Abstract
Introduction:
To understand the influence of age and comorbidities, this study analyzed the incidence and risk factors for post-hepatectomy morbidity/mortality in patients with “borderline” (BL) operability, defined as age≥75, dependent function, lung disease, ascites/varices, myocardial infarction, stroke, steroids, weight loss>10%, and/or sepsis.
Methods:
All elective hepatectomies were identified in the 2005–13 ACS-NSQIP database. Predictors of 30-day morbidity/mortality in BL patients were analyzed.
Results:
3,574/15,920 (22.4%) patients met BL criteria. Despite non-BL and BL patients undergoing similar magnitude hepatectomies (p>0.4), BL patients had higher severe complication (SC, 23.3% vs. 15.3%) and mortality rates (3.7% vs. 1.2%, p<0.001). BL patients with any SC experienced a 14.1% mortality rate (vs. 7.3%, non-BL, p<0.001). The mortality disparity was more pronounced with ≥2 and ≥3 SC (24.6% vs. 14.1%; 34.4% vs. 23.4%, p<0.001). Independent risk factors for SC in BL patients included anesthesia score>3 (odds ratio, OR-1.29), smoking (OR-1.41), albumin<3.5g/dL (OR-1.36), bilirubin >1 (OR-2.21), operative time>240min (OR-1.58), additional colorectal procedure (OR-1.78), and concurrent procedure (OR-1.73, all p<0.05). Independent predictors of mortality included disseminated cancer (OR-0.44), albumin<3.5g/dL (OR-1.94), thrombocytopenia (OR-1.95), and extended/right hepatectomy (OR-2.81, all p<0.01).
Conclusions:
Hepatectomy patients meeting BL criteria have an overall post-hepatectomy mortality rate that is triple that of non-BL patients. With less clinical reserve, BL patients who suffer SC are at greater risk of post-hepatectomy death, reflecting their low tolerance for physiologic insults. To improve outcomes, hepatobiliary surgeons should emphasize the preoperative identification of BL operable patients in order to optimize modifiable medical risk factors and to choose appropriate magnitude operations.
Keywords: Comorbidity, Surgical Outcomes, Patient Selection
INTRODUCTION
Over the past 2–3 decades, liver surgery has become significantly safer due to improved patient selection,[1–3] optimization of preoperative risk factors (anatomic[4] and physiologic[5,6]), advanced surgical techniques,[7,8] parenchyma-sparing operations,[9–14] targeted perioperative care,[15] personalized treatment sequencing,[16–19] and modern strategies for clinical rescue after complications.[1,20–22] With these advances, surgeons are pushing the envelope and expanding the limits of both technical resectability[22] and medical operability,[2] thus offering surgery to medically borderline (BL) patients who likely would not have been offered surgery prior to the contemporary era.[23]
BL operability has been previously described by the current authors in the context of pancreatic surgery.[24] Within this context, the population of BL patients defined as a subset of patients who have modifiable comorbidities (with exception of age), which can be targeted for intervention before a hepatectomy. These surgical risk factors can be addressed with medical optimization, immunonutrition programs, and formal prehabilitation, before a hepatectomy to potentially prevent morbidity and improve the failure to rescue rate in BL patients.
This study hypothesized that BL patients are at greater risk for post-hepatectomy morbidity/mortality compared to their non-BL counter parts. To address this question, the current study analyzed the national rates of post-hepatectomy morbidity/mortality in the growing cohort of BL patients from the most recent version of the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database. Furthermore, the relationship between BL status and ability/failure to rescue these patients after major morbidity was examined. Within this context, the primary aim was to identify risk factors for severe complications and mortality in order to find potentially modifiable risk factors in the borderline patient population undergoing hepatectomy.
PATIENTS AND METHODS
Patients and data collection
From the 2005–2013 NSQIP participant use file, all hepatectomy procedures were initially extracted. Emergency operations and wedge biopsies (current procedural terminology [CPT] code 47100) were eliminated to focus on elective and substantive liver resections. The extent of hepatectomy was classified by the primary CPT code and included the following liver resections by order of increasing magnitude: partial (CPT 47120), left (47125), right (47130), and extended (47122) hepatectomies. The patients with the following preoperative conditions were excluded in order to select a population the authors felt could reasonably undergo elective surgery under non-urgent circumstances: ventilator dependence, coma, altered mental status, congestive heart failure in the past month, peripheral vascular disease with rest pain or requiring operation, dialysis or current acute renal failure, bleeding disorder, angina in past month, and dyspnea at rest. Risk factors for major morbidity/mortality were derived from analyses of NSQIP-collected perioperative clinical factors, as previously described.[1,2,25,26]
Preoperative variables assessed included age, sex, race, weight, body mass index, hematocrit, platelet count, white blood cell count, partial thrombin time, international normalized ratio, blood urea nitrogen, creatinine, albumin, aspartate aminotransferase, alkaline phosphatase, bilirubin, independent function, American Society of Anesthesiologists (ASA) class, chronic obstructive pulmonary disease (COPD), smoking, pneumonia, sepsis, disseminated cancer, diabetes, ascites, previous operation within 30 days, preoperative hospitalization, preoperative chemotherapy, and preoperative radiation therapy.
Intraoperative variables included extent of hepatectomy, operative time, concurrent major operation, and radiofrequency ablation. Concurrent major operations included gastrointestinal resection/anastomosis, biliary resection/reconstruction, thoracic operation, and ventral hernia repair. Concurrent major operations excluded cholecystectomy, lymphadenectomy, vena cava repair, diaphragm repair, and diagnostic laparoscopy.[2]
Postoperative variables included any venous thromboembolism (VTE, deep vein thrombosis and/or pulmonary embolus),[25,26] renal insufficiency/failure, respiratory failure, return to operating room (ROR), cardiac arrest, stroke, coma, myocardial infarction, postoperative sepsis/septic shock, pneumonia, surgical site infection, organ space infection (OSI), fascial dehiscence, length of stay, and 30-day mortality (or death during first hospitalization if longer than 30 days).
