Abstract
Fat cells (adipocytes) develop from adipocyte precursor cells (preadipocytes) that themselves derive from mesenchymal progenitors. Although the events controlling preadipocyte differentiation into mature adipocytes have been largely explored, the mechanisms that direct mesenchymal progenitors down the adipocyte pathway remain unknown. Similarly, although adipocytes are generally thought to derive from mesoderm, key information is lacking regarding the origin and the development of the adipose tissue during embryogenesis. The aim of this study was to gain insight into the ontogeny of fat cells, both in mouse embryonic stem (mES) cell-derived cultures and during normal development. We first used genetically engineered mES cells to produce and select ES cell-derived neuroepithelial progenitors and showed that neuroectoderm, rather than mesoderm, may be a source of adipocytes in mES cell-derived cultures. We then used primary and secondary cultures of developing quail neural crest (NC) cells to demonstrate that NC cells are able, upon stimulation with defined factors, to differentiate into adipocytes, thus providing a powerful system to study the earliest stages of adipocyte differentiation. Finally, we mapped NC derivatives in vivo using Cre-mediated recombination in transgenic mice and demonstrated that a subset of adipocytes originates from the NC during normal development.
Keywords: Adipocyte, Differentiation, Development, Origin, Neural crest, Mouse, Quail
Introduction
Obesity has become a serious worldwide health problem driving an ever-growing interest for the study of adipose tissue development. Obesity is the result of an imbalance between energy intake and expenditure, and is often characterised by an increase in both adipocyte size and numbers. In addition to storing and mobilizing neutral lipids in response to various hormones, adipocytes are endowed with intracrine, autocrine/paracrine and endocrine properties, such as the secretion of leptin and adiponectin (Ailhaud and Hauner, 2003). The adipocyte lineage originates from mesenchymal progenitors, which by yet unknown mechanisms form adipocyte precursor cells or preadipocytes, that then differentiate into mature, lipid-containing adipocytes. This overall process is termed adipogenesis and can occur during the whole lifespan of an organism (Ailhaud et al., 1992; Hausman et al., 2001; Rosen and MacDougald, 2006).
The differentiation of preadipocytes into adipocytes has been extensively studied in vitro (reviewed by Otto and Lane, 2005; Rosen and MacDougald, 2006). This is mostly because of the establishment of immortal, preadipocyte cell lines that were selected from disaggregated mouse embryos or from adult adipose tissue for their ability to accumulate cytoplasmic triacylglycerols (Green and Kehinde, 1975; Green and Kehinde, 1976; Green and Meuth, 1974; Negrel et al., 1978). These cell lines are believed to be faithful models of preadipocyte differentiation and they have provided important insights into the control of the late steps of adipogenesis. Preadipocyte differentiation is governed by the sequential expression of a set of key transcription factors, including members of the CCAAT/enhancer binding protein (C/EBP) and the peroxisome proliferator-activated receptor (PPAR) families (Mandrup and Lane, 1997; Tontonoz et al., 1995), as well as the adipocyte determination and differentiation factor-1/sterol response element binding protein 1c (ADD1/SREBP1c) (Ailhaud et al., 1992; Rosen et al., 2000). Terminal differentiation is accompanied by changes in the expression of cytoskeletal and extracellular matrix proteins (Spiegelman and Farmer, 1982) and by dramatic increases in the fat cell-specific expression of PPARγ, adipocyte fatty acid binding protein (FABP4) and several lipid-synthesizing enzymes such as glycerophosphate deshydrogenase (GPDH) (MacDougald and Lane, 1995). However, established preadipocyte cell lines are limited for studying early events of differentiation as they represent cells that are already committed to the adipogenic lineage.
The initial commitment of mesenchymal progenitors to the adipocyte lineage is much less understood, mostly because there are no specific cell surface markers available so far to identify and isolate mesenchymal progenitors or preadipocytes in vivo. Furthermore, because adipose tissue cannot be detected macroscopically during mammalian embryogenesis, minimal information is available regarding the ontogeny of fat cells. In the trunk and limbs, where most of the adipose tissues will finally form, mesenchyme is of mesodermal origin and therefore adipocytes are thought to derive from mesoderm only. However, it is worth noting that during development of higher vertebrates, the mesoderm is not the only germ layer source of mesenchymal cells. In the head, the facial bones, jaw and associated connective tissues have been shown to derive from the neural crest (NC). The NC is a vertebrate cell population that arises from the neuroectoderm. After neural tube closure, NC cells undergo an epithelio-mesenchyme transition and migrate to diverse regions in the developing embryo. They then become widely distributed in numerous sites, where they differentiate into diverse cell types. NC-derivatives include pigment cells, neurons and glial cells of the peripheral nervous system (PNS), as well as some endocrine cells. In the head and neck, the NC also yields mesectodermal cells, which are ectoderm-derived mesenchymal cells differentiating into connective tissue cells, vascular smooth muscle cells, tendons, dermis, odontoblasts, cartilages and bones (reviewed by Dupin et al., 2006; Le Douarin and Kalcheim, 1999; Le Douarin et al., 2004). In contrast to other mesenchymal cells, lineage relationships between the NC and adipocytes have not been carefully explored in the past. Seminal grafting experiments performed in the 1970s and 1980s in the avian embryo indicated, however, that the NC might generate adipocyte-like cells in some areas of the face and the neck (Le Lievre and Le Douarin, 1975).
Mouse embryonic stem (mES) cells might provide an alternative system for studying the early steps of adipocyte development. mES cells are proliferating, pluripotent stem cells that have been isolated from blastocyst-stage mouse embryos (Bradley et al., 1984; Brook and Gardner, 1997; Evans and Kaufman, 1981; Martin, 1981). They can be propagated indefinitely in an undifferentiated state in vitro, and they can be easily genetically modified (Smith et al., 1988; Williams et al., 1988). When ES cells are cultured without leukaemia inhibitory factor (LIF) on a non-adherent surface, they aggregate to form embryoid bodies (EBs) in which the cells form ectodermal, mesodermal and endodermal derivatives, thus offering a unique cell culture model to study early steps of development (Keller, 1995). Extra-embryonic endoderm, cardiac, endothelial and haematopoietic cell types normally predominate during differentiation of mES cells in EBs (Doetschman et al., 1985; Fehling et al., 2003). However, exposure of developing EBs to all-trans retinoic acid (RA) induces alternative lineages (Rohwedel et al., 1999). At high doses, RA promotes neural differentiation (Bain et al., 1995; Okabe et al., 1996). By contrast, early and transient treatment with intermediate levels of RA seems to favour the emergence of mesenchymal progenitors capable, when subsequently exposed to appropriate signal molecules, of generating adipocytes, osteoblasts or chondrocytes (Dani et al., 1997; Kawaguchi et al., 2005; Phillips et al., 2003). It is unknown whether these different mesenchymal cell types develop independently or from a common multipotent precursor. In addition, the cellular and molecular mechanisms underlying formation of mesenchymal precursors from ES cells remain obscure. Recent data by Kawaguchi et al. suggest that either a mesodermal subset or NC-like cells, or both, may be the source(s) of mesenchymal precursors in mES cell-derived cultures (Kawaguchi et al., 2005).
