Abstract
Objectives
To identify areas of agreement and disagreement in the implementation of multi‐parametric magnetic resonance imaging (mpMRI) of the prostate in the diagnostic pathway.
Materials and Methods
Fifteen UK experts in prostate mpMRI and/or prostate cancer management across the UK (involving nine NHS centres to provide for geographical spread) participated in a consensus meeting following the Research and Development Corporation and University of California‐Los Angeles (UCLA‐RAND) Appropriateness Method, and were moderated by an independent chair. The experts considered 354 items pertaining to who can request an mpMRI, prostate mpMRI protocol, reporting guidelines, training, quality assurance (QA) and patient management based on mpMRI levels of suspicion for cancer. Each item was rated for agreement on a 9‐point scale. A panel median score of ≥7 constituted ‘agreement’ for an item; for an item to reach ‘consensus’, a panel majority scoring was required.
Results
Consensus was reached on 59% of items (208/354); these were used to provide recommendations for the implementation of prostate mpMRI in the UK. Key findings include prostate mpMRI requests should be made in consultation with the urological team; mpMRI scanners should undergo QA checks to guarantee consistently high diagnostic quality scans; scans should only be reported by trained and experienced radiologists to ensure that men with unsuspicious prostate mpMRI might consider avoiding an immediate biopsy.
Conclusions
Our consensus statements demonstrate a set of criteria that are required for the practical dissemination of consistently high‐quality prostate mpMRI as a diagnostic test before biopsy in men at risk.
Keywords: consensus methods, multi‐parametric MRI, prostate cancer, recommendations
Introduction
The prostate diagnostic pathway is currently based on carrying out a TRUS‐guided biopsy in men with an elevated serum PSA. TRUS‐guided biopsy involves taking 10–12 needle core biopsies from the prostate without prior knowledge as to whether the man has cancer, and if he does, where the cancer resides. This leads to over‐diagnosis of clinically insignificant cancers and missing cancers that are clinically significant, as well as the harms of deploying needles through the rectum.
Multi‐parametric MRI (mpMRI) of the prostate could transform the prostate cancer clinical pathway 1, 2, 3, 4, 5, 6, 7. A recently published prospective multi‐centre study (PROstate MRI Imaging Study [PROMIS]) comparing the diagnostic accuracy of mpMRI and TRUS biopsy demonstrated that mpMRI outperforms TRUS biopsy as a diagnostic tool for the detection of clinically significant cancer. Further, PROMIS showed that a quarter of men at risk could avoid immediate biopsy as a result of unsuspicious mpMRI 8.
However, not all UK institutions have the ability to deliver the benefits of mpMRI to the extent reported within the PROMIS 8. At present, from a Freedom of Information dataset acquired by Prostate Cancer UK, only 50% of centres across UK offer mpMRI to the standard reported within the PROMIS 9.This is because firstly, mpMRI scan quality is variable across centres (of note, ~50% of scanners would need replacement over the next 5 years 9). Secondly, sufficiently experienced radiologists are required to interpret these complex scans. Lastly, there is a lack of detailed guidance for clinicians in how to use mpMRI reports in making decisions in clinic. Sub‐optimal performance in any part of the pathway reduces the potential benefits of introducing mpMRI before first prostate biopsy, risking under‐detection of clinically significant cancers if biopsies are avoided or over‐calling of scans, preventing men from benefiting from mpMRI's triage characteristics of avoiding a biopsy.
To address these challenges, a formal consensus process to determine areas of agreement and disagreement within a panel of UK experts in the field of prostate cancer and/or mpMRI was organised. The aims were to define criteria for requesting, performing and reporting mpMRI scans, addressing quality assurance (QA) of mpMRI, establishing the requirements for mpMRI training, and guiding patient management using mpMRI.
Materials and Methods
Design, Setting and Participants
A modified Research and Development Corporation and University of California‐Los Angeles (RAND‐UCLA) Appropriateness Method (RAM) was followed 10. A questionnaire containing 376 items was constructed by six core panelists (H.A., C.A., A.B., A.K., C.M., S.P.) and revisions were made following consultation with other members. The items were identified based on differences in practice across the UK and abroad without duplicating the aims of previous consensus processes. The questionnaire was divided into six sections: (i) Who can request prostate mpMRI, (ii) Prostate mpMRI acquisition protocol updates, (iii) Prostate mpMRI reporting, (iv) QA/quality control (QC) of prostate mpMRI diagnostic process, (v) Management of patients based on prostate mpMRI reports, and (vi) Training in prostate mpMRI.
The diagnostic role of mpMRI in a pre‐biopsy setting in men at risk of prostate cancer was considered and the uses of mpMRI for active surveillance of low‐risk disease or post‐therapy follow‐up were not addressed.
Panelists were selected due to their peer‐reviewed publications and expertise in prostate mpMRI and/or prostate cancer management whilst ensuring a geographical spread. The questionnaire was sent to 21 experts (eight radiologists, seven urologists, two oncologists, three radiographers, and a physicist). Eighteen participated in round 1 and fifteen attended the meeting. An independent non‐scoring moderator with significant experience in leading consensus meetings acted as chair.
Round 1: Individual Questionnaire Completion
Panelists were asked to rate their agreement with questionnaire statements for which they considered they had sufficient expertise on a 9‐point scale (ranging from 1 ‘strongly disagree’ to 9 ‘strongly agree’). If they lacked expertise for a particular item, they scored ‘0’ to indicate that they were non‐scoring experts for that item.
Round 2: Face‐to‐Face Meeting Discussion
Fifteen attending panel members were shown the first‐round score distribution for each questionnaire statement. After each statement discussion, the panelists rescored the item. Items scored by at least eight panel members were included in the results. Nine consensus statements were added, 23 removed and 39 statements reworded for clarity. Eight items responded to by less than eight panelists were excluded reducing the number of consensus statements to 354.
Interpretation of the Results
We considered that there was ‘agreement’ with an individual statement for a panel median score of ≥7 and ‘disagreement’ for a panel median score of ≤3. A score between 4 and −6 reflected ‘uncertainty’. Consideration for a particular item to reach ‘consensus’ depended on the number of scoring panel members as elaborated in the RAM 10.
Results
Pre‐meeting consensus was reached in 127 of 376 items (34%). During the meeting, consensus was reached in 208 of 354 items (59%). Table 1 shows the percentage of items reaching consensus for each section of the questionnaire before and after the face‐to‐face meeting. The Appendix S1 includes the detailed results for each questionnaire item.
Table 1.
