Abstract
After been exposed to the visual input, in the first year of life, the brain experiences subtle but massive changes apparently crucial for communicative/emotional and social human development. Its lack could be the explanation of the very high prevalence of autism in children with total congenital blindness. The present theory postulates that the superior colliculus is the key structure for such changes for several reasons: it dominates visual behavior during the first months of life; it is ready at birth for complex visual tasks; it has a significant influence on several hemispheric regions; it is the main brain hub that permanently integrates visual and non-visual, external and internal information (bottom–up and top–down respectively); and it owns the enigmatic ability to take non-conscious decisions about where to focus attention. It is also a sentinel that triggers the subcortical mechanisms which drive social motivation to follow faces from birth and to react automatically to emotional stimuli. Through indirect connections it also activates simultaneously several cortical structures necessary to develop social cognition and to accomplish the multiattentional task required for conscious social interaction in real life settings. Genetic or non-genetic prenatal or early postnatal factors could disrupt the SC functions resulting in autism. The timing of postnatal biological disruption matches the timing of clinical autism manifestations. Astonishing coincidences between etiologies, clinical manifestations, cognitive and pathogenic autism theories on one side and SC functions on the other are disclosed in this review. Although the visual system dependent of the SC is usually considered as accessory of the LGN canonical pathway, its imprinting gives the brain a qualitatively specific functions not supplied by any other brain structure.
Keywords: autism spectrum disorders (ASD), autism pathogenesis, human development, congenital blindness, pulvinar, visual pathways, superior colliculus (SC)
Introduction
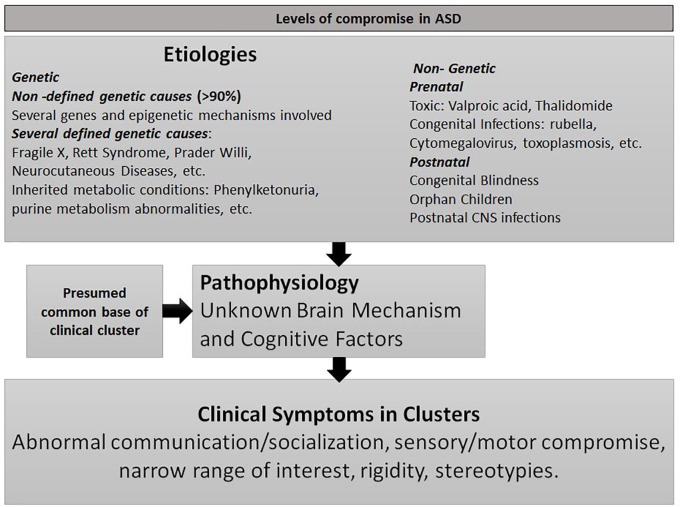
Autism spectrum disorder (ASD) is not a disease; it is a syndrome with hundreds of genetics and non-genetics etiologies (see Figure 1) and with broad clinical manifestations. Its pathogenesis, scarcely known, is also presumed to be heterogeneous (Waterhouse et al., 2016). The coherence of the syndrome lies in the presence of the core symptoms in cluster (Hobson, 2014): ASD individuals with extremely varied etiologies, cognitive levels, and cultures share a broad range of specific symptoms in social, communicative, cognitive, motor and sensitive domains. This strongly suggests that diverse pathogenic mechanisms could have a common final pathway which, in turn, compromises several brain networks resulting in disparate symptoms. The unsuccessful efforts to find it could be due to the limitations of in vivo investigation on the developing human brain (Courchesne et al., 2007) and to the fact that social cognition and communication are mainly non-consciously acquired during early life. Hence, little is known about the functional and cognitive processes that govern its development.
FIGURE 1.
Levels of compromise in ASD.
An often overlooked fact that could be a clue for pathogenic research is that a complete lack of vision during the first year of life results very frequently in a full-blown ASD syndrome. During early life, visual input is dominated by the SC and the Pul (Bridge et al., 2015). The SC is an extremely ancient and complex subcortical structure, not only essential for simultaneous visual attention and eye movements as it is usually believed. It also has a crucial role in the integration of external and internal senses with emotional, autonomic and endocrine functions, as well as in visual/motor transformation, target selection and goal-directed motor responses (Merker, 2007; Krauzlis et al., 2013). It is also the neurologic substrate of innate behaviors (Furigo et al., 2010) like automatic attention to faces and biologic movement (Nguyen et al., 2014; Rosa Salva et al., 2015; Mares et al., 2016), social motivation, and innate fear (e.g., to snakes) (Liddell et al., 2005; Wei et al., 2015). Due to its strategic location as a first line visual and multisensory structure to receive environmental input and its multiple direct connections from the cortex, the SC is in the interphase of several complex processes: (1) the bottom–up and top–down attentional interplay (Merker, 2013), (2) the shift from covert to overt attention (Sato et al., 2016), and (3) the interaction of medial and lateral cortical brain networks that regulate and integrate endogenous with externally drive attention respectively (Menon and Uddin, 2010; Katyal and Ress, 2014; Mysore and Knudsen, 2014; Xuan et al., 2016). These dimensions are essential not only to process ongoing information but also to mental time travel, to mentalize abilities (Corbetta et al., 2008) and to develop complex representations of the self (Fabbro et al., 2015). But, perhaps, the most relevant aspect for ASD pathogenesis is the evidence that the SC has a plastic role in the postnatal microstructure of the brain in an exclusive way that favors social/communicative abilities (Maior et al., 2012; Rosa Salva et al., 2015; Soares et al., 2017).
The aim of this work is to show that different lines of research on ASD etiologies and pathogenesis, including cognitive theories, functional studies and anatomic evidence converge on the SC.
ASD and Congenital Blindness
From the first report of autism in blind children made 60 years ago (Keeler, 1956), a review of 12 different studies yields a ∼50% of prevalence of ASD in early blindness (Jure et al., 2016). The prevalence is even higher when only children with total CB are considered. In a school for the blind, ASD was found in 18 of 25 (72%) students with CB and in only 1 of 13 (8%) with partial or acquired blindness. Statistical analysis showed that CB was the main responsible factor. No other variable, such as etiology of blindness or socioeconomic family status, accounted for this very high ASD prevalence (Jure et al., 2016).
Another similarity lies in the evolution of symptoms; besides the most common chronic clinical course of ASD, two other subgroups have been described in children with CB: one that showed an early autism regression and a second one with a late autism recovery. Both occur at the same age window as in the sighted: regression takes place between 15 and 30 months (Cass et al., 1994; Rosenbloom, 1994; Dale and Sonksen, 2002; Luyster et al., 2005; Jure et al., 2016) and recovery occurs beyond 10 years in sighted/ASD (Fein et al., 2013) and CB/ASD individuals (Hobson and Lee, 2010; Jure et al., 2016). However, regression is ∼3000 times more frequent in CB than in the sighted (Dale, 2005) and recovery in CB was not only limited to high functioning ASD individuals (Hobson and Lee, 2010; Jure et al., 2016) as it was described in sighted ASD subjects (Fein et al., 2013).
The high prevalence of autism in early blindness has been overlooked until the present (Hobson and Bishop, 2003; Tager-Flusberg, 2005). Several reasons might explain this lack of attention (Jure et al., 2016). Perhaps, the strongest is the automatic assumption that it is not a “true” autism because it is secondary to blindness. However, autism is exclusively defined clinically (DSM or ICD criteria). Comparatively, a similar clinical syndrome (e.g., spastic hemiparesis) in different individuals usually reflects similar brain compromise (pyramidal tract), independent of the etiology (cerebral infarct, brain malformation, etc.). To exclude CB as one etiologic factor of ASD would be similar to exclude tuberous sclerosis, fragile X, fetal valproate syndrome as well as hundreds of other ASD etiologies.
Diffuse postnatal brain changes occur during the first year of life by visual influence and the microstructure and functionality of the brain is different in CB, resulting in atypical neurodevelopment (Coullon et al., 2015; Hasson et al., 2016; Reislev et al., 2017). Humans studies point to the SC as the main structure responsible of those changes (Coullon et al., 2015).
The Role of Vision in Development
Visual input has a pervasive influence on sensorimotor coherence, cognition, behavior and social development and this is an area of active research (Itier and Batty, 2009; Young et al., 2009; Graham and LaBar, 2012; Landgraf and Osterheider, 2013; Leigh and Zee, 2015; Zihl and Dutton, 2015). The brain is almost continuously and automatically creating a visual representation of the world in a self-centered perspective (Merker, 2013; Zihl and Dutton, 2015). Non-conscious integration of vision with somatosensory input allows the differentiation between the self and the environment (Fraiberg, 1977). After birth, vision is the main tool to understand the meaning of the surrounding world including objects and people, the notion of object permanence, and the cause-effect relationships that govern actions.
Social development is the result of an interaction between innate forces -social motivation-, and late developed abilities environmentally influenced -social cognition. These behaviors are supported by extensive but selective subcortico/cortical brain networks named “the social brain network” (Senju and Johnson, 2009b; Graham and LaBar, 2012; Mares et al., 2016) which is automatically activated when a face enters in our visual field (Elgar and Campbell, 2001; Fletcher-Watson et al., 2008; Klin et al., 2015). At 10 min of life the visual attention of the baby is not random. Innate forces govern an attraction for tracking faces or face-like patterns (Goren et al., 1975). This behavior declines at the end of the first month to enable a more developed face processing (Johnson et al., 1991), but a strong bias for ultra-rapid automatic detection of faces remains lifelong (Fletcher-Watson et al., 2008). The child also pays special attention to human motion and visual communication (Klin et al., 2015). Non-verbal pragmatics like eye contact, reciprocal smiling, and taking turns in sound exchanges are one of the first behaviors to emerge, and the earliest mouth movements to produce sounds are led by vision (Skipper, 2014). Attention to the mouth predicts larger vocabulary size in normal children (Young et al., 2009) and infants frequently focus on the speaker’s mouth (Lewkowicz and Hansen-Tift, 2012). Gaze direction is highly informative of the other’s intention and is pivotal for Joint Attention, essential to encode a new label for an object. The eyes are the most attended feature of the face as they give several fundamental clues for surviving and well-being: emotion, gender and identity recognition (Itier and Batty, 2009). Some authors proposed that this innate eye and face detector system (Baron-Cohen, 1997; Itier and Batty, 2009) is more sensitive to emotional expressions than identity or gender features (Lundqvist et al., 2014; Neath and Itier, 2015; Palanica and Itier, 2015; Neath-Tavares and Itier, 2016). Abnormalities in visual attention and gaze behavior are universally present in the ASD population and have been highlighted in its first description by Kanner (1943). Growing evidence suggests that automatic, non-conscious attention to faces, eyes and biologic movement is mediated by the SC-Pul-Amy complex (Senju and Johnson, 2009a; Tamietto and de Gelder, 2010; Campatelli et al., 2013).
Full communication in real life settings requires the integration of several verbal and visual features that are processed separately (Bolis and Schilbach, 2017). Mother/infant social-motor body and emotional synchrony have an important influence on the development of intersubjectivity, social and language learning. “Interpersonal synchronization” in time and content encompasses joined attention, imitation (Marsh et al., 2013), turn-taking, non-verbal social communicative exchanges, affect sharing and engagement (Brenner et al., 2007; Zhao et al., 2017).
Regarding postnatal visual influence on brain development, it was proposed that the subcortical SC-Pul-Amy complex “tutors” slow developing cortical networks, allowing further specialization on social abilities (Johnson et al., 2015). Indirect evidence and animal studies suggest that early social visual experiences have an epigenetic influence on subcortical experience-expectant visual circuits, turning on and off specific genes resulting in plastic effects on experience-dependent subcortico-cortical networks conforming the social brain (Johnson et al., 2005; Klin et al., 2015; Rosa Salva et al., 2015). The microscopic changes that occur during the first months of life are perhaps the epitome of the complexity of the interactions between genetic and epigenetic environmental factors necessary to shape the human brain. The peak of synaptic growth is reached approximately at 12 months old, followed by a widespread pruning in early childhood; long-range brain connections increase from birth to early adulthood (Vértes and Bullmore, 2015).
Two Visual Pathways
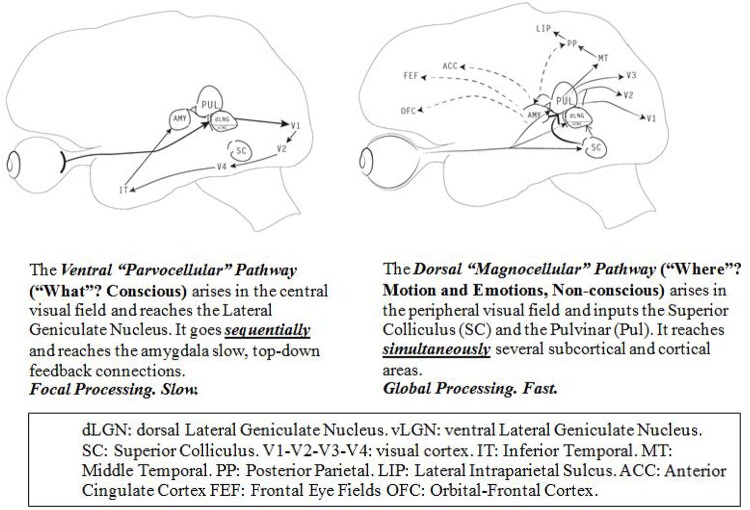
Although we perceive vision as a unitary function, there are two visual pathways with different but highly complementary and interconnected functions (Figure 2). The classic ventral pathway receives parvocellular input from numerous small cones, which are responsible for the central, foveal retinal vision. It represents ∼90% of the visual input, and it is unifocal and slower than the dorsal pathway. It allows a conscious, fine resolution, and a detailed and colorful analysis of the stimuli. After reaching the primary visual striate occipital cortex (V1) directly through the dorsal lateral geniculate nucleus (dLGN), it goes mainly sequentially through the extrastriate cortex (V2-V4) up to the infero-temporal area. Any object or event that is under conscious scrutiny occupies the central vision and flows through this pathway.
FIGURE 2.
Two visual pathways.
The dorsal pathway, extremely fast but of low resolution, receives magnocellular and koniocellular input from the rods, located in the peripheral visual field. It is multifocal, mainly non-conscious or pre-attentive and very sensitive to any movement or change in contrast luminescence. It allows recognition of where things are located in space as a result of the permanent input from both visual fields; its main first stations are the SC, the Pul and the vLGN. It reaches simultaneously, the extrastriate (V2-V3), the striate visual cortex (V1), and the middle-temporal cortex (MT). This last structure provides ongoing information about the visual environment to the posterior parietal (PP) and the LIP, essential to register sequences of movements (Lamme and Roelfsema, 2000).
Although both networks interact and contribute to non-conscious and conscious vision (Breitmeyer, 2014), the dorsal pathway is by far the most prevalent for pre-attentive and non-conscious processing as it is faster and it is continuously screening the peripheral visual field through the SC, a brain sentinel specially trained to select any biological relevant information such as motion, emotions and novelty (Tamietto and de Gelder, 2010; Tamietto, 2011; Soares et al., 2017). Most evidence indicates that the dorsal pathway is responsible for the visual behavior in the first months of life (Hammarrenger et al., 2003; Johnson, 2005; Bridge et al., 2015).
