Abstract
Background and Purpose:
Focal cerebral arteriopathy (FCA), a common cause of arterial ischemic stroke (AIS) in previously healthy children, often progresses over days to weeks, increasing risk of recurrent stroke. We developed a novel severity scoring system designed to quantify FCA progression and correlate with clinical outcomes.
Methods:
The Vascular effects of Infection in Pediatric Stroke (VIPS) study prospectively enrolled 355 children with AIS (2010–2014), including 41 with centrally confirmed FCA. Two neuroradiologists independently reviewed FCA cerebrovascular imaging, assigning a graded severity score of zero (no involvement) to four (occlusion) to individual arterial segments. The FCA severity score (FCASS) was the unweighted sum. In an iterative process, we modeled scores derived from different combinations of arterial segments to identify the model that optimized correlation with clinical outcome, simplicity and reliability.
Results:
The optimal FCASS summed scores from five arterial segments: supraclinoid internal carotid artery, A1, A2, M1, and M2. The median (interquartile range [IQR]) baseline FCASS was 4 (2, 6). Of 33 children with follow-up imaging, the maximum FCASS (at any time point) was 7 (5, 9). Twenty-four (73%) had FCA progression on follow-up with their maximum FCASS at a median of 8 (5, 35.5) days post-stroke; their median FCASS increase was 4 (2.5, 6). FCASS did not correlate with recurrent AIS. Maximum (but not baseline) FCASS correlated with 1-year Pediatric Stroke Outcome Measures (p=0.037).
Conclusions:
Our novel scoring system for FCA severity correlates with neurologic outcomes in the VIPS cohort, and provides a tool for FCA treatment trials under development.
Keywords: ischemic stroke, children, pediatric
Introduction:
Focal cerebral arteriopathy of childhood (FCA), also known as transient cerebral arteriopathy (TCA), is an acute, monophasic disease causing unilateral stenosis of the intracranial cerebral arteries, mainly involving the anterior circulation. One of the most common causes of arterial ischemic stroke (AIS) in a previously healthy child, FCA has also been described in young adults.1–4 It confers a high risk of recurrent stroke (up to 25% within 1 year), compounding post-stroke lifelong disability.5, 6 Most cases are presumed to be inflammatory; vessel wall imaging (VWI) demonstrates enhancement of affected arterial segments.7 In its early phase, FCA can rapidly progress over days to weeks. Children with progressive arteriopathies have a higher risk of recurrent ischemia,6, 8, 9 and a single study suggests a link between elevated inflammatory biomarkers (hsCRP and serum amyloid A), arteriopathy progression, and stroke recurrence.8 Varicella zoster virus (VZV) is a long-established cause of FCA,10–12 yet other pathogens, including other herpes viruses, likely play a role as FCA continues to occur in VZV-vaccinated children.13
Corticosteroids are increasingly used to treat FCA, although in the absence of clinical trial data.14, 15 Equipoise remains: steroids might suppress the presumed focal inflammatory process, but might also worsen the underlying infectious pathogenesis. A Delphi consensus identified this issue as the highest priority for a clinical trial in the field of childhood stroke.16 Investigators currently developing FCA treatment trial protocols have realized the need for a quantitative measure of FCA severity to serve as a clinical trial surrogate endpoint and quantify temporal evolution of the arteriopathy. Using the subcohort of children with FCA enrolled in the Vascular effects of Infection in Pediatric Stroke (VIPS) study, our goals were (1) to develop an FCA Severity Score (FCASS) that correlates with clinical outcomes, and (2) to apply the score to describe the natural history of FCA. We also performed exploratory analyses to determine whether the final FCASS correlated with previously reported VIPS study biomarkers of inflammation and infection.8, 17
Methods:
In accordance with the NIH-approved data sharing policy for the VIPS study, the data supporting this study are available from the corresponding author upon request. The VIPS study was a prospective cohort study that enrolled 355 children (29 days to 18 years of age) with AIS at 37 international centers and collected clinical and imaging data and serum samples.2, 4 Ethics approval was obtained by the institutional review board at each center, and written informed consent was obtained for each enrollment. We centrally reviewed clinical data and all clinically obtained cerebral and vascular imaging to confirm the AIS diagnosis, estimate the infarct volume (using ABC/2), and classify cervical and cerebral arteriopathies.2, 4 A board-certified neuroradiologist (M.W.) delineated the infarct contour and the brain contour on FLAIR images and, taking into account FLAIR slice thickness, calculated the absolute infarct volume and brain volume. We then calculated the relative infarct volume (percent of total brain volume) by dividing the absolute volume of the infarct by the absolute volume of the brain. As previously reported, we defined FCA as “unifocal and unilateral stenosis/irregularity of the large intracranial arteries of the anterior circulation (distal internal carotid artery [ICA] and/or its proximal branches),” and then sub-classified as FCA-inflammation type (FCA-i), FCA-dissection type (FCA-d), and FCA-undetermined (FCA-u).4