The aim of this study was to gain insight into the ontogeny of fat cells, both in ES cell-derived cultures and during normal development. We first used genetically engineered mES cells to produce and select mES cell-derived neuroepithelial progenitors and we show that the neuroectoderm/NC, rather than the mesoderm, may be a source of adipocytes in mES cell-derived cultures. Most importantly, we then isolated primary NC cells from developing quail embryos and we demonstrated that these cells can be induced to differentiate into mature adipocytes upon stimulation with defined growth factors and hormones. Finally, we used Cre-lox fate mapping in Sox10-Cre transgenic mice to demonstrate that a subset of adipocytes originate from the NC during normal development.
Materials and Methods
ES cell culture and adipocyte differentiation
All reagents were from Sigma unless otherwise indicated. All cells were incubated at 37°C in a humidified 5% CO2 95% air atmosphere. CGR8 (Mountford et al., 1994) and OSG (Billon et al., 2002) mES cells were maintained without feeders in Glasgow modification of Eagle’s medium (GMEM, Invitrogen) supplemented with foetal calf serum (FCS; 10%, Dutscher), non-essential amino acids (Invitrogen), glutamine (10 mM, Invitrogen), sodium pyruvate (100 mM, Invitrogen), 2-mercaptoethanol (1 mM) and LIF, as described (Wdziekonski et al., 2006).
EB formation and adipocyte differentiation were performed according to the protocol described by Dani et al. (Dani et al., 1997) with minor modifications. ES cell culture medium without LIF was used throughout and changed every day during suspension culture and every 2 days after plating. The hanging drop method (Hole, 1999) was employed to aggregate 103 cells per 20 μl of medium for 3 days. Cell aggregates were then pooled and cultured in suspension with RA (10−7 M) for 3 days. For subsequent differentiation, EBs were plated on gelatin-coated dishes. After 24 hours, adipocyte differentiation was induced by the addition of insulin (170 nM), triiodothyronine (T3, 2 nM), and roziglitazone (0.5 μM), a treatment referred to herein as DIF1.
Neural differentiation protocol on genetically engineered ES cells
Genetically engineered, selectable sox2-βgeo/oct4-tk ES cells (OSG) have been described elsewhere (Billon et al., 2002). Basically, these cells have a βgeo gene inserted into the Sox2 locus and a hygromycin-thymidine-kinase (tk) fusion gene inserted into the Oct4 locus. As Sox2 is specifically expressed in neuroepithelial cells and Oct4 is expressed in undifferentiated ES cells, treatment of these doubly targeted ES cells with both Ganciclovir and G-418 allows selection of neuroepithelial cells, while eliminating residual undifferentiated ES cells. Neural differentiation and selection of neuroepithelial precursors were performed as described (Billon et al., 2002): on day 0, differentiation was induced by growing the cells in suspension without LIF in order to induce formation of EBs, and RA (10−6 M) was added on day 4 to promote neural development. After 2 days, the medium was changed to a 50:50 mixture of Dulbecco’s modified Eagle’s medium (DMEM)-F12 containing N2 supplement and Neurobasal medium containing B27 supplement (Invitrogen). FGF-2 (PreproTech, Rocky Hill, NJ, USA) was added at 20 ng/ml together with G-418 (100 μg/ml) and Ganciclovir (2.5 μM) to select for neuroepithelial cells and against undifferentiated ES cells, respectively. At day 8, EBs were dissociated with trypsin and replated in the same medium on poly-D-lysine (PDL; 10 μg/ml)- and laminin (10 μg/ml)-coated tissue culture flasks. After 2 more days (day 10; 4 days of selection), adipocyte development was promoted by the addition of DIF1 treatment (see above) in ES cell medium without LIF.
Quail NC cell cultures
Fertile quail eggs, obtained from commercial sources, were incubated at 38°C and staged according to Hamburger and Hamilton (HH) (Hamburger and Hamilton, 1951) or according to the number of pairs of somites formed. Cephalic neural crest cells (CNCCs) were obtained from explants of mesencephalic-rhombencephalic neural tubes that were microsurgically removed from quail embryos at the 6-8 somite stage (stage 9 HH). Trunk neural crest cells (TNCCs) were isolated similarly from neural tubes dissected at the 18-25 somite stage from the level of the last 10 somites formed. Explanted neural tubes (including premigratory NC) were cultured in cloning medium, as described (Trentin et al., 2004). After 48 hours, the neural tubes were removed, leaving behind the outgrowth of migratory NC cells, which constitute the primary cultures.
After five days of primary culture, CNCC or TNCC were either left in cloning medium (control) or switched to various combinations of media known to be permissive for adipocyte development (Rodriguez et al., 2004; Student et al., 1980): L1 medium contains DMEM (Invitrogen) supplemented with 10% FCS (Dutscher). Hmads medium is a 50:50 mixture of DMEM and Ham’s-F12 (Invitrogen) supplemented with 10 μg/ml transferrin. Adipocyte differentiation was induced using DIF1 (see above) or DIF2 treatments. In DIF2 treatment, cells were first treated with dexamethasone (1 μM), 1-methyl-3-isobutylmethyl-xanthine (IBMX, 0.5 mM), insulin (170 nM), T3 (2 nM) and roziglitazone (0.5 μM) for 2 days and then switched to DIF1 treatment (i.e. dexamethasone and IBMX were omitted).
To perform secondary cultures, CNCC or TNCC from 2-day primary cultures (see above) were harvested by treatment with trypsin-EDTA solution (Trentin et al., 2004) and seeded in four-well culture plates (Nunc) at a density of 5×103 cells/well (20 μl) in cloning medium. After 4 more days, adipocyte differentiation was induced using various combinations of the media described above.