Definitions
“Borderline operable” patients were defined as those with any of the following preoperative conditions: age ≥75 years, lack of functional independence (as defined by NSQIP), chronic obstructive pulmonary disease, ascites/varices, myocardial infarction in last 6 months, stroke or TIA history, steroid use in last 30 days, weight loss >10% in last 6 months, and preoperative sepsis or systemic inflammatory response syndrome. These variables (with exception of age) were chosen because they have the potential to be optimized, based on previous work in the preoperative assessment of patients undergoing pancreatic surgery.[24,27] The age cutoff was based on past NSQIP studies on older cancer surgery patients.[2,28,29] Post-hepatectomy severe complications included the following NSQIP occurrences: OSI, ROR, dehiscence, re-intubation, ventilator dependence or failure to wean >48 hours, acute renal insufficiency or failure, stroke or coma, cardiac arrest or myocardial infarction, VTE, sepsis or septic shock, and pneumonia. In accordance with the NSQIP definition, postoperative mortality was defined as death within 30 days of surgery or death during first hospitalization if longer than 30 days.
Statistical analysis
The association between pre- and intra-operative risk factors (BL operability, comorbidities, and extent of resection) and morbidity/mortality were compared. Mann-Whitney U-tests were used for comparison of nonparametric continuous data. Chi-squared test or Fisher’s exact test were used for comparison of nonparametric categorical data. After univariate analysis, significant risk factors (p<0.05 and >1% of all patients) were entered into a multivariate logistic regression model to determine independent associations with morbidity/mortality. Statistical analyses were performed using SPSS Statistics 21 (IBM, Armonk, NY). All tests were two-sided. Multivariate statistical significance was defined as p<0.05.
RESULTS
Patients, age distribution, comorbidities, and extent of hepatectomy
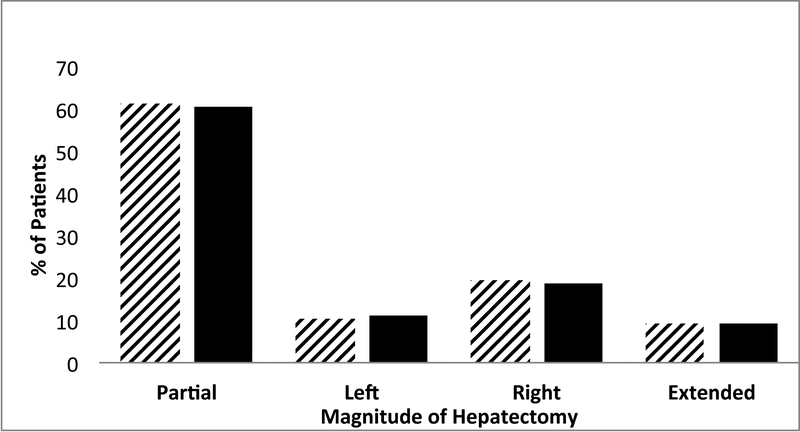
BL operable patients represented 22.5% (n=3574) of the 15,920 patients who met inclusion criteria. This population was more likely to have the following comorbidities: diabetes, medical hypertension, elevated bilirubin, elevated INR, elevated white blood cell count, dyspnea on exertion, cardiac disease, hypoalbuminemia, hematocrit < 39%, uremia, elevated creatinine, thrombocytopenia, and ASA class ≥3 (Table 1). Across all extents of hepatectomy, there was no statistical difference in the proportions of non-BL vs. BL patients undergoing resection. From 2005–2013, there was no statistical difference in the proportion of patients who met BL criteria (2010=22.5%, 2011=21.4%, 2012=22.8%, and 2013=20.8%; p=0.078).
Table 1.
Differences Between Borderline and Non-Borderline Operable Patients
| All Patients (n=15920) | Non-Borderline Operable | Borderline Operable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Characteristic | n or median | % or range | n or median | % or range | n or median | % or range | P | |||
| n | 15920 | 100% | 12346 | 77.6 % | 3574 | 22.4% | ||||
| Preoperative factors | ||||||||||
| Age | 60 | 17–90 | 57 | 18–74 | 74 | 17–90 | <0.001 | |||
| Gender, male | 7582 | 47.6% | 5677 | 46.0% | 1905 | 53.3% | <0.001 | |||
| BMI ≥30 kg/m2 | 5057 | 31.8% | 4110 | 33.3% | 947 | 26.5% | <0.001 | |||
| Diabetes | 2470 | 15.5% | 1718 | 13.9% | 752 | 21.0% | <0.001 | |||
| Dyspnea on exertion | 1095 | 6.9% | 659 | 5.3% | 436 | 12.2% | <0.001 | |||
| Previous coronary stent | 333 | 2.1% | 187 | 1.5% | 146 | 4.1% | <0.001 | |||
| Previous cardiac surgery | 289 | 1.8% | 153 | 1.2% | 136 | 3.8% | <0.001 | |||
| Medical hypertension | 7352 | 46.2% | 5165 | 41.8% | 2187 | 61.2% | <0.001 | |||
| Albumin <3.5 g/dL | 1910 | 12.0% | 1166 | 9.4% | 744 | 20.8% | <0.001 | |||
| Alkaline phosphatase >93IU/L | 6814 | 42.8% | 5128 | 41.5% | 1686 | 47.2% | <0.001 | |||
| AST ≥30 IU/L | 6039 | 37.9% | 4617 | 37.4% | 1422 | 39.8% | 0.009 | |||
| Bilirubin >1 mg/dL | 1734 | 10.9% | 1249 | 10.1% | 485 | 13.6% | <0.001 | |||
| Sodium <135 mEq/L | 1403 | 8.8% | 986 | 8.0% | 417 | 11.7% | <0.001 | |||
| White blood cells >11,000/μL | 862 | 5.4% | 564 | 4.6% | 298 | 8.3% | <0.001 | |||
| INR >1 | 5627 | 35.3% | 4050 | 32.8% | 1577 | 44.1% | <0.001 | |||
| PTT >29 sec | 5342 | 33.6% | 4078 | 33.0% | 1264 | 35.4% | 0.009 | |||