Development of FCASS:
For the current study, we designed an initial FCASS based on the vascular imaging appearance of nine unilateral arterial segments: cervical, petrous, cavernous, and supraclinoid segments of the ICA; M1 and M2 segments of the middle cerebral artery; A1 and A2 segments of the anterior cerebral artery; and the posterior cerebral artery (PCA; either P1 or P2). Because FCA is a unilateral disease, we measured only the involved (symptomatic) side of the intracranial circulation. Two neuroradiologists (M.W., N.S.) independently re-examined the vascular imaging for all 41 FCA cases in VIPS and applied the initial scoring system. Each segment was assigned a graded severity score: 0=no involvement; 1=irregularity or banding with no stenosis; 2=stenosis, <50% reduction in diameter; 3=stenosis, >50% reduction in diameter; 4=occlusion. The small size of M2 and A2 branches precluded reliable scoring of stenosis severity, so any stenosis of those segments was initially scored as “3” (with no option to score as “2”); hence, these segments were classified into one of only four categories (scored 0, 1, 3, or 4). The scores for the individual arterial segments were summed without weighting to provide an initial total score. These methods would not capture changes over time that were within scoring categories (e.g., for progression from 60% to 90% stenosis; both would be coded as “3”). Hence, we devised the “delta point”: a single point that could be added/subtracted to indicate any interval worsening (+1) or improvement (−1) not otherwise captured by the sum total of the scored segments. (Only a single delta point could be applied, regardless of whether a single segment or multiple segments changed.) We defined the “maximum FCASS” for each case as the highest total score at any time point. If only a single FCASS score was available (n=8), that score was also considered to be the maximum, unless otherwise indicated. One neuro-radiologist (M.W.) scored all imaging twice in two separate sessions.
Optimization of the FCASS:
We then optimized the scoring system with the a priori goals of maximizing correlation with clinical outcomes while maintaining simplicity (“user-friendliness”) and achieving a high degree of intra-rater and inter-rater reliability. Clinical outcomes included 1-year cumulative stroke recurrence rates, as previously defined,5 and 1-year Pediatric Stroke Outcome Measures (PSOM). The PSOM is a validated outcome measure for childhood AIS which quantifies neurological exam findings and can be estimated via a telephone parental questionnaire; scores range from 0 (no deficits) to 10 (profound deficits).18, 19 The VIPS study performed 1-year PSOMs (12 months +/− 3 months post-stroke) in clinic for the majority, and via telephone when in-clinic assessments were not feasible (Jordan LC, et al., Neurology, in press, 2018).
In an iterative process, we created different FCASS models by serially removing arterial segments (Online Supplemental Methods). We assessed each individual model, measuring the correlation of the FCASS (baseline and maximum scores) with clinical outcomes and determining intra- and inter-rater reliability. . We carried forward the optimal FCASS model to the final refinement step: determining whether it was possible to further collapse the numeric scoring system (0 to 4, for each arterial segment) into fewer categories. Instructions for the final (optimal) FCASS system are in the Online Supplemental Methods. We then analyzed the final FCASS in our cohort to describe its correlation with serum biomarkers and, in those with follow-up imaging, its evolution over time.
Data Analysis:
We computed intraclass correlation coefficients (ICC) as estimates of intra-rater and inter-rater reliability for the total FCASS and individual components using standard regression methods. We utilized Spearman’s rank-order correlation to assess for correlation between FCASS and relative infarct volume. In analyses of correlation with clinical outcomes, our predictor variables included both baseline and maximum FCASS, both including and excluding those without follow-up data. Using methods previously described for the VIPS cohort, we used Cox proportional hazards models to measure risk of stroke recurrence in children with FCA,5 and Spearman correlation coefficients to measure potential association with 1-year PSOM scores.20 Because our specific purpose was to compare multiple models in order to find the one that best correlated with our chosen outcomes (rather than testing a particular hypothesis), we made no multiple comparison adjustments.