CNCC and TNCC cultures were recorded as positive for adipocyte differentiation only when they included at least 10 adipocytes per culture.
Animals
All animals were housed in the University College London (UCL) animal facility. Animal experiments were performed according to the UK Animal Act 1986 and approved by the UCL Care Committee. Rosa26R-YFP mice were purchased from the Jackson Laboratory (Bar Harbor, Maine, USA). These mice were crossed with Sox10-Cre mice expressing Cre recombinase under the control of the Sox10 promoter (Matsuoka et al., 2005). Offspring with the genotype Sox10-Cre/Rosa26R-YFP were used in this study. Eight-week-old mice were asphyxiated with CO2 and tissues were immediately removed and fixed in 4% (w/v) paraformaldehyde (PFA) in phosphate-buffered saline (PBS) at room temperature for 1 hour. All tissues were cryoprotected overnight in 20% (w/v) sucrose in PBS, embedded in Tissue-Tek optimum cutting temperature (OCT) compound (R. A. Lamb, Eastbourne, UK) and frozen on dry ice. Sections of 25 μm thickness were cut on a cryostat and mounted on SuperFrost® Plus glass slides (VWR International).
RT-PCR analysis
Total RNA was extracted using the TRI-Reagent kit (Euromedex, Souffel Weyersheim, France) according to the manufacturer’s instructions. cDNAs were synthesised using SuperScript Reverse Transcriptase (Invitrogen) according to the supplier’s instructions and were used as templates for the polymerase chain reaction (PCR). All primer sequences are detailed in Table 1. For semi-quantitative PCR, parameters were 94°C for 30 seconds for the denaturing step, 60°C for 30 seconds for the annealing step and 72°C for 1 minute for the elongation step. Real-time PCR assays were run on an ABI Prism 7000 real-time PCR machine (PerkinElmer Life Sciences). Reactions were performed according to the manufacturer’s instructions using SYBR green master mix (Eurogentec, Angers, France). PCR conditions were as follows: 2 minutes at 50°C, 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Gene expression was quantified using the comparative-ΔCt method. Data were normalised relative to Gapdh amplification and the highest expression was defined as 100%.
Table 1. PCR primer sequences.
| Gene | Specificity | Forward primer (5′-3′) | Reverse primer (5′-3′) | Size (bp) | Accession number |
|---|---|---|---|---|---|
| (A) RT-PCR | |||||
| FABP4 | Mouse | TTGGTCACCATCCGGTCAGA | TTCCACCACCAGCTTGTCAC | 207 | NM_024406.1 |
| β-actin | Mouse | GGCCCAGAGCAAGAGAGGTATCC | ACGCACGATTTCCCTCTCAGC | 460 | NM_007393.1 |
| CEBPα | Avian | GTGCTTCATGGAGCAAGCCAA | TGTCGATGGAGTGCTCGTTCT | 191 | X66844 |
| PPARγ | Avian | TACATAAAGTCCTTCCCGCTGACC | TCCAGTGCGTTGAACTTCACAGC | 470 | AF163811 |
| FABP4 | Avian | GAGTTTGATGAGACCACAGCAGA | ATAACAGTCTCTTTGCCATCCCA | 330 | AF432507 |
| 18s | Avian | TAGATAACCTCGAGCCGATCGCA | GACTTGCCCTCCAATGGATCCTC | 312 | AF173612 |
| (B) Quantitative RT-PCR | |||||
| GAPDH | Mouse | CATGGCCTTCCGTGTTCCTA | TGCCTGCTTCACCACCTTCT | 106 | NM_008084 |
| Oct4 | Mouse | GTTGGAGAAGGTGGAACCAA | CTCCTTCTGCAGGGCTTTC | 61 | NM_013633.1 |
| SOX1 | Mouse | GCCGAGTGGAAGGTCATGT | TGTAATCCGGGTGTTCCTTCAT | 97 | NM_009233.1 |
| SOX1 | Mouse | GCCGAGTGGAAGGTCATGT | TGTAATCCGGGTGTTCCTTCAT | 97 | NM_009233.1 |
| SOX2 | Mouse | GTGTTTGCAAAAAGGGAAAAGT | TCTTTCTCCCAGCCCTAGTCT | 60 | NM_011443.2 |
| SOX9 | Mouse | CAGCAAGACTCTGGGCAAG | TCCACGAAGGGTCTCTTCTC | 63 | NM_011448.2 |
| SOX10 | Mouse | CACATCGACTTCGGCAACGT | CCGTTGGGTGGCAGGTATT | 107 | XM_128139.5, XM_900992.2 |
| FOXD3 | Mouse | AGCGCGATGTAAGAGTAGGG | AGGTCTGACCCCGAACAAG | 63 | NM_010425.3 |
Immunocytochemistry and stainings
mES cells were cultured on PDL-coated glass coverslips and fixed in 4% PFA in PBS for 5 minutes at room temperature. After washing with PBS, cells were incubated for 30 minutes in 10% normal goat serum/0.1% Triton X-100 to block non-specific staining. They were then incubated for 1 hour in the first antibody, washed in PBS, and incubated for 1 hour in FITC-coupled goat anti-mouse immunoglobulin (Ig) or goat anti-rabbit Ig antibodies (diluted 1:100; Jackson ImmunoResearch Laboratories) and bisbenzamide (5 ng/ml; Hoechst No. 33342; Sigma). Coverslips were mounted in Citifluor mounting medium (CitiFluor, London, UK) and examined with a Zeiss fluorescence microscope. The following antibodies were used: monoclonal anti-rat nestin antibody (diluted 1:100, Pharmingen), polyclonal anti-Sox9, anti-Sox10 and anti-FoxD3 antibodies (diluted 1:100, Chemicon).
Quail NC cell cultures were fixed at day 20 in 4% PFA in PBS for 30 minutes and cell phenotypes were analysed by immunocytochemistry as described previously (Trentin et al., 2004). Briefly, glial cells and myofibroblasts were identified using anti-Schwann cell myelin protein (SMP) (Dulac et al., 1988) and an antibody to α-smooth muscle actin (Sigma), respectively; neurons and adrenergic cells were stained with anti-βIII-tubulin (Chemicon) and anti-quail tyrosine hydroxylase (Fauquet and Ziller, 1989), respectively; melanocytes were recognised by the presence of pigment granules whereas unpigmented melanoblasts were labelled with anti-melanocyte/melanoblast early marker (Nataf et al., 1993). Secondary FITC- and Texas Red-conjugated antibodies were purchased from Southern Biotech (Birmingham, AL, USA). Fluorescence was observed under an X70 Olympus inverted microscope.