Blindsight
Blindsight is the ability to accurately use visual information without involvement of the primary visual cortex (V1), in absence of conscious visual perception (PöPpel et al., 1973; Weiskrantz et al., 1995). It is not a unitary phenomenon: “attentional blindsight” refers to the ability to make a visual discrimination in the blind hemifield, while “action blindsight” is the ability to respond with saccades, smooth eye pursuits, pointing or grasping to a new target or to a moving stimulus present in the blind hemifield (Danckert and Rossetti, 2005), specially to looming images (Hervais-Adelman et al., 2015). A surprising finding was the demonstration of “emotional blindsight” (de Gelder et al., 1999) that denotes the non-conscious capacity to correctly respond to emotional salient visual stimuli as fear, happy or angry faces, or whole-body emotional expressions in the blind hemifield (Van den Stock et al., 2011; Celeghin et al., 2015; Diano et al., 2017). These findings strongly suggest the existence of automatic, non-conscious activation of several input-processing-output subcortical circuits (SC-Pul-Amy) of the dorsal visual pathway (Tamietto and de Gelder, 2010; Diano et al., 2017; Soares et al., 2017). Based on animal and human studies, most authors maintain that blindsight is exclusively mediated by the SC (Leh, 2006; Leh et al., 2006; Tamietto et al., 2010), while a few claim that it is driven by the LGN (Breitmeyer, 2014; Ajina et al., 2015). The most convincing evidence of the exclusivity of the SC on blindsight is that a selective inactivation of the visual SCs in monkeys severely compromised non-conscious guided behavior (Kato et al., 2011).
Through the SC the dorsal pathway reaches several alternative (non-canonical) visual structures simultaneously allowing a panoramic processing of space with special sensitivity to faces, eyes, biological movement and emotional clues. This fast detection of relevant stimuli (Soares et al., 2017) is followed by a selection, also provided by the SC, which results in a shift from peripheral to central vision up to five times in a second if necessary (orienting attention) (Fecteau and Munoz, 2006).
Previous ASD Theories
Visual abilities are superior to language in most ASD individuals; hence, it seems paradoxical to look for a pathogenic theory based on the high prevalence of ASD in CB. Nevertheless, as it will be exposed in this section, most of the current ASD pathogenic theories are based on visual processing and visual attention.
Magnocellular Pathway Dysfunction Theories
Compromise on motion perception in ASD individuals (Gepner et al., 1995) and abnormal face processing from early life to adulthood has been proposed to be secondary to magnocellular dysfunction (Milne et al., 2002; McCleery et al., 2007). A direct relationship has been found in the degree of compromise on peripheral vision secondary to dysfunctions on the magnocellular pathway and the degree of autism severity (Sutherland and Crewther, 2010; Crewther and Crewther, 2014; Crewther et al., 2015; Vanmarcke and Wagemans, 2016). Greenaway et al. (2013) remark that previous evidence against selective and marked compromise of the magnocellular pathway in ASD individuals is the result of methodological issues in assessment. The only histological evidence of abnormal sensory input in ASD is the description of complete lack of normal magnocellular neurons on the LGN in Fragile X/ASD individuals (Kogan, 2003). Unfortunately, this excellent work did not include SC magnocellular investigation.
Global vs. Local Visual Perception Theories
The WCC was initially proposed as a diminished ability in the global top–down processing, essential for the integration of detailed features in a coherent whole, to explain social and non-social deficits in ASD individuals (Frith and Happé, 1994). Years later the same authors suggested that instead of a deficit in global processing, a bias for local processing resulted in superiority in visual perception of details (Happé and Frith, 2006). Similarly, the authors of a related theory – the EPF – (Mottron et al., 1999; Mottron et al., 2006) that initially postulated a stronger local processing in ASD individuals, then suggested an automatic attention bias for local visual information (Guy et al., 2016). This is in consonance with an extensive meta-analysis review showing that typical individuals perceived automatically the whole picture before conscious attention to details, a pattern that seems to be reversed in ASD (Van der Hallen et al., 2015). Additionally, drawing tasks comparing typical children with those with ASD did not show better local performance supporting the theory of bias on visual attention (Smith et al., 2016).
Innate Motivation to Social Attention
There is a growing body of research focusing on innate motivational aspects that bias social learning in an atypical way (Dawson et al., 2004). The Social Motivation theory of ASD focuses on a prenatal compromise in the experience expectant circuits (Chevallier et al., 2012) prepared to attend to faces and biologic stimuli as soon as the child is born (Klin et al., 2015; Rosa Salva et al., 2015). Both, lack of social motivation as a result of hypoarousal, or an excess of negative feelings provoked by hyperarousal states have been proposed to explain ASD social abnormalities (Senju and Johnson, 2009a; Chevallier et al., 2012). Emerging evidence seems to support this approach, at least in a subgroup of ASD individuals (Gamer et al., 2010; Assaf et al., 2013; Skuse et al., 2014).
Face Processing
Face and gaze processing has been one of the most active research topic in ASD for its prevalent role on socialization and communication and due to the observance of its abnormal developmental pattern in autistic children (Elgar and Campbell, 2001; Senju and Johnson, 2009a; Senju et al., 2011; Elsabbagh et al., 2012; Weigelt et al., 2012; Jones and Klin, 2013). After reviewing extensive behavioral and neurophysiologic studies on ASD individuals, Senju and Johnson proposed a “fast track modulator model” theory based on an early dysfunction in the subcortical face and eye contact detection route: the SC-Pul-Amy complex (Senju and Johnson, 2009a; Senju et al., 2011). Similarly, Campatelli et al. (2013) remark the existence of weakly coordinated interacting networks for normal face processing in ASD: the subcortical “face detection network” (SC-Pul-Amy) activates the “gaze/action representation network” (STS, sensori-motor cortex and IFG), the “emotional evaluation network” (Amy, insula and limbic system) and the “face identification network” (fusiform gyrus and inferior occipital gyrus). MEG images corroborate that normal face processing is mediated by an automatic visual pathway that triggers diverse extra-striate cortical visual areas, a pattern of activation that is different in ASD individuals (Bailey et al., 2005). Human fMRI studies also suggest a dysfunction on the automatic face detection subcortical system involving the SC-Pul-Amy in autistic individuals (Kleinhans et al., 2011), or an exaggerated subcortical activation that results in eye avoidance (Hadjikhani et al., 2017).
While some authors described that ASD individuals actively avoid looking at fearful faces (Corden et al., 2008), others found a direct relationship between autism severity and lack of attention to happy faces (Lassalle and Itier, 2015). Contradictory results could be explained by basic attentional factors such as the “arousal effect”: emotional potency or perceptual saliency (Lundqvist et al., 2014). A compromise of the normal attentional early orienting bias for emotional faces was significantly related to autism severity and not with ADHD and anxiety symptoms in children (Antezana et al., 2016).
A clinical study of high risk population infants revealed that the earlier manifestations of ASD were abnormalities in eye contact, visual tracking and visual attention (Zwaigenbaum et al., 2005). Neurophysiological studies showed abnormal sensitivity to dynamic eye gaze and atypical patterns of processing faces in those that later developed ASD (Elsabbagh et al., 2012; Keehn et al., 2015). An abnormal curve of development in eye contact was found in a prospective follow up study on infants later diagnosed as autistic (Jones and Klin, 2013). Klin et al. (2015) found that toddlers with ASD fail to recognize and to orient to biological motion perception, a skill present in newborns and even in lower vertebrates like hatched chicks. They suggest focusing future research on children between 4 and 12 weeks of life, when the transition from subcortical to cortical control seems to occur.
Attention in ASD
Described from the beginning (Kanner, 1943), attentional abnormalities are usually presented as associated symptoms of ASD. Instead, there is a renewed interest in the initial proposal of the role of early attention and its disruption as a core dysfunction with seminal influence on ASD symptomatology (Gold and Gold, 1975). According to different authors, even a slight compromise on basic attentional processing on early life could have an exponential effect on joint attention, affecting language and social development (Zwaigenbaum et al., 2005; Mundy and Newell, 2007; Chawarska et al., 2013; Elsabbagh et al., 2013; Sacrey et al., 2014) overfocusing, restrictive range of interest and repetitive behaviors (Keehn et al., 2013; Stevenson et al., 2014). It was found that attention to the speaker’s mouth and eyes influenced the development of language in typical and ASD children (Young et al., 2009), but not in children with language disorders, suggesting that the language compromise on children with autism may be driven in part by abnormal social attention (Tenenbaum et al., 2014). Longitudinal studies of ASD children showed beneficial effects of early stimulation of joint attention on verbal language development (Gulsrud et al., 2014).
Social and Non-social Attentional Compromise
Impairments in shifting attention to novel visual and auditory stimuli have been proposed as the basic compromise on ASD’ individuals by Courchesne et al. (1985); they point to the cerebellum as responsible for these abnormalities (Courchesne et al., 1994; Fatemi et al., 2012). A general decreased spontaneous visual attention to both, faces and objects, has been found in autistic individuals of different ages compared with non-autistic, and the degree of attentional deficit was related to autism severity (Guimard-Brunault et al., 2013). Similarly, a compromise of visual attention and saccadic reaction to faces and objects was found in a population of high risk 7 month-old infants who later developed ASD. The authors conclude that an atypical visual orienting may represent an early manifestation of ASD (Elison et al., 2013). Oculomotor abnormalities as unusual visual search or saccade production have been also proposed as the basis of abnormal socio-communicative development (Johnson et al., 2012).
Disengagement and ASD
In an extensive review on the impact of attention on core ASD symptoms and cognitive visual strengths, Keehn et al. (2013) highlight that previous reports of atypicalities on either the alerting, orienting or executive attention interacting systems could be secondary to a primary deficit in disengaging attention. This is in consonance with abnormal disengagement observed as the earliest symptom in ASD infants (Elsabbagh et al., 2013; Sacrey et al., 2014) and with ERP studies in adults with ASD that showed abnormalities in attention disengagement (Kawakubo et al., 2007).
Multiple attention and ASD
At present, most prevailing autism theories are based on deficits on simultaneous multiple attention and they state that in order to disclose it, real life evaluation settings should be used (Murray et al., 2005; Fletcher-Watson et al., 2009; Guillon et al., 2014; Jaworski and Eigsti, 2015; Keehn and Joseph, 2016; Unruh et al., 2016; Bolis and Schilbach, 2017). A subgroup of these theories point to deficits in Simultaneous Attention to the Self and Others to allow joint attention (Mundy and Newell, 2007; Just et al., 2014; Skorich et al., 2017).
Summary of ASD theories and its implications with the SC (see Table 1)
Table 1.
Correspondence between seminal symptoms of each ASD proposed pathogenesis and SC functions∗ (see text for references).
| ASD proposed theory | Endophenotype/Seminal symptom | Possible SC function compromised |
|---|---|---|
| Magnocellular visual pathway dysfunction. | Compromise on visual attention and/or compromise on motion perception and face processing. | Main (or exclusive) gate of the Magnocellular Visual Pathway. |
| Local over global visual non-conscious (WCC-EPF Model). | Automatic bias to local instead of global visual processing. | Exclusive structure described for automatic bias for global visual processing. |
| Innate social motivation compromise. | Emotional hypoarousal or hyperarousal to social stimuli. | First line experience-expectant sentinel structure that triggers the “social brain network.” |
| Abnormal emotional regulation. | Compromise on the alarm system or emotion system to social and non-social detection and reaction | Detector System and gate to trigger the emotional brain networks (e.g., fight or flight reactions, fear to snakes, etc.) |
| Face processing abnormalities (emotional or non-emotional processing). | Abnormalities in subcortical face detection network (SC/Pul/Amy). | First line specific neurons with shorter latencies for face recognition (25-ms). |
| Abnormal attention to social and non-social stimuli. | Atypical visual orienting to faces and objects. | First Sentinel/reactive system to novelty (biologic or non-biologic). |
| Attention disengagement. | Neurons exclusively prepared to disengagement functions. | |
| Compromise of simultaneous or multiple attention. | Elected structure to explain human multiple attention in real word scenes (e.g., MASC model). | |
| Mirror neuron system dysfunction. Abnormal motor and social synchrony (imitation). Compromise on the representation of the Self. | Compromise on visual implicit attention that activates the sensory-motor- emotional networks. Simultaneous Attention to the Self and others to allow joint attention (it also requires simultaneous synchrony between sensory, motor, cognitive, emotional and autonomic variables). | SC functions allow visual implicit attention, non-conscious decisions, visuomotor transformation and simultaneous triggering of sensory-motor-emotional networks. It is the main structure involved on the on each developmental stage of the representation of the Self. |
| Abnormal eye movements and saccade production. | Idem (abnormal saccades). | Main hub for visual search and saccade movements. |
| Abnormal multisensory integration. | Abnormal visual, auditory and somatosensory integration. | Sentinel multisensory single neurons that integrate visual, auditory and somatosensory input. |
| Intense world theory | Diminished prepulse inhibition (PPI) | Diminished PPI is exclusively associated with SC compromise. |
∗The authors of each theory proposed that the full clinical ASD picture is secondary to the compromise of the seminal symptom described. However the great diversity of ASD symptoms and the high presence of associated disorders could only be explained by a compromise of a hub with sensory, motor, social, emotional, autonomic, communicative integrate functions.
The previous theories reveal an attempt to disclose a single endophenotype responsible for the clinical manifestations of ASD. Although each proposal is different – magnocellular dysfunction, local over a global processing bias, difficulty to pay attention to multiple stimuli or to a novel stimulus, abnormalities in disengaging attention, lack of social motivation to attend to faces or biologic movement, or abnormalities in saccadic movements- there is one common factor in all these theories: the automatic or non-conscious visual attention is atypical in ASD individuals.
The finding of a unified cognitive endophenotype leads to the search of a dysfunction of a specific brain structure. The complexity of the attentional process makes this task difficult; several circuits could be involved. Even the brain’s default network, which is selectively activated by internal mentation, has been proposed as responsible for ASD symptoms (Andrews-Hanna, 2012). Additionally, exclusive cognitive dimensions explain neither the full clinical syndrome nor the comorbidities (dyspraxia, anxiety, emotional abnormalities, sleep disorders, autonomic symptoms, epilepsy, etc.).
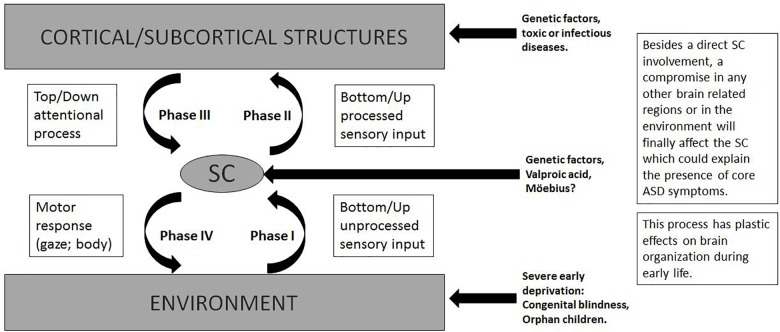
The complexity of SC and its influence on the developing brain seem to explain every previous theory and level of ASD compromise. Figure 3 shows the central role of the SC in different phases of these interactions and the impact of different etiologies that can disrupt each step resulting in ASD. A summary of its complex functions is necessary to understand the present theory.
FIGURE 3.
The SC is in the interface of bottom–up/top–down attentional process. Relevant information is sent to higher structures. Motor responses could be conscious or non conscious, limited to eye movement (redirection of the attention) or all body goal oriented response.
The SC as a “HUB”
The SC is a very ancient structure correspondent to the optic tectum (OT) in lower vertebrates (amphibians and fish). In mammals, it has a significant influence on early synaptogenesis and on plastic reorganization of higher structures after an injury in adults (Rushmore, 2006; Takaura et al., 2011). It contains seven layers that can be functionally and anatomically divided into two parts: The SCs (layers I-III) exclusively visual and SCid (layers IV-VII) with multisensory and motor functions. The “cortex like” anatomic organization (May, 2006), the direct or indirect connections with most other parts of the brain, and the strategic localization as a first line structure to environmental (visual/auditory) and body sensory input renders the SC in a unique center implicated in qualitative functions not described on any other brain region (Wurtz and Albano, 1980; May, 2006; Field et al., 2008; Cerkevich et al., 2014). It is a central structure of most subcortical Brainstem/Basal Ganglia loops which are the foundation of higher Thalamic/Cortical Networks (Redgrave, 2010).
Visual information recorded at the SCs is sent through rich connections to the SCid. This small center is perhaps the most integrative area of the brain. It transforms multisensory information and emotions in complex motor commands: gaze movements, head and body turning (Gandhi and Katnani, 2011), arm goal directed movements (Philipp and Hoffmann, 2014) and complex primitive reactions like “freezing,” “hunting,” or “approach” (Furigo et al., 2010; Comoli et al., 2012).