In exploratory analyses, we calculated Spearman correlation coefficients between FCASS (baseline and maximum) and the inflammatory and infectious biomarkers previously measured and reported in the VIPS cohort. Inflammatory markers included serum concentrations of high sensitivity C-reactive protein (hsCRP), serum amyloid A (SAA), myeloperoxidase (MPO), and tumor necrosis factor alpha (TNF-α).8 Infectious biomarkers included serum IgM/IgG antibodies for herpes simplex virus (HSV) 1 and 2, cytomegalovirus (CMV), Epstein Barr virus (EBV), and VZV.17 We employed our previously published algorithm for using acute and convalescent serologies to classify “serologic evidence of acute herpesvirus infection” as a dichotomous variable;17 we used Wilcoxon rank sum tests to compare FCASS scores in those with versus without infection. All analyses were done using Stata v14 (Stata Corp., College Station, TX) with alpha set at 0.05.
Results:
The 41 FCA cases in the VIPS cohort (25 FCA-i, 7 FCA-d, and 9 FCA-u) had a median age of 11.3 years (IQR 7.4, 14.3 years; range 5 months, 17.7 years) and were 46% male. The overall VIPS cohort had a median age of 7.2 (IQR 2.8, 14.3) years and was 56% male. FCA cases were racially diverse: 27 (66%) white, 4 (10%) Asian, 3 (7%) Hispanic, 2 (5%) black, 5 (12%) other/unknown. Baseline vascular imaging was performed on the same day as the stroke in 41% of cases: median of 1 day (IQR 0, 2 days; range 0, 14 days) after the stroke ictus. MRA was the most common imaging modality, performed in 38 cases (93%) at baseline and 40 cases (98%) overall (including follow-up imaging); conventional angiography was performed in 14 (34%) and CTA in 15 (37%). At baseline, the median absolute infarct volume was 14.7 ml (IQR 6.5, 33.0 mL; range 0.08, 251 mL); the median relative infarct volume was 1.1 % (IQR 0.5, 2.3%; range 0.005, 15.2 %).
Development of the FCASS:
Because we identified no PCA abnormalities, we excluded this segment from the model. Only 34 cases (83%) had cervical vascular imaging (at any time point), and 25 (73%) of these had no cervical arterial involvement; we therefore also excluded the cervical ICA from the model. Model A included all the remaining segments (petrous, cavernous, and supraclinoid segments of the ICA; M1, M2, A1 and A2), which were then removed in an iterative fashion (Models B-E). We modeled both baseline and maximum FCASS; for this analysis, the baseline score of those with no follow-up imaging was also defined as the maximum score. Inter-rater and intra-rater reliability was high for all models (Online Table I). None of the models correlated with recurrent stroke (Table 1). In all models, the maximum FCASS score was more closely associated with 1-year PSOMs than the baseline score; Model B (supraclinoid ICA, M1, M2, A1, and A2 segments) for the maximum FCASS score was the only model that demonstrated a significant correlation with 1-year PSOMs (Table 1). Model B was therefore carried forward to the final refinement step of maximizing simplicity. We created additional models collapsing the numeric scoring system (0 to 4) into fewer categories: Model B1 combining scores 1 through 4; Model B2 combining scores 1&2 and scores 3&4; Model B3 combining scores 1 through 3. Each of these models demonstrated a weaker correlation with 1-year PSOMs (On-line Table II). Hence, we designated Model B with the initial numeric scoring system (0 to 4, for each arterial segment) as the final (optimal) FCASS (On-line Supplemental Methods). We used this model for all subsequent analyses.
Table 1.