Number (%) of items reaching consensus in each section of the questionnaire
| Section | Pre‐meeting | Post‐meeting |
|---|---|---|
| n/N (%) consensus items | n/N (%) consensus items | |
| I. mpMRI requests | 6/12 | 10/12 |
| II. mpMRI acquisition protocol updates | 12/41 (29) | 25/41 (61) |
| III. mpMRI clinical reporting | 43/141 (30) | 85/131 (65) |
| IV. QA/QC of mpMRI | 44/100 (44) | 47/89 (53) |
| V. Management of patients | 12/56 (21) | 24/54 (44) |
| VI. mpMRI training | 10/26 (38) | 17/27 (63) |
| Total | 127/376 (34) | 208/354 (59) |
Statements for which consensus was reached are summarised below. Some statements for which consensus majority was not reached are also discussed while mentioning that this was ‘agreement without consensus’.
Section I: Who Can Request Prostate mpMRI?
The panel agreed in consensus that mpMRI requests should be made by urologists, uro‐oncologists, and specialist urology nurses. The latter would act mostly as a filter to determine the appropriateness of all incoming requests. In the current healthcare environment, any other clinical team may also be able to request prostate mpMRI provided that there is prior urological consultation, to ensure effective communication of mpMRI results and continuity of care. There was consensus that GPs should not directly request prostate mpMRI and patients should not self‐refer for prostate mpMRI.
It was also unanimously agreed that mpMRI should not be offered to all men prior to clinical assessment and that an elevated PSA should be assessed with other clinical factors such as age, family history, DRE findings, PSA kinetics and previous TRUS biopsy to determine referral for a prostate mpMRI examination.
Section II: Updates on Prostate mpMRI Acquisition Protocol
The panel focused on differences between previous UK recommendations 11 and other international consensus guidance such as Prostate Imaging and Reporting and Data System (PI‐RADS_v1 and _v2) 12, 13. The outcomes are summarised in Table 2, with some elaborated on below.
Table 2.
Prostate mpMRI acquisition protocol updates
| Protocol updates |
|---|
|
|
|
|
Consensus was reached on orientating axial imaging to the patient and not to the position of the prostate gland. Although the latter is the orientation of choice for the correspondence of MRI scans to prostatectomy specimens particularly used in research, in the setting of men undergoing surveillance with repeat scans to monitor any interval change in lesion size, axial imaging to the patient was considered helpful for improving consistency and reproducibility of scans and lesion measurements albeit this requires validation. Also direct intervention of radiologists during the scan is reduced.
T2‐Weighted Imaging (T2W)
The panel endorsed the previous statement 12 that this sequence should be acquired in all three planes (the sagittal plane being useful for pre‐surgical planning and improved visualisation of the bladder neck). In particular, T2W should be obtained as three separate acquisitions (axial, coronal and sagittal, two‐dimensional [2D], fast‐spin echo [FSE], multi‐slice) instead of single 3D acquisition until further research on direct comparison of diagnostic quality and cancer conspicuity of 2D vs 3D T2W for both peripheral zone (PZ) and transition zone (TZ) are available 14, 15. The maximum voxel size in‐plane resolution of T2 sequences should be ≤0.7 mm, in keeping with previous recommendations 11, 12. The use of T2 sequences with a large field‐of‐view to cover abdominal nodes outside the pelvis was questioned and was not considered as an essential requirement, as MRI has a poor performance for detection of nodal disease compared to functional imaging techniques such as choline or prostate‐specific membrane antigen positron emission tomography (PET), especially when there is clinical concern of nodal metastatic spread 16, 17.
Diffusion‐Weighted Imaging (DWI) Sequences
The minimum high‐b value for diffusion sequences should be b = 1 400 s/mm2 at 1.5 T and b = 2 000 s/mm2 at 3 T 11, 12. Although consensus was not reached, the majority supported the preference for a separately acquired high b‐sequence over an extrapolated/calculated high b‐value images. Further evidence on the comparison between ‘extrapolated’ vs ‘separate’ high‐b value image acquisitions for histology‐validated prostate cancer detection would be of value 18. The maximum voxel size in‐plane resolution of DWI should as far as possible be kept at ≤2 mm as per previous UK guidelines 11.
Dynamic Contrast‐Enhanced Imaging (DCE)
The panel recognised that DCE‐MRI is an essential component of prostate mpMRI for detection, staging and treatment planning 19, 20. DCE‐MRI acts as a ‘safety net’ or a ‘back‐up’ mpMRI sequence especially when DWI images are degraded, which is not uncommon in routine practice (e.g. due to rectal gas). DCE‐MRI analysis should be performed visually, with anatomical evaluation in the early arterial enhancement images of the prostate. Quantitative pharmacokinetic DCE modelling or curve shape parametric evaluation was deemed unnecessary. This means that the temporal resolution can be up to 15 s between scans to allow for a high spatial resolution and anatomical interpretation of DCE‐MRI images.
Section III: Standards for Prostate mpMRI Clinical Reports
Table 3 summarises the outcomes of this section.
Table 3.
Consensus recommendations on clinical mpMRI reports
| Recommendations on clinical mpMRI reports |
|---|
|
|
|
Who Can Report Prostate mpMRI?
Given the expertise required to report mpMRI, the panel recommended that only uro‐radiologists or radiologists with a specialist interest in prostate cancer imaging should produce prostate mpMRI reports. They should report at least 100 mpMRI examinations per year with the requirement of an active participation in multi‐disciplinary team (MDT) meetings of at least twice a month. Other specialists (general radiologists, MR radiographers, urologists or uro‐oncologists) should only be able to review/demonstrate prostate mpMRI findings within the scope of their practice (e.g. a urologist would review an MRI prior to performing a targeted biopsy, but the images would already have been formally reported by a radiologist with prostate MRI expertise).
Significant Cancer Definition Thresholds for mpMRI Assessment
Acknowledging differences in opinions on the definitions of significant prostate cancer, there was consensus agreement to align with the definitions of clinically significant cancer as described in PI‐RADS_v2 12. Specifically, mpMRI should be scored to rule out Gleason score ≥3+4 and/or volume ≥0.5 mL, and/or extraprostatic extension/seminal vesicle invasion.
Prostate mpMRI Clinical Reports
There was consensus on the use of a 5‐point Likert‐impression scale (based on the radiologist's overall opinion and experience without the use of a dominant MRI sequence) to rate likelihood of clinically significant disease in routine reporting, as prospectively validated in the multi‐centre PROMIS study 8. There was no consensus on the routine use of the current ‘lesion‐based only’ assessment PI‐RADS_v2 scoring system or a concurrent use of PI‐RADS_v2 and the subjective Likert assessment in the UK. The panel acknowledged the lack of direct comparisons between subjective Likert assessment and PI‐RADS_v2 scoring, as comparisons to date involved Likert assessment and PI‐RADS_v1 21, 22. The majority of the panel disagreed with current PI‐RADS_v2 reporting recommendations that lesion size should be the only factor differentiating between a score 4 and 5 for the likelihood of tumour.
