from 57 patients were obtained from plasma samples using an ELISA kit. In the subset of GEP-NETs, I-P serotonin was measured in 45, and 5-HIAA in 52 patients respectively. Table 1 demonstrates that biomarkers were elevated in the majority of patients. CgA levels were elevated (> 100 ng/ml) in 53%. Similarly, I-P serotonin and 5-HIAA levels were increased (> 800 ng/109 platelets and > 100 μmol/h, respectively) in 73% and 60% of cases, respectively. Finally, patient surgery was categorized as R0 when no macroscopic disease was visible post-surgery; in our cohort 124 out of 166 patients had surgery (74.6%); within this group, 52% were classified as R0.
Survival Rates
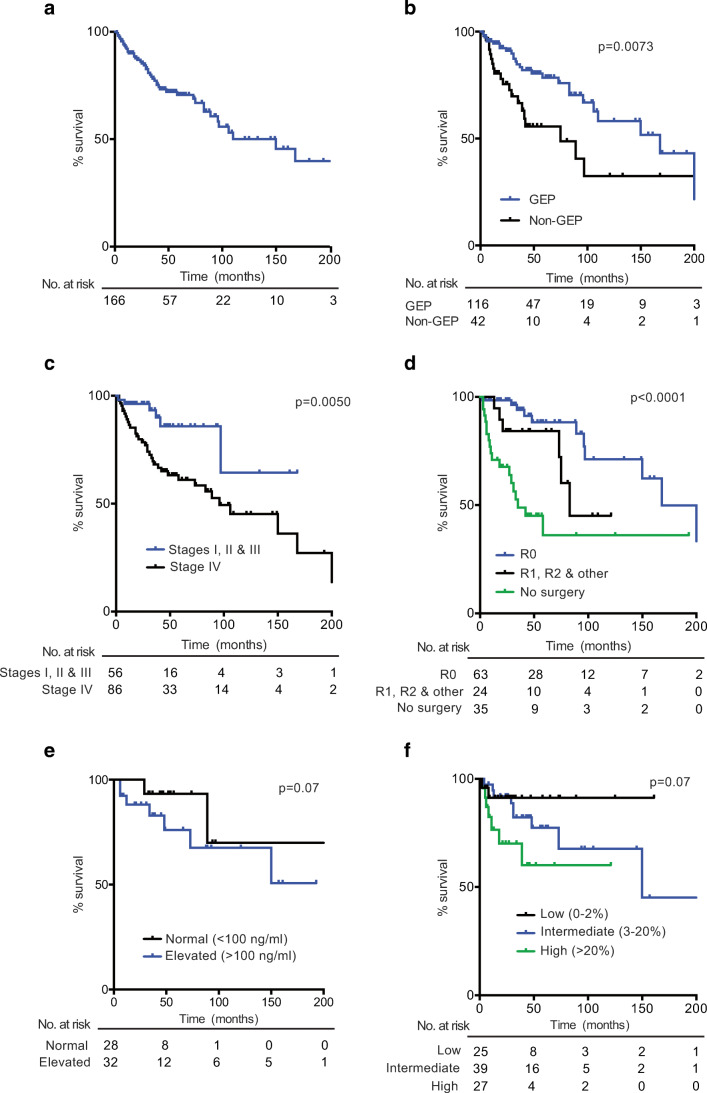
Median overall survival (OS) for all registered patients was 110 months with a 5-year survival rate of 71% (see Table 1 and Fig. 1a). When analyzed by tumor primary site, the most favorable prognosis was observed in the small bowel (median OS 168 months, 5-year survival 86.5%) and the least among gastric tumors with a median OS of 31 months. Figure 1 shows Kaplan–Meier estimate curves: for all patients (Fig. 1a), by tumor primary site (GEP versus non-GEP; Fig. 1b, log rank test p = 0.0073), by cancer stage (Fig. 1c, log rank test p = 0.0050), by quality of surgery (Fig. 1d, log rank test p < 0.0001), by serum CgA concentration (Fig. 1e) and by Ki67 index (Fig. 1f). Briefly, patients with GEP tumors had significantly better OS rates than non-GEP (Fig.1b). As expected, OS rates were lower in stage IV patients than with stage I, II, and III combined (Fig. 1c). Patients with optimal surgery classified as R0 had significantly better OS (Fig. 1d) and patients with elevated CgA levels or high Ki67 had worse OS (Fig. 1e, f); however, these differences did not reach statistical significance (p = 0.07). The number of patients at risk over time, in every case, is indicated at the bottom of every graph.