| Hematocrit<39 | 7601 | 47.7% | 5628 | 45.6% | 1973 | 55.2% | <0.001 | |||
| BUN ≥20 mg/dL | 2404 | 15.1% | 1535 | 12.4% | 869 | 24.3% | <0.001 | |||
| Creatinine >1.3mg/dL | 779 | 4.9% | 462 | 3.7% | 317 | 8.9% | <0.001 | |||
| Platelets <150,000/μL | 1968 | 12.4% | 1466 | 11.9% | 502 | 14.0% | 0.001 | |||
| Chemotherapy within 30d | 934 | 5.9% | 762 | 6.2% | 172 | 4.8% | 0.002 | |||
| ASA class ≥3 | 10888 | 68.4% | 7993 | 64.7% | 2895 | 81.0% | <0.001 | |||
| ASA class ≥4 | 692 | 4.3% | 409 | 3.3% | 283 | 7.9% | <0.001 | |||
| Admitted ≥1day before operation | 1154 | 7.2% | 705 | 5.7% | 449 | 12.6% | <0.001 | |||
| Intraoperative | ||||||||||
| Operative time, min | 222 | 7–1045 | 223 | 7–1045 | 217 | 19–1029 | 0.178 | |||
| Operative time >240 min | 6910 | 43.4% | 5405 | 43.8% | 1505 | 42.1% | 0.076 | |||
| Extent of hepatectomy | 0.441 | |||||||||
| Partial | 9728 | 61.1% | 7562 | 61.3% | 2166 | 60.6% | ||||
| Left | 1673 | 10.5% | 1272 | 10.3% | 401 | 11.2% | ||||
| Right | 3052 | 19.2% | 2378 | 19.3% | 674 | 18.9% | ||||
| Extended | 1467 | 9.2% | 1134 | 9.2% | 333 | 9.3% | ||||
| Partial vs. Left/Right/Extended | 9728 | 61.1% | 7562 | 61.3% | 2166 | 60.6% | ||||
| Right/Extended vs. Left/Partial | 0.752 | |||||||||
| Right/Extended | 4159 | 28.4% | 3512 | 28.4% | 1007 | 28.2% | ||||
| Left/Partial | 11401 | 71.6% | 8834 | 71.6% | 2567 | 71.8% | ||||
| Biliary repair/reconstruction | 772 | 4.8% | 531 | 4.3% | 241 | 6.7% | <0.001 | |||
| Another abdominal organ | 2073 | 13.0% | 1515 | 12.3% | 558 | 15.6% | <0.001 | |||
| Postoperative | ||||||||||
| Postoperative pneumonia | 448 | 2.8% | 277 | 2.2% | 171 | 4.8% | <0.001 | |||
| Reintubation | 477 | 3.0% | 278 | 2.3% | 199 | 5.6% | <0.001 | |||
| Ventilator >48hrs | 489 | 3.1% | 289 | 2.3% | 202 | 5.7% | <0.001 | |||
| Stroke | 50 | 0.3% | 30 | 0.2% | 20 | 0.6% | 0.003 | |||
| Renal insufficiency/Failure | 305 | 1.9% | 211 | 1.7% | 94 | 2.6% | <0.001 | |||
| Urinary tract infection | 516 | 3.2% | 368 | 3.0% | 148 | 4.1% | 0.001 | |||
| Cardiac arrest | 125 | 0.8% | 74 | 0.6% | 51 | 1.4% | <0.001 | |||
| Myocardial infarction | 75 | 0.5% | 36 | 0.3% | 39 | 1.1% | <0.001 | |||
| Sepsis | 805 | 5.1% | 572 | 4.6% | 233 | 6.5% | <0.001 | |||
| Septic shock | 292 | 1.8% | 168 | 1.4% | 124 | 3.5% | <0.001 | |||
| Return to OR (ROR) | 603 | 3.8% | 419 | 3.4% | 184 | 5.1% | <0.001 | |||
| Deep SSI or dehiscence | 269 | 1.7% | 184 | 1.5% | 85 | 2.4% | 0.079 | |||
| Dehiscence | 125 | 0.8% | 86 | 0.7% | 39 | 1.1% | 0.019 | |||
| VTE (DVT and/or PE) | 421 | 2.6% | 306 | 2.5% | 115 | 3.2% | 0.015 | |||
| Any severe complication | 2715 | 17.1% | 1884 | 15.3% | 831 | 23.3% | <0.001 | |||
| Postop LOS, days (IQ Range) | 6 | (4–8) | 6 | (4–7) | 6 | (5–9) | <0.001 | |||
| Death within 30 days | 280 | 1.8% | 148 | 1.2% | 132 | 3.7% | <0.001 | |||
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; OR, operating room; SSI, surgical site infection; LOS, length of stay; PVD peripheral vascular disease
Not significant: race, smoker, alcohol use, radiation or operation in preceding 30 days, chief resident involvement; simultaneous colorectal operation, additional RFA, any SSI or wound disruption, organ space infection
Not analyzed in the univariate analysis because <0.9% total cases: preoperative open wound, postoperative coma
Post-hepatectomy major morbidity
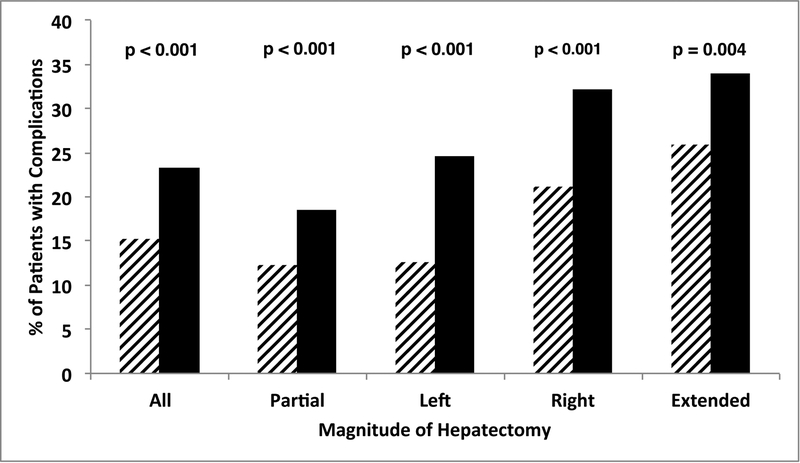
Despite undergoing hepatectomies of similar anatomic magnitude, BL patients more frequently experienced severe complications across all extents of hepatectomy (Figure 1A). Severe complication rates were strongly associated with the magnitude of hepatectomy in BL patients [33.9% (113/333) for extended, 32.2% (217/674) for right, 24.7% (99/401) for left, and 18.6% (402/2166) for partial hepatectomies, Figure 1A]. The univariate analysis of risk factors associated with severe complications among BL patients is detailed in Table 2. In multivariate analysis, risk factors independently associated with severe complications included both preoperative and intraoperative risk factors: ASA class >3 (odds ratio, OR, 1.30, 95% confidence interval, CI, 1.03–1.63, p=0.026), smoking (OR 1.41, 95% CI 1.14–1.75, p=0.002), albumin <3.5g/dL (OR 1.36, 95% CI 1.11–1.67, p=0.003), operative time >240 min (OR 1.58, 95% CI 1.33–1.89, p=0.001), concurrent colorectal procedure (OR 1.78, 95% CI 1.10–2.87, p=0.019), and major concurrent abdominal operation (OR 1.73, 1.25–2.39, p<0.001).