The Sprague Effect
The SC was usually considered just a passive responder of frontal/parietal orders to produce eye movements. This misconception changed after the description of the “Sprague effect,” a hemineglect syndrome produced by a SC unilateral lesion in cats (Sprague and Meikle, 1965; Sprague, 1966) or the resolution of the hemineglect in a patient with a frontal lesion after the inactivation of the contralateral SC (Weddell, 2004) showing the significant influence of the SC on hemispheric functions.
The confrontation of the blindsight phenomenon with the Sprague effect highlights the silent but crucial functions of the SC. In blindsight the primary visual cortex is compromised but the SC and the magnocellular visual pathway are spared and the individual is able to react to stimuli. Conversely, the Sprague effect results in a severe neglect and makes the individual unable to react, even when the LGN and the primary visual cortex are not compromised. This is an evidence of the role of SC in the continuous, non-conscious multi-attention, emotional processing, including autonomic and motor reactions.
The Role of the SC on Gaze and Attention
At every moment we draw our attention to different targets and the most conspicuous behavior of our attentional interest is gaze direction. Non-conscious or conscious, driven by extrinsic motivations to promote survival or by intrinsic motivation guided by the pleasure to learn new skills (Caligiore et al., 2015), a simple gaze shift is always the result of a complex mechanism. The SC actively participates in each level of these processes (Corrigan et al., 2015). Every cortical and subcortical region involved in eye movements connect directly or indirectly with the SC (Merker, 2013).
The “MASC” -Model of Attention in the SC- proposed to understand multiattentional human tasks is based on real world scenes, combining computational and behavioral neural responses (Adeli et al., 2017). It seems to be the best suited physiologic model to explain the new theories of ASD based on compromise on simultaneous multiple attention (see above).
SC Inner Functions
First Sentinel/Reactive System
Animal and human studies demonstrated that the SC has the intrinsic ability to recognize figure configuration (Girman and Lund, 2007; Georgy et al., 2016), to follow an object in movement (Dash et al., 2015; Inayat et al., 2015), and to select a new target (Phongphanphanee et al., 2014; Taouali et al., 2015). In rats, the inactivation of the primary visual cortex only increased the SC response to moving objects (Zhao et al., 2014). Pre-attentive discrimination between two relevant visual stimuli is accomplished exclusively at the SC (Krauzlis et al., 2013).
Also relevant to ASD theories is the finding in mammals of several groups of specialized SC cells, for example, neurons in visual layers with the shortest latencies throughout the brain to respond to faces (25-ms) (Nguyen et al., 2014); Visuomotor neurons in intermediary layers (Krauzlis et al., 2013) that allow the enigmatic transformation of visual images into motor commands; Multisensory neurons, specially trained to respond to space-temporal congruent stimuli (i.e., biologic events) (Stein et al., 2009); and Disengagement neurons, essential to change focus (Ngan et al., 2015). These findings strongly suggest that the SC is highly involved in pivotal operations of attention orienting: recognition of a new relevant clue, disengagement, visuomotor transformation for shifting, and new engagement (de Araujo et al., 2015). Disruptions of each of these functions have been linked to ASD pathogenesis (Keehn et al., 2013) (see above and see Table 1).
SC/Cortex Interactions
The SC functions described above allow only primitive reactions to exogenous stimuli. The existence of Bottom–Up indirect multiple connections from SC to the cortex and Top–Down direct from the cortex to the SC allow other multiple roles on attentional and goal directed higher functions. Animal studies show that postnatal environmental influence shapes the microstructure of the brain through the SC (Rosa Salva et al., 2015) and top–down connections from the cortex prepare special SC neurons as sentinels for future relevant information (Stein et al., 2014). This double input continues to be functional during all life and indicates that the SC requires both, bottom–up environmental and top-down cortical influence to function properly.
Bottom–Up Indirect SC/Cortical Connections
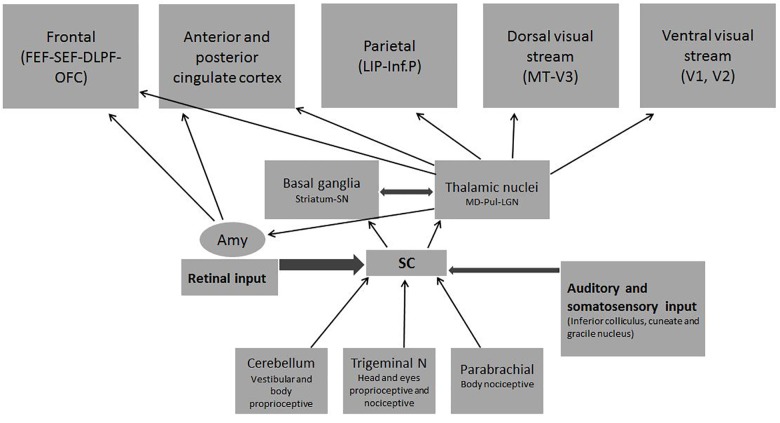
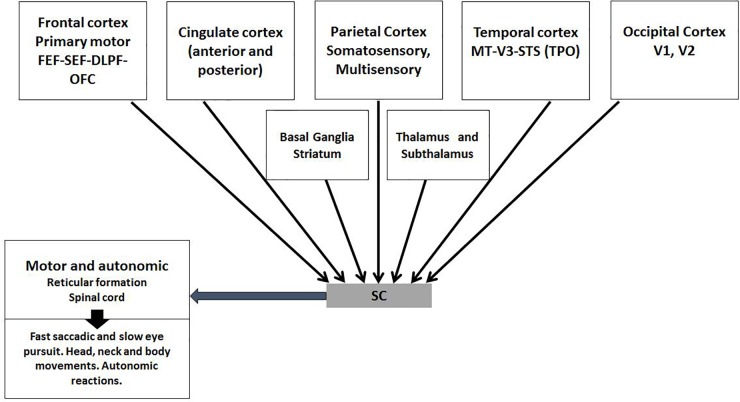
Somatosensory and motor maps are established inside the SC according to visual input. In turn it organizes parietal and premotor cortical regions through several multisensory and motor SC/cortical connections (Figure 4). New evidence indicates that several SC/brainstem/thalamic /cerebellar pathways are essential to cover visual spatial attention (Krauzlis et al., 2013). The present author suggests other more detailed sources for the interested reader (Wurtz and Albano, 1980; Sommer, 2003; May, 2006).
FIGURE 4.
Indirect bottom–up multisensory input from the SC to the cortex: Almost all sensory inputs (visual, auditory, somatosensory, proprioceptive and nociceptive) converge at the Deep SC. The visual input from the superficial SC is the most massive and leads to the correspondence of the rest (somatosensory, auditory, etc.) Several thalamic nuclei receive deep SC input to further reach the cortex.
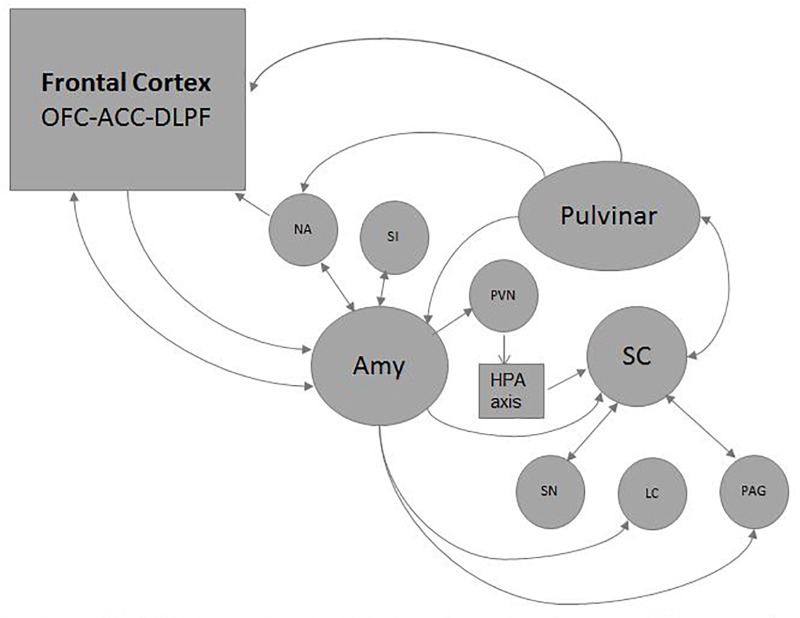
Affective and motivational aspects of visual attention and saccade control activates the SC’ “Emotion System” or “Alarm System” (Figure 5) (see Vuilleumier, 2015 for a review). It simultaneously triggers attentional, autonomic, endocrine and cognitive functions through the reward dopaminergic system, the alerting adrenergic system, the cholinergic system, and the endocrine system (Liddell et al., 2005; May et al., 2009; Redgrave, 2010; Tamietto and de Gelder, 2010; Van den Stock et al., 2011; Almeida et al., 2015). Some basic emotions like innate fear to snakes are mediated by SC/brainstem connections without the participation of the Amy (Merker, 2007; Soares et al., 2017). The Pul and the Amy, also considered crucial Hubs for emotional and social-communicative development, are activated by the SC before reaching cortical structures (Bickart et al., 2014; Bridge et al., 2015; Diano et al., 2017) (see Figures 4, 5).
FIGURE 5.
Emotional circuits triggered by the SC: visual and non-visual stimuli carrying emotional content could be processed exclusively by subcortical circuits or to reach the frontal cortex resulting or not in a motor non-conscious or conscious response respectively. The reward dopaminergic system is mediated by the Susbtantia njgra (SN)and the nucleo accubens (NA); the alerting adrenergic system by the locus ceruleous (LC);the cholinergic system by the substantia innomminata (SI). The hypothalamic-pituitary-adrenal axis (HPA) is reached through the paraventricular nucleus (PVN). Some basic emotions like defensive, reproductive, maternal or pain related behaviors are mediated by connections with the periacueductal gray matter (PAG). Through these ways the SC activates simultaneously several attentional, autonomic, endocrine and cognitive functions.
Multisensory/motor networks that process imitation abilities conform the “mirror neuron system,” which was also postulated as responsible for ASD pathogenesis (Williams J.H.G. et al., 2001). The mirror system is mainly influenced by emotional and motivational aspects of social and goal directed learning triggered by visual implicit attention (Vivanti and Rogers, 2014). As it was remarked, the SC seems to be the only sentinel structure prepared to trigger automatically and simultaneously sensory/motor/emotional networks.
Top/Down Direct Cortical/SC Connections
The SC receives back direct input from several visual and multimodal sensitive and motor cortical regions (Figure 6) (May, 2006). These connections are the most complex known model of top–down influence. During early life it has plastic effects. For example, it has been proved in newborn cats that SC neurons only become multisensory after few months of the double training from the environment and the multisensory cortex. These neurons are not only sentinels for very subtle space-temporal congruent events, they also send motor information (Alvarado et al., 2008; Stein et al., 2009; Stein et al., 2014). This sensory/motor integration could explain the attention to the mouth and the imitative movements that infants display in early life, apparently crucial to phonologic discrimination and its influence on language development (Lewkowicz and Hansen-Tift, 2012; Skipper, 2014; Kuhl, 2015).
FIGURE 6.
Top–down direct multisensory, motor, and emotion related input from the cortex to the SC. The SC receives direct cortical input mainly from V layer that concentrates all elaborated sensory-motor information from most cortical neurons. Temporal-Parietoccipital (TPO) associated area and frontal lobe regions -premotor mirror networks, emotional networks (OFC) and eye movements areas (FEF-SEF)-should be integrated for mentalize abilities. With this direct input, the SC functions as a contiguous cortical layer. Subcortical nuclei process non-conscious sensory-motor functions and are essential to SC functions.
Endogenous attention also activates the SC by top–down influence (Katyal and Ress, 2014) even in absence of the primary visual cortex (Yoshida et al., 2017). Monkey studies suggest that the interaction between vision, memory, self- motion and top–down spatial attention to conscious and non-conscious tracking of multiple visual cues during eye movements is coordinated by the SC (Dash et al., 2016). These functions are essential for mental time travel, for imaginative play and language development (Corballis, 2010). A poor imaginative play and abnormalities in understanding and using language referred to distant facts in time or space are frequently present even in high functioning ASD preschoolers (Rapin and Allen, 1983).
The input from the cortex to the SC is provided mainly by the pyramidal motor cells of the V layer that concentrate all the elaborate sensory-motor information of most cortical neurons. Although anatomically distant, the direct SC-cortex connections are functionally similar to connections between contiguous cortical layers (Merker, 2013).
The SC: A Decision Making Structure
Inside the SC there is a struggle of different forces of incoming stimuli before the selection of an attentional target (Mysore and Knudsen, 2011; Furlan et al., 2015; Inayat et al., 2015; Pretegiani et al., 2015; Wolf et al., 2015; Jagadisan and Gandhi, 2016). The exact mechanism of this competition is a subject of active investigation in bio-cybernetic to understand how a visual stimulus is transformed into a response (Brandt et al., 2012; Mysore and Knudsen, 2014; Phongphanphanee et al., 2014; Taouali et al., 2015; Adeli et al., 2017; Veale et al., 2017). This internal activity is influenced by extracollicular input process (Merker, 2007; Thurat et al., 2015; Khan et al., 2016).
The SC follows top–down orders from higher structures (Bauer et al., 2015; Paneri and Gregoriou, 2017) but it is prepared to take the control of any event which could be a source of interest or a potential threat (Maior et al., 2011; Mysore and Knudsen, 2011; Krauzlis et al., 2013, 2014; Phongphanphanee et al., 2014; Schröder and Carandini, 2014). The reaction is not always limited to a gaze change (Lovejoy and Krauzlis, 2010); sometimes a whole body, very fast non-conscious lifesaving reaction is needed and the SC must take the control. In this perspective SC decisions are superordinate even to cortex decision (Merker, 2007). Thus, we can suggest that the attentional decisions of the infant influenced by emotional and cognitive dimensions operate through the SC. Then, it is plausible to theorize that the most proposed endophenotype of ASD –the automatic attentional bias- is determined by the SC.
The SC and the Representation of the Self
Different levels of complexity of brain activity have been proposed to explain basic to sophisticated representations of the self (Merker, 2007; Fabbro et al., 2015). Apparently, the primary self is sustained purely by a subcortical system (Fabbro et al., 2015) called the “optic brain” (Merker, 2007). This conglomeration of nuclei, whose center is the SC, concentrates complex innate behaviors: the hypothalamus drives exploratory, ingestive, aggressive, defensive, social, sexual, and parenting basic goal directed behaviors and the PAG is involved in pain and powerful emotional reactions (Merker, 2007). These subcortical nuclei seem to be essential for further development of reflective knowledge based on self-consciousness, sustained by frontal, parietal and temporal structures. With the contribution of these cortical networks, babies start to recognize themselves in a mirror at 18 months of age. Few months later (∼24 months) they achieve the most complex representation of the self, exclusive of humans -the narrative-self-, supported by three cognitive aspects: (1) the ability to mental time travel to reconstruct past events and imagine possible future scenarios, (2) mentalizing abilities (theory of mind) and (3) language abilities that allow sharing it with other congeners (Fabbro et al., 2015).
The functional link between widespread bilateral cortical regions required to sustain this complex function is based on synchronized gamma band (∼40 Hz) neuronal activity which is highly coordinated with activity recorded at the SC (Fabbro et al., 2015) and the Pul (Soares et al., 2017). Recent MEG studies in humans support the existence of an impaired gamma band synchrony during eye-gaze processing in ASD (Richard et al., 2013). A direct relationship has been found in the severity of ASD and the compromise of shared attention secondary to a poor self-categorization (Skorich et al., 2017).