Fig. 1.
Kaplan–Meier survival curves in Chilean NET patients. a For all patients recorded in this registry (n = 166). b According to tumor primary site, comparing GEP versus non-GEP (Log Rank test p = 0.0073). c By tumor stage, comparing stage I, II and III against stage IV (log-rank test p = 0.0050). d By quality of surgery, comparing no surgery, R0, or R1+R2+other grouped (log-rank test p < 0.0001). e By CgA levels comparing normal (< 100 ng/ml) versus elevated (≥ 100 ng/ml) (log-rank test p = 0.07). f By Ki67, comparing low (0–2%), intermediate (3–20%), and high (> 20%) levels (log-rank p = 0.07). The number of patients at risk over time, in each case, is indicated at the bottom of every graph
NET Primary Site Distribution in Chilean in Comparison to Other Registries
Table 2 shows the distribution by primary site across various NET registries. First, Table 2A compares primary site distribution only in GEP-NET cohorts in China [13], Argentina [12], and Chile. Second, Table 2B compares primary site distribution excluding pancreas (GI-NET) in cohorts from England [6], Japan [14], and Chile. Then, Table 2C compares the distribution of primary sites among cohorts that include all NETs from the USA (the SEER database) [3], Spain [5], Norway [15], and Chile. The Brazilian NET registry reported tumor sites as thoracic was not included in Table 2.
Table 2.
Comparative table of Chilean data compared to published NET registries. Distribution by tumor primary site
| A. GEP-NETs cohorts: primary site proportions | B. GI-NETs cohorts: primary site proportions | C. All NETs cohorts: primary site proportions | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primary site | China | Argentina | Chile | England | Japan | Chile | USA | Spain | Norway | Chile |
| Stomach | 27% | 7% | 8% | 12% | 15% | 11% | 5% | 6% | 3% | 5% |
| Small bowel | 6% | 29% | 46% | 29% | 19% | 65% | 23% | 16% | 12% | 32% |
| Colon | 3% | 12% | 10% | 13% | 2% | 16% | 4% | 5% | 4% | 7% |
| Appendix | 2% | # | 4% | 38% | NR | 3% | 3% | 9% | 4% | 2% |
| Rectum | 30% | # | # | 8% | 55% | # | 15% | 6% | 2% | # |
| Pancreas | 31% | 25% | 30% | – | – | – | 7% | 34% | 3% | 21% |
# Indicates these were recorded as colon, NR no reported value
Discussion
To the best of our knowledge, this is the first NET registry in the Pacific South American region that describes epidemiologic and clinico-pathologic characteristics of patients. To date, the Chilean registry contains a relatively low number of recorded cases (n = 166); however, it is important to note that Chile has a population of approximately 17.5 million which is notably lower than other countries with NET registries [16]. While the largest NET database is currently maintained by the SEER program in the USA [3], there exist only two reports of NET registries in Latin America [11, 12], and from these, only an observational study from Argentina reports on 532 NET cases which include relevant clinical data.
Overall, the Chilean registry confirms that NETs are a highly heterogeneous group of neoplasms with a wide variety of clinical presentations and outcomes. NETs are commonly considered indolent tumors, especially when compared to carcinomas; however, the prognosis among Chilean patients was highly variable with median OS values that ranged from 168 months in small-bowel tumors, down to 31 months in gastric tumors. Despite this, the median OS for our study was 110 months, which is in line with Spanish and Argentinean NET registries that reported 144 and 121 months OS, respectively [5, 12], and possibly reflecting the recent common heritage between these nations. Likewise, the median age at diagnosis in Chilean patients was 53 years, a value comparable to registries in China (53 years) [13] and Argentina (53.2 years) [12]. The Chilean registry also demonstrated a slight preponderance of males over females, a trend similar to that reported in a Spanish national NET registry [5], yet differing from other reports that show a higher NET prevalence among the female population [3, 17].
Chromogranin A (CgA) is widely used as a biomarker for NET diagnosis and monitoring [18–20]. Although there are several methods to measure CgA levels, the only clinically validated method available in Chile is by ELISA; for consistency, all CgA measurements in our registry were performed at the same center using this method. Also, general consensus establishes ~ 100 ng/ml as a cutoff for normal CgA levels. However, as occurs with NET prevalence and incidence, the normal range of CgA levels in Latin American patients is somewhat uncertain. Currently, our research group is working on the assessment of CgA levels in Chilean healthy subjects and NET patients in order to obtain a more accurate CgA cutoff value.