In addition to lesion‐based assessment, the remainder of the prostate should also be scored on a subjective 5‐point Likert‐assessment scale, to assess the significance of diffuse ‘background’ signal change within the gland, which may potentially mask significant tumour and prompt biopsy (as illustrated in Fig. 1). Of note, whole‐gland assessment is not addressed in PI‐RADS_v2. Prostate and tumour volume should be reported (the sagittal plane to measure the antero‐posterior diameter and height of the gland, and the axial plane for the width of gland were found to be more accurate for gland volume estimation 23; of note, the use of the semi‐ellipsoid formula for lesion volume estimation is practical albeit not yet validated). It is not necessary to report the apparent diffusion coefficient (ADC) values of lesions given the variability between scanners and centres 24, 25. When calculating PSA density, the panel recommended the use of MR‐based volumes over TRUS‐based volume, for greater accuracy 26.
Figure 1.
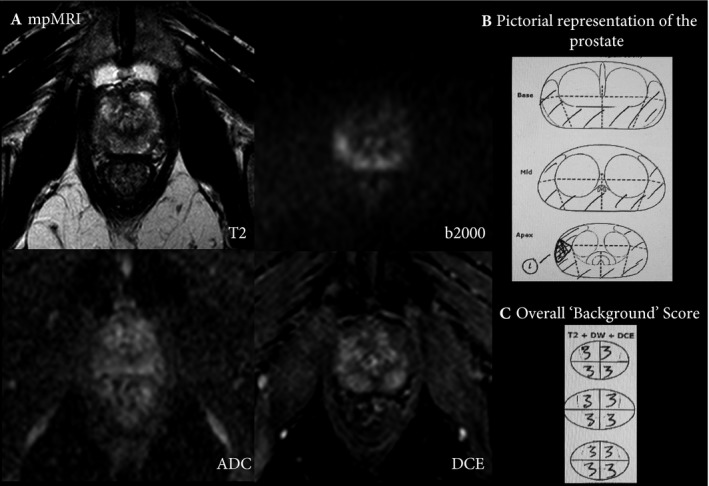
(A) Shows the mpMRI of a 62‐year‐old man, with a PSA level of 4.4 ng/mL and a gland volume of 25 mL at the level of the mid‐gland to apex region. On T2W imaging, there is diffuse and patchy low T2 signal and a lower T2 signal at the right lateral gland, with an equivocal high signal focus on diffusion high b value at 9 o'clock and corresponding equivocal low ADC signal with bilateral enhancement on DCE. The focal lesion (represented by number 1 in 1. (B) was reported with a Likert‐assessment of 3/5. Besides, the remainder of the gland was also assessed with the whole prostate divided into quarters for Likert assessment (C). Each quarter was reported as a ‘Likert‐assessment’ 3/5. The background changes scored 3 are represented by the shaded area in B. Upon transperineal template mapping biopsy, the prostate was found to harbour adenocarcinoma Gleason 3+4, (40% biopsy core involvement) at the right posterior apex, focal high‐grade prostatic intraepithelial neoplasia at the left posterior apex and Gleason 3+3, at eight different sites within the prostate (10–40% biopsy core involvement).
For pictorial prostate diagrams, there was uncertainty over routine use of either a minimum of 12‐sector or extensive 36‐sector PIRADS_v2 diagram. However, the panel all emphasised the benefits of a clear identification and an effective description of all mpMRI visible lesion(s) – these may be either: drawn on any sectored prostate template diagram (either hand‐drawn or computer‐generated); indicated/contoured on the sequence it is best visible in picture archiving communications system; screenshot as key images; saved as annotated images or indicated within the narrative text by sequence and slice numbers.
Double‐Reporting
While there is not a need to double‐read all mpMRI, there was agreement in consensus that equivocal prostate mpMRI (Likert‐impression 3) should be double‐read, if avoiding biopsy is under consideration. Also, discordant mpMRI scores with biopsy results should be retrospectively re‐read by a different radiologist. Any uro‐radiologist or radiologist with a specialist interest in prostate mpMRI imaging meeting the minimum requirements for independent reporting would be deemed appropriate to double‐read the scans.
Table 4 shows areas lacking consensus in the mpMRI reporting section.
Table 4.
Shows areas lacking consensus in clinical mpMRI reporting
| Areas lacking consensus in clinical mpMRI reporting |
|---|
| • PI‐RADS_v2 scoring system may be used during training/gaining experience before switching to the use of subjective Likert‐impression once experienced. |
| • The narrative report should refer to the sectors as named in the PI‐RADS_v2 pictorial report used: e.g. sectors named PZpl (postero‐lateral PZ), PZpm (posteromedial PZ), TZp (posterior TZ), TZa (anterior TZ), etc.). |
| • In the pictorial report, the prostate diagram should be represented in all three planes. |
| • mpMRI suspicious lesions contouring should be performed only when targeted biopsy or focal treatment is planned. |
| • Tumour volume should be calculated by summation of contoured areas on each slice of the tumour/software rendering. |
| • Transition zone (TZ) tumour should be measured from T2 only (as in PI‐RADS_v2). |
Section IV: QA/QC of Prostate mpMRI
Setting national quality standards to perform prostate mpMRI was envisaged in this section, to parallel breast imaging, where national quality standards are established to ensure safe, reliable, and accurate imaging services at accredited facilities.
However, as prostate cancer is not part of a national screening programme, consensus was not achieved to define stringent QA standards equivalent to breast imaging. This would ultimately be within the remit and expertise of accreditation bodies to be implemented in the future. Nevertheless, some areas of the panel discussion covering site‐specific, scanner‐specific, image‐specific and radiologist‐specific QA aspects are highlighted below.
Although consensus was not reached, a majority of 67% supported accreditation for sites performing prostate mpMRI, which would be administered by a national body. An accredited centre should be able to perform T2W, DWI and DCE‐MRI to the latest national guidelines and perform or refer patients for biopsy, MDT‐meeting discussions, and treatment.
Moreover, it was agreed in consensus that every scanner should undergo regular QA/QC procedures in order to perform prostate mpMRI. This is already a routine scanner QA requirement within NHS institutions. Detailed guidance is available on the American College of Radiology (ACR)/Association of American Physicists in Medicine (AAPM) website 27.
For diagnostic image‐quality assessment, qualitative and quantitative assessments were discussed. Whilst the usefulness of quantitative image assessment was unclear, qualitative assessment through visual image assessment by a radiologist analysing the images (e.g. looking for artefacts to prompt correction, assessing lesion conspicuity etc.) was recognised as adequate to determine diagnostic acceptability.
For radiologists to maintain reporting performance over time, the panel supported the use of combined self‐performance tests, external performance assessments, and institution‐based audits. The development of any online performance assessment tools could feature non histology‐validated MRI cases to compare the radiologist's performance to experts and/or use histology‐validated cases to evaluate the radiologist's sensitivity, specificity, false negatives/positives, and accuracy for significant cancer.