Clinical outcome correlation of iterative models of the FCA Severity Score (FCASS). Data for maximum FCASS are shown. Model A includes: petrous, cavernous, and supraclinoid segments of the ICA; M1 and M2 segments of the middle cerebral artery (MCA); A1 and A2 segments of the anterior cerebral artery (ACA). Subsequent models exclude arterial segments in an iterative fashion. Final (optimal) model shown in bold.
| Model | Arterial segments excludeda | All FCA subjects (n=41) |
Subjects with follow-up (n=33) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year PSOMb |
Recurrent stroke |
1-year PSOMb |
Recurrent stroke |
||||||
| HR | 95% CI | p-valuec | HR | 95% CI | p-valuec | ||||
| A | None excluded | 0.13 | 1.1 | (0.97, 1.14) | 0.19 | 0.19 | 1.05 | (0.97, 1.13) | 0.24 |
| B | Proximal (petrous/cavernous) ICA | 0.037 | 1.1 | (0.91, 1.23) | 0.47 | 0.037 | 1.05 | (0.90, 1.24) | 0.53 |
| C | Proximal ICA, A1 | 0.089 | 1.10 | (0.92, 1.32) | 0.30 | 0.12 | 1.10 | (0.91, 1.34) | 0.32 |
| D | Proximal ICA, A1, A2 | 0.087 | 1.1 | (0.92, 1.35) | 0.26 | 0.14 | 1.10 | (0.91, 1.34) | 0.34 |
| E | Proximal ICA, A1, A2, M2 | 0.11 | 1.2 | (0.89, 1.59) | 0.25 | 0.21 | 1.18 | (0.86, 1.61) | 0.31 |
PCA was normal in all cases and not included in any model
P-values for trend test for correlation between maximum FCASS and 1-year PSOM (pediatric stroke outcome measure; categorical variable)
Cox proportional hazard for correlation between maximum FCASS and time to first recurrent AIS
Change in FCASS over Time:
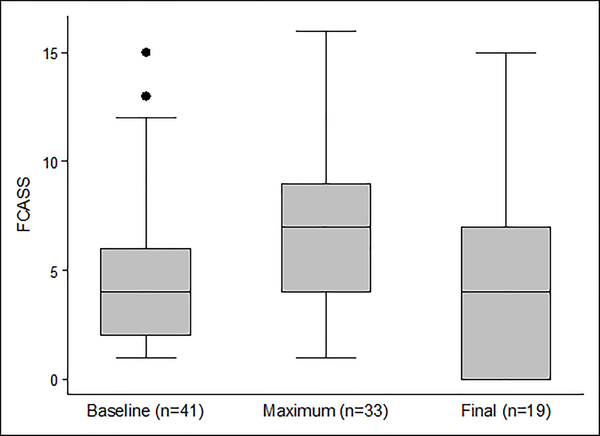
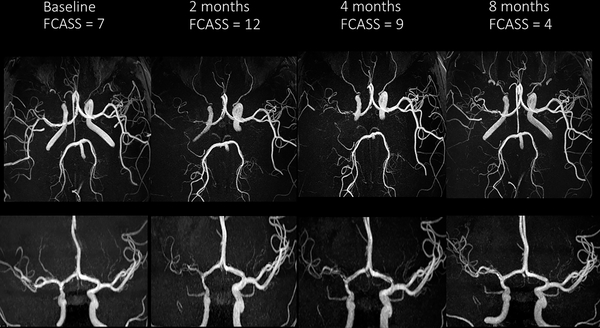
The baseline FCASS was a median of 4 (IQR 2, 6; range 1, 15; Figure 1). Of the 41 cases, 33 (80%) had at least one follow-up vascular imaging study, and 28 (68%) had more than one. Those with versus without follow-up vascular imaging had similar absolute and relative infarct volumes, baseline FCASS scores, and 1-year PSOMs (Online Supplemental Table III). The median time from stroke to first follow-up imaging was 10 days (IQR 5, 52; range 1, 405), and from stroke to last follow-up was 112 days (IQR 4, 349; range 0, 674; days). The change in FCASS is summarized in Figure 1; representative imaging is shown in Figure 2. Our imaging reviewers did not apply the “delta point” for any cases in this cohort. The maximum FCASS among all 41 cases, including those with a single assessment, was a median of 7 (IQR 4, 9; range 1, 16); the maximum FCASS among those 33 cases with follow-up vascular imaging was similar (median 7; IQR 5, 9; range 2, 16). Among those 33 with multiple FCASS measurements, the score increased (indicating worsening arteriopathy) in 24 (73%), remained unchanged in 8 (24%), and decreased (without an initial increase) in 1 (3%). Among the 24 with increased scores, the median change was +4 (IQR 2.5, 6; range 1, 9), and the median time from stroke to the imaging with the maximum FCASS was 8 days (IQR 5, 35.5; range 2, 189). The greatest change observed was in a case of FCA-d: baseline score of 7 and a maximum score of 16 two days later. Of 19 patients with their last follow-up imaging six months or longer after the stroke, the last FCASS was a median of 4 (IQR 0, 7; range 0, 15); 26% of patients had complete normalization of their vascular imaging (final FCASS of 0) (Figure 1).