For immunofluorescence staining of yellow fluorescent protein (YFP) and perilipin, cryostat sections of transgenic mice were air-dried and immersed in PBS, pH 7.4, containing 0.1% Triton X-100 for 5 minutes to remove excess OCT compound. Non-specific binding sites were blocked by incubating slides with 10% heat-inactivated sheep serum/0.5% Triton X-100/PBS for at least 1 hour at room temperature. After blocking, sections were incubated overnight at 4°C with rabbit anti-GFP polyclonal antibody (ab290, Abcam; diluted 1:8000) and guinea pig anti-perilipin polyclonal antibody (PROGP20, RDI, Concord, MA, USA; diluted 1:2000). After washing the sections several times in PBS containing 0.1% Triton X-100, they were incubated with a secondary antibody mixture of fluorescein-conjugated anti-rabbit IgG and rhodamine-conjugated anti-guinea pig IgG (both at 1:200; Pierce Biotechnology) for 1 hour at room temperature. After several washes in PBS containing 0.1% Triton X-100, cell nuclei were counterstained with Hoechst (0.01 mg/ml; Sigma). Sections were coverslipped with an antifade mounting medium (Dako Cytomation Fluorescent) and observed under a confocal microscope (Leica TCS SP).
Neutral lipid accumulation was assessed in mES cells and NC cell cultures by Oil Red O staining as previously described (Abderrahim-Ferkoune et al., 2003).
Results
Early steps of adipogenesis in mES cells are correlated with downregulation of mesoderm markers and upregulation of neural/NC markers
To explore the origin of adipocytes derived from mES cells, we first induced adipogenesis using a standard protocol involving treatment of early differentiating EBs with RA (Dani et al., 1997). We treated EBs with RA between day 3 and day 6 of differentiation, a period known to be permissive for adipocyte development, and we examined expression of mesodermal (Brachyury), mesenchymal (Sox9), neuroepithelial (Sox1) and NC (FoxD3, Sox9 and Sox10) marker genes. Consistent with previous reports, we observed that, whereas treatment of nascent EBs with RA resulted in marked reduction of Brachyury expression (Fehling et al., 2003; Kawaguchi et al., 2005), Sox1, Sox9, Sox10 and FoxD3 mRNAs were all detected in RA-treated EBs (data not shown) (Kawaguchi et al., 2005). Together, these data support the idea that RA treatment, largely used to induce adipocyte development in mES cells, reduces mesoderm formation and favours neuroepithelial/NC development in EBs.
Adipocytes can be obtained from a purified population of mES cell-derived neuroepithelial progenitors
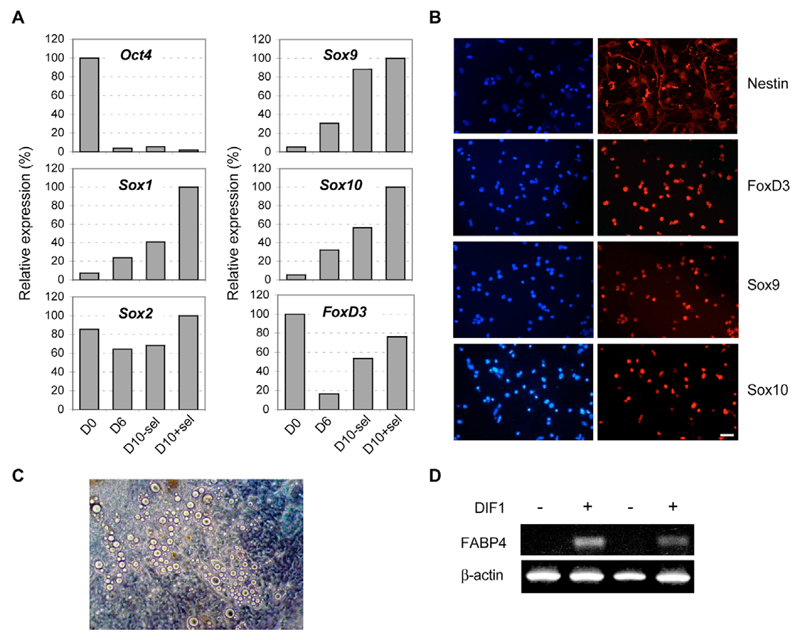
We then tested the hypothesis that neuroectoderm, rather than mesoderm, could be a source of adipocytes in mES cell-derived cultures. We used genetically engineered, selectable Sox2-βgeo/oct4-tk mES cells that allow enrichment for neuroepithelial progenitors (Billon et al., 2002; Li et al., 1998). We first used a standard, RA treatment protocol to promote neural development of these selectable mES cells (Bain et al., 1995; Billon et al., 2002; Li et al., 1998). After 6 days, we selected EBs with G418 and Ganciclovir to enrich for neuroepithelial cells and eliminate residual undifferentiated mES cells, respectively. After 4 days of selection (Fig. 1A, Day 10+selection), Oct4 mRNA could barely be detected, suggesting that no residual undifferentiated ES cells were present in the culture. By contrast, Sox1 and Sox2 mRNAs were readily detected (Fig. 1A), and more than 85% of the cells expressed the neuroepithelial marker nestin (Fig. 1B), indicating that a combined negative and positive selection strategy was extremely efficient to generate highly enriched populations of neuroepithelial cells (Billon et al., 2002). This treatment also resulted in a significant increase in Sox9, Sox10 and FoxD3 mRNAs (Fig. 1A), suggesting that NC-like cells might develop within the selected population of neuroepithelial cells. These data were further supported by the finding that a high percentage of the cells also expressed FoxD3, Sox9 and Sox10 proteins (Fig. 1B).
Fig. 1. Development of adipocytes from ES cell-derived neuroepithelial precursors.