The SC and the Building of the Social Brain
The newborn’s ability to follow biologic motion and faces (Greenough et al., 1987) is sustained by experience-expectant circuits. Apparently, similar to the early imprinting phenomenon on birds, this event triggers simultaneously neurotransmitters and genes inducing qualitative changes on several brain regions responsible for the future social and cognitive style of the individual (Klin et al., 2015; Rosa Salva et al., 2015). The SC ascending connections are the candidate for an initial plastic effect on more “experience-dependent” higher circuits of the social brain. Prenatal and early postnatal animal studies have shown that the first neurotransmitter responsible for synaptic prune and specialization is GABA (Hensch, 2016). Evidence shows that the SC contains the largest amounts of GABA and the highest density of GABAergic synaptic terminals in the brain (Grantyn et al., 2011). Alterations in early GABA expression secondary to gene defects, perinatal trauma or toxic effects during pregnancy could result in neurodevelopmental disorders (ASDs, Rett Syndrome) and/or epilepsy (Abrahams and Geschwind, 2008; Takesian and Hensch, 2013), and repetitive circling behavior in rats (Velíšek et al., 2005). Not only does GABA play a role in early brain organization, but also almost all neurotransmitters (including nitric oxide) are involved in SC functions indicating its ubiquitous influence on several brain structures necessary to accomplish complex functions (Fuentes-Santamaria et al., 2008).
Effects of SC Lesions on Behavior in Humans and Animals
Few descriptions of motor or saccadic eye movement secondary to isolated unilateral SC lesion in adults were found in the literature (Heywood and Ratcliff, 1975; Girotti et al., 1979; Paidakakos et al., 2012).
More than a half century ago Denny-Brow described behavior abnormalities in monkeys and in a 17 year old girl with SC lesion. The author was impressed by how this small area inactivates the elaborate hemispheric organization for reaction to the external world. At that time he proposed that the SC was essential for social development (Denny-Brown, 1962).
Animal Studies
Bilateral lesions of the SC in infant capuchin monkeys impaired fear of snakes (Maior et al., 2011) and, on follow-up, they showed a transitory compromise of social behaviors. The authors proposed that the SC may play a key role in early stages of social development, but after this period its neural substrate involves larger networks (Maior et al., 2012). Despite the fact that the SC has connection with all brain areas involved in gaze direction, its ablation in rats does not result in absence of eye movements. Instead, it severely affects the ability to select relevant information to survival (ex.: to respond to a threat) and to establish priorities from several clues in covert attention to decide where the next gaze should be oriented (Merker, 2013) (which is an endophenotype of ASD) (see Table 1).
Beyond Social/Communicative Compromise: Stereotypies and Motor Abnormalities
Based on the ubiquitous presence of hyper-reactivity to nociceptive responses, acoustic startle response, and diminished prepulse inhibition (PPI) Markram and Markram (2010) proposed the “Intense World Theory” to explain ASD. They point to a prenatal compromise of the brainstem resulting in hyper-functioning of local neural microcircuits. Diminished PPI has been specifically associated with SC compromise (Soares et al., 2017) and it is in consonance with its extremely excitatory role that needs continuous gabaergic inhibition from several surrounding nuclei, intrinsic interneurons (Merker, 2007) and the cortex (Yu et al., 2013). Prenatal exposure to valproic acid in humans results in Fetal Valproate syndrome, associated with a high ASD prevalence (Williams G. et al., 2001; Schneider and Przewłocki, 2005) and a rodent model of autism with striking behavioral, anatomical and pathological, similarities was created by exposure of rat fetuses to valproic acid (VPA) (Schneider and Przewłocki, 2005). This is in concordance with the specific compromise of gabaergic SC’ neurons in rats exposed to the drug that not only showed difficulties in social and exploratory behavior but also abnormal sensory patterns of hypo or hyper-reactivity to nociceptive responses, to acoustic startle response, and diminished PPI (Dendrinos et al., 2011; Nakamura et al., 2015). Similarly, increased seizure activity secondary to hyperconnected networks in the OT has been found in tadpole prenatally exposed to valproic acid (James et al., 2015). Less circumscribed (not limited to the SC) prenatal focal brainstem lesions that result in Möebius syndrome or Thalidomide syndrome were also associated with high ASD prevalence, abnormalities in sensory processing, and epilepsy (Miller et al., 2005; Miller and Strömland, 2011).
All previous listed findings seem to be in consonance with brain abnormalities observed in ASD individuals such as increased local subcortical hyperconnection and abnormalities of early pre and post-natal synaptic organization (Abrahams and Geschwind, 2008; Markram and Markram, 2010).
Motor abnormalities in synchrony and imitation are among the most common earlier ASD’ manifestations (Marsh et al., 2013) and have been attributed to abnormal brain connectivity (Fishman et al., 2015) or magnocellular dysfunction (Ronconi et al., 2012). Social synchrony development is influenced by several variables like innate motivation and emotions, mimetic desire, visuomotor integration, implicit and explicit attention (Fitzpatrick et al., 2017). As it was previously exposed, the SC has a central role in the integration of sensory, motor, and emotional variables, necessary for mentalizing functions and the representation of the self.
ASD Etiologies, Timing of Clinical Manifestation and Neurodevelopmental Course
Non-defined genetic causes represent the most prevalent etiology of ASD and several hundred of genetic markers have been proposed (Willsey et al., 2013). This complexity is increased by prenatal and postnatal genes-environment interactions (Fein and Helt, 2017). For that reason, this line of research could be useful to recognize subtypes of autisms but unfruitful to explain the presence of the cluster of symptoms in the entire spectrum including children with non-genetic etiologies. It is more rational to propose that multiple etiologies confluence on specific networks in a specific age window, provoking a dysfunction responsible for the myriad of core symptoms (see Figure 1). In this section, etiologic genetic and non-genetics factors will be discussed under the perspective of prenatal and early postnatal SC involvement.
Prenatal Etiologies
It is clear that experience-expectant subcortical SC circuits that drive social motivation are almost exclusively influenced by genetic aspects. It is probable that a single compromise of any gen responsible for diverse interacting substances like oxytocin (OXT), dopamine, GABA, glutamine, acetylcholine, endogenous opioid, serotonin, etc., could jeopardize the prenatal emergence of social motivation.
Two conspicuous examples are, on one side, at the very beginning of the pathway, the finding of autism-relevant social abnormalities in Engrailed-2 knockout mice, a gene responsible for retino-tectal (SC) axon guidance and for proper development of monoaminergic hindbrain circuits (Brielmaier et al., 2012) and on the final top–down influence, the results of a multidisciplinary study revealing that several ASD associated genes converge over midfetal layer 5/6 cortical projection neurons (Willsey et al., 2013) which, from every cortical lobe, directly connect with the SC (Merker, 2013).
There are other possible mechanisms, to name a few due to the complexity of structures and functions involved. For example, a single gene defect compromising a peptide like OXT or its receptors have been linked to a reduced ability to face recognition, face emotion, or direction of gaze detection in members of families with an autistic child (Skuse et al., 2014). OXT was specifically linked with the SC in monkeys (Freeman et al., 2014) and, fMRI images in humans showed Amy and SC activation with enhanced attention to faces by OXT administration (Gamer et al., 2010). Serotonin has been highly implicated in abnormal emotional regulation, stereotypies, and obsessive symptoms of ASD individuals (Tuchman, 2006; Johnston, 2011). Serotonin transporter gen markers were linked to anatomic and functional abnormalities in a specific emotion related SC-Pul-Amy pathway activated by eye contact and, apparently essential to the development of social cognition (Skuse, 2006). In rodents, the influence of the SC on cortical serotonin regulation in response to visual and non-visual stimuli has been shown (Dringenberg et al., 2003; Bezdudnaya and Castro-Alamancos, 2014). An fMRI study showed bilateral dysfunction of dopaminergic networks during reward related social games (Assaf et al., 2013). Genetic studies pointed to abnormalities in dopaminergic receptors involved in the rewarding value of joint attention in ASD’ individuals (Gangi et al., 2016); several dopamine networks are activated by the SC (see above for references). Most of these genetic mechanisms are still unknown, which could explain the frequent non-defined genetic etiologies that result in ASD.
Prenatal neuroinflammation is also a frequently proposed etiology of ASD (Bilbo et al., 2018; Ohja et al., 2018). A selective compromise of social behavior mediated by the OT was observed in tadpoles exposed to elevated levels of pro-inflammatory cytokines (PIC) during early development (Lee et al., 2010). Additionally, it has been previously described that non-genetic prenatal conditions like fetal valproic acid or thalidomide syndrome directly compromise the SC (see above).
SC/Cortex Postnatal Mutual Influence
The postnatal appearance of the symptoms after 2 months of normal eye gaze in some children (Jones and Klin, 2013) or the occurrence of an autistic regression between 1 and 3 years old in almost a third of ASD’ children is suggestive of an active postnatal pathogenic compromise. If early interactions between environment and the genes shape several brain circuits and the “bottleneck” or the “interphase” between both is the SC, any significant genetic or non-genetic etiologic event will impact directly or indirectly on it. The postnatal complexity of environmental-gene interactions increases the vulnerability for pathogenic factors, mainly on genes that participate in neurotransmitter physiology, and brain microstructure. Most of the genetic markers of ASD are related to synaptic formation (Abrahams and Geschwind, 2008; Adviento et al., 2014; Sala et al., 2015; Sanders et al., 2015), cell adhesion molecules (Klin et al., 2015), axon guidance (Suda et al., 2011) or the metabolism of several neurotransmitters including GABA (Grantyn et al., 2011) or oxide nitric (Campbell et al., 2013; Sweeten et al., 2004).
It is worth noting that the synapses are diffuse but their compromise in ASD affects selectively some circuits, sparing or even enhancing others. This observation strengthens the theory of a lack of visual imprinting provided by the magnocellular pathway through the SC. Neuroanatomical and neurofunctional imaging studies disclose a selective compromise of networks that supports faces and motion perception, and emotional processing (Patriquin et al., 2016). White matter tract studies reveal hypo-connectivity of the large-scale tracts (Courchesne et al., 2007), more specifically the bilateral uncinate and superior longitudinal fasciculi (Jou et al., 2011). All these networks support social and communicative development and are activated by the SC after birth.
The compromise of brain connectivity is not limited to the cortex. A large scale multidisciplinary evaluation of diverse images studies not only confirmed the selective long range cortico-cortical hypoconnectivity in areas related to the social brain, but also highlighted a new finding: hyperconnectivity in subcortical thalamic structures (Di Martino et al., 2014) This is in consonance with the animal models of ASD prenatally exposed to valproic acid that also showed abnormal response to sensory stimuli and stereotypies (see above for references). Cortical top-down direct connections exert mostly inhibitory effects on SC neurons (Yu et al., 2013). Hence, it is possible that genetic and non-genetic factors which compromise distant but connected regions with the SC including the cortex could be a potential source of ASD pathogenesis; for example: Tuberous sclerosis, West Syndrome or electrical status epilepticus during slow sleep (McVicar et al., 2005; Tuchman et al., 2009).
Non-genetic Postnatal Environmental Factors
A high prevalence of autistic behavior, including repetitive and restricted range of interest, was found in orphan children with severe early deprivation and in adopted children after early care breakdown. The main determinant factor was the occurrence of the deprivation during the first 6 months of life or longer (Rutter et al., 1999; Kreppner et al., 2010; Nelson et al., 2011; Green et al., 2016). Diverse functional and anatomic brain studies on severely deprived orphans showed abnormalities in the Amy, the hippocampus, the ventral striatum, the orbitofrontal cortex and reduced white matter organization of the bilateral uncinate and superior longitudinal fasciculi (Nelson et al., 2011). These findings also support the existence of a critical period that needs the contribution of the environment to produce the synaptic and neural changes necessary for the development of the social networks. This could explain the very high prevalence of autism regression between 15 and 30 months of age, even in children with CB that showed a visual improvement after the first year of life (Dale, 2005) and the significant difference of ASD in children with CB compared with partial or acquired blindness (Hobson and Bishop, 2003; Jure et al., 2016).
Developmental Trajectories
Based on the interplay of “social motivation” and “social cognition,” subserved by “experience-expectant” and “experience-dependent” brain circuits respectively, this model could also explain the different developmental trajectories described in ASD children (Fountain et al., 2012). During the first months of life, “experience-dependent” higher circuits are modeled by “experience-expectant” networks. A change of this dominant role in the opposite way (i.e., cortical over subcortical structures) from the first months through adulthood seems to occur gradually. The adult phenotype seems to be the result of both: the intensity of ASD’s initial symptoms and the late appearing cognitive resources fed by cultural influence. Autism recovery or optimal outcome has been described in a minority of sighted individuals (∼20%) after 8 years of age, with mild ASD before 5 years old and above average level of cognition. The authors argued that this compensation could be secondary to the use of explicit, higher order, resources to substitute weak implicit social processing (Fein et al., 2013). The most dramatic autism recovery observed in an important minority of children with CB (Hobson and Lee, 2010) could be explained by a presumed normal genetic background for social abilities and a great need of verbal communication regarding its unique role as a vehicle for learning in blind individuals. On the other hand, the very high prevalence of Autism regression in CB before 3 years of age (Jure et al., 2016) could be the result of a secondary degeneration (diaschisis) of selective circuits as a result of a total lack of visual input during the first months of life.
Associated Language Disorders and Intellectual Deficit (ID)
Regarding that most of the numerous genes involved in the pathogenesis of ASD are related with early axon, synapsis formation and neurotransmitters, the most probable scenario is that different groups of those genes participate in different developmental stages of brain organization and function. If the genetic compromise affects the connections diffusely, the most likely clinical result will be the association of ASD with ID (which is present in ∼50% of ASD individuals) and more severe language disorders. If genes with a more selective function are affected, i.e., some of those that are activated by the SC in the postnatal period, the clinical manifestations will be more selective, and perhaps limited to ASD symptoms without intellectual compromise.
Additionally, the SC houses several types of neurons, dependent of several unknown genetic markers (Byun et al., 2015) that could be selectively affected resulting in different ASD phenotypes. For example, hypothetically, a compromise of multisensory SC neurons will result in less extended multisensory cortex and more cortical neurons recruited to both, isolated auditory and visual sensory processing respectively. This could explain not only most of ASD symptoms (including language disorders) according to some authors (Stein et al., 2014; Stevenson et al., 2014) but also the higher ability to visual local processing and the higher frequency of absolute pitch in ASD population (Heaton et al., 2008). A dysfunction of neurons that process facial configuration could explain the attentional tendencies to objects instead of congeners in early life. On the other hand, the rigidity, narrow interest and selectivity for some particular topics could be secondary to a compromise on disengagement neurons.
To understand the influence of attention on language development it is essential to clarify the variability in the attentional compromise: attentional neglect as in “Sprague effect” means that the individual is unable to visualize or to process the presented information despite all the efforts made by the interlocutor (e.g., to share attention over a proposed activity); this severe neglect from infancy could result in a complete lack of language development. Children with a selective attentional interest, e.g., on numbers, letters, songs, or TV programs can be able to repeat the alphabet, several songs with excellent phonology, or numbers in two different languages, but will display a serious compromise in communication. A mild compromise, limited to multiattentional tasks, can explain the ability of some high functioning ASD individuals to sustain a formal dialog with one or two persons but the inability (or avoidance) to do it informally, in a group of several individuals. As it was previously mentioned, new evidence points to abnormal social attention to explain the language compromise on children with ASD (Tenenbaum et al., 2014).