Figure 1.
Box-and-whisker plots summarizing baseline, maximum, and final FCASS scores. Maximum FCASS includes only those with follow-up imaging (n=33). Final FCASS includes only those 19 cases with follow-up imaging ≥6 months post-stroke. [Box represents interquartile range (IQR); line within box represents median; whiskers represent upper adjacent (75th percentile + 1.5*IQR) and lower adjacent (25th percentile – 1.5*IQR) values; dots represent outliers.]
Figure 2.
Cerebral MRA images demonstrating evolution of a typical case of FCAi: axial views (upper row) and frontal views (lower row) at four time points. Baseline images demonstrate mild irregularity of the right supraclinoid ICA and M1 segment of the middle cerebral artery. The 2-month images show progression to severe stenosis; the 4- and 8-month images demonstrate subsequent improvement.
Correlation with Infarct Volume and Neurological Outcomes:
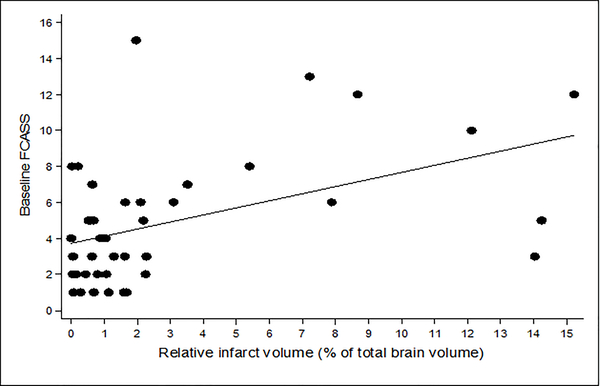
Larger relative infarct volumes on baseline imaging correlated with higher FCASS scores: Spearman’s rho 0.41 (p=0.008) for baseline FCASS (Figure 3); and 0.50 (p=0.0008) for maximum FCASS. Children with (N=8) versus without (N=33) recurrent AIS during the study period had similar FCASS scores (Table 2). The median 1-year PSOM in our cohort (N=39 with 1-year outcomes) was 1 (IQR 0, 1.5; range 0, 4.5). Higher 1-year PSOMs (indicating greater neurological deficits) correlated with higher maximum FCASS scores (p=0.037, Table 2).
Figure 3.
Scatter plot demonstrating the correlation between baseline FCASS and relative infarct volume (% of total brain volume), also measured at baseline.
Table 2.
Natural history of FCASS over time stratified by FCA subtype, recurrence, and 1-year neurological outcomes (PSOM).
| Baseline |
Maximum* |
Final** |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median | (Range) | n | Median | (Range) | n | Median | (Range) | |
| Overall | 41 | 4 | (1, 15) | 33 | 7 | (2, 16) | 19 | 4 | (0, 15) |
| Subtype‡ | |||||||||
| FCAi | 25 | 3 | (1, 8) | 22 | 6 | (2, 13) | 12 | 3.5 | (0, 7) |
| FCAd | 7 | 10 | (5, 13) | 5 | 14 | (5, 16) | 3 | 5 | (0, 9) |
| FCAu | 9 | 3 | (1, 15) | 6 | 8 | (2, 15) | 4 | 3.5 | (0, 15) |
| Recurrent stroke | |||||||||
| Yes | 8 | 4.5 | (1, 8) | 8 | 7 | (3, 16) | 4 | 4.5 | (0, 9) |
| No | 33 | 4 | (1, 15) | 25 | 7 | (2, 16) | 15 | 4 | (0, 15) |
| 1-year PSOM† | |||||||||
| 0–1 (no/mild deficits) | 25 | 4 | (1, 15) | 21 | 6 | (2, 15) | 13 | 3 | (0, 15) |
| 1.5–3 (moderate) | 12 | 5 | (1, 13) | 9 | 9 | (3, 16) | 5 | 7 | (1, 9) |
| 3.5–6 (severe) | 2 | 7 | (2, 12) | 2 | 12 | (8, 16) | 1 | 5 | (5, 5) |
| 6.5–10 (profound) | 0 | - | (-) | 0 | - | (-) | 0 | - | (-) |
Time from index stroke to imaging with maximum FCASS: median 5 days (range 0, 189 days; IQR 2, 15); includes all of those with follow-up (n=33), with or without progression
The last FCASS score for those cases with follow-up imaging ≥6 months post-stroke.