Sox2-βgeo/oct4-tk genetically engineered ES cells were treated with RA and then selected between day 6 (D6) and day 10 (D10) to enrich for neuroepithelial cells and to eliminate residual undifferentiated ES cells. They were then induced to differentiate towards the adipocyte lineage. (A) At various times, the cells were processed for quantitative PCR analysis using Oct4, Sox1, Sox2, Sox9, Sox10, FoxD3 or GAPDH probes. Data were normalised relative to GAPDH amplification and the highest expression was defined as 100%. Similar results were obtained in two independent experiments. +sel, with selection; –sel, no selection. (B) After selection (D10), neuroepithelial precursors were stained with anti-FoxD3, anti-Sox9 or anti-Sox10 antibody (red) to identify NC-like cells, and with bisbenzimide to identify cell nuclei (blue). 14 days after induction of adipocyte differentiation (+), adipocytes were identified either using their characteristic morphology (C) or by RT-PCR to detect FABP4 mRNA (D). The results in D are shown for two independent experiments. Scale bar: 100 μm.
To investigate whether selected, mES cell-derived neuroepithelial cells could develop towards adipogenesis, we plated these cells and cultured them in the presence of factors known to promote adipocyte differentiation in ES cell-derived cultures (DIF1) (Dani et al., 1997). We used accumulation of lipid droplets within the cells, as well as fat cell-specific expression of FABP4 mRNA, to monitor adipocyte differentiation. As shown in Fig. 1C, mature adipocytes containing lipid droplets could easily be observed after 14 days of treatment with adipogenic factors. Furthermore, FABP4 mRNA was readily detected in cells treated with adipogenic factors (Fig. 1D). By contrast, in the absence of adipogenic factors, mES cell-derived neuroepithelial cell cultures showed neither lipid accumulation nor FABP4 expression (data not shown).
Together, these data suggest that, in vitro, adipocytes can develop from a highly enriched population of mES cell-derived neuroepithelial cells, possibly through a NC pathway.
Adipocytes can differentiate from quail cephalic and truncal NC cells in vitro
We then checked whether adipocytes could develop from primary NC cells isolated from a normal developing embryo. We used in vitro cultures of quail NC cells because they have been instrumental in establishing the developmental potentialities of cephalic and trunk NC cells (Baroffio et al., 1988; Baroffio et al., 1991; Dupin et al., 1990; Lahav et al., 1998; Trentin et al., 2004).
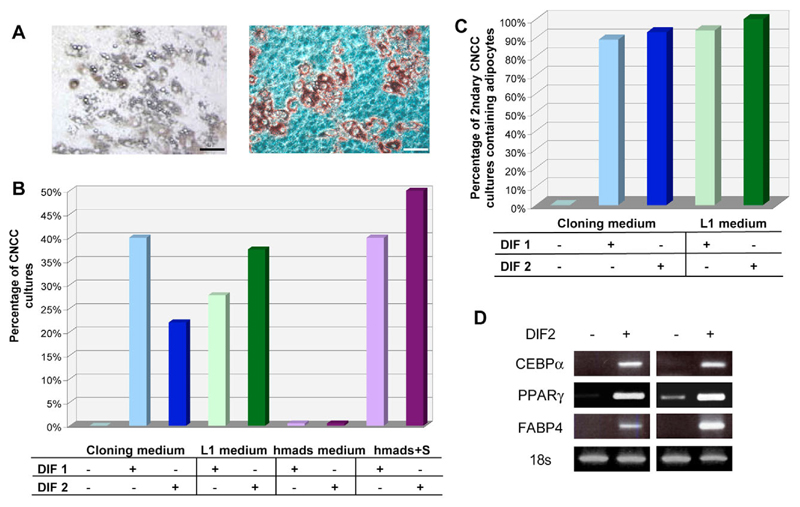
We first isolated neural tubes (including the premigratory NC) from the cephalic region of quail embryos at HH stage 9 (Hamburger and Hamilton, 1951), and allowed cephalic NC cells (CNCC) to migrate away from the neural tubes for 48 hours in explant cultures. We then removed neural tubes and grew migrating CNCC in culture medium permissive for differentiation along the main NC derivatives (i.e. cloning medium) (Trentin et al., 2004). Four days later (at day 6 of culture), we either maintained CNCC under these conditions or switched them to culture media known to be permissive for adipocyte differentiation of either mouse preadipocyte cell lines (i.e. L1 medium) (Student et al., 1980) or human adipose tissue-derived stem cells (i.e. serum-free hmads medium) (Rodriguez et al., 2004). We induced adipocyte differentiation in each of these three media using two well-described adipogenic protocols (i.e. DIF1 and DIF2, see Materials and methods section). After 15 more days, we assessed for the presence of adipocytes using their characteristic morphological feature (lipid droplet-filled cytoplasm) and by staining the cultures with Oil Red O to reveal neutral lipid droplets. As shown in Fig. 2A, typical mature adipocytes could readily be detected in CNCC cultures stimulated to differentiate in cloning medium, as well as in L1 medium (data not shown). By contrast, hmads medium could not sustain CNCC survival, resulting in the death of most of the cultures. Quantification of the number of CNCC primary cultures containing adipocytes in each medium condition revealed that up to 40% of adipocyte-containing cultures could be obtained in cloning medium, and 37% in L1 medium (Fig. 2B). No adipocyte developed in the presence of hmads medium. The addition of 1% serum to this medium (hmads+S medium), however, enhanced CNCC survival and formation of up to 50% of adipocyte-containing cultures in differentiating conditions (Fig. 2B). Together, these data suggest that adipocytes can readily develop from primary cultures of CNCC under various culture conditions.
Fig. 2. Development of adipocytes in primary cultures of quail cephalic NC cells (CNCC).
(A,B) Primary cultures of CNCC were obtained from mes-rhombencephalon of HH stage 9 quail embryo in explant culture and expanded for 5 days in cloning medium. Adipocyte differentiation was then induced using different media (cloning, L1 and hmads) and adipogenic treatments (DIF1 or DIF2). Adipocytes were identified after 15 days. (A) Typical adipocytes show lipid droplet-filled cytoplasm (left) and are stained with Oil Red O, which reveals neutral lipids (right). Scale bar: 100 μm. (B) Quantification of CNCC primary cultures containing adipocytes after treatment with the mentioned media and adipogenic treatments. A total of 100 cultures were analysed. (C,D) Secondary cultures of quail CNCC were isolated from 48-hour primary cultures. Adipocyte differentiation was then induced at day 6 using the mentioned media and adipogenic treatments. (C) Quantification of secondary CNCC cultures containing adipocytes after 15 days of treatment. A total of 84 cultures were analysed. (D) Expression of CEBPα, PPARγ, FABP4 and 18s RNAs after 3 days in cloning medium+DIF1. The results are shown for two independent CNCC cultures out of 10 distinct experiments.