Summary and Conclusion
This review on two parallel lines of research, ASD pathogenesis and SC functions, discloses several common points worth investigating (see Table 1). The three levels of ASD’ compromise (clinical, etiological and pathogenic) could be explained by the role of the SC as a hub for developing brain networks that support social, emotional and communicative behaviors (see Figures 1, 3). Genetic and epigenetic and non-genetic prenatal or postnatal etiologies can affect the role of SC to transform early visual experiences into microstructural cerebral changes. Different pathogenic lines compromising the SC and affecting neuronal differentiation, synaptic formation, pruning, neurotransmitters, neurotrophic factors, or neurohormones (see text above and see Table 2) could explain the diversity of clinical subgroups. The timing of postnatal biological disruption parallels the timing of clinical manifestation. Most ASD cognitive theories, instead of being rejected, seem to adequately fit in this framework to explain all ASD symptoms in one individual and qualitative differences between subgroups. Every seminal symptom or endophenotype of each previous proposed theory is explained by a disruption of one of the several SC functions (see Table 1). Only the integration in this very small structure of all orienting senses with attentional, autonomic, endocrine, emotional, motivational variables and its transformation on motor commands, could explain the repetition of such variety of symptoms -social, communicative, autonomic, sensory-motor, emotional, etc.- in almost each individual with the disorder. Most evidence shows that this simultaneous integration led by vision and its influence on several cortical and subcortical structures (by gamma band activity?) is necessary to accomplish the synchrony needed to multiple attention of several variables required on real life social environments (see above for references). The interplay of a continuum set of abilities, or deficits, of the two broad cognitive styles: those provided by the SC on one side -non-conscious, peripheral, automatic, simultaneous, global-, and the more focal, conscious, analytic and detailed system -independent of the SC- on the other side, could explain the broad spectrum of the clinical compromise and the uneven skills usually observed in ASD individuals. The lack of the inhibitory action of cortical structures over the SC seems to be the best explanation to the abnormal response of sensory stimuli, the frequently associated anxiety disorders, obsessive thinking and the stereotypies.
Table 2.
Etiologies and brain mechanism proposed for ASD and its coincidence with SC compromise (see text for references).
| Etiologies or brain mechanism of ASD | SC functions |
|---|---|
| Genetic markers of ASD are related to synaptic formation, cell adhesion molecules, axon guidance, etc. | The SC is the main brain organizer (by early visual input) during the first months of life. Several specific genes related with retino-tectal axon guidance and cortical-SC connections are related with ASD etiology. |
| GABA is the main synaptogenic neurotransmitter during early life. | The SC is the main GABAergic brain center with a generalized influence on sensitive, motor and emotional circuits. |
| Fetal valproate syndrome | Specific compromise of gabaergic SC’ neurons in rats exposed to the valproic acid: they showed difficulties in social and exploratory behavior, abnormal sensory patterns and diminished prepulse inhibition (PPI). |
| Others neurotransmitters and hormones (OXT, testosterone, dopamine, glutamate, nitric oxide, choline, etc.) have been implicated in the pathogenesis of ASD. | Most reward related dopaminergic circuits are activated by the SC. The interaction between OXT and testosterone has an influence on the gaze behavior toward faces (aversion or indifference)∗ which are closely related with the SC. Most neurotransmitters and neurotrophic factors have input and/or output influence on the SC. |
| Impaired gamma band synchrony in ASD. | Widespread cortical synchronized gamma band activity is triggered by SC activation. |
| New images studies found selective long range cortico-cortical hypoconnectivity in areas related to the social brain, and hyperconnectivity in subcortical thalamic structures. | The SC could be responsible of the hypoconnectivity of the social brain network regarding its specific role on these brain areas during early human development. The recent finding of subcortical hyperconnectivity reinforces the theory of subcortical network compromise (whose center is the SC) and explains several symptoms such as the stereotypies, the abnormal PPI, obsessions, etc. |
| Prenatal neuroinflammation is a proposed etiology of ASD. | Impaired social behavior by compromise of the optic tectum was observed in tadpoles exposed to elevated levels of pro-inflammatory cytokines (PIC) during early development. |
The interaction of experience-expectant circuits that drive innate social motivation and late maturing experience-dependent networks that support social cognitive development is the best framework to explain the different neurodevelopmental courses (regression or optimal outcome) and the social subtypes. For example, an innate lack of social motivation would result in an aloof or passive social style. The very frequent social inappropriate subtype could be secondary to a high innate desire of interaction but without the resources (attentional, cognitive, etc.) provided by higher order cognitive processes that require frequent and long-lasting bottom–up and top–down SC-cortical interaction to allow a fully developed social cognition. It could be concluded that it seems unlikely to find another brain structure with the complexity of SC suitable to explain the complexity of all ASD variables.
Future Research
Regarding the SC anatomic location, its complexity, the plastic changes it suffers during the first months and the limitations for in vivo studies in infants, images and functional studies represent a significant challenge. Animal studies are limited due to their lack of higher order social and communicative abilities compared to humans. New technologies are indispensable for revealing the intricate mechanisms that govern the qualitative brain changes after the exposure of visual experiences. However, it is also necessary to change the focus of research from cortical to subcortical structures regarding its prominent role on hemispheric organization during early life. A search for anatomic and physiologic differences in the SC of non-autistic vs. autistic individuals could provide an initial clue for further research.
While the previous description was centered on the SC, it should be stressed that it is closely joined to the Pul. The inferior and the lateral Pul have a key role in emotional and social functions and are more developed in humans than in other primates. The main questions are: is the SC essential for social and communicative development during the first months of life? Could it be replaced by another structure like the Pul? Which one of the two structures is more relevant in ASD pathogenesis? Is it possible that selective compromises of SC functions could explain different pathogenic ASD pathways? What are the genetic-epigenetic interactions mediated by the SC necessary to support the development of social/communicative behaviors? (For example: “what are the effects of visual experiences and social interactions on the SC during the first 6 months of life to turn on and off different genes that activate brain mechanisms to allow social and communicative development?”). To respond to these questions clinical and biological researchers must work in collaboration.
Author Contributions
The author, a child neurologist interested in neurodevelopmental disorders, started a review research prompted by his previous work that showed that congenital blindness conveys a huge prevalence of autism. Two parallel lines of research, one on previous pathogenic theories and etiologies of autism, and the second on superior colliculus functions, were confronted to elaborate this hypothesis on autism pathogenesis.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Special thanks to my mentor Prof. Isabelle Rapin, who helped and encouraged in every step of this work before she passed away on May 27, 2017. I am grateful to Dr. Solomon Moshe and Dr. Deborah Fein for their helpful revisions and comments on earlier versions of the manuscript. To my son Dr. Pablo L. Jure who guided me from the beginning.
Abbreviations
- Amy
amygdala
- ASD
autism spectrum disorders or autism
- CB
congenital blindness
- EPF
enhanced perceptual functioning model
- FEF
frontal eye fields
- fMRI
functional MRI
- IFG
inferior frontal gyrus
- LIP
lateral intraparietal area
- MD
mediodorsal thalamus
- MEG
magnetoencephalography
- MT
middle temporal cortex
- MRI
magnetic resonance imaging
- OXT
oxytocin
- PAG
pariacueductal gray matter
- PVN
paraventricular nucleus
- Pul
pulvinar
- SC
superior colliculus
- SCs
superficial SC
- SCid
intermediate/deep SC
- SEF
supplementary eye fields
- SN
susbtantia nigra
- STS
superior temporal sulcus
- TMS
transcranial magnetic stimulation
- V1
primary visual cortex
- V2-V3-V4
secondary visual cortex
- WCC
weak central coherence theory
References
- Abrahams B. S., Geschwind D. H. (2008). Advances in autism genetics: on the threshold of a new neurobiology. Nat. Rev. Genet. 9 341–355. 10.1038/nrg2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli H., Vitu F., Zelinsky G. J. (2017). A model of the superior colliculus predicts fixation locations during scene viewing and visual search. J. Neurosci. 37 1453–1467. 10.1523/JNEUROSCI.0825-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adviento B., Corbin I. L., Widjaja F., Desachy G., Enrique N., Rosser T., et al. (2014). Autism traits in the RASopathies. J. Med. Genet. 51 10–20. 10.1136/jmedgenet-2013-101951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajina S., Pestilli F., Rokem A., Kennard C., Bridge H. (2015). Human blindsight is mediated by an intact geniculo-extrastriate pathway. Elife 4:e08935. 10.7554/eLife.08935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida I., Soares S. C., Castelo-Branco M. (2015). The distinct role of the amygdala, superior colliculus and pulvinar in processing of central and peripheral snakes. PLoS One 10:e0129949. 10.1371/journal.pone.0129949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado J. C., Rowland B. A., Stanford T. R., Stein B. E. (2008). A neural network model of multisensory integration also accounts for unisensory integration in superior colliculus. Brain Res. 1242 13–23. 10.1016/j.brainres.2008.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R. (2012). The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18 251–270. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antezana L., Mosner M. G., Troiani V., Yerys B. E. (2016). Social-emotional inhibition of return in children with autism spectrum disorder versus typical development. J. Autism Dev. Disord. 46 1236–1246. 10.1007/s10803-015-2661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M., Hyatt C. J., Wong C. G., Johnson M. R., Schultz R. T., Hendler T., et al. (2013). Mentalizing and motivation neural function during social interactions in autism spectrum disorders. Neuroimage Clin. 3 321–331. 10.1016/j.nicl.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A. J., Braeutigam S., Jousmäki V., Swithenby S. J. (2005). Abnormal activation of face processing systems at early and intermediate latency in individuals with autism spectrum disorder: a magnetoencephalographic study. Eur. J. Neurosci. 21 2575–2585. 10.1111/j.1460-9568.2005.04061.x [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. (1997). Mindblindness: An Essay on Autism and Theory of Mind. Cambridge, MA: MIT Press. [Google Scholar]
- Baron-Cohen S. (2017). The eyes as window to the mind. Am. J. Psychiatry 174 1–2. 10.1176/appi.ajp.2016.16101188 [DOI] [PubMed] [Google Scholar]
- Bauer J., Magg S., Wermter S. (2015). Attention modeled as information in learning multisensory integration. Neural Netw. 65 44–52. 10.1016/j.neunet.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T., Castro-Alamancos M. A. (2014). Neuromodulation of whisking related neural activity in superior colliculus. J. Neurosci. 34 7683–7695. 10.1523/JNEUROSCI.0444-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart K. C., Dickerson B. C., Feldman Barrett L. (2014). The amygdala as a hub in brain networks that support social life. Neuropsychologia 63 235–248. 10.1016/j.neuropsychologia.2014.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo S. D., Block C. L., Bolton J. L., Hanamsagar R., Tran P. K. (2018). Beyond infection - Maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. 299 241–251. 10.1016/j.expneurol.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolis D., Schilbach L. (2017). Observing and participating in social interactions: action perception and action control across the autistic spectrum. Dev. Cogn. Neurosci. 29 168–175. 10.1016/j.dcn.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt T., Dieterich M., Strupp M., Glasauer S. (2012). Model approach to neurological variants of visuo-spatial neglect. Biol. Cybernet. 106 681–690. 10.1007/s00422-012-0517-3 [DOI] [PubMed] [Google Scholar]
- Breitmeyer B. G. (2014). Contributions of magno- and parvocellular channels to conscious and non-conscious vision. Philo. Trans. R. Soc. B Biol. Sci. 369 20130213–20130213. 10.1098/rstb.2013.0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner L. A., Turner K. C., Müller R.-A. (2007). Eye movement and visual search: are there elementary abnormalities in autism? J. Autism Dev. Disord. 37 1289–1309. 10.1007/s10803-006-0277-9 [DOI] [PubMed] [Google Scholar]
- Bridge H., Leopold D. A., Bourne J. A. (2015). Adaptive pulvinar circuitry supports visual cognition. Trends Cogn. Sci. 20 146–157. 10.1016/j.tics.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brielmaier J., Matteson P. G., Silverman J. L., Senerth J. M., Kelly S., Genestine M., et al. (2012). Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One 7:e40914. 10.1371/journal.pone.0040914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun H., Kwon S., Ahn H.-J., Liu H., Forrest D., Demb J. B., et al. (2015). Molecular features distinguish ten neuronal types in the mouse superficial superior colliculus. J. Comp. Neurol. 524 2300–2321. 10.1002/cne.23952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiore D., Mustile M., Cipriani D., Redgrave P., Triesch J., De Marsico M., et al. (2015). Intrinsic motivations drive learning of eye movements: an experiment with human adults. PLoS One 10:e0118705. 10.1371/journal.pone.0118705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campatelli G., Federico R. R., Apicella F., Sicca F., Muratori F. (2013). Face processing in children with ASD: literature review. Res. Autism Spectr. Disord. 7 444–454. 10.1016/j.rasd.2012.10.003 [DOI] [Google Scholar]
- Campbell M. G., Kohane I. S., Kong S. W. (2013). Pathway-based outlier method reveals heterogeneous genomic structure of autism in blood transcriptome. BMC Med. Genom. 6:34. 10.1186/1755-8794-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass H. D., Sonksen P. M., McConachie H. R. (1994). Developmental setback in severe visual impairment. Arch. Dis. Child. 70 192–196. 10.1136/adc.70.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeghin A., de Gelder B., Tamietto M. (2015). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Conscious. Cogn. 36 414–425. 10.1016/j.concog.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerkevich C., Lyon D., Balaram P., Kaas J. (2014). Distribution of cortical neurons projecting to the superior colliculus in macaque monkeys. Eye Brain 2014 121–137. 10.2147/EB.S53613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawarska K., Macari S., Shic F. (2013). Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol. Psychiatry 74 195–203. 10.1016/j.biopsych.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E. S., Schultz R. T. (2012). The social motivation theory of autism. Trends Cogn. Sci. 16 231–239. 10.1016/j.tics.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoli E., Das Neves Favaro P., Vautrelle N., Leriche M., Overton P. G., Redgrave P. (2012). Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front. Neuroanat. 6:9. 10.3389/fnana.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis M. C. (2010). Mirror neurons and the evolution of language. Brain Lang. 112 25–35. 10.1016/j.bandl.2009.02.002 [DOI] [PubMed] [Google Scholar]
- Corbetta M., Patel G., Shulman G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58 306–324. 10.1016/j.neuron.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden B., Chilvers R., Skuse D. (2008). Emotional modulation of perception in asperger’s syndrome. J. Autism Dev. Disord. 38 1072–1080. 10.1007/s10803-007-0485-y [DOI] [PubMed] [Google Scholar]