PSOM=Pediatric Stroke Outcome Measure; missing for 2 cases; trend test for correlation, p=0.68 for baseline, 0.037 for maximum, and 0.16 for final FCASS score
FCAi=inflammation type; d=dissection type; u=undetermined
Differences between FCAi and FCAd are significant for baseline (p=0.001) and maximum (p=0.0007) but not for final FCASS (p=0.11)
Correlation with Inflammatory Biomarkers and Herpesvirus Exposure:
Acute serum samples were collected for 38 children at a median 5 days post-stroke (IQR 3, 10 days); 25 had convalescent serum samples collected at median 20 days post-stroke (IQR 14, 32) days). Baseline and maximum FCASS scores (utilizing all subjects, including those without follow-up imaging) correlated with acute serum levels of hsCRP, but not other levels of inflammatory biomarkers (Table 3). The median (IQR) hsCRP was 0.86 (0.48, 1.56) for the quartile with the highest maximum FCASS scores, compared to 0.16 (0.16, 0.25) for the lowest quartile. FCASS did not differ significantly by serological evidence of acute herpesvirus infection. Of 25 FCA cases with paired (acute and convalescent) serologies, 10 (40%) had evidence of an acute herpesvirus infection: eight positive for HSV (1 or 2) alone, one positive for VZV, and one positive for HSV and VZV. The median (IQR) baseline FCASS was 5 (1, 6) for the 10 herpes-positive cases and 3 (2, 7) for the 15 herpes-negative cases (p=0.98); maximum FCASS was 8 (6, 12) for herpes-positive and 6 (2, 8) for herpes-negative cases (p=0.13).
Table 3.
Correlation of FCA Severity Score (FCASS) with acute serum levels of inflammatory markers measured in the VIPS study*
| Baseline FCASS | Maximum FCASS | |||
|---|---|---|---|---|
| n=36 |
n=36** |
|||
| Serum Inflammatory Marker | Spearman’s rho | p-value | Spearman’s rho | p-value |
| hsCRP | 0.35 | 0.036 | 0.47 | 0.004 |
| MPO | 0.30 | 0.08 | 0.28 | 0.10 |
| SAA | 0.20 | 0.24 | 0.27 | 0.11 |
| TNF-α | −0.05 | 0.75 | −0.16 | 0.34 |
hsCRP=human C-reactive protein; SAA=serum amyloid A; MPO=myeloperoxidase; TNF-α=tumor necrosis factor alpha
Inflammatory markers were not available for 5 of the 41 FCA patients.
For those with no follow-up imaging, the baseline score was considered to be the maximum score.
Discussion:
By developing a novel tool for quantifying the severity, extent, and evolution of arterial disease in children with FCA—the FCASS—we demonstrated a wide range of disease severity within our FCA cohort, and found dynamic changes over time with doubling of the score over a matter of days in the most severely progressive cases. Children with higher maximum FCASS scores had significantly larger infarct volumes and worse neurological outcomes at 1-year. FCASS severity also correlated with one acute serum inflammatory biomarker, hsCRP, providing some additional evidence that FCA is an inflammatory disease. We could not confirm an association with evidence of acute herpesvirus infection, however.