Figure 1.
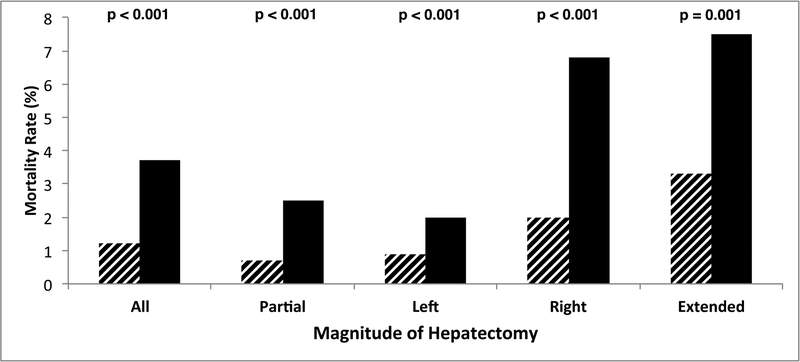
Comparison of magnitude of hepatectomy, borderline status, morbidity (A) and mortality (B) demonstrates that rates of severe complications and death increased with magnitude of hepatectomy and were significantly worse in borderline patients for each extent of hepatectomy.
Table 2.
Factors Associated with Severe Complications in Borderline Operable Patients
| All Borderline Patients | No Severe Complications | Severe Complications | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Characteristic | n or median | % or range | n or median | % or range | n or median | % or range | P | |||
| n | 3574 | 100% | 2743 | 76.7% | 831 | 23.3% | ||||
| Preoperative factors | ||||||||||
| Gender, male | 1905 | 53.3% | 1416 | 50.7% | 489 | 58.8% | <0.001 | |||
| Diabetes | 752 | 21.0% | 559 | 20.0% | 193 | 24.7% | 0.078 | |||
| Smoker | 574 | 16.1% | 409 | 14.6% | 165 | 21.1% | 0.001 | |||
| Previous cardiac surgery | 136 | 3.8% | 94 | 3.4% | 42 | 5.4% | 0.087 | |||
| Albumin <3.5 g/dL | 744 | 20.8% | 490 | 17.5% | 254 | 32.5% | <0.001 | |||
| Alkaline phosphatase >93 IU/L | 1686 | 47.2% | 1207 | 43.2% | 479 | 61.3% | <0.001 | |||
| AST ≥30 IU/L | 1422 | 39.8% | 1038 | 37.2% | 384 | 49.2% | <0.001 | |||
| Bilirubin >1 mg/dL | 485 | 13.6% | 328 | 11.7% | 157 | 20.1% | <0.001 | |||
| INR >1 | 1577 | 44.1% | 1144 | 41.0% | 433 | 55.4% | <0.001 | |||
| PTT >29 sec | 1264 | 35.4% | 906 | 32.4% | 358 | 45.8% | <0.001 | |||
| Hematocrit <39% | 1973 | 55.2% | 1455 | 52.1% | 518 | 66.3% | <0.001 | |||
| Open wound | 49 | 1.4% | 29 | 1.0% | 20 | 2.6% | 0.003 | |||
| ASA class ≥3 | 2895 | 81.0% | 2187 | 78.3% | 708 | 90.7% | <0.001 | |||
| Admitted ≥1day before operation | 449 | 12.6% | 292 | 10.5% | 157 | 20.1% | <0.001 | |||
| Intraoperative | ||||||||||
| Operative time, min | 217 | 19–1029 | 205 | 19–869 | 270 | 25–1029 | <0.001 | |||
| Operative time >240 min | 1505 | 42.1% | 1022 | 36.6% | 483 | 61.8% | <0.001 | |||
| Extent of hepatectomy | <0.001 | |||||||||
| Partial | 2166 | 60.6% | 1764 | 63.2% | 402 | 51.5% | ||||
| Left | 401 | 11.2% | 302 | 10.8% | 99 | 12.7% | ||||
| Right | 674 | 18.9% | 457 | 16.4% | 217 | 27.8% | ||||
| Extended | 333 | 9.3% | 220 | 7.9% | 113 | 14.5% | ||||
| Partial vs. Left/Right/Extended | 2166 | 60.6% | 1764 | 63.2% | 402 | 51.5% | <0.001 | |||
| Right/Extended vs. Left/Partial | <0.001 | |||||||||
| Right/Extended | 1007 | 28.2% | 677 | 24.2% | 330 | 42.3% | ||||
| Left/Partial | 2567 | 71.8% | 2066 | 74.0% | 501 | 64.1% | ||||
| Biliary repair/reconstruction | 241 | 6.7% | 119 | 4.3% | 122 | 15.6% | <0.001 | |||
| Colorectal operation | 124 | 3.5% | 69 | 2.5% | 55 | 7.0% | <0.001 | |||
| Another major abdominal operation | 558 | 15.6% | 317 | 11.3% | 241 | 29.0% | <0.001 | |||
| Postoperative | ||||||||||
| Postoperative LOS, days (IQ Range) | 6 | (5–9) | 6 | (4–9) | 11 | (7–19) | <0.001 | |||
| Death within 30 days | 132 | 3.7% | 15 | 0.5% | 117 | 15.0% | <0.001 | |||
Abbreviations: AST, aspartate aminotransferase; INR, international normalized ratio; PTT, partial thrombin time; ASA, American Society of Anesthesiologists; LOS, length of stay
Not significant: age, race, year of operation, body mass index, alcohol use, previous coronary stent/angioplasty, medical hypertension, disseminated cancer, chemotherapy within 30 days, radiation therapy within 90 days, sodium, blood urea nitrogen, creatinine, platelets, white blood cells, operation in preceding 30 days
Post-hepatectomy mortality
The overall (both BL and non-BL) post-hepatectomy mortality rate was 1.8%, which correlated with the magnitude of hepatectomy (extended, 4.2%, 62/1,467; right, 3.0%, 93/3,052; left, 1.1%, 19/1,673; and partial, 1.1%, 106/9,728; p<0.001). BL patients experienced a higher overall 30-day mortality rate (3.7%, 132/3,574 vs. 1.2%, 148/12,346 in non-BL, p<0.001, Figure 1B) and worse mortality rates at each extent of hepatectomy. The mortality rate difference was most pronounced after extended (7.5%, 25/333, vs. 3.3%, 37/1,134) and right (6.8%, 46/674, vs. 2.0%, 47/2,378) hepatectomies (p<0.001, Figure 1B). The univariate analysis of risk factors associated with mortality among BL patients is listed in Table 3. Independently associated risk factors for mortality among BL patients identified in multivariate analysis included the following preoperative and intraoperative variables (all potentially modifiable): albumin <3.5 g/dL (OR 1.94, 95% CI 1.31–2.86, p=0.001), platelets <150,000/μL (OR 1.95, 95% CI 1.25–3.42, p=0.003), bilirubin >1.0 mg/dL (OR 2.21, 95% CI 1.47–3.34,p<0.001), ASA ≥4 (OR 1.84, 95% CI 1.10–3.08, p=0.02), disseminated cancer (OR 0.44, 95% CI 0.28–0.70, p<0.001), and magnitude of anatomic resection (extended/right vs. left/partial, OR 2.81, 95% CI 1.93–4.08, p<0.001).