It was recognised that these performance characteristics would not only reflect the expertise of the biopsy operator but also the reporting radiologist. These assessments would help identify under‐performing radiologists to motivate self‐improvement, such as increasing the number of mpMRI reporting backed by continuous feedback from experts or peer‐reviewers, second‐reading by an experienced radiologist for a set period, increasing mpMRI to pathology correlations, e.g. over the next 6–12 months, before any re‐evaluation.
Section V: Management of Patients After Prostate mpMRI Results
Whilst not formally considering all clinical factors as items for consensus voting, it was clear that pre‐biopsy mpMRI scoring should not be the only factor guiding decisions about whether to biopsy. Other factors such as age, family history, use of 5α‐reductase inhibitors, comorbidities, total PSA, PSA kinetics, PSA density, urine dipstick tests (to exclude infection), prior biopsy results, and patient preference, might be considered in conjunction.
When pre‐biopsy mpMRI is scored 1–2 (not suspicious for clinically significant cancer) and the PSA density is below an agreed threshold, it was agreed in consensus that the patient can be discharged to the GP with PSA follow‐up, i.e. no immediate biopsy is required. However, where PSA density is above the agreed threshold, then biopsy should be discussed with the patient as part of a shared decision‐making process discussing risks of disease being present (~0–24% of negative mpMRI harbour significant cancer depending on the definition used 2, 8, 28, 29, 30, 31, 32, 33, 34 and the risks related to the biopsy procedure. The recommended biopsy technique for men who on clinical grounds, are advised or choose to have a biopsy despite a unsuspicious mpMRI is by any transperineal systematic biopsy (10/12, 83% panelists) due to no visible MR target; systematic TRUS biopsy would only increase the detection rate by 1–2% 31, 32 and was not deemed worthwhile in the presence of unsuspicious mpMRI.
For equivocal mpMRI impressions (Likert‐assessment 3), a biopsy is recommended when the PSA density is above an agreed threshold (unanimous consensus). There was agreement but not in consensus that young patients (seven of 12 panelists) and with positive family history (eight of 12 panelists) could also undergo biopsy if the mpMRI was scored 3. It was agreed but not in consensus that biopsy options in equivocal mpMRI should include MR‐guided biopsy (visually estimated, image fusion or in‐bore; eight of 12 panelists), transperineal systematic biopsy (seven of 12 panelists) or combined targeted and systematic sampling (seven of 12 panelists) of the gland. Emerging evidence shows no statistical difference between the use of combined targeted and systematic biopsy vs each biopsy technique alone for significant cancer detection in equivocal lesions 35.
Immediate biopsy is recommended for suspicious pre‐biopsy mpMRI (Likert‐assessment 4–5); Suspicious mpMRI followed by negative targeted biopsy should be discussed as part of an MDT meeting for collective management decision, where the possibility of a missed targeted biopsy, or of a false‐positive mpMRI report would both be considered. Table 5 summarises this section's discussion.
Table 5.
Recommendations for incorporation of mpMRI scores in patient's management
| Recommendations for incorporation of mpMRI scores in patient's management |
|---|
| mpMRI scores 1–2 |
| • No immediate biopsy is recommended. |
| • Biopsy can be considered as part of a shared decision process with the patient if • PSA density is elevated or clinical concerns persist. |
| mpMRI score 3 |
| • Immediate biopsy if PSA density is elevated. |
| mpMRI scores 4–5 |
| • Immediate biopsy. |
| mpMRI scores 4–5 and targeted biopsy is negative |
| • Discuss in MDT meeting. |
Section VI: Training in Prostate mpMRI Reporting
It was unanimously agreed that prostate mpMRI reporting cannot be self‐taught. Before commencing independent mpMRI reporting, radiologists should undertake a combination of core theoretical prostate mpMRI course, hands‐on practice at workstations with supervised reporting, and should also participate in MDT meetings or attend MDT‐type workshops where patient‐based clinical scenarios are discussed. Training should be dispensed and certified by national bodies such as the Royal College of Radiologists. Table 6 highlights the areas of agreement of the panel within this section.
Table 6.
Summarises the recommendations in the training section
| Agreement in consensus |
|---|
|
|
Figure 2 summarises key recommendations from this process.
Figure 2.
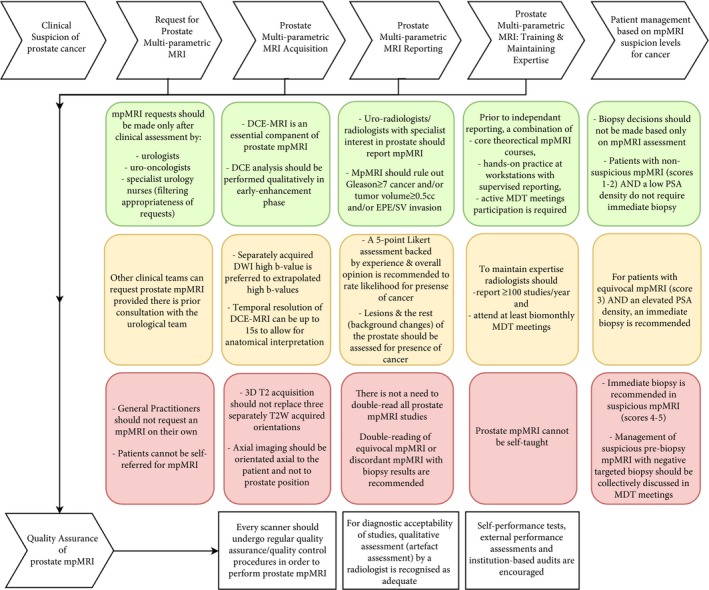
Summarises the key recommendations across the early prostate cancer diagnosis pathway to deliver consistently high‐quality mpMRI studies.
Discussion
The present paper reports formal UK recommendations from a RAM consensus process to guide the practice of pre‐biopsy prostate mpMRI in expert and non‐expert centres. Recommendations have been made to ensure consistently high‐quality mpMRI scans and improve standards in reporting to offer better guidance in management decisions.
Clinical Implications
There are a number of key statements that we believe will be of significant impact in the UK prostate cancer diagnostic pathway.
First, we have laid down the minimum conditions for a prostate mpMRI. This includes the added value of DCE‐MRI, which has been lately questioned 36, 37, 38, 39, 40, 41. A recent UK audit revealed that 24% of NHS centres do not conduct prostate MRI with DCE 9; it was found that the main reason for non‐compliance with the UK and PI‐RADS guidelines 11, 12 was due to capacity problems. However, the panel still recommends the use of DCE as integral to prostate MRI, i.e. which evolves from bi‐parametric (T2W and DWI) to mpMRI, with the addition of DCE‐MRI. The benefits of DCE‐MRI include the fact that it acts as a back‐up to overcome technical failures from DWI and artefacts (such as susceptibility artefact from rectal gas and distortion from hip replacement), which hinder diagnostic accuracy 19, 42, 43, 44, 45. Besides, DCE‐MRI is helpful for the less experienced radiologists 46 and also beneficial in differentiating the anterior fibromuscular stroma from anterior tumours 47. DCE‐MRI can also act as a ‘safety’ sequence in patients where diffusion images are significantly distorted by susceptibility artefact from air within the rectum or total hip replacements. Furthermore, DCE also improves the confidence regarding identified lesions particularly for those of an indeterminate nature 19, 48, 49.