- Corrigan F. M., Grand D., Raju R. (2015). Brainspotting: sustained attention, spinothalamic tracts, thalamocortical processing, and the healing of adaptive orientation truncated by traumatic experience. Med. Hypotheses 84 384–394. 10.1016/j.mehy.2015.01.028 [DOI] [PubMed] [Google Scholar]
- Coullon G. S. L., Jiang F., Fine I., Watkins K. E., Bridge H. (2015). Subcortical functional reorganization due to early blindness. J. Neurophysiol. 113 2889–2899. 10.1152/jn.01031.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E., Lincoln A. J., Kilman B. A., Galambos R. (1985). Event-related brain potential correlates of the processing of novel visual and auditory information in autism. J. Autism Dev. Disord. 15 55–76. 10.1007/BF01837899 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Pierce K., Schumann C. M., Redcay E., Buckwalter J. A., Kennedy D. P., et al. (2007). Mapping early brain development in Autism. Neuron 56 399–413. 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Courchesne E., Townsend J., Akshoomoff N. A., Saitoh O., Yeung-Courchesne R., Lincoln A. J., et al. (1994). Impairment in shifting attention in autistic and cerebellar patients. Behav. Neurosci. 108 848–865. 10.1037/0735-7044.108.5.848 [DOI] [PubMed] [Google Scholar]
- Crewther D. P., Crewther D., Bevan S., Goodale M. A., Crewther S. G. (2015). Greater magnocellular saccadic suppression in high versus low autistic tendency suggests a causal path to local perceptual style. R. Soc Open Sci. 2:150226. 10.1098/rsos.150226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crewther D. P., Crewther D. P. (2014). Peripheral global neglect in high vs. low autistic tendency. Front. Psychol. 5:284. 10.3389/fpsyg.2014.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N. (2005). “Early signs of developmental setback and autism in infants with severe visual impairment,” in Autism and Blindness: Research and Reflections, ed. Pring L. (London: Whurr; ), 74–98. [Google Scholar]
- Dale N., Sonksen P. (2002). Developmental outcome, including setback, in young children with severe visual impairment. Dev. Med. Child Neurol. 44 613–622. 10.1111/j.1469-8749.2002.tb00846.x [DOI] [PubMed] [Google Scholar]
- Danckert J., Rossetti Y. (2005). Blindsight in action: What can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci. Biobehav. Rev. 29 1035–1046. 10.1016/j.neubiorev.2005.02.001 [DOI] [PubMed] [Google Scholar]
- Dash S., Nazari S. A., Yan X., Wang H., Crawford J. D. (2016). Superior colliculus responses to attended, unattended, and remembered saccade targets during smooth pursuit eye movements. Front. Syst. Neurosci. 10:34. 10.3389/fnsys.2016.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash S., Yan X., Wang H., Crawford J. D. (2015). Continuous updating of visuospatial memory in superior colliculus during slow eye movements. Curr. Biol. 25 267–274. 10.1016/j.cub.2014.11.064 [DOI] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., et al. (2004). Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 40 271–283. 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- de Araujo M. F. P., Matsumoto J., Ono T., Nishijo H. (2015). An animal model of disengagement: temporary inactivation of the superior colliculus impairs attention disengagement in rats. Behav. Brain Res. 293 34–40. 10.1016/j.bbr.2015.07.031 [DOI] [PubMed] [Google Scholar]
- de Gelder B., Vroomen J., Pourtois G., Weiskrantz L. (1999). Non-conscious recognition of affect in the absence of striate cortex. Neuroreport 10 3759–3763. 10.1097/00001756-199912160-00007 [DOI] [PubMed] [Google Scholar]
- Dendrinos G., Hemelt M., Keller A. (2011). Prenatal VPA exposure and changes in sensory processing by the superior colliculus. Front. Integr. Neurosci. 5:68. 10.3389/fnint.2011.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny-Brown D. (1962). The midbrain and motor integration. Proc. R. Soc. Med. 55 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Yan C.-G., Li Q., Denio E., Castellanos F. X., Alaerts K., et al. (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 19 659–667. 10.1038/mp.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M., Celeghin A., Bagnis A., Tamietto M. (2017). Amygdala response to emotional stimuli without awareness: facts and interpretations. Front. Psychol. 7:2029. 10.3389/fpsyg.2016.02029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg H. C., Vanderwolf C., Noseworthy P. A. (2003). Superior colliculus stimulation enhances neocortical serotonin release and electrocorticographic activation in the urethane-anesthetized rat. Brain Res. 964 31–41. 10.1016/S0006-8993(02)04062-3 [DOI] [PubMed] [Google Scholar]
- Elgar K., Campbell R. (2001). Annotation: the cognitive neuroscience of face recognition: implications for developmental disorders. J. Child Psychol. Psychiatry 42 705–717. 10.1111/1469-7610.00767 [DOI] [PubMed] [Google Scholar]
- Elison J. T., Paterson S. J., Wolff J. J., Reznick J. S., Sasson N. J., Gu H., et al. (2013). White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 170 899–908. 10.1176/appi.ajp.2012.12091150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Fernandes J., Jane Webb S., Dawson G., Charman T., Johnson M. H. (2013). Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry 74 189–194. 10.1016/j.biopsych.2012.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M., Mercure E., Hudry K., Chandler S., Pasco G., Charman T., et al. (2012). Infant neural sensitivity to dynamic eye gaze is associated with later emerging Autism. Curr. Biol. 22 338–342. 10.1016/j.cub.2011.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro F., Aglioti S. M., Bergamasco M., Clarici A., Panksepp J. (2015). Evolutionary aspects of self- and world consciousness in vertebrates. Front. Hum. Neurosci. 9:157. 10.3389/fnhum.2015.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi S. H., Aldinger K. A., Ashwood P., Bauman M. L., Blaha C. D., Blatt G. J., et al. (2012). Consensus paper: pathological role of the cerebellum in Autism. Cerebellum 11 777–807. 10.1007/s12311-012-0355-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau J. H., Munoz D. P. (2006). Salience, relevance, and firing: a priority map for target selection. Trends Cogn. Sci. 10 382–390. 10.1016/j.tics.2006.06.011 [DOI] [PubMed] [Google Scholar]
- Fein D., Barton M., Eigsti I.-M., Kelley E., Naigles L., Schultz R. T., et al. (2013). Optimal outcome in individuals with a history of autism. J. Child Psychol. Psychiatry 54 195–205. 10.1111/jcpp.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein D., Helt M. (2017). Facilitating Autism Research. J. Int. Neuropsychol. Soc. 23 903–915. 10.1017/S1355617717001096 [DOI] [PubMed] [Google Scholar]
- Field C. B., Johnston K., Gati J. S., Menon R. S., Everling S. (2008). Connectivity of the primate superior colliculus mapped by concurrent microstimulation and event-related fMRI. PLoS One 3:e3928. 10.1371/journal.pone.0003928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I., Datko M., Cabrera Y., Carper R. A., Müller R.-A. (2015). Reduced integration and differentiation of the imitation network in autism: a combined functional connectivity magnetic resonance imaging and diffusion-weighted imaging study: imitation Network Connectivity. Ann. Neurol. 78 958–969. 10.1002/ana.24533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick P., Romero V., Amaral J. L., Duncan A., Barnard H., Richardson M. J., et al. (2017). Social motor synchronization: insights for understanding social behavior in autism. J. Autism Dev. Disord. 47 2092–2107. 10.1007/s10803-017-3124-2 [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S., Findlay J. M., Leekam S. R., Benson V. (2008). Rapid detection of person information in a naturalistic scene. Perception 37 571–583. 10.1068/p5705 [DOI] [PubMed] [Google Scholar]
- Fletcher-Watson S., Leekam S. R., Benson V., Frank M. C., Findlay J. M. (2009). Rapid detection of person information in a naturalistic scene. Neuropsychologia 47 248–257. 10.1016/j.neuropsychologia.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Fountain C., Winter A. S., Bearman P. S. (2012). Six developmental trajectories characterize children with Autism. Pediatrics 129 e1112–e1120. 10.1542/peds.2011-1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiberg S. (1977). Insights from the Blind: Comparative Studies of Blind and Sighted Infants. New York, NY: Basic Books. [Google Scholar]
- Freeman S. M., Inoue K., Smith A. L., Goodman M. M., Young L. J. (2014). The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology 45 128–141. 10.1016/j.psyneuen.2014.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U., Happé F. (1994). Autism: beyond “theory of mind.”. Cognition 50 115–132. 10.1016/0010-0277(94)90024-8 [DOI] [PubMed] [Google Scholar]
- Fuentes-Santamaria V., Alvarado J. C., Stein B. E., McHaffie J. G. (2008). Cortex contacts both output neurons and nitrergic interneurons in the superior colliculus: direct and indirect routes for multisensory integration. Cereb. Cortex 18 1640–1652. 10.1093/cercor/bhm192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo I. C., de Oliveira W. F., de Oliveira A. R., Comoli E., Baldo M. V. C., Mota-Ortiz S. R., et al. (2010). The role of the superior colliculus in predatory hunting. Neuroscience 165 1–15. 10.1016/j.neuroscience.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Furlan M., Smith A. T., Walker R. (2015). Activity in the human superior colliculus relating to endogenous saccade preparation and execution. J. Neurophysiol. 114 1048–1058. 10.1152/jn.00825.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Buchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. U.S.A. 107 9400–9405. 10.1073/pnas.1000985107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi N. J., Katnani H. A. (2011). Motor functions of the superior colliculus. Annu. Rev. Neurosci. 34 205–231. 10.1146/annurev-neuro-061010-113728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangi D. N., Messinger D. S., Martin E. R., Cuccaro M. L. (2016). Dopaminergic variants in siblings at high risk for autism: associations with initiating joint attention: Dopaminergic variants and joint attention. Autism Res. 9 1142–1150. 10.1002/aur.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgy L., Celeghin A., Marzi C. A., Tamietto M., Ptito A. (2016). The superior colliculus is sensitive to gestalt-like stimulus configuration in hemispherectomy patients. Cortex 81 151–161. 10.1016/j.cortex.2016.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner B., Mestre D., Masson G., de Schonen S. (1995). Postural effects of motion vision in young autistic children. Neuroreport 6 1211–1214. 10.1097/00001756-199505300-00034 [DOI] [PubMed] [Google Scholar]
- Girman S. V., Lund R. D. (2007). Most superficial sublamina of rat superior colliculus: neuronal response properties and correlates with perceptual figure-ground segregation. J. Neurophysiol. 98 161–177. 10.1152/jn.00059.2007 [DOI] [PubMed] [Google Scholar]
- Girotti F., Cefalà A., Sghirlanzoni A., Savoiardo M., Avanzini G. (1979). Oculomotor abnormalities in patients with lesions involving the tectal region. Ital. J. Neurol. Sci. 1 139–148. 10.1007/BF02335843 [DOI] [PubMed] [Google Scholar]
- Gold M. S., Gold J. R. (1975). Autism and attention: theoretical considerations and a pilot study using set reaction time. Child Psychiatry Hum. Dev. 6 68–80. 10.1007/BF01438301 [DOI] [PubMed] [Google Scholar]
- Goren C. C., Sarty M., Wu P. Y. (1975). Visual following and pattern discrimination of face-like stimuli by newborn infants. Pediatrics 56 544–549. [PubMed] [Google Scholar]
- Graham R., LaBar K. S. (2012). Neurocognitive mechanisms of gaze-expression interactions in face processing and social attention. Neuropsychologia 50 553–566. 10.1016/j.neuropsychologia.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R., Henneberger C., Jüttner R., Meier J. C., Kirischuk S. (2011). Functional hallmarks of GABAergic synapse maturation and the diverse roles of neurotrophins. Front. Cell. Neurosci. 5:13. 10.3389/fncel.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Leadbitter K., Kay C., Sharma K. (2016). Autism spectrum disorder in children adopted after early care breakdown. J. Autism Dev. Disord. 46 1392–1402. 10.1007/s10803-015-2680-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenaway R., Davis G., Plaisted-Grant K. (2013). Marked selective impairment in autism on an index of magnocellular function. Neuropsychologia 51 592–600. 10.1016/j.neuropsychologia.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Black J. E., Wallace C. S. (1987). Experience and brain development. Child Development 58 539–559. 10.2307/1130197 [DOI] [PubMed] [Google Scholar]
- Guillon Q., Hadjikhani N., Baduel S., Rogé B. (2014). Visual social attention in autism spectrum disorder: insights from eye tracking studies. Neurosci. Biobehav. Rev. 42 279–297. 10.1016/j.neubiorev.2014.03.013 [DOI] [PubMed] [Google Scholar]
- Guimard-Brunault M., Hernandez N., Roché L., Roux S., Barthélémy C., Martineau J., et al. (2013). Back to basic: Do children with autism spontaneously look at screen displaying a face or an object? Autism Res. Treat. 2013 1–7. 10.1155/2013/835247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulsrud A. C., Hellemann G. S., Freeman S. F. N., Kasari C. (2014). Two to ten years: developmental trajectories of joint attention in children with asd who received targeted social communication interventions: developmental trajectories of joint attention. Autism Res. 7 207–215. 10.1002/aur.1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J., Mottron L., Berthiaume C., Bertone A. (2016). A developmental perspective of global and local visual perception in autism spectrum disorder. J. Autism Dev. Disord. 10.1007/s10803-016-2834-1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N., Åsberg Johnels J., Zürcher N. R., Lassalle A., Guillon Q., Hippolyte L., et al. (2017). Look me in the eyes: constraining gaze in the eye-region provokes abnormally high subcortical activation in autism. Sci. Rep. 7:3163. 10.1038/s41598-017-03378-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarrenger B., Leporé F., Lippé S., Labrosse M., Guillemot J.-P., Roy M.-S. (2003). Magnocellular and parvocellular developmental course in infants during the first year of life. Doc. Ophthalmol. 107 225–233. 10.1023/B:DOOP.0000005331.66114.05 [DOI] [PubMed] [Google Scholar]
- Happé F., Frith U. (2006). The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36 5–25. 10.1007/s10803-005-0039-0 [DOI] [PubMed] [Google Scholar]
- Hasson U., Andric M., Atilgan H., Collignon O. (2016). Congenital blindness is associated with large-scale reorganization of anatomical networks. Neuroimage 128 362–372. 10.1016/j.neuroimage.2015.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton P., Davis R. E., Happé F. G. E. (2008). Research note: exceptional absolute pitch perception for spoken words in an able adult with autism. Neuropsychologia 46 2095–2098. 10.1016/j.neuropsychologia.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Hensch T. K. (2016). The power of the infant brain. Sci. Am. 314 64–69. 10.1038/scientificamerican0216-64 [DOI] [PubMed] [Google Scholar]
- Hervais-Adelman A., Legrand L. B., Zhan M., Tamietto M., de Gelder B., Pegna A. J. (2015). Looming sensitive cortical regions without V1 input: evidence from a patient with bilateral cortical blindness. Front. Integr. Neurosci. 9:51. 10.3389/fnint.2015.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S., Ratcliff G. (1975). “Long-term oculomotor consequences of unilateral colliculectomy in man,” in Basic Mechanisms of Ocular Motility and their Clinical Implications, eds Lennerstrand G., Bach-y-Rita P. (Oxford: Pergamon Press; ), 561–564. [Google Scholar]
- Hobson R. P. (2014). The coherence of autism. Autism 18 6–16. 10.1177/1362361313497538 [DOI] [PubMed] [Google Scholar]
- Hobson R. P., Bishop M. (2003). The pathogenesis of autism: insights from congenital blindness. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 358 335–344. 10.1098/rstb.2002.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson R. P., Lee A. (2010). Reversible autism among congenitally blind children? A controlled follow-up study. J. Child Psychol. Psychiatry 51 1235–1241. 10.1111/j.1469-7610.2010.02274.x [DOI] [PubMed] [Google Scholar]