Table 3.
Factors Associated with Post-Hepatectomy Death in Borderline Patients
| All Borderline Patients | No Death in 30 days |
Postoperative Death (30 days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Characteristic | n or median | % or range | n or median | % | n or median | % or range | P | |||
| n | 3574 | 100% | 3442 | 96.3% | 132 | 3.7% | ||||
| Preoperative factors | ||||||||||
| Age | 74 | 17–90 | 74 | 17–90 | 76 | 29–90 | 0.830 | |||
| Previous cardiac surgery | 136 | 3.8% | 122 | 3.5% | 14 | 10.6% | <0.001 | |||
| Sodium <135 mEq/L | 417 | 11.7% | 391 | 11.4% | 26 | 19.7% | 0.003 | |||
| Albumin <3.5 g/dL | 744 | 20.8% | 687 | 20.0% | 57 | 43.2% | <0.001 | |||
| Alkaline phosphatase >93 IU/L | 1686 | 47.2% | 1601 | 46.5% | 85 | 64.4% | <0.001 | |||
| AST ≥30 IU/L | 1422 | 39.8% | 1346 | 39.1% | 76 | 57.6% | <0.001 | |||
| Bilirubin >1 mg/dL | 485 | 13.6% | 438 | 12.7% | 47 | 35.6% | <0.001 | |||
| WBC >11,000/μL | 298 | 8.3% | 283 | 8.2% | 15 | 11.4% | 0.200 | |||
| INR >1 | 1577 | 44.1% | 1501 | 43.6% | 76 | 57.6% | 0.002 | |||
| Platelets <150,000/μL | 502 | 14.0% | 473 | 13.7% | 29 | 22.0% | 0.008 | |||
| ASA class ≥3 | 2895 | 81.0% | 2779 | 80.7% | 116 | 87.9% | 0.040 | |||
| Disseminated cancer | 1297 | 36.3% | 1272 | 37.0% | 25 | 18.9% | <0.001 | |||
| Admitted ≥1day before operation | 449 | 12.6% | 417 | 12.1% | 32 | 24.2% | <0.001 | |||
| Intraoperative | ||||||||||
| Operative time, min | 217 | 19–1029 | 222 | 7–1045 | 268 | 67–891 | <0.001 | |||
| Operative time >240 min | 1505 | 42.1% | 1428 | 41.5% | 77 | 58.3% | <0.001 | |||
| Extent of hepatectomy | <0.001 | |||||||||
| Partial | 2166 | 60.6% | 2113 | 61.4% | 53 | 40.2% | ||||
| Left | 401 | 11.2% | 393 | 11.4% | 8 | 6.1% | ||||
| Right | 674 | 18.9% | 628 | 18.2% | 46 | 34.8% | ||||
| Extended | 333 | 9.3% | 308 | 8.9% | 25 | 18.9% | ||||
| Partial vs. Left/Right/Extended | 2166 | 60.6% | 2113 | 61.4% | 53 | 40.2% | <0.001 | |||
| Right/Extended vs. Left/Partial | <0.001 | |||||||||
| Right/Extended | 1007 | 28.2% | 936 | 27.2% | 71 | 53.8% | ||||
| Left/Partial | 2567 | 71.8% | 2506 | 72.8% | 61 | 46.2% | ||||
| Biliary repair/reconstruction | 241 | 6.7% | 219 | 6.4% | 22 | 16.7% | <0.001 | |||
| Another major abdominal operation | 558 | 15.6 | 527 | 15.3% | 31 | 23.5% | 0.011 | |||
| Postoperative | ||||||||||
| Any severe complication | 831 | 23.3% | 710 | 20.6% | 114 | 86.4% | <0.001 | |||
| ≥2 severe complications | 431 | 12.1% | 325 | 9.4% | 106 | 80.3% | <0.001 | |||
| ≥3 severe complications | 227 | 6.4% | 149 | 4.3% | 78 | 59.1% | <0.001 | |||
| Postoperative LOS, days (IQ Range) | 6 | (5–9) | 6 | (5–9) | 9 | (5–16.8) | <0.001 | |||
Abbreviations: AST, aspartate aminotransferase; WBC, white blood cells; INR, international normalized ratio; ASA, American Society of Anesthesiologists; LOS, length of stay
Not significant: gender, race, year of operation, body mass index, diabetes, smoker, alcohol use, dyspnea on exertion, previous coronary stent/angioplasty, medical hypertension, chemotherapy within 30 days, radiation therapy within 90 days, blood urea nitrogen, creatinine, hematocrit, partial thrombin time, chief resident involvement, operation in preceding 30 days, open wound
Failure to rescue: mortality after severe complications
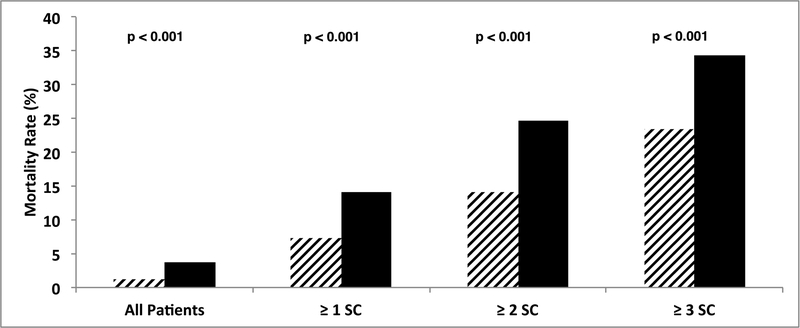
Having at least one severe complication was associated with a 14.1% (117/831) mortality rate in BL patients vs. 7.3% (137/1884) in non-BL patients (p<0.001, Figure 2). Of the 831 BL patients who suffered at least one severe complication, 51.9% (431/831) experienced a second and 27.3% (227/831) experienced a third. Those who experienced this cascade were least likely to be rescued. To emphasize the downward spiral that often led to death, the mortality rate for 1 SC= 14.1% (117/831), 2 SC=24.6% (106/431), and ≥3 SC=34.4% (78/227) in BL patients (Figure 2). Mortality rates were at least 7 absolute percentage points greater in BL vs. non-BL patients at each level of SC (e.g. 34.4% (78/227) vs. 23.4% (93/398), with ≥3 severe complications, p=0.004).
Figure 2.