Two published studies describe severity scores for childhood arteriopathies, although neither was specific to FCA.9, 21 Both examined the correlation with recurrent stroke. The “Cerebrovascular Stenosis Score (CVSS)” used a similar quantitative scoring system (“1 for low-grade stenosis, 2 for high-grade stenosis, or 3 for occlusion”), scoring 18 bilateral arterial segments and summing the individual scores.21 When applied to a cohort of 49 children with AIS and a variety of arteriopathies, the CVSS demonstrated good inter-rater reliability (ICC 0.77; 95% CI 0.63, 0.87), although lower than that for FCASS (0.86; 0.81, 0.90); CVSS intra-rater reliability was not assessed. Higher CVSS predicted stroke recurrence amongst children with moyamoya, but not other forms of arteriopathy. A British study of 43 children with AIS and abnormal MRA categorized the MRA abnormalities into three grades without scoring individual arterial segments; the authors did not assess intra- or inter-rater reliability.9 Although limited by variable timing of follow-up vascular imaging, they found that children with progressive arteriopathies (of any subtype) had triple the risk of recurrent ischemic events (stroke, transient ischemic attack, or silent infarcts) compared to those without evidence of arteriopathy progression.
In our overall VIPS cohort, including all types of cervical and cerebral arteriopathy (e.g., FCA, moyamoya, cervical dissection, genetic arteriopathies), we similarly found that arteriopathy progression predicted recurrent AIS.8 In the FCA subcohort, however, FCASS scores did not predict recurrence, although higher maximum FCASS scores correlated with both larger infarct size and poorer 1-year neurological outcomes. We speculate that this finding may reflect the nature of progressive brain injury in children with accelerating FCA. Because FCA is a focal disease, its progression may result in expansion of the core infarct in the same ICA distribution and a slow worsening of the original deficits (e.g., worsened hemiparesis) that may not be distinct enough from the index stroke to meet criteria for a recurrent stroke. However, we did not have adequate follow-up brain imaging to measure infarct expansion and test this hypothesis.
Limitations include our small sample size; we may have been underpowered to detect an association between FCASS and stroke recurrence, for example. Our cohort had only clinically-obtained imaging, hence combining a mix of imaging modalities and variable performance and timing of follow-up imaging. The FCASS was developed primarily using MRA, which can over-estimate arterial stenosis relative to conventional angiography and CTA; we were unable to study how different modalities may yield different results. The clinical decision to perform follow-up imaging at a particular time may itself reflect greater severity of the disease and/or worse clinical outcomes. (We may have been underpowered to detect a difference between those with and without follow-up imaging.) We may have missed periods of arteriopathy progression, leading to misclassification of cases with versus without progression. Future studies would be improved by imaging collection at systematic time points among all patients. We developed the tool in a single patient cohort; however, a validation study is now underway, testing FCASS in an independent FCA cohort from the Swiss Neuropaediatric Stroke Study.22
Despite these limitations, the FCASS demonstrated high intra- and inter-rater reliability and correlated with neurological outcomes in the VIPS cohort. After validation in an independent cohort, we anticipate it could serve as a clinically meaningful measurement and endpoint for FCA treatment trials. Our natural history data provide further evidence that FCA is a dynamic arteriopathy. Frequent progression in the first days to weeks suggests a window for therapeutic intervention to improve neurological outcomes. The recent Swiss/Australian observational study of FCA found that children who received corticosteroid therapy had better 6-month PSOMs than those who did not;14 this compelling finding should now be tested in clinical trials.
Supplementary Material
ACKNOWLEDGMENTS:
The authors wish to acknowledge the important contributions of the research coordinators at VIPS sites, and of the patients and their families.
FUNDING: NIH R01 NS062820 (PIs Fullerton, DeVeber); Marc and Lynne Benioff for statistical support; AHA Bugher Award (Bernard).
Footnotes
DISCLOSURES: The authors have no commercial interests related to this project. VIPS investigators (Online Appendix) received NIH funding for this project.