Second, we also questioned the widespread use of PI‐RADS_v2 scoring system and recommended the use of a subjective 5‐point Likert‐assessment of mpMRI scans in the UK pending higher‐level validation and further evolution of the PI‐RADS scoring system (noting that PI‐RADS_v3 is currently under development). Although PI‐RADS_v2 promotes an objective lesion‐based scoring approach 12, it does not include routine assessment of the whole prostate, e.g. the significance of diffuse ‘background’ signal change within the whole gland is often not addressed 50. Even though the dominance of a sequence in PZ/TZ lesion evaluation was acknowledged, tumours exhibiting strong cancer suspicion on the non‐dominant sequence (e.g. T2 in the PZ or ADC in the TZ) could be missed with PI‐RADS_v2. Also, the latter does not fit in other areas, i.e. central zone, anterior stroma and zonal interface where zonal origin unclear. These are areas where a ‘Likert‐impression’ score can be more useful. Nevertheless, radiologists can use the descriptive scoring characteristics as elaborated in PI‐RADS_v2 to guide their opinions and supplement them with their own experience, as well as features outside of PI‐RADS_v2 criteria to form an overall subjective Likert‐impression. The panel also acknowledged that mpMRI descriptive features in the PIRADS_v2 guidelines are particularly useful for the less experienced and for research. Furthermore, they discussed the need for a histologically‐validated pictorial guide to illustrate subjective Likert‐impressions and this could be delivered through datasets acquired during clinical trials.
Third, in order to maintain quality mpMRI reporting and guarantee accurate and safe prostate mpMRI reports, minimum standards for reporting radiologists were tackled. Although the effect of dedicated training on the accuracy of prostate cancer localisation on mpMRI, the effect of continual feedback on reporting confidence and a ‘learning curve’ effect have been documented, the establishment of a threshold number of prostate mpMRI required during training, to reach independent reporting and to maintain expertise are lacking 51, 52, 53, 54. While some may not agree with the concept of quantitative metrics to gauge quality or experience, the majority of the panel agreed that an independent radiologist should report >100 prostate mpMRI scans per year with regular attendance to MDT meetings of at least twice a month. Also, prior to independent reporting, supervised reporting of at least 100 mpMRI studies were deemed appropriate. Moreover, centres carrying out at least 250 cases per year were regarded as best suited to dispense training. These numbers are under the proviso that the scans also meet the minimum quality requirement as per the latest protocol guidelines. Furthermore, it was stressed that general radiologists are not to report prostate mpMRI unless they have a specialist interest in prostate mpMRI, and like uro‐radiolgists are prepared to meet the necessary minimum requirements in terms of training and experience, prior to autonomous reporting.
This expert group initiated the discussion regarding prostate mpMRI‐specific QA, but it was recognised that more specialist technical groups with specific QA expertise are required to set‐up relevant QA requirements across the whole prostate cancer pathway including QA procedures for pathology, surgery, and data collection. QA requirements for breast cancer diagnosis pathway 55 could be used as an exemplar approach.
Last, we considered who should be biopsied based on mpMRI reports. There was consensus that mpMRI report should be used to determine whether a man should be biopsied, capitalising on the high sensitivity and high negative‐predictive values for mpMRI in excluding clinically significant prostate cancer and between one‐quarter and one‐third of men would be given the opportunity to avoid an immediate biopsy. Growing literature on the combined use of PSA density with mpMRI as an additional factor to reduce the false negatives of mpMRI 50, 56, 57, 58, 59, 60, 61 was endorsed by the panel to better select patients for biopsy after non‐suspicious and equivocal mpMRI 35, 57, 62. Whilst various PSA density thresholds have been previously suggested 63, 64, 65, the threshold of 0.15 ng/mL/mL is proving to be useful in the diagnostic setting 57, 58, 59, 66, although individual centres may choose to be more conservative in using lower PSA density threshold (e.g. 0.12 ng/mL/mL) alongside other risk factors in deciding which men can avoid a biopsy until more robust evidence is available.
Research Implications
Some areas did not reach consensus, due to conflicting results or lack of data in the literature to guide discussions. Areas of further research include: lesion detection/conspicuity comparisons from dedicated vs extrapolated/computed long b‐values at 3 T and 1.5 T without endorectal coils; quantitative image quality assessments; specific MR‐prostate phantom development; threshold number of mpMRI studies required during and after prostate mpMRI training and to reach autonomous reporting; long‐term clinical risk of cancer and outcomes of high‐grade prostatic intraepithelial neoplasia (HGPIN), atypical small acinar proliferation (ASAP), atrophy or inflammation upon diagnosis for mpMRI directed management options 67, 68; and combining mpMRI with molecular/genomic biomarkers, risk‐calculators (other than TRUS‐biopsy validated ones) across the prostate cancer pathway for diagnosis.
Methodological Limitations
Expert group discussions are prone to biases, but latest available evidence was used and an independent chair ensured balanced debates. Even if one or two panelists dominated the discussion, they had only one vote. Besides, some members of the panel scored the items before the meeting but were not present in the face‐to‐face meeting. Whilst a GP representative on our panel would have been beneficial to address the initial questions involving GPs, the contribution to the remainder of the document would be limited. Finally, this process does not aim to reach consensus in areas of disagreement or minimise uncertainties in clinical areas but it has helped to identify areas warranting additional research.
Conclusions
The promise of mpMRI is reflected by the rapid uptake of this investigation into clinical practice and the growing demand to offer this test across the UK. Our consensus statements demonstrate a set of criteria that are required for the reliable dissemination of prostate mpMRI as a diagnostic test prior to biopsy in men at risk. It is of utmost importance that quality should be maintained across the whole prostate pathway in all healthcare settings for prostate mpMRI to be used as a tool to rule in and rule out clinically significant prostate cancer.
Participating Members and Author Contributions
Meeting Chair: Jan van der Meulen.
Panelists who agreed to be part of the process: eight radiologists (Clare Allen, Tristan Barrett, Alexander P.S. Kirkham, Phil Haslam, Anwar R. Padhani, Amit Patel, Jonathan Richenberg, Shonit Punwani), seven urologists (Jim Adshead, Hashim U. Ahmed, John Graham, Christof Kastner, Alan McNeill, Caroline M. Moore, Ghulam Nabi), two oncologists (Chris Parker, John Staffurth), three radiographers (Darren Walls, Jacqueline Pursey, Alexandra Lipton) and a physicist (Alan Bainbridge).