- Inayat S., Barchini J., Chen H., Feng L., Liu X., Cang J. (2015). Neurons in the most superficial lamina of the mouse superior colliculus are highly selective for stimulus direction. J. Neurosci. 35 7992–8003. 10.1523/JNEUROSCI.0173-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itier R. J., Batty M. (2009). Neural bases of eye and gaze processing: the core of social cognition. Neurosci. Biobehav. Rev. 33 843–863. 10.1016/j.neubiorev.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadisan U. K., Gandhi N. J. (2016). Disruption of fixation reveals latent sensorimotor processes in the superior colliculus. J. Neurosci. 36 6129–6140. 10.1523/JNEUROSCI.3685-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James E. J., Gu J., Ramirez-Vizcarrondo C. M., Hasan M., Truszkowski T. L. S., Tan Y., et al. (2015). Valproate-induced neurodevelopmental deficits in Xenopus laevis tadpoles. J. Neurosci. 35 3218–3229. 10.1523/JNEUROSCI.4050-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J. L. B., Eigsti I.-M. (2015). Low-level visual attention and its relation to joint attention in autism spectrum disorder. Child Neuropsychol. 23 316–331. 10.1080/09297049.2015.1104293 [DOI] [PubMed] [Google Scholar]
- Johnson B. P., Rinehart N. J., Papadopoulos N., Tonge B., Millist L., White O., et al. (2012). A closer look at visually guided saccades in autism and Asperger’s disorder. Front. Integr. Neurosci. 6:99 10.3389/fnint.2012.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. H. (2005). Subcortical face processing. Nat. Rev. Neurosci. 6 766–774. 10.1038/nrn1766 [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Dziurawiec S., Ellis H., Morton J. (1991). Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40 1–19. 10.1016/0010-0277(91)90045-6 [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Griffin R., Csibra G., Halit H., Farroni T., De Haan M., et al. (2005). The emergence of the social brain network: evidence from typical and atypical development. Dev. Psychopathol. 17 599–619. 10.1017/S0954579405050297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. H., Senju A., Tomalski P. (2015). The two-process theory of face processing: modifications based on two decades of data from infants and adults. Neurosci. Biobehav. Rev. 50 169–179. 10.1016/j.neubiorev.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Johnston M. V. (2011). Education of a child neurologist: developmental neuroscience relevant to child neurology. Semin. Pediatr. Neurol. 18 133–138. 10.1016/j.spen.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W., Klin A. (2013). Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature 504 427–431. 10.1038/nature12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou R. J., Mateljevic N., Kaiser M. D., Sugrue D. R., Volkmar F. R., Pelphrey K. A. (2011). Structural neural phenotype of autism: preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am. J. Neuroradiol. 32 1607–1613. 10.3174/ajnr.A2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jure R., Pogonza R., Rapin I. (2016). Autism Spectrum Disorders (ASD) in blind children: very high prevalence, potentially better outlook. J. Autism Dev. Disord. 46 749–759. 10.1007/s10803-015-2612-5 [DOI] [PubMed] [Google Scholar]
- Just M. A., Cherkassky V. L., Buchweitz A., Keller T. A., Mitchell T. M. (2014). Identifying autism from neural representations of social interactions: neurocognitive markers of Autism. PLoS One 9:e113879. 10.1371/journal.pone.0113879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. (1943). Autistic disturbances of affective contact. Nervous Child 2 217–250. [PubMed] [Google Scholar]
- Kato R., Takaura K., Ikeda T., Yoshida M., Isa T. (2011). Contribution of the retino-tectal pathway to visually guided saccades after lesion of the primary visual cortex in monkeys: role of retino-tectal pathway in blindsight. Eur. J. Neurosci. 33 1952–1960. 10.1111/j.1460-9568.2011.07729.x [DOI] [PubMed] [Google Scholar]
- Katyal S., Ress D. (2014). Endogenous attention signals evoked by threshold contrast detection in human superior colliculus. J. Neurosci. 34 892–900. 10.1523/JNEUROSCI.3026-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y., Kasai K., Okazaki S., Hosokawa-Kakurai M., Watanabe K., Kuwabara H., et al. (2007). Electrophysiological abnormalities of spatial attention in adults with autism during the gap overlap task. Clin. Neurophysiol. 118 1464–1471. 10.1016/j.clinph.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Keehn B., Joseph R. M. (2016). Slowed search in the context of unimpaired grouping in autism: evidence from multiple conjunction search: slowed visual search in ASD. Autism Res. 9 333–339. 10.1002/aur.1534 [DOI] [PubMed] [Google Scholar]
- Keehn B., Müller R.-A., Townsend J. (2013). Atypical attentional networks and the emergence of autism. Neurosci. Biobehav. Rev. 37 164–183. 10.1016/j.neubiorev.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B., Vogel-Farley V., Tager-Flusberg H., Nelson C. A. (2015). Atypical hemispheric specialization for faces in infants at risk for autism spectrum disorder: atypical lateralization in high-risk infants. Autism Res. 8 187–198. 10.1002/aur.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeler W. R. (1956). “Autistic patterns and defective communication in blind children with retrolental fibroplasia,” in Proceedings of the Annual Meeting of the American Psychopathological Association, New York, NY, 64–83. [PubMed] [Google Scholar]
- Khan A. Z., Munoz D. P., Takahashi N., Blohm G., McPeek R. M. (2016). Effects of a pretarget distractor on saccade reaction times across space and time in monkeys and humans. J. Vis. 16:5. 10.1167/16.7.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N. M., Richards T., Johnson L. C., Weaver K. E., Greenson J., Dawson G., et al. (2011). fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage 54 697–704. 10.1016/j.neuroimage.2010.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Shultz S., Jones W. (2015). Social visual engagement in infants and toddlers with autism: early developmental transitions and a model of pathogenesis. Neurosci. Biobehav. Rev. 50 189–203. 10.1016/j.neubiorev.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan C. S. (2003). Differential impact of the FMR1 gene on visual processing in fragile X syndrome. Brain 127 591–601. 10.1093/brain/awh069 [DOI] [PubMed] [Google Scholar]
- Krauzlis R. J., Bollimunta A., Arcizet F., Wang L. (2014). Attention as an effect not a cause. Trends Cogn. Sci. 18 457–464. 10.1016/j.tics.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis R. J., Lovejoy L. P., Zénon A. (2013). Superior colliculus and visual spatial attention. Annu. Rev. Neurosci. 36 165–182. 10.1146/annurev-neuro-062012-170249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner J., Kumsta R., Rutter M., Beckett C., Castle J., Stevens S., et al. (2010). IV. Developmental course of deprivation-specific psychological patterns: early manifestations, persistence to age 15, and clinical features. Monogr. Soc. Res. Child Dev. 75 79–101. 10.1111/j.1540-5834.2010.00551.x [DOI] [PubMed] [Google Scholar]
- Kuhl P. K. (2015). Baby Talk. Sci. Am. 313 64–69. 10.1038/scientificamerican1115-64 [DOI] [PubMed] [Google Scholar]
- Lamme V. A., Roelfsema P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 23 571–579. 10.1016/S0166-2236(00)01657-X [DOI] [PubMed] [Google Scholar]
- Landgraf S., Osterheider M. (2013). “To see or not to see: that is the question.” The “Protection-Against-Schizophrenia” (PaSZ) model: evidence from congenital blindness and visuo-cognitive aberrations. Front. Psychol. 4:352. 10.3389/fpsyg.2013.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassalle A., Itier R. J. (2015). Autistic traits influence gaze-oriented attention to happy but not fearful faces. Soc. Neurosci. 10 70–88. 10.1080/17470919.2014.958616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. H., Mills E. A., Schwartz N., Bell M. R., Deeg K. E., Ruthazer E. S., et al. (2010). Neurodevelopmental effects of chronic exposure to elevated levels of pro-inflammatory cytokines in a developing visual system. Neural Dev. 5:2. 10.1186/1749-8104-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh S. E. (2006). Unconscious vision: new insights into the neuronal correlate of blindsight using diffusion tractography. Brain 129 1822–1832. 10.1093/brain/awl111 [DOI] [PubMed] [Google Scholar]
- Leh S. E., Mullen K. T., Ptito A. (2006). Absence of S-cone input in human blindsight following hemispherectomy. Eur. J. Neurosci. 24 2954–2960. 10.1111/j.1460-9568.2006.05178.x [DOI] [PubMed] [Google Scholar]
- Leigh R. J., Zee D. S. (2015). The Neurology of Eye Movements. Oxford: Oxford University Press; 10.1093/med/9780199969289.001.0001 [DOI] [Google Scholar]
- Lewkowicz D. J., Hansen-Tift A. M. (2012). Infants deploy selective attention to the mouth of a talking face when learning speech. Proc. Natl. Acad. Sci. U.S.A. 109 1431–1436. 10.1073/pnas.1114783109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell B. J., Brown K. J., Kemp A. H., Barton M. J., Das P., Peduto A., et al. (2005). A direct brainstem-amygdala-cortical “alarm” system for subliminal signals of fear. Neuroimage 24 235–243. 10.1016/j.neuroimage.2004.08.016 [DOI] [PubMed] [Google Scholar]
- Lovejoy L. P., Krauzlis R. J. (2010). Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat. Neurosci. 13 261–266. 10.1038/nn.2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D., Juth P., Öhman A. (2014). Using facial emotional stimuli in visual search experiments: the arousal factor explains contradictory results. Cogn. Emot. 28 1012–1029. 10.1080/02699931.2013.867479 [DOI] [PubMed] [Google Scholar]
- Luyster R., Richler J., Risi S., Hsu W.-L., Dawson G., Bernier R., et al. (2005). Early regression in social communication in autism spectrum disorders: a CPEA study. Dev. Neuropsychol. 27 311–336. 10.1207/s15326942dn2703_2 [DOI] [PubMed] [Google Scholar]
- Maior R. S., Hori E., Barros M., Teixeira D. S., Tavares M. C. H., Ono T., et al. (2011). Superior colliculus lesions impair threat responsiveness in infant capuchin monkeys. Neurosci. Lett. 504 257–260. 10.1016/j.neulet.2011.09.042 [DOI] [PubMed] [Google Scholar]
- Maior R. S., Hori E., Uribe C. E., Saletti P. G., Ono T., Nishijo H., et al. (2012). A role for the superior colliculus in the modulation of threat responsiveness in primates: toward the ontogenesis of the social brain. Rev. Neurosci. 23 697–706. 10.1515/revneuro-2012-0055 [DOI] [PubMed] [Google Scholar]
- Mares I., Smith M. L., Johnson M. H., Senju A. (2016). Direct gaze facilitates rapid orienting to faces: evidence from express saccades and saccadic potentials. Biol. Psychol. 121 84–90. 10.1016/j.biopsycho.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram K., Markram H. (2010). The intense world theory – a unifying theory of the neurobiology of Autism. Front. Hum. Neurosci. 4:224. 10.3389/fnhum.2010.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K. L., Isenhower R. W., Richardson M. J., Helt M., Verbalis A. D., Schmidt R. C., et al. (2013). Autism and social disconnection in interpersonal rocking. Front. Integr. Neurosci. 7:4. 10.3389/fnint.2013.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P. J. (2006). The mammalian superior colliculus: laminar structure and connections. Progr. Brain Res. 151 321–378. 10.1016/S0079-6123(05)51011-2 [DOI] [PubMed] [Google Scholar]
- May P. J., McHaffie J. G., Stanford T. R., Jiang H., Costello M. G., Coizet V., et al. (2009). Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur. J. Neurosci. 29 575–587. 10.1111/j.1460-9568.2008.06596.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J. P., Allman E., Carver L. J., Dobkins K. R. (2007). Abnormal magnocellular pathway visual processing in infants at risk for Autism. Biol. Psychiatry 62 1007–1014. 10.1016/j.biopsych.2007.02.009 [DOI] [PubMed] [Google Scholar]
- McVicar K. A., Ballaban-Gil K., Rapin I., Moshe S. L., Shinnar S. (2005). Epileptiform EEG abnormalities in children with language regression. Neurology 65 129–131. 10.1212/01.wnl.0000167193.53817.0f [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B. (2007). Consciousness without a cerebral cortex: a challenge for neuroscience and medicine. Behav. Brain Sci. 30 63–81. 10.1017/S0140525X07000891 [DOI] [PubMed] [Google Scholar]
- Merker B. (2013). The efference cascade, consciousness, and its self: naturalizing the first person pivot of action control. Front. Psychol. 4:501. 10.3389/fpsyg.2013.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. T., Strömland K., Ventura L., Johansson M., Bandim J. M., Gillberg C. (2005). Autism associated with conditions characterized by developmental errors in early embryogenesis: a mini review. Int. J. Dev. Neurosci. 23 201–219. 10.1016/j.ijdevneu.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Miller M. T., Strömland K. K. (2011). What can we learn from the thalidomide experience: an ophthalmologic perspective. Curr. Opin. Ophthalmol. 22 356–364. 10.1097/ICU.0b013e3283499f24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne E., Swettenham J., Hansen P., Campbell R., Jeffries H., Plaisted K. (2002). High motion coherence thresholds in children with autism. J. Child Psychol. Psychiatry 43 255–263. 10.1111/1469-7610.00018 [DOI] [PubMed] [Google Scholar]
- Mottron L., Burack J. A., Stauder J. E., Robaey P. (1999). Perceptual processing among high-functioning persons with autism. J. Child Psychol. Psychiatry 40 203–211. 10.1111/1469-7610.00433 [DOI] [PubMed] [Google Scholar]
- Mottron L., Dawson M., Soulières I., Hubert B., Burack J. (2006). Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J. Autism Dev. Disord. 36 27–43. 10.1007/s10803-005-0040-7 [DOI] [PubMed] [Google Scholar]
- Mundy P., Newell L. (2007). Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 16 269–274. 10.1111/j.1467-8721.2007.00518.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D., Lesser M., Lawson W. (2005). Attention, monotropism and the diagnostic criteria for autism. Autism 9 139–156. 10.1177/1362361305051398 [DOI] [PubMed] [Google Scholar]
- Mysore S., Knudsen E. (2014). Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron 84 214–226. 10.1016/j.neuron.2014.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore S. P., Knudsen E. I. (2011). The role of a midbrain network in competitive stimulus selection. Curr. Opin. Neurobiol. 21 653–660. 10.1016/j.conb.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Matsumoto J., Takamura Y., Ishii Y., Sasahara M., Ono T., et al. (2015). Relationships among parvalbumin-immunoreactive neuron density, phase-locked gamma oscillations, and autistic/schizophrenic symptoms in PDGFR-ββ knock-out and control mice. PLoS One 10:e0119258. 10.1371/journal.pone.0119258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neath K. N., Itier R. J. (2015). Fixation to features and neural processing of facial expressions in a gender discrimination task. Brain Cogn. 99 97–111. 10.1016/j.bandc.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neath-Tavares K. N., Itier R. J. (2016). Neural processing of fearful and happy facial expressions during emotion-relevant and emotion-irrelevant tasks: a fixation-to-feature approach. Biol. Psychol. 119 122–140. 10.1016/j.biopsycho.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. A., Bos K., Gunnar M. R., Sonuga-Barke E. J. S. (2011). V. The neurobiological toll of early human deprivation: the neurobiological toll of early human deprivation. Monogr. Soc. Res. Child Dev. 76 127–146. 10.1111/j.1540-5834.2011.00630.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngan N. H., Matsumoto J., Takamura Y., Tran A. H., Ono T., Nishijo H. (2015). Neuronal correlates of attention and its disengagement in the superior colliculus of rat. Front. Integr. Neurosci. 9:9. 10.3389/fnint.2015.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. N., Matsumoto J., Hori E., Maior R. S., Tomaz C., Tran A. H., et al. (2014). Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Front. Behav. Neurosci. 8:85. 10.3389/fnbeh.2014.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohja K., Gozal E., Fahnestock M., Cai L., Cai J., Freedman J. H., et al. (2018). Neuroimmunologic and neurotrophic interactions in autism spectrum disorders: relationship to neuroinflammation. Neuromolecular Med. 20 161–173. 10.1007/s12017-018-8488-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paidakakos N. A., Rokas E., Theodoropoulos S., Dimogerontas G., Konstantinidis E. (2012). Posttraumatic benedikt’s syndrome: a rare entity with unclear anatomopathological correlations. World Neurosurg. 78 715.e13–715.e15. 10.1016/j.wneu.2012.03.028 [DOI] [PubMed] [Google Scholar]
- Palanica A., Itier R. J. (2015). Eye gaze and head orientation modulate the inhibition of return for faces. Attent. Percept. Psychophys. 77 2589–2600. 10.3758/s13414-015-0961-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneri S., Gregoriou G. G. (2017). Top-down control of visual attention by the prefrontal cortex. Functional specialization and long-range interactions. Front. Neurosci. 11:545. 10.3389/fnins.2017.00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin M. A., DeRamus T., Libero L. E., Laird A., Kana R. K. (2016). Neuroanatomical and neurofunctional markers of social cognition in autism spectrum disorder: social Brain in Autism. Hum. Brain Mapp. 37 3957–3978. 10.1002/hbm.23288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp R., Hoffmann K.-P. (2014). Arm movements induced by electrical microstimulation in the superior colliculus of the macaque monkey. J. Neurosci. 34 3350–3363. 10.1523/JNEUROSCI.0443-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongphanphanee P., Marino R. A., Kaneda K., Yanagawa Y., Munoz D. P., Isa T. (2014). Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. Eur. J. Neurosci. 40 2329–2343. 10.1111/ejn.12579 [DOI] [PubMed] [Google Scholar]