Comparison of number of severe complications, mortality and borderline status demonstrates that rates of failure to rescue worsen with increasing cascade (number) of severe complications. At each extent of hepatectomy, borderline patients were more susceptible to postoperative death after severe complication(s).
DISCUSSION
This analysis of a multi-institutional sample of hepatectomy patients was able to define a cohort of patients who were considered to have BL operability, irrespective of their anatomic tumor resectability. The finding that 22.4% of patients met the BL operability definition indicates that a substantial number of patients can be identified as high-risk prior to surgery. The study also defined the magnitude of risk engendered by the BL status. The results determined that BL patients experienced significantly more SC at each magnitude of hepatectomy and triple the 30-day mortality rate of non-BL patients, indicating that an inability to rescue these patients after a severe complication was responsible for the significantly higher mortality rate.
When reviewing overall outcomes that group all types of patients and all extents of resection, the “safety” of liver surgery can be overestimated. While it is true that 30-day mortality rates have been reduced to 1% or less at major academic centers and 1.8% among the 2005–2013 NSQIP patients, this national database analysis reveals that mortality rates remain high for a right hepatectomy (3.0%) and for extended hepatectomy (4.2%). In BL patients undergoing a major hepatectomy, the mortality rates double those of the whole population at 6.8% for right hepatectomy and 7.5% for extended hepatectomy.
The finding that there was no difference in surgical magnitude between BL and healthier patients, despite the availability of the BL criteria data in the preoperative setting, suggests that surgeons are not currently using these data to modify operative planning. Given that the BL patients were older and had a greater number of baseline comorbidities, it appears that there is an opportunity to improve outcomes with better patient selection, more specific preoperative optimization, and modulation of surgical approach and extent.[2] With regard to prehabilitation, the positive impact of these programs has been well demonstrated in pancreatic cancer surgery patients receiving multi-modality therapy.[24,27] Prehabilitation programs can address advanced ASA class, smoking, and hypoalbuminemia with smoking cessation, nutritional support, and physiologic conditioning. With over 85% of elective liver resections in the NSQIP sample being done for malignancies,[2] most cancer surgeons will acknowledge that a short delay to optimize comorbidities is unlikely to affect long-term oncologic outcomes. The greater risk comes from rushing into surgery and having severe complications which hinder return to intended oncologic therapy (RIOT).[31] Either through an inability to RIOT, or other immunologic mechanisms, postoperative complications have clearly been shown to decrease survival after abdominal cancer resections.[32–34] Based on this paradigm, to the extent that the disease process allows, preoperative outpatient nutritional support and medical optimization are likely to reduce complications and prolong cancer-specific survivals.[35,36]
Severe complication and mortality rates have been correlated with the magnitude of operation and its covariates including transfusions, longer operations, combination procedures, and major anatomic resections. The data from this study suggest that longer and more complex (including combination) procedures raise the risk of severe complications and death for BL patients. For example, BL patients who underwent combination liver/colorectal operations had a 44.4% (55/124) rate of severe complications. These data offer a “reality check” in regard to the significantly increased risk of severe complications and failure to rescue faced by BL patients, particularly those undergoing extensive hepatic resections. By using creative treatment sequencing,[16,18,19,37] avoiding synchronous gastrointestinal tract resections,[17,38] limiting chemotherapy-associated liver injury,[5,39] maximizing future liver remnant,[40–42] and performing parenchyma-sparing resections,[7,8] surgeons can limit the anatomic magnitude and physiologic sequelae of hepatectomy in BL patients, potentially improving on an otherwise unacceptable risk/benefit ratio for a particular patient needing a hepatectomy.