Panelists who completed the questionnaire: Clare Allen, Tristan Barrett, Alexander P.S. Kirkham, Phil Haslam, Amit Patel, Jonathan Richenberg, Shonit Punwani, Hashim U. Ahmed, Jim Adshead, Christof Kastner, Caroline M. Moore, Ghulam Nabi, Chris Parker, John Staffurth, Darren Walls, Jacqueline Pursey, Alexandra Lipton, Alan Bainbridge.
Panelists who participated in face‐to‐face meeting: Clare Allen, Tristan Barrett, Alexander P.S. Kirkham, Phil Haslam, Amit Patel, Jonathan Richenberg, Shonit Punwani, Hashim U. Ahmed, Christof Kastner, Caroline M. Moore, Chris Parker, John Staffurth, Darren Walls, Jacqueline Pursey, Alan Bainbridge.
Meeting Coordinator: Mrishta Brizmohun Appayya.
Critical revision of the questionnaire and manuscript for important intellectual content: Jim Adshead, Hashim U. Ahmed, John Graham, Christof Kastner, Alan McNeill, Caroline M. Moore, Ghulam Nabi, Chris Parker, John Staffurth; Darren Walls, Jacqueline Pursey, Alexandra Lipton, Alan Bainbridge; Clare Allen, Tristan Barrett, Francesco Giganti, Edward W. Johnston, Alexander P.S. Kirkham, Phil Haslam, Anwar R. Padhani, Amit Patel, Jonathan Richenberg, Jan van der Meulen, Shonit Punwani.
Elaboration of questionnaire: Mrishta Brizmohun Appayya, Hashim U. Ahmed, Clare Allen, Alan Bainbridge, Alexander P.S. Kirkham, Caroline M. Moore, Shonit Punwani.
Study concept and design: Mrishta Brizmohun Appayya, Shonit Punwani.
Analysis and interpretation of data: Mrishta Brizmohun Appayya.
Statistical analysis: Mrishta Brizmohun Appayya.
Obtaining funding: Shonit Punwani.
Administrative, technical, or material support: Edward W. Johnston, Francesco Giganti, Larissa Moniz, Chris Whittle, Karen Stalbow.
Supervision: Shonit Punwani.
Conflict of Interest
None.
Abbreviations
- (2(3)D
two (three)‐dimensional
- ADC
apparent diffusion coefficient
- DCE
dynamic contrast‐enhanced imaging
- DWI
diffusion‐weighted imaging
- MDT
multi‐disciplinary team
- mpMRI
multi‐parametric MRI
- PI‐RADS (_v1)(_v2)(_v3)
Prostate Imaging and Reporting and Data System (version 1) (version 2) (version 3)
- PROMIS
PROstate MRI Imaging Study
- PZ
peripheral zone
- QA
quality assurance
- QC
quality control
- RAM
RAND‐UCLA Appropriateness Method
- RAND‐UCLA
Research and Development Corporation and University of California‐Los Angeles
- T2W
T2‐weighted imaging
- TZ
transition zone
Supporting information
Appendix S1. Detailed results for each questionnaire item.
Acknowledgements and Funding
We are most grateful to Prostate Cancer UK and London Cancer for their support of the meeting. We are grateful to Karen Stalbow, Larissa Moniz and Chris Whittle for assistance and administrative support. Prostate Cancer UK provided funding for travel and accommodation of panelists; and the venue for the meeting. Mrishta Brizmohun Appayya received funding support from Prostate Cancer UK and London Cancer. Jan van der Meulen is partly supported by the NHS National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care North Thames. Hashim U. Ahmed is supported by the NIHR Imperial Biomedical Research Centre and the Wellcome Trust. Shonit Punwani receives funding support from the NIHR University College London Hospitals (UCLH; http://www.uclh.nhs.uk/cancercollab) Biomedical Research Centre, London Cancer (http://www.uclh.nhs.uk/londoncancer) and the Cancer Research UK (CRUK) King's College London (KCL)/University College London (UCL) Cancer Imaging Centre. London Cancer received funding from NHS England as part of the New Care Models programme. The funders had no role in the data collection, analysis or in the preparation of the manuscript.
References
- 1. Fütterer JJ, Briganti A, De Visschere P et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol 2015; 68: 1045–53 [DOI] [PubMed] [Google Scholar]
- 2. Siddiqui MM, Rais‐Bahrami S, Turkbey B et al. Comparison of MR/ultrasound fusion‐guided biopsy with ultrasound‐guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoots IG, Petrides N, Giganti F et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol 2015; 67: 627–36 [DOI] [PubMed] [Google Scholar]
- 4. National Institute for Health and Care Excellence . Prostate Cancer: Diagnosis and Management, Clinical Guideline [CG175], 2014. Available at: http://www.nice.org.uk/guidance/cg175/chapter/1-recommendations. Accessed April 2018
- 5. Dickinson L, Ahmed HU, Hindley RG et al. Prostate‐specific antigen vs. magnetic resonance imaging parameters for assessing oncological outcomes after high intensity‐focused ultrasound focal therapy for localized prostate cancer. Urol Oncol 2017; 35: 30.e9–e15 [DOI] [PubMed] [Google Scholar]
- 6. Panebianco V, Barchetti F, Sciarra A et al. Prostate cancer recurrence after radical prostatectomy: the role of 3‐T diffusion imaging in multi‐parametric magnetic resonance imaging. Eur Radiol 2013; 23: 1745–52 [DOI] [PubMed] [Google Scholar]
- 7. Hegde JV, Demanes DJ, Veruttipong D et al. Pretreatment 3T multiparametric MRI staging predicts for biochemical failure in high‐risk prostate cancer treated with combination high‐dose‐rate brachytherapy and external beam radiotherapy. Brachytherapy 2017; 16: 1106–12 [DOI] [PubMed] [Google Scholar]
- 8. Ahmed HU, El‐Shater Bosaily A, Brown LC et al. Diagnostic accuracy of multi‐parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017; 389: 815–22 [DOI] [PubMed] [Google Scholar]
- 9. Cooper A, Prostate Cancer UK . FOI Request – Multi‐Parametric MRI Usage Data for Your Trust/Health Board/Health & Social Care Trust 2016. Available at: https://public.tableau.com/profile/ali.cooper-!/vizhome/mpMRIFOIpublicdashboard-ProstateCancerUK_0/FullresultsStory. Accessed April 2018
- 10. Fitch K, Bernstein SJ, Aguilar MD et al. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, CA: RAND, 2001. [Google Scholar]
- 11. Kirkham AP, Haslam P, Keanie JY et al. Prostate MRI: who, when, and how? Report from a UK consensus meeting. Clin Radiol 2013; 68: 1016–23 [DOI] [PubMed] [Google Scholar]
- 12. Weinreb JC, Barentsz JO, Choyke PL et al. PI‐RADS prostate imaging – reporting and data system: 2015, Version 2. Eur Urol 2016; 69: 16–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barentsz JO, Richenberg J, Clements R et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012; 22: 746–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenkrantz AB, Neil J, Kong X et al. Prostate cancer: comparison of 3D T2‐weighted with conventional 2D T2‐weighted imaging for image quality and tumor detection. AJR Am J Roentgenol 2010; 194: 446–52 [DOI] [PubMed] [Google Scholar]