- PöPpel E., Held R., Frost D. (1973). Residual visual function after brain wounds involving the central visual pathways in man. Nature 243 295–296. 10.1038/243295a0 [DOI] [PubMed] [Google Scholar]
- Pretegiani E., Astefanoaei C., Daye P. M., FitzGibbon E. J., Creanga D.-E., Rufa A., et al. (2015). Action and perception are temporally coupled by a common mechanism that leads to a timing misperception. J. Neurosci. 35 1493–1504. 10.1523/JNEUROSCI.2054-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin I., Allen D. A. (1983). “Developmental language disorders: nosologic considerations,” in Neuropsychology of Language, Reading, and Spelling, ed. Kirk U. (San Diego, CA: Academic Press; ), 155–184. [Google Scholar]
- Redgrave P. (2010). Interactions between the midbrain superior colliculus and the basal ganglia. Front. Neuroanat. 4:132 10.3389/fnana.2010.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reislev N. H., Dyrby T. B., Siebner H. R., Lundell H., Ptito M., Kupers R. (2017). Thalamocortical connectivity and microstructural changes in congenital and late blindness. Neural Plast. 2017:9807512. 10.1155/2017/9807512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard A. E., Lajiness-O’Neill R. R., Bowyer S. M. (2013). Impaired prefrontal gamma band synchrony in autism spectrum disorders during gaze cueing. Neuroreport 24 894–897. 10.1097/WNR.0000000000000015 [DOI] [PubMed] [Google Scholar]
- Ronconi L., Gori S., Ruffino M., Franceschini S., Urbani B., Molteni M., et al. (2012). Decreased coherent motion discrimination in autism spectrum disorder: the role of attentional zoom-out deficit. PLoS One 7:e49019. 10.1371/journal.pone.0049019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa Salva O., Mayer U., Vallortigara G. (2015). Roots of a social brain: developmental models of emerging animacy-detection mechanisms. Neurosci. Biobehav. Rev. 50 150–168. 10.1016/j.neubiorev.2014.12.015 [DOI] [PubMed] [Google Scholar]
- Rosenbloom L. (1994). Developmental setback in severe visual impairment. Arch. Dis. Child. 71 179–180. 10.1136/adc.71.2.179-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore R. J. (2006). Functional circuitry underlying visual neglect. Brain 129 1803–1821. 10.1093/brain/awl140 [DOI] [PubMed] [Google Scholar]
- Rutter M., Andersen-Wood L., Beckett C., Bredenkamp D., Castle J., Groothues C., et al. (1999). Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. J. Child Psychol. Psychiatry 40 537–549. 10.1111/1469-7610.00472 [DOI] [PubMed] [Google Scholar]
- Sacrey L.-A. R., Armstrong V. L., Bryson S. E., Zwaigenbaum L. (2014). Impairments to visual disengagement in autism spectrum disorder: a review of experimental studies from infancy to adulthood. Neurosci. Biobehav. Rev. 47 559–577. 10.1016/j.neubiorev.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Sala C., Vicidomini C., Bigi I., Mossa A., Verpelli C. (2015). Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J. Neurochem. 135 849–858. 10.1111/jnc.13232 [DOI] [PubMed] [Google Scholar]
- Sanders S. J., He X., Willsey A. J., Ercan-Sencicek A. G., Samocha K. E., Cicek A. E., et al. (2015). Insights into Autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron 87 1215–1233. 10.1016/j.neuron.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Uono S., Toichi M. (2016). Neural mechanisms underlying conscious and unconscious attentional shifts triggered by eye gaze. Neuroimage 124 118–126. 10.1016/j.neuroimage.2015.08.061 [DOI] [PubMed] [Google Scholar]
- Schneider T., Przewłocki R. (2005). Behavioral alterations in rats prenatally exposed to valproic acid: animal model of Autism. Neuropsychopharmacology 30 80–89. 10.1038/sj.npp.1300518 [DOI] [PubMed] [Google Scholar]
- Schröder S., Carandini M. (2014). A cortical rein on the tectum’s gain. Neuron 84 6–8. 10.1016/j.neuron.2014.09.021 [DOI] [PubMed] [Google Scholar]
- Senju A., Johnson M. H. (2009a). Atypical eye contact in autism: models, mechanisms and development. Neurosci. Biobehav. Rev. 33 1204–1214. 10.1016/j.neubiorev.2009.06.001 [DOI] [PubMed] [Google Scholar]
- Senju A., Johnson M. H. (2009b). The eye contact effect: mechanisms and development. Trends Cogn. Sci. 13 127–134. 10.1016/j.tics.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Senju A., Kikuchi Y., Akechi H., Hasegawa T., Tojo Y., Osanai H., et al. (2011). Atypical modulation of face-elicited saccades in autism spectrum disorder in a double-step saccade paradigm. Res. Autism Spectr. Disord. 5 1264–1269. 10.1016/j.rasd.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J. I. (2014). Echoes of the spoken past: how auditory cortex hears context during speech perception. Philos. Trans. R. Soc. B Biol. Sci. 369:20130297. 10.1098/rstb.2013.0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorich D. P., Gash T. B., Stalker K. L., Zheng L., Haslam S. A. (2017). Exploring the cognitive foundations of the shared attention mechanism: evidence for a relationship between self-categorization and shared attention across the autism spectrum. J. Autism Dev. Disord. 47 1341–1353. 10.1007/s10803-017-3049-9 [DOI] [PubMed] [Google Scholar]
- Skuse D. (2006). Genetic influences on the neural basis of social cognition. Philos. Trans. R. Soc. B Biol. Sci. 361 2129–2141. 10.1098/rstb.2006.1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse D. H., Lori A., Cubells J. F., Lee I., Conneely K. N., Puura K., et al. (2014). Common polymorphism in the oxytocin receptor gene ( OXTR ) is associated with human social recognition skills. Proc. Natl. Acad. Sci. U.S.A. 111 1987–1992. 10.1073/pnas.1302985111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Kenny L., Rudnicka A., Briscoe J., Pellicano E. (2016). Drawing firmer conclusions: autistic children show no evidence of a local processing bias in a controlled copying task. J. Autism Dev. Disord. 46 3481–3492. 10.1007/s10803-016-2889-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S. C., Maior R. S., Isbell L. A., Tomaz C., Nishijo H. (2017). Fast detector/first responder: interactions between the superior colliculus-pulvinar pathway and stimuli relevant to primates. Front. Neurosci. 11:67. 10.3389/fnins.2017.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M. A. (2003). What the brain stem tells the frontal cortex. i. oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J. Neurophysiol. 91 1381–1402. 10.1152/jn.00738.2003 [DOI] [PubMed] [Google Scholar]
- Sprague J. M. (1966). Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153 1544–1547. 10.1126/science.153.3743.1544 [DOI] [PubMed] [Google Scholar]
- Sprague J. M., Meikle T. H. (1965). The role of the superior colliculus in visually guided behavior. Exp. Neurol. 11 115–146. 10.1016/0014-4886(65)90026-9 [DOI] [PubMed] [Google Scholar]
- Stein B. E., Stanford T. R., Rowland B. A. (2009). The neural basis of multisensory integration in the midbrain: its organization and maturation. Hear. Res. 258 4–15. 10.1016/j.heares.2009.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. E., Stanford T. R., Rowland B. A. (2014). Development of multisensory integration from the perspective of the individual neuron. Nat. Rev. Neurosci. 15 520–535. 10.1038/nrn3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson R. A., Siemann J. K., Schneider B. C., Eberly H. E., Woynaroski T. G., Camarata S. M., et al. (2014). Multisensory temporal integration in Autism spectrum disorders. J. Neurosci. 34 691–697. 10.1523/JNEUROSCI.3615-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda S., Iwata K., Shimmura C., Kameno Y., Anitha A., Thanseem I., et al. (2011). Decreased expression of axon-guidance receptors in the anterior cingulate cortex in autism. Mol. Autism 2:14. 10.1186/2040-2392-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland A., Crewther D. P. (2010). Magnocellular visual evoked potential delay with high autism spectrum quotient yields a neural mechanism for altered perception. Brain 133 2089–2097. 10.1093/brain/awq122 [DOI] [PubMed] [Google Scholar]
- Sweeten T. L., Posey D. J., Shankar S., McDougle C. J. (2004). High nitric oxide production in autistic disorder: a possible role for interferon-γ. Biol. Psychiatry 55 434–437. 10.1016/j.biopsych.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. (2005). in Autism and blindness: research and reflections, ed. Pring L. London: Whurr. [Google Scholar]
- Takaura K., Yoshida M., Isa T. (2011). Neural substrate of spatial memory in the superior colliculus after damage to the primary visual cortex. J. Neurosci. 31 4233–4241. 10.1523/JNEUROSCI.5143-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian A. E., Hensch T. K. (2013). Balancing plasticity/stability across brain development. Progr. Brain Res. 207 3–34. 10.1016/B978-0-444-63327-9.00001-1 [DOI] [PubMed] [Google Scholar]
- Tamietto M. (2011). Sentinels in the visual system. Front. Behav. Neurosci. 5:6. 10.3389/fnbeh.2011.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamietto M., Cauda F., Corazzini L. L., Savazzi S., Marzi C. A., Goebel R., et al. (2010). Collicular vision guides nonconscious behavior. J. Cogn. Neurosci. 22 888–902. 10.1162/jocn.2009.21225 [DOI] [PubMed] [Google Scholar]
- Tamietto M., de Gelder B. (2010). Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11 697–709. 10.1038/nrn2889 [DOI] [PubMed] [Google Scholar]
- Taouali W., Goffart L., Alexandre F., Rougier N. P. (2015). A parsimonious computational model of visual target position encoding in the superior colliculus. Biol. Cybernet. 109 549–559. 10.1007/s00422-015-0660-8 [DOI] [PubMed] [Google Scholar]
- Tenenbaum E. J., Amso D., Abar B., Sheinkopf S. J. (2014). Attention and word learning in autistic, language delayed and typically developing children. Front. Psychol. 5:490. 10.3389/fpsyg.2014.00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurat C., N’Guyen S., Girard B. (2015). Biomimetic race model of the loop between the superior colliculus and the basal ganglia: subcortical selection of saccade targets. Neural Netw. 67 54–73. 10.1016/j.neunet.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Tuchman R. (ed.). (2006). Autism: a Neurological Disorder of Early Brain Development. London: MacKeith Press for the International Child Neurology Association. [Google Scholar]
- Tuchman R., Moshé S. L., Rapin I. (2009). Convulsing toward the pathophysiology of autism. Brain Dev. 31 95–103. 10.1016/j.braindev.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh K. E., Sasson N. J., Shafer R. L., Whitten A., Miller S. J., Turner-Brown L., et al. (2016). Social orienting and attention is influenced by the presence of competing nonsocial information in adolescents with Autism. Front. Neurosci. 10:586. 10.3389/fnins.2016.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Stock J., Tamietto M., Sorger B., Pichon S., Grezes J., de Gelder B. (2011). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proc. Natl. Acad. Sci. U.S.A. 108 16188–16193. 10.1073/pnas.1107214108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Hallen R., Evers K., Brewaeys K., Van den Noortgate W., Wagemans J. (2015). Global processing takes time: a meta-analysis on local–global visual processing in ASD. Psychol. Bull. 141 549–573. 10.1037/bul0000004 [DOI] [PubMed] [Google Scholar]
- Vanmarcke S., Wagemans J. (2016). Individual differences in spatial frequency processing in scene perception: the influence of autism-related traits. Vis. Cogn. 24 115–131. 10.1080/13506285.2016.1199625 [DOI] [Google Scholar]
- Veale R., Hafed Z. M., Yoshida M. (2017). How is visual salience computed in the brain? Insights from behaviour, neurobiology and modelling. Philos. Trans. R. Soc. B Biol. Sci. 372:20160113. 10.1098/rstb.2016.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velíšek L., Velíšková J., Ravizza T., Giorgi F. S., Moshé S. L. (2005). Circling behavior and [14C]2-deoxyglucose mapping in rats: possible implications for autistic repetitive behaviors. Neurobiol. Dis. 18 346–355. 10.1016/j.nbd.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Vértes P. E., Bullmore E. T. (2015). Annual research review: growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. J. Child Psychol. Psychiatry 56 299–320. 10.1111/jcpp.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivanti G., Rogers S. J. (2014). Autism and the mirror neuron system: insights from learning and teaching. Philos. Trans. R. Soc. B Biol. Sci. 369 20130184–20130184. 10.1098/rstb.2013.0184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. (2015). Affective and motivational control of vision. Curr. Opin. Neurol. 28 29–35. 10.1097/WCO.0000000000000159 [DOI] [PubMed] [Google Scholar]
- Waterhouse L., London E., Gillberg C. (2016). ASD Validity. Rev. J. Autism Dev. Disord. 3 302–329. 10.1007/s40489-016-0085-x [DOI] [Google Scholar]
- Weddell R. A. (2004). Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain Cogn. 55 497–506. 10.1016/j.bandc.2004.02.075 [DOI] [PubMed] [Google Scholar]
- Wei P., Liu N., Zhang Z., Liu X., Tang Y., He X., et al. (2015). Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat. Commun. 6:6756. 10.1038/ncomms7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt S., Koldewyn K., Kanwisher N. (2012). Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci. Biobehav. Rev. 36 1060–1084. 10.1016/j.neubiorev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Weiskrantz L., Barbur J. L., Sahraie A. (1995). Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1). Proc. Natl. Acad. Sci. U.S.A. 92 6122–6126. 10.1073/pnas.92.13.6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G., King J., Cunningham M., Stephan M., Kerr B., Hersh J. H. (2001). Fetal valproate syndrome and autism: additional evidence of an association. Dev. Med. Child Neurol. 43:202. 10.1017/S001216220100038X [DOI] [PubMed] [Google Scholar]
- Williams J. H. G., Whiten A., Suddendorf T., Perrett D. I. (2001). Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev. 25 287–295. 10.1016/S0149-7634(01)00014-8 [DOI] [PubMed] [Google Scholar]
- Willsey A. J., Sanders S. J., Li M., Dong S., Tebbenkamp A. T., Muhle R. A., et al. (2013). Coexpression networks implicate human midfetal deep cortical projection neurons in the pathogenesis of Autism. Cell 155 997–1007. 10.1016/j.cell.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A. B., Lintz M. J., Costabile J. D., Thompson J. A., Stubblefield E. A., Felsen G. (2015). An integrative role for the superior colliculus in selecting targets for movements. J. Neurophysiol. 114 2118–2131. 10.1152/jn.00262.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz R. H., Albano J. E. (1980). Visual-motor function of the primate superior colliculus. Annu. Rev. Neurosci. 3 189–226. 10.1146/annurev.ne.03.030180.001201 [DOI] [PubMed] [Google Scholar]
- Xuan B., Mackie M.-A., Spagna A., Wu T., Tian Y., Hof P. R., et al. (2016). The activation of interactive attentional networks. Neuroimage 129 308–319. 10.1016/j.neuroimage.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Hafed Z. M., Isa T. (2017). Informative cues facilitate saccadic localization in blindsight monkeys. Front. Syst. Neurosci. 11:5. 10.3389/fnsys.2017.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young G. S., Merin N., Rogers S. J., Ozonoff S. (2009). Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Dev. Sci. 12 798–814. 10.1111/j.1467-7687.2009.00833.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Xu J., Rowland B. A., Stein B. E. (2013). Development of cortical influences on superior colliculus multisensory neurons: effects of dark-rearing. Eur. J. Neurosci. 37 1594–1601. 10.1111/ejn.12182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Uono S., Yoshimura S., Kubota Y., Toichi M. (2017). Atypical Gaze cueing pattern in a complex environment in individuals with ASD. J. Autism Dev. Disord. 47 1978–1986. 10.1007/s10803-017-3116-2 [DOI] [PubMed] [Google Scholar]
- Zhao X., Liu M., Cang J. (2014). Visual cortex modulates the magnitude but not the selectivity of looming-evoked responses in the superior colliculus of awake mice. Neuron 84 202–213. 10.1016/j.neuron.2014.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J., Dutton G. N. (2015). “Development and neurobiological foundations of visual perception,” in Cerebral Visual Impairment in Children, eds Zihl J., Dutton G. N. (Vienna: Springer; ), 11–49. 10.1007/978-3-7091-1815-3_2 [DOI] [Google Scholar]
- Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., Szatmari P. (2005). Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 23 143–152. 10.1016/j.ijdevneu.2004.05.001 [DOI] [PubMed] [Google Scholar]