The last point to emphasize is the alarmingly high rate of failure to rescue in BL patients. The rate of failure to rescue rose steeply with the additive burden of severe complications, with mortality rates of 14.1%, 24.6%, and 34.4%, after ≥1, ≥2, and ≥3 severe complications, respectively. When evaluating patients with increasing number of severe complications, BL patients’ rates of failure to rescue were equivalent to that of non-BL patients with one less complication (Figure 2). In other words, BL patients enter the operating room with one strike against them and, therefore, have less clinical reserve to recover from physiologic insults. Furthermore, they are more susceptible to having adverse events postoperatively. This emphasizes the significance of preoperative patient selection (and identification of who is medically BL), medical optimization, and choosing operations of lesser magnitude when oncologically appropriate. Vigilance in the postoperative course should be aimed at preventing severe complications and watching for any early signs to mitigate the complication cascade once a severe complication develops.[43]
The intent of this study is not to discourage surgeons from operating on patients who meet this study’s BL operability definition. With the population aging, BL patients will constitute an increasingly larger proportion of patients being referred for hepatectomy.[23],[44] Preventive strategies to identify and remedy the comorbid conditions that define the BL criteria are needed to address this increasing demand for complex procedures.[40]
Basing this analysis on the NSQIP database has both strengths and weaknesses.[45] Although not tailored for liver surgery, NSQIP-recorded variables were effective for this study’s extensive analysis of morbidity/mortality in BL hepatectomy patients. Due to its large scale and inclusion of the most recent available data (n=15,920 patients) this dataset facilitated a detailed analysis of morbidity/mortality predictors unavailable in single-institution studies.[46–48] While single-institution studies have more granular data, their results are biased toward well-published academic medical centers, which may not be applicable to national practice. Additionally, NSQIP data does not record occurrences after 30 days. Evidence in cancer surgery literature indicates that 90-day morbidity/mortality is more reflective of the true sequelae after oncologic resections. For example, as many as one-third of deaths from postoperative hepatic insufficiency occur after 30 days.[39] In addition, NSQIP does not record data on cancer-specific or overall survival, making an analysis of the potential overall benefit to patients from hepatectomy as an oncologic treatment impossible. Nonetheless, the NSQIP database facilitated a realistic view of nationwide trends, including the fact that BL patients comprise greater than one-fifth of hepatectomy patients.
In conclusion, BL operable patients have an overall post-hepatectomy mortality rate that is triple that of non-BL patients. With less clinical reserve, BL patients who suffer severe complications are at greater risk of post-hepatectomy death, reflecting their low tolerance for physiologic insults. To improve surgical outcomes, hepatobiliary surgeons should emphasize the preoperative identification of BL operable patients in order to optimize modifiable medical risk factors and to choose appropriate magnitude operations.
Footnotes
Publisher's Disclaimer: ACS-NSQIP Disclaimer for Participant Use File Research: The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
REFERENCES
- 1.Aloia TA, Fahy BN, Fischer CP, et al. : Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzeng CW, Cooper AB, Vauthey JN, et al. : Predictors of morbidity and mortality after hepatectomy in elderly patients: analysis of 7621 NSQIP patients. HPB (Oxford) 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RB, Aloia TA, Loyer E, et al. : Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madoff DC, Abdalla EK, Gupta S, et al. : Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol 2005;16:215–225. [DOI] [PubMed] [Google Scholar]
- 5.Kishi Y, Zorzi D, Contreras CM, et al. : Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010;17:2870–2876. [DOI] [PubMed] [Google Scholar]
- 6.Tzeng CW, Aloia TA: Colorectal liver metastases. J Gastrointest Surg 2013;17:195–201; quiz p 201–192. [DOI] [PubMed] [Google Scholar]
- 7.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN: Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg 2005;242:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palavecino M, Kishi Y, Chun YS, et al. : Two-surgeon technique of parenchymal transection contributes to reduced transfusion rate in patients undergoing major hepatectomy: analysis of 1,557 consecutive liver resections. Surgery 2010;147:40–48. [DOI] [PubMed] [Google Scholar]
- 9.Gold JS, Are C, Kornprat P, et al. : Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 2008;247:109–117. [DOI] [PubMed] [Google Scholar]
- 10.Karanjia ND, Lordan JT, Quiney N, et al. : A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur J Surg Oncol 2009;35:65–70. [DOI] [PubMed] [Google Scholar]
- 11.Redaelli CA, Wagner M, Krahenbuhl L, et al. : Liver surgery in the era of tissue-preserving resections: early and late outcome in patients with primary and secondary hepatic tumors. World J Surg 2002;26:1126–1132. [DOI] [PubMed] [Google Scholar]
- 12.Sarpel U, Bonavia AS, Grucela A, et al. : Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis? Ann Surg Oncol 2009;16:379–384. [DOI] [PubMed] [Google Scholar]
- 13.Stewart GD, O’Suilleabhain CB, Madhavan KK, et al. : The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur J Surg Oncol 2004;30:370–376. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Shimada H, Matsumoto C, et al. : Impact of the Degree of Liver Resection on Survival for Patients with Multiple Liver Metastases from Colorectal Cancer. World Journal of Surgery 2008;32:2057–2069. [DOI] [PubMed] [Google Scholar]
- 15.Farges O, Goutte N, Bendersky N, Falissard B: Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg 2012;256:697–704; discussion 704–695. [DOI] [PubMed] [Google Scholar]