To reduce the variability of cell differentiation between the different primary CNCC cultures, we next investigated the adipogenic potentialities of CNCC in secondary cultures, prepared by replating primary CNCC at the initial density of 2.5×105 cells per ml. As shown in Fig. 2C, 89-100% of day 20 secondary cultures contained adipocytes when induced to differentiate in either cloning or L1 medium. Furthermore, several markers of adipocyte differentiation, including CEBPα, PPARγ and FABP4 mRNAs, could readily be detected in these conditions after 9 days of culture, when the first differentiating adipocytes were detected under microscopy (Fig. 2D). Therefore, our results clearly indicate that, at least in vitro, CNCC can differentiate into adipocytes with a high efficiency. Analysis of CNCC cultures at day 20 with lineage-specific markers indicated that other CNCC derivatives, such as glial cells, neurons, melanocytes and myofibroblasts, also differentiated in adipocyte-containing cultures (data not shown).
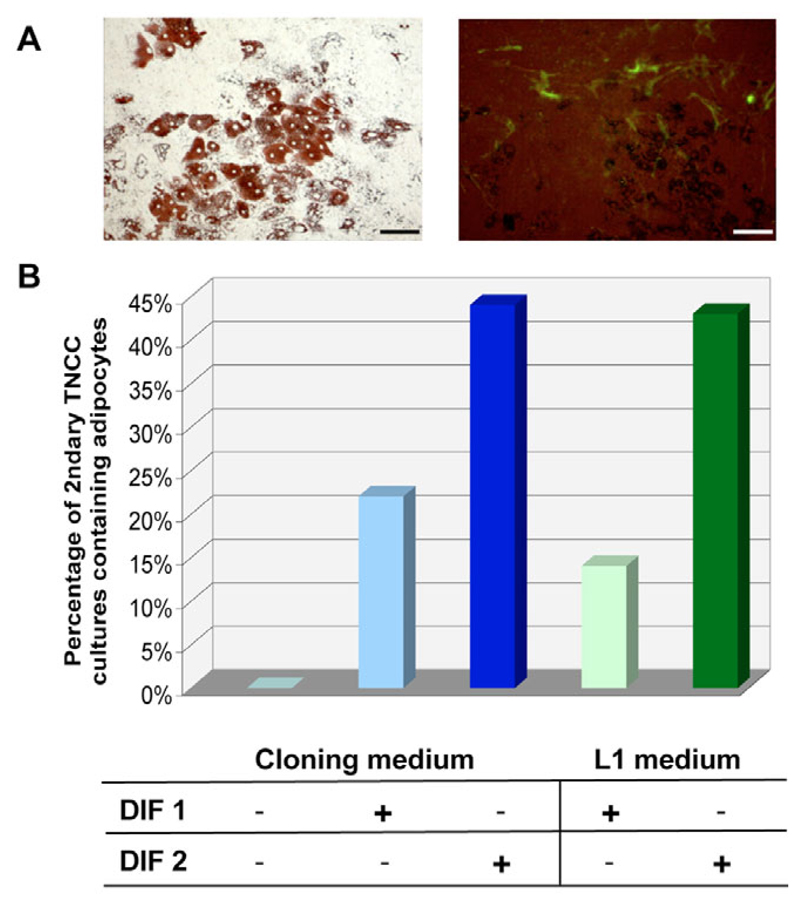
To investigate whether the trunk NC cells (TNCC) can differentiate into adipocytes in vitro, we isolated quail NC cells from the thoracic level, replated them into secondary cultures and treated them as above. As shown in Fig. 3A, adipocytes could readily be observed after 20 days in adipogenic conditions. These cultures also comprised SMP-positive Schwann cells (Fig. 3A), as well as other cell types known to arise from TNCC (data not shown). Quantification of TNCC cultures containing adipocytes revealed that more than 40% of TNCC cultures contained adipocytes when submitted to DIF2 treatment in cloning or L1 media (Fig. 3B). Therefore, trunk NC cells in culture exhibit adipogenic developmental potential, although with a lower frequency than cephalic NC cells in similar conditions.
Fig. 3. Development of adipocytes in secondary cultures of quail trunk NCC (TNCC).
TNCC that had migrated from cultured thoracic neural tubes were replated into secondary cultures and treated as in Fig. 2. (A) Typical adipocytes showing Oil Red O-stained lipid droplets (left) and SMP-positive glial cells (right) were identified after 20 days in adipogenic conditions. Scale bar: 100 μm. (B) Quantification of TNCC cultures containing adipocytes after treatment with the mentioned media and adipogenic treatments. A total of 42 cultures were analysed.
Some adipocytes are derived from the NC during mouse development
To investigate whether subsets of adipocytes originate from the NC during normal development, we adopted a recombinase-mediated lineage labelling strategy in transgenic mice. We used Sox10-Cre transgenic mice to map NC derivatives because to date, Sox10 is considered as the best bona fide NC marker (Matsuoka et al., 2005). Indeed, Sox10 is expressed strongly in premigratory and migratory NC cells at all rostro-caudal levels of the neural axis during early mouse embryonic development (Kuhlbrodt et al., 1998). Most importantly, it is not expressed in cephalic and somitic mesoderm (Ferguson and Graham, 2004). We crossed Sox10-Cre transgenic founders to a Cre-conditional R26-YFP reporter line (Srinivas et al., 2001) to identify the YFP+ NC-derived population at a single-cell resolution. Extensive analysis of Cre and Sox10 expression on the double transgenic offspring has been previously conducted by Matsuoka et al. to ascertain that Sox10 activation of the Cre transgene accurately reflects endogenous gene expression (Matsuoka et al., 2005).