REFERENCES:
- 1.Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sebire G. Transient cerebral arteriopathy: A disorder recognized by serial angiograms in children with stroke. J Child Neurol. 1998;13:27–32 [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Hills NK, deVeber GA, Barkovich AJ, Elkind MS, Sear K, et al. Arteriopathy diagnosis in childhood arterial ischemic stroke: Results of the Vascular effects of Infection in Pediatric Stroke Study. Stroke. 2014;45:3597–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bulder MM, Braun KP, Leeuwis JW, Lo RT, van Nieuwenhuizen O, Kappelle LJ, et al. The course of unilateral intracranial arteriopathy in young adults with arterial ischemic stroke. Stroke. 2012;43:1890–1896 [DOI] [PubMed] [Google Scholar]
- 4.Wintermark M, Hills NK, DeVeber GA, Barkovich AJ, Bernard TJ, Friedman NR, et al. Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: Results of the VIPS Study. AJNR Am J Neuroradiol. 2017;38:2172–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fullerton HJ, Wintermark M, Hills NK, Dowling MM, Tan M, Rafay MF, et al. Risk of recurrent arterial ischemic stroke in childhood: A prospective international study. Stroke. 2016;47:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun KP, Bulder MM, Chabrier S, Kirkham FJ, Uiterwaal CS, Tardieu M, et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain. 2009;132:544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stence NV, Pabst LL, Hollatz AL, Mirsky DM, Herson PS, Poisson S, et al. Predicting progression of intracranial arteriopathies in childhood stroke with vessel wall imaging. Stroke. 2017;48:2274–2277 [DOI] [PubMed] [Google Scholar]
- 8.Fullerton HJ, deVeber GA, Hills NK, Dowling MM, Fox CK, Mackay MT, et al. Inflammatory biomarkers in childhood arterial ischemic stroke: Correlates of stroke cause and recurrence. Stroke. 2016;47:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626 [DOI] [PubMed] [Google Scholar]
- 10.Kamholz J, Tremblay G. Chickenpox with delayed contralateral hemiparesis caused by cerebral angiitis. Ann Neurol. 1985;18:358–360 [DOI] [PubMed] [Google Scholar]
- 11.Sebire G, Meyer L, Chabrier S. Varicella as a risk factor for cerebral infarction in childhood: A case-control study. Ann Neurol. 1999;45:679–680 [DOI] [PubMed] [Google Scholar]
- 12.Lanthier S, Armstrong D, Domi T, deVeber G. Post-varicella arteriopathy of childhood: Natural history of vascular stenosis. Neurology. 2005;64:660–663 [DOI] [PubMed] [Google Scholar]
- 13.Fullerton HJ, Hills NK, Elkind MS, Dowling MM, Wintermark M, Glaser CA, et al. Infection, vaccination, and childhood arterial ischemic stroke: Results of the VIPS Study. Neurology. 2015;85:1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steinlin M, Bigi S, Stojanovski B, Gajera J, Regenyi M, El-Koussy M, et al. Focal cerebral arteriopathy: Do steroids improve outcome? Stroke. 2017;48:2375–2382 [DOI] [PubMed] [Google Scholar]
- 15.Elbers J, Armstrong D, Yau I, Benseler S. Vascular imaging outcomes of childhood primary angiitis of the central nervous system. Pediatr Neurol. 2016;63:53–59 [DOI] [PubMed] [Google Scholar]
- 16.Steinlin M, O’Callaghan F, Mackay MT. Planning interventional trials in childhood arterial ischaemic stroke using a delphi consensus process. Dev Med Child Neurol. 2017;59:713–718 [DOI] [PubMed] [Google Scholar]
- 17.Elkind MS, Hills NK, Glaser CA, Lo WD, Amlie-Lefond C, Dlamini N, et al. Herpesvirus infections and childhood arterial ischemic stroke: Results of the VIPS Study. Circulation. 2016;133:732–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324 [DOI] [PubMed] [Google Scholar]
- 19.Lo WD, Ichord RN, Dowling MM, Rafay M, Templeton J, Halperin A, et al. The pediatric stroke recurrence and recovery questionnaire: Validation in a prospective cohort. Neurology. 2012;79:864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan LC, Hills NK, Fox CK, Ichord RN, Pergami P, deVeber GA, et al. Socioeconomic determinants of outcome after childhood arterial ischemic stroke. Neurology. 2018;91:e509–e516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sultan SM, Beslow LA, Vossough A, Elkind MS, Kasner SE, Mirsky DM, et al. Predictive validity of severity grading for cerebral steno-occlusive arteriopathy in recurrent childhood ischemic stroke. Int J Stroke. 2015;10:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinlin M, Pfister I, Pavlovic J, Everts R, Boltshauser E, Capone Mori A, et al. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): A population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36:90–97 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.