- 15. Westphalen AC, Noworolski SM, Harisinghani M et al. High‐resolution 3‐T endorectal prostate MRI: a multireader study of radiologist preference and perceived interpretive quality of 2D and 3D T2‐weighted fast spin‐echo MR images. AJR Am J Roentgenol 2016; 206: 86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mapelli P, Picchio M. Initial prostate cancer diagnosis and disease staging–the role of choline‐PET‐CT. Nat Rev Urol 2015; 12: 510–8 [DOI] [PubMed] [Google Scholar]
- 17. Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA‐PET in prostate cancer management. Nat Rev Urol 2016; 13: 226–35 [DOI] [PubMed] [Google Scholar]
- 18. Bittencourt LK, Attenberger UI, Lima D et al. Feasibility study of computed vs measured high b‐value (1400 s/mm²) diffusion‐weighted MR images of the prostate. World J Radiol 2014; 6: 374–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greer MD, Shih JH, Lay N et al. Validation of the dominant sequence paradigm and role of dynamic contrast‐enhanced imaging in PI‐RADS Version 2. Radiology 2017; 285: 859–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan CH, Hobbs BP, Wei W, Kundra V. Dynamic contrast‐enhanced MRI for the detection of prostate cancer: meta‐analysis. AJR Am J Roentgenol 2015; 204: W439–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Renard‐Penna R, Mozer P, Cornud F et al. Prostate Imaging Reporting and Data System and Likert scoring system: multiparametric MR imaging validation study to screen patients for initial biopsy. Radiology 2015; 275: 458–68 [DOI] [PubMed] [Google Scholar]
- 22. Rosenkrantz AB, Lim RP, Haghighi M, Somberg MB, Babb JS, Taneja SS. Comparison of interreader reproducibility of the prostate imaging reporting and data system and likert scales for evaluation of multiparametric prostate MRI. AJR Am J Roentgenol 2013; 201: W612–8 [DOI] [PubMed] [Google Scholar]
- 23. Jeong CW, Park HK, Hong SK, Byun SS, Lee HJ, Lee SE. Comparison of prostate volume measured by transrectal ultrasonography and MRI with the actual prostate volume measured after radical prostatectomy. Urol Int 2008; 81: 179–85 [DOI] [PubMed] [Google Scholar]
- 24. Litjens GJ, Hambrock T, Hulsbergen‐van de Kaa C, Barentsz JO, Huisman HJ. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 2012; 265: 260–6 [DOI] [PubMed] [Google Scholar]
- 25. Jafar MM, Parsai A, Miquel ME. Diffusion‐weighted magnetic resonance imaging in cancer: reported apparent diffusion coefficients, in‐vitro and in‐vivo reproducibility. World J Radiol 2016; 8: 21–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paterson NR, Lavallée LT, Nguyen LN et al. Prostate volume estimations using magnetic resonance imaging and transrectal ultrasound compared to radical prostatectomy specimens. Can Urol Assoc J 2016; 10: 264–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Price R, Allison J, Clarke G et al. Magnetic Resonance Imaging Quality Control Manual. American College of Radiology. 2015. Available at: https://www.acr.org/~/media/ACRNoIndex/Documents/QCManual/2015_MR_QCManual_Book.pdf. Accessed April 2018
- 28. Panebianco V, Barchetti F, Sciarra A et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015; 33: 17.e1–7 [DOI] [PubMed] [Google Scholar]
- 29. Wysock JS, Mendhiratta N, Zattoni F et al. Predictive value of negative 3T multiparametric magnetic resonance imaging of the prostate on 12‐core biopsy results. BJU Int 2016; 118: 515–20 [DOI] [PubMed] [Google Scholar]
- 30. Lu AJ, Syed JS, Nguyen KA et al. Negative multiparametric magnetic resonance imaging of the prostate predicts absence of clinically significant prostate cancer on 12‐core template prostate biopsy. Urology 2017; 105: 118–22 [DOI] [PubMed] [Google Scholar]
- 31. Itatani R, Namimoto T, Atsuji S et al. Negative predictive value of multiparametric MRI for prostate cancer detection: outcome of 5‐year follow‐up in men with negative findings on initial MRI studies. Eur J Radiol 2014; 83: 1740–5 [DOI] [PubMed] [Google Scholar]
- 32. Pokorny MR, de Rooij M, Duncan E et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound‐guided biopsy versus magnetic resonance (MR) imaging with subsequent MR‐guided biopsy in men without previous prostate biopsies. Eur Urol 2014; 66: 22–9 [DOI] [PubMed] [Google Scholar]
- 33. Filson CP, Natarajan S, Margolis DJ et al. Prostate cancer detection with magnetic resonance‐ultrasound fusion biopsy: the role of systematic and targeted biopsies. Cancer 2016; 122: 884–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moldovan PC, Van den Broeck T, Sylvester R et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta‐analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur Urol 2017; 72: 250–66 [DOI] [PubMed] [Google Scholar]
- 35. Hansen NL, Barrett T, Kesch C et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy‐naïve men with suspicion of prostate cancer. BJU Int 2018; 122: 40–9 [DOI] [PubMed] [Google Scholar]
- 36. Kuhl CK, Bruhn R, Krämer N, Nebelung S, Heidenreich A, Schrading S. Abbreviated biparametric prostate MR imaging in men with elevated prostate‐specific antigen. Radiology 2017; 285: 493–505 [DOI] [PubMed] [Google Scholar]
- 37. De Visschere P, Lumen N, Ost P, Decaestecker K, Pattyn E, Villeirs G. Dynamic contrast‐enhanced imaging has limited added value over T2‐weighted imaging and diffusion‐weighted imaging when using PI‐RADSv2 for diagnosis of clinically significant prostate cancer in patients with elevated PSA. Clin Radiol 2017; 72: 23–32 [DOI] [PubMed] [Google Scholar]
- 38. Thestrup KC, Logager V, Baslev I, Møller JM, Hansen RH, Thomsen HS. Biparametric versus multiparametric MRI in the diagnosis of prostate cancer. Acta Radiol Open 2016; 5: 2058460116663046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vargas HA, Hötker AM, Goldman DA et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole‐mount pathology as standard of reference. Eur Radiol 2016; 26: 1606–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scialpi M, Prosperi E, D'Andrea A et al. Biparametric versus multiparametric MRI with non‐endorectal coil at 3T in the detection and localization of prostate cancer. Anticancer Res 2017; 37: 1263–71 [DOI] [PubMed] [Google Scholar]
- 41. Stanzione A, Imbriaco M, Cocozza S et al. Biparametric 3T magnetic resonance imaging for prostatic cancer detection in a biopsy‐naïve patient population: a further improvement of PI‐RADS v2? Eur J Radiol 2016; 85: 2269–74 [DOI] [PubMed] [Google Scholar]