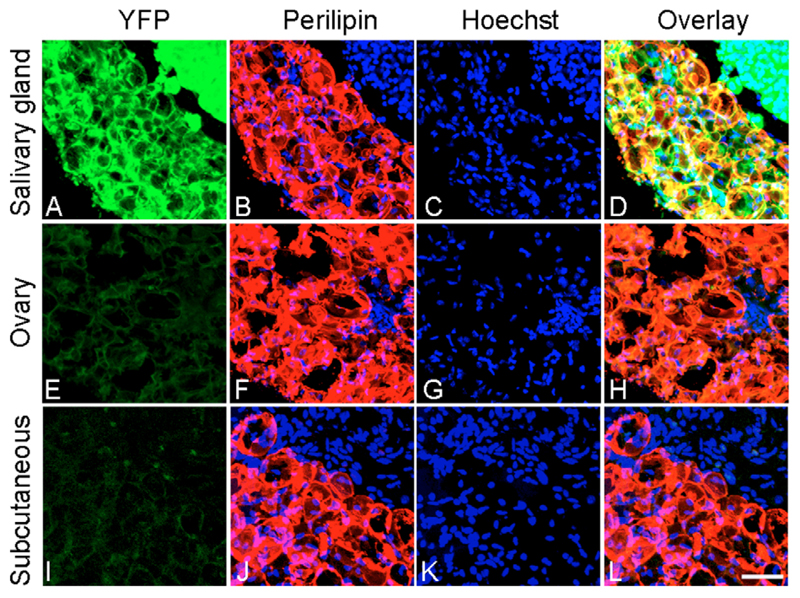
We examined Sox10-Cre/R26-YFP offspring for the presence of YFP+ adipocytes at postnatal day 28 (P28), because at this stage the adipose tissue can be detected at both cephalic (jaws) and trunk (subcutaneous and perigonadal depots) levels. We confirmed adipocyte identity by immunolabelling sections with perilipin, an adipocyte marker that is specifically expressed at the periphery of lipid droplets (Greenberg et al., 1991). As shown in Fig. 4A-D, YFP+, perilipin+ adipocytes could be detected in the area around the salivary gland and ears in Sox10-Cre/R26-YFP mice. Perilipin showed complete coexpression with YFP, suggesting that the majority of adipocytes in this site are derived from NC, and not from mesoderm. By contrast, YFP expression could not be detected in either peri-ovarial (Fig. 4E-H) or subcutaneous (Fig. 4I-L) adipocytes, suggesting that these two truncal fat depots do not arise from NC. Consistent with these findings, staining for β-galactosidase activity in 18-month-old Sox10-Cre/R26-lacZ males revealed β-galactosidase-positive adipocytes around the salivary gland, but not in truncal anatomic regions including subcutaneous, perirenal, periepididymal and interscapular adipose depots (data not shown).
Fig. 4. Development of adipocytes from the NC during mouse development.
Permanent genetic lineage labelling of pre- and post-migratory NC was achieved by crossing transgenic mice carrying a Sox10-Cre construct into a R26-YFP reporter. Double immunolabelling of P28 Sox10-Cre/R26-YFP offspring with anti-GFP (green) and anti-perilipin (red) antibodies was used to identify NC derivatives and adipocytes, respectively. Bisbenzimide was used to identify cell nuclei (blue). Sections show salivary gland and ear (A-D), peri-ovarial (E-H) and trunk subcutaneous (I-L) regions. There is almost complete colocalisation of YFP and perilipin in the salivary gland area, whereas no overlapping can be detected in the ovary and trunk subcutaneous adipose depots.
Scale bar: 50 μm.
Discussion
Throughout the past century, observations about the development of adipose tissue have been recorded in a variety of species. Early on, it was noticed that fat depots develop in many regions during mammalian postnatal life, generally in areas composed of loose connective tissues such as the subcutaneous layers between muscles and dermis, but also around internal organs (Rosen et al., 2000). Extensive histological and histochemical studies in pig foetuses have revealed that immature fat cells, containing small lipid droplets, appear in subcutaneous areas from the last third of the gestation period, always in close association with blood vessels (Hausman and Kauffman, 1986; Hausman et al., 1990). Several mesodermal cell types within the foetus have been proposed to be adipocyte precursors, including fibroblasts and endothelial cells (reviewed by Hausman et al., 1980). However, the diffuse nature of adipose tissues, as well as the lack of molecular markers to prospectively identify adipocyte precursors, have left the origin of the adipocyte lineage uncertain. In the present study, we have used several approaches to address this issue in mice and avians and we provide direct evidence for the contribution of the NC to the adipocyte lineage.
To gain insight into the ontogeny of fat cells, we first used mouse ES cells, because they provide a powerful system to model the earliest stages of mammalian development (Keller, 2005). Early and transient treatment with RA turned out to be crucial for quantitative induction of adipocytes and other mesenchymal cell types from ES cells (Dani et al., 1997; Kawaguchi et al., 2005). However, the basis for this effect of RA is uncertain. It has been proposed that RA might posteriorise nascent mesoderm in developing EBs, without promoting somitogenesis nor sclerotome development (Kawaguchi et al., 2005). RA treatment also favours the formation of neuroectoderm derivatives within EBs, including NC-like cells expressing Sox9, Sox10 and FoxD3 (this study) (see also Kawaguchi et al., 2005). Because some mesenchymal cells are known to develop from the NC during normal development (reviewed by Dupin et al., 2006; Le Douarin et al., 2004; Le Douarin and Kalcheim, 1999), it has been proposed that either a mesodermal subset or NC, or both, may be the source(s) of mesengenesis in ES cells (Kawaguchi et al., 2005). In support of the second hypothesis, we show here, using genetically engineered ES cells, that it is possible to derive adipocytes from a highly enriched population of ES cell-derived neuroepithelial precursors. These precursors express NC markers, suggesting that adipocytes developing from RA-treated ES cells in vitro might follow a NC, rather than a mesodermal, differentiation pathway. However, the elucidation of the molecular events involved in RA-induced adipogenesis in mES cells is unclear at present and requires further investigation. Interestingly, RA-independent formation of major neuroectodermal derivatives, including NC cells, has been reported from mouse and primate ES cells in vitro (Mizuseki et al., 2003). NC induction involved exposure to stromal cell-derived inducing activity (SDIA) and BMP4, and allowed the generation of PNS neurons and smooth muscle cells. The formation of other mesenchymal derivatives, however, was not assessed in this study. Furthermore, in this system too, the molecular pathways underlying NCC induction and differentiation remain to be clarified (Mizuseki et al., 2003).