In relation to the primary tumor sites, we found that the majority of recorded tumors were reported as GEP-NETs, in accordance with previous registries [5, 17]. However, when analyzed in further detail, the percentages of this classification of tumor displayed discrepancies across different geographic areas, for example, the percentage of small-bowel tumors in our cohort was 46%, a value higher than the 29% reported in Argentina [12] and strikingly different from the 6% in a Chinese registry [13]. Conversely, the proportion of stomach (27%) and rectum (30%) tumors in the Chinese registry was notoriously higher than that reported in the present study (8% and < 10% respectively, see comparative Table 2A). The high proportion of small-bowel NETs in our population study was even more evident when we compared other registries reporting only GI-NETs from England [6] and Japan [14] (see comparative Table 2B). Furthermore, comparative Table 2C shows that the proportion of small-bowel tumors in our cohort (32%) is twofold the reported value in the Spanish registry (16%) and higher than the SEER database (23%). Undoubtedly, a definitive explanation for this phenomenon remains pending; however, we speculate this is likely derived from a combination of ethnic and environmental influences, as genetically the Chilean population possess a high European ancestry component combined with Native American [21] that might explain some similarities with the Spanish registry [5] and the remarkable differences with Asian registries [13, 14]. The comparative GEP-NET data alone (Table 2A) illustrates the need for national and regional databases. Regarding origin, Argentina and Chile in South America tend to have a lower percentage of stomach tumors and a higher proportion of small bowel; notably, an inverse distribution is observed in the available data from China. Furthermore, the combined proportion of GEP-NET/colon (this includes colon, appendix, and rectum) in Argentina (12%) and Chile (10%) are quite similar, in sharp contrast with the GEP-NET/colon percentage in China (30%). Apart from ethnic differences, an alternative explanation for these regional discrepancies is that several members of the medical team that elaborated this Chilean registry are gastroenterologist surgeons, and therefore, the high percentage of small-bowel tumors could be overestimated due to a registration bias. Similarly, we noticed a relatively small percentage of thoracic NETs in our registry (6.6%) that includes bronchial (n = 8) and thymus (n = 3) tumors. The SEER database indicates up to 23% of thoracic NETs among Caucasian males [3]. Our cancer center is a national reference center for breast and gastric cancer patients, and therefore, the lack of thoracic NETs could also be attributed to a registration bias. Prospectively, our registry will seek to incorporate more centers in order to avoid this ascertainment bias.
Another interesting finding of our study is the significant proportion of stage IV metastatic patients (62%, Table 1); this is also notoriously different from other registries including the SEER database (21%) or the national registry from Spain (44%). Several factors could potentially explain these numbers: firstly, the Chilean registry is led by oncologists that frequently deal with more advanced disease patients compared to other medical professionals in the area such as endocrinologists or gastroenterologists, and as mentioned above, this could be another registration bias in our study. Second, this could be attributed to the late diagnosis of patients; in general, NETs are difficult to diagnose; as explained above, these tumors can sometimes remain asymptomatic for several years until the onset of metastatic disease. Finally, this could be attributed to a socioeconomic factor in Chile, the relatively high costs of treatment and diagnostic tests along with lack of medical insurance coverage may result in many patients receiving diagnosis and medical attention at a more advanced stage.
In summary, our study presents for the first time a Chilean NET registry including a wide range of primary sites, confirming the previously reported high heterogeneity of NETs. As discussed above, reported demographics and basic clinico-pathological characteristics of patients are in line with other NET registries. Remarkably, our study shows an unusual high proportion of small-bowel NETs and advanced stage IV tumors. The regional differences in NET primary site proportions and sub-types highlight the need for country-based NET databases in order to identify population-specific bias and may provide the basis for a better understanding of the regional discrepancies in incidence and distribution. A definitive explanation for these discrepancies remains pending and further registry-guided investigation will add to the knowledge on this seemingly rare and highly heterogeneous disease.
Electronic supplementary material
(PNG 285 kb)
(AI 1467 kb)
Acknowledgements
Novartis, Chile, S.A.
Funding
NOVARTIS CHILE S.A.; FONDECYT Grant Nos. 1180173 (MG) and 1180241 (GO). CONICYT-FONDAP 15130011 (GO). IMII P09/016-F (GO).