- 42. Caglic I, Hansen NL, Slough RA, Patterson AJ, Barrett T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur J Radiol 2017; 90: 174–80 [DOI] [PubMed] [Google Scholar]
- 43. Mazaheri Y, Vargas HA, Nyman G, Akin O, Hricak H. Image artifacts on prostate diffusion‐weighted magnetic resonance imaging: trade‐offs at 1.5 Tesla and 3.0 Tesla. Acad Radiol 2013; 20: 1041–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barrett T, Vargas HA, Akin O, Goldman DA, Hricak H. Value of the hemorrhage exclusion sign on T1‐weighted prostate MR images for the detection of prostate cancer. Radiology 2012; 263: 751–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tamada T, Sone T, Jo Y et al. Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology 2008; 248: 531–9 [DOI] [PubMed] [Google Scholar]
- 46. Fütterer JJ, Engelbrecht MR, Huisman HJ et al. Staging prostate cancer with dynamic contrast‐enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology 2005; 237: 541–9 [DOI] [PubMed] [Google Scholar]
- 47. Ward E, Baad M, Peng Y et al. Multi‐parametric MR imaging of the anterior fibromuscular stroma and its differentiation from prostate cancer. Abdom Radiol (NY) 2017; 42: 926–34 [DOI] [PubMed] [Google Scholar]
- 48. Druskin SC, Ward R, Purysko AS et al. Dynamic contrast enhanced MRI improves classification of prostate lesions: a study of pathologic outcomes on targeted prostate biopsy. J Urol 2017; 198: 1301–8 [DOI] [PubMed] [Google Scholar]
- 49. Rosenkrantz AB, Babb JS, Taneja SS, Ream JM. Proposed adjustments to PI‐RADS version 2 decision rules: impact on prostate cancer detection. Radiology 2017; 283: 119–29 [DOI] [PubMed] [Google Scholar]
- 50. Brizmohun Appayya M, Sidhu HS, Dikaios N et al. Characterizing indeterminate (Likert‐scored 3/5) peripheral zone prostate lesions with PSA density, PI‐RADS scoring and qualitative descriptors on multi‐parametric MRI. Br J Radiol 2017; 91: 20170645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akin O, Riedl CC, Ishill NM, Moskowitz CS, Zhang J, Hricak H. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol 2010; 20: 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Garcia‐Reyes K, Passoni NM, Palmeri ML et al. Detection of prostate cancer with multiparametric MRI (mpMRI): effect of dedicated reader education on accuracy and confidence of index and anterior cancer diagnosis. Abdom Imaging 2015; 40: 134–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosenkrantz AB, Ayoola A, Hoffman D et al. The learning curve in prostate MRI interpretation: self‐directed learning versus continual reader feedback. AJR Am J Roentgenol 2017; 208: W92–100 [DOI] [PubMed] [Google Scholar]
- 54. Gaziev G, Wadhwa K, Barrett T et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI‐transrectal ultrasonography (TRUS) fusion‐guided transperineal prostate biopsies as a validation tool. BJU Int 2016; 117: 80–6 [DOI] [PubMed] [Google Scholar]
- 55. Perry N, Broeders M, de Wolf C, Törnberg S, Holland R, von Karsa L eds. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th edn 2006. Health & Consumer Protection. Available at: http://www.euref.org/downloads?download=24:european-guidelines-for-quality-assurance-in-breast-cancer-screening-and-diagnosis-pdf. Accessed April 2018 [DOI] [PubMed] [Google Scholar]
- 56. Rais‐Bahrami S, Siddiqui MM, Vourganti S et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate‐specific antigen (PSA)‐based detection of prostate cancer in men without prior biopsies. BJU Int 2015; 115: 381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Distler FA, Radtke JP, Bonekamp D et al. The value of PSA density in combination with PI‐RADS™ for the accuracy of prostate cancer prediction. J Urol 2017; 198: 575–82 [DOI] [PubMed] [Google Scholar]
- 58. Hansen NL, Barrett T, Koo B et al. The influence of prostate‐specific antigen density on positive and negative predictive values of multiparametric magnetic resonance imaging to detect Gleason score 7‐10 prostate cancer in a repeat biopsy setting. BJU Int 2017; 119: 724–30 [DOI] [PubMed] [Google Scholar]
- 59. Washino S, Okochi T, Saito K et al. Combination of Prostate Imaging Reporting and Data System (PI‐RADS) score and prostate‐specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naïve patients. BJU Int 2017; 119: 225–33 [DOI] [PubMed] [Google Scholar]
- 60. Sim KC, Sung DJ, Kang KW et al. Magnetic resonance imaging‐based prostate‐specific antigen density for prediction of Gleason score upgrade in patients with low‐risk prostate cancer on initial biopsy. J Comput Assist Tomogr 2017; 41: 731–6 [DOI] [PubMed] [Google Scholar]
- 61. Alberts AR, Roobol MJ, Drost FH et al. Risk‐stratification based on magnetic resonance imaging and prostate‐specific antigen density may reduce unnecessary follow‐up biopsy procedures in men on active surveillance for low‐risk prostate cancer. BJU Int 2017; 120: 511–9 [DOI] [PubMed] [Google Scholar]
- 62. Hansen NL, Kesch C, Barrett T et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image‐fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int 2017; 120: 631–8 [DOI] [PubMed] [Google Scholar]
- 63. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994; 271: 368–74 [PubMed] [Google Scholar]
- 64. Ha YS, Yu J, Salmasi AH et al. Prostate‐specific antigen density toward a better cutoff to identify better candidates for active surveillance. Urology 2014; 84: 365–71 [DOI] [PubMed] [Google Scholar]
- 65. van den Bergh RC, Vasarainen H, van der Poel HG et al. Short‐term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. BJU Int 2010; 105: 956–62 [DOI] [PubMed] [Google Scholar]
- 66. Venderink W, van Luijtelaar A, Bomers JG et al. Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal, likely or highly likely to be clinically significant prostate cancer. Eur Urol 2017; pii: S0302‐2838(17)30110‐0; [Epub ahead of print]. 10.1016/j.eururo.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 67. Godoy G, Huang GJ, Patel T, Taneja SS. Long‐term follow‐up of men with isolated high‐grade prostatic intra‐epithelial neoplasia followed by serial delayed interval biopsy. Urology 2011; 77: 669–74 [DOI] [PubMed] [Google Scholar]
- 68. Epstein JI. Editorial comment. Long‐term follow‐up of men with isolated high‐grade prostatic intra‐epithelial neoplasia followed by serial delayed interval biopsy. Urology 2011; 77: 674–6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed results for each questionnaire item.