- 16.Brouquet A, Abdalla EK, Kopetz S, et al. : High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouquet A, Mortenson MM, Vauthey JN, et al. : Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 2010;210:934–941. [DOI] [PubMed] [Google Scholar]
- 18.Mentha G, Majno PE, Andres A, et al. : Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 2006;93:872–878. [DOI] [PubMed] [Google Scholar]
- 19.Mentha G, Roth AD, Terraz S, et al. : ‘Liver first’ approach in the treatment of colorectal cancer with synchronous liver metastases. Dig Surg 2008;25:430–435. [DOI] [PubMed] [Google Scholar]
- 20.Andres A, Toso C, Moldovan B, et al. : Complications of elective liver resections in a center with low mortality: a simple score to predict morbidity. Arch Surg 2011;146:1246–1252. [DOI] [PubMed] [Google Scholar]
- 21.Jarnagin WR, Gonen M, Fong Y, et al. : Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397–406; discussion 406–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmitti G, Roses RE, Andreou A, et al. : Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg 2013;17:57–64; discussion p 64–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dokmak S, Fteriche FS, Borscheid R, et al. : 2012 Liver resections in the 21st century: we are far from zero mortality. HPB (Oxford) 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzeng CW, Katz MH, Fleming JB, et al. : Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg 2014;18:146–155; discussion 155–146. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng CW, Curley SA, Vauthey JN, Aloia TA: Distinct predictors of pre- versus post-discharge venous thromboembolism after hepatectomy: analysis of 7621 NSQIP patients. HPB (Oxford) 2013;15:773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzeng CW, Katz MH, Fleming JB, et al. : Risk of venous thromboembolism outweighs post-hepatectomy bleeding complications: analysis of 5651 National Surgical Quality Improvement Program patients. HPB (Oxford) 2012;14:506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzeng CW, Fleming JB, Lee JE, et al. : Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Ann Surg Oncol 2012;19:2045–2053. [DOI] [PubMed] [Google Scholar]
- 28.Al-Refaie WB, Parsons HM, Henderson WG, et al. : Major cancer surgery in the elderly: results from the American College of Surgeons National Surgical Quality Improvement Program. Ann Surg 2010;251:311–318. [DOI] [PubMed] [Google Scholar]
- 29.Kurian AA, Wang L, Grunkemeier G, et al. : Defining “the elderly” undergoing major gastrointestinal resections: receiver operating characteristic analysis of a large ACS-NSQIP cohort. Ann Surg 2013;258:483–489. [DOI] [PubMed] [Google Scholar]
- 30.Dindo D, Demartines N, Clavien PA: Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzeng CW, Vauthey JN: Postoperative complications and oncologic outcomes after resection of colorectal liver metastases: the importance of staying on track. Ann Surg Oncol 2013;20:2457–2459. [DOI] [PubMed] [Google Scholar]
- 32.Khuri SF, Henderson WG, DePalma RG, et al. : Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005;242:326–341; discussion 341–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scaife CL, Hartz A, Pappas L, et al. : Association between postoperative complications and clinical cancer outcomes. Ann Surg Oncol 2013;20:4063–4066. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda A, Matsumoto S, Seya T, et al. : Does Postoperative Complication Have a Negative Impact on Long-Term Outcomes Following Hepatic Resection for Colorectal Liver Metastasis?: A Meta-Analysis. Ann Surg Oncol 2013. [DOI] [PubMed] [Google Scholar]
- 35.Braga M, Gianotti L, Vignali A, Carlo VD: Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002;132:805–814. [DOI] [PubMed] [Google Scholar]
- 36.Braga M, Gianotti L, Vignali A, et al. : Hospital resources consumed for surgical morbidity: effects of preoperative arginine and omega-3 fatty acid supplementation on costs. Nutrition 2005;21:1078–1086. [DOI] [PubMed] [Google Scholar]
- 37.Tzeng CW, Aloia TA, Vauthey JN, et al. : Morbidity of Staged Proctectomy After Hepatectomy for Colorectal Cancer: A Matched Case-Control Analysis. Ann Surg Oncol 2012. [DOI] [PubMed] [Google Scholar]
- 38.Reddy SK, Pawlik TM, Zorzi D, et al. : Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481–3491. [DOI] [PubMed] [Google Scholar]
- 39.Vauthey JN, Pawlik TM, Ribero D, et al. : Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065–2072. [DOI] [PubMed] [Google Scholar]
- 40.Shindoh J, Truty MJ, Aloia TA, et al. : Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg 2013;216:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shindoh J, Tzeng CW, Aloia TA, et al. : Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg 2014;18:45–51. [DOI] [PubMed] [Google Scholar]
- 42.Shindoh J, Tzeng CW, Aloia TA, et al. : Optimal future liver remnant in patients treated with extensive preoperative chemotherapy for colorectal liver metastases. Ann Surg Oncol 2013;20:2493–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sukumar S, Roghmann F, Trinh VQ, et al. : National trends in hospital-acquired preventable adverse events after major cancer surgery in the USA. BMJ open 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegel R, Ma J, Zou Z, Jemal A: Cancer statistics, 2014. CA Cancer J Clin 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 45.Pitt HA, Kilbane M, Strasberg SM, et al. : ACS-NSQIP has the potential to create an HPB-NSQIP option. HPB (Oxford) 2009;11:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagano Y, Nojiri K, Matsuo K, et al. : The impact of advanced age on hepatic resection of colorectal liver metastases. J Am Coll Surg 2005;201:511–516. [DOI] [PubMed] [Google Scholar]
- 47.Fong Y, Blumgart LH, Fortner JG, Brennan MF: Pancreatic or liver resection for malignancy is safe and effective for the elderly. Ann Surg 1995;222:426–434; discussion 434–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsujita E, Utsunomiya T, Yamashita Y, et al. : Outcome of hepatectomy in hepatocellular carcinoma patients aged 80 years and older. Hepatogastroenterology 2012;59:1553–1555. [DOI] [PubMed] [Google Scholar]