References
- 1.Klöppel G. Oberndorfer and his successors: from carcinoid to neuroendocrine carcinoma. Endocr Pathol. 2007;18:141–144. doi: 10.1007/s12022-007-0021-9. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015;121:589–597. doi: 10.1002/cncr.29099. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Carbonero R, Capdevila J, Crespo-Herrero G, Diaz-Perez JA, Martinez del Prado MP, Alonso Orduna V, Sevilla-Garcia I, Villabona-Artero C, Beguiristain-Gomez A, Llanos-Munoz M, Marazuela M, Alvarez-Escola C, Castellano D, Vilar E, Jimenez-Fonseca P, Teule A, Sastre-Valera J, Benavent-Vinuelas M, Monleon A, Salazar R. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): results from the National Cancer Registry of Spain (RGETNE) Ann Oncol. 2010;21:1794–1803. doi: 10.1093/annonc/mdq022. [DOI] [PubMed] [Google Scholar]
- 6.Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563–2569. doi: 10.1038/ajg.2010.341. [DOI] [PubMed] [Google Scholar]
- 7.Lepage C, Bouvier A-M, Manfredi S, Dancourt V, Faivre J. Incidence and management of primary malignant small bowel cancers: a well-defined French population study. Am J Gastroenterol. 2006;101:2826–2832. doi: 10.1111/j.1572-0241.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 8.Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol Oncol Res. 2011;17:759–763. doi: 10.1007/s12253-011-9382-y. [DOI] [PubMed] [Google Scholar]
- 9.Tsai H-J, Wu C-C, Tsai C-R, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. 2013;8:e62487. doi: 10.1371/journal.pone.0062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT, Takayanagi R, Shimatsu A. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58–64. doi: 10.1007/s00535-014-0934-2. [DOI] [PubMed] [Google Scholar]
- 11.Younes RN, RN Y. Neuroendocrine tumors: a registry of 1,000 patients. Rev Assoc Med Bras. 2008;54:305–307. doi: 10.1590/S0104-42302008000400014. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor J, Marmissolle F, Bestani C, et al. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in Argentina: results from the large database of a multidisciplinary group clinical multicenter study. Mol Clin Oncol. 2014;2:673–684. doi: 10.3892/mco.2014.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J-H, Zhang Y-Q, Shi S-S, Chen YJ, Yuan XH, Jiang LM, Wang SM, Ma L, He YT, Feng CY, Sun XB, Liu Q, Deloso K, Chi Y, Qiao YL. A nation-wide retrospective epidemiological study of gastroenteropancreatic neuroendocrine neoplasms in China. Oncotarget. 2017;8:71699–71708. doi: 10.18632/oncotarget.17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K, Doi R, Shimatsu A, Nishida T, Komoto I, Hirata Y, Nakamura K, Igarashi H, Jensen RT, Wiedenmann B, Imamura M. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234–243. doi: 10.1007/s00535-009-0194-8. [DOI] [PubMed] [Google Scholar]
- 15.Cetinkaya RB, Aagnes B, Thiis-Evensen E, et al. Trends in incidence of neuroendocrine neoplasms in Norway: a report of 16,075 cases from 1993 through 2010. Neuroendocrinology. 2016;104:1–10. doi: 10.1159/000442207. [DOI] [PubMed] [Google Scholar]
- 16.Instituto Nacional de Estadística (INE) (2017) Primeros resultados definitivos del Censo 2017: un total de 17.574.003 personas fueron efectivamente censadas. In: INE. http://www.ine.cl/prensa/detalle-prensa/2017/12/22/primeros-resultados-definitivos-del-censo-2017-un-total-de-17.574.003-personas-fueron-efectivamente-censadas
- 17.Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AKC, Modlin IM. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 18.Vinik AI, Woltering EA, Warner RRP, Caplin M, O'Dorisio TM, Wiseman GA, Coppola D, Go VL, North American Neuroendocrine Tumor Society (NANETS) NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 19.Pape UF, Perren A, Niederle B, Gross D, Gress T, Costa F, Arnold R, Denecke T, Plöckinger U, Salazar R, Grossman A, Barcelona Consensus Conference participants ENETS consensus guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology. 2012;95:135–156. doi: 10.1159/000335629. [DOI] [PubMed] [Google Scholar]
- 20.Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, Meyer T, Newell-Price J, Poston G, Reed N, Rockall A, Steward W, Thakker RV, Toubanakis C, Valle J, Verbeke C, Grossman AB, UK and Ireland Neuroendocrine Tumour Society Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyheramendy S, Martinez FI, Manevy F, Vial C, Repetto GM. Genetic structure characterization of Chileans reflects historical immigration patterns. Nat Commun. 2015;6:6472. doi: 10.1038/ncomms7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PNG 285 kb)
(AI 1467 kb)