Regardless of the uncertainty about NCC and adipocyte formation in mES cell cultures, independent observations indicate that some adipocytes might normally develop through a NC pathway in vivo. Indeed, quail-chick grafting experiments performed by Le Lievre and Le Douarin indicated that the cephalic NC generates adipose cells in the skin over the calvarium and in the ventral part of the neck (Le Lievre and Le Douarin, 1975). In the present report, we revisited these observations through modern cell fate mapping experiments in the mouse. Using Sox10-Cre/R26 transgenic mice (Matsuoka et al., 2005), we have demonstrated that a subset of adipocytes in the face (salivary gland and ear) indeed originate from NC, and not from mesoderm, during normal development. The contribution of NC to mesenchymal cell types during development is not restricted to the adipocyte lineage and has been established in various classes of vertebrates. The replacement of the cephalic NC by its quail counterpart in chick embryos showed that the facial and visceral skeleton, including the hyoid cartilages, as well as the frontal, parietal and squamosal bones, are NC derived; only the occipital and otic (partly) regions of the skull are of mesodermal origin. Moreover, much of the dermis, all of the connective components of facial musculature and the wall (except endothelium) of the blood vessels that irrigate the face and forebrain have a NC origin (reviewed in Le Douarin et al., 2004). The contribution of cephalic NC cells to building the head skeleton and the cardiac outflow tract has been confirmed in the mouse, using genetic Wnt1-Cre fate mapping (Chai et al., 2000; Jiang et al., 2000; Santagati and Rijli, 2003). Our findings that mature adipocytes in the ear region arise from the NC during mouse development provide further evidence of the crucial role of the cephalic NC in generating the various mesenchymal derivatives forming head structures and tissues, and identify the adipose tissue as being one of them.
NCC fate is not the same along the neural axis, as established by mapping the anteroposterior origin of NC derivatives in quail-chick chimera (Le Douarin et al., 2004). The generation of mesenchymal derivatives was found to be restricted to the cephalic NC region (from mid-diencephalon to somite 4 inclusive) in higher vertebrates. Indeed, when the quail trunk NC is orthotopically implanted in the chick, no quail mesenchymal cells are ever present in the host (Le Douarin and Teillet, 1974; Nakamura and Ayer-le Lievre, 1982). In order to confirm these data, Matsuoka et al. analysed 23 truncal skeletal structures known to derive entirely from mesoderm (including the pelvic bone, ilium, humerus, ulna, tibia, fibula, digits and sacral vertebrae) in sox10-Cre/R26-GFP mice (Matsuoka et al., 2005). They found that none of these elements were GFP positive, whereas dorsal root ganglia and Schwann cells surrounding motor axons, both of which have a trunk NC origin, were GFP labelled. In accordance with these observations, we report here that NC-derived adipocytes could be found in the cephalic region, but not in truncal, subcutaneous and perigonadal fat depots of Sox10-Cre/R26-YFP mice. These findings suggest that truncal adipocytes do not arise from NC. They further indicate that, similarly to other mesenchymal cells such as chondrocytes and osteocytes, adipocytes have a different origin along the anteroposterior axis: in the trunk they derive from the mesoderm, whereas in the cephalic region adipocytes exhibit an alternative origin in the NC. Whether these different origins reflect functional differences is unknown at present. Of note, morphological and functional differences have been reported for different fat depots in rodents and humans: visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), for instance, differ in various biochemical properties, such as insulin and adrenergic response (Lafontan and Berlan, 2003; Montague and O’Rahilly, 2000). Whether cephalic versus truncal adipose depots also present site-specific regulation remains to be assessed. It also remains to be ascertained whether, in addition to the NC, the cephalic mesoderm and/or the anteriormost somites also contribute to the formation of some adipose tissues in the head and neck.
We report here that quail NCC, upon stimulation with defined adipogenic factors, can efficiently differentiate into adipocytes in vitro. It is worth noting that the ability to generate adipocytes in vitro was not restricted to CNCC, but was also shared by TNCC, although adipogenic potential was higher in CNCC. In vivo, trunk NC cells give rise to melanocytes, PNS neurons and glial cells, and to adrenal medullary cells. By contrast, the trunk NC does not form an axial and appendicular trunk skeleton nor associated connective tissues (Le Douarin et al., 2004). A recent study, however, suggested that trunk NC cells provide a small contribution to mesenchymal tissues by generating a subset of fibroblasts in the mouse sciatic nerve (Joseph et al., 2004). Therefore, although during normal development the property of NC to form mesenchyme is almost restricted to the cephalic part of the neural axis, our finding that TNCC in culture can generate adipocytes suggests that a hidden capacity of trunk NC to yield mesenchymal cells can be revealed by appropriate environmental cues. In support of this hypothesis, it has been reported that small subsets of mesenchymal cells can be derived from TNCC after unilateral heterotopic grafting, provided that these cells develop in close relationship with host cephalic migratory NC cells (Nakamura and Ayer-le Lievre, 1982). In addition, long-term in vitro culture of avian TNCC can trigger their differentiation into chondrocytes (Abzhanov et al., 2003; McGonnell and Graham, 2002). Moreover, mouse TNC explants yield dentine and bone when recombined with branchial arch 1 epithelium in intraocular grafts (Lumsden, 1988). Finally, clonogenic cells from avian and mammalian TNC generate myofibroblasts in addition to neural and melanocytic cell types in vitro (Shah et al., 1996; Trentin et al., 2004). Together, these data support the idea that the trunk NC of higher vertebrates has cryptic mesenchymal differentiation potentials.
Our findings that quail NCC can generate adipocytes in vitro opens exciting new opportunities to study the events regulating the earliest stages of adipocyte lineage induction and specification from the NC. Adipocytes produced from CNCC accumulate intracellular lipids as multiple droplets. Furthermore, they express key adipocyte differentiation regulators such as CEBPα and PPARγ, as well as the specific adipocyte molecular marker FABP4. This pattern of gene expression is entirely consistent with that observed upon adipogenesis of murine clonal preadipocyte cell lines (Student et al., 1980) and human adipose tissue-derived stem cells (Rodriguez et al., 2004), suggesting that the regulatory pathways involved in adipocyte terminal differentiation are conserved between these three species.
As a conclusion, we present here several lines of evidence showing that adipocytes differentiate from the NC in vivo and in vitro, thus providing important insight into the developmental origin of the adipocyte lineage. First, the derivation of adipocytes from mES cells was found to involve a neuroepithelial/NC-like, rather than mesodermal, differentiation pathway. Second, we have shown that quail cephalic and trunk NC cells can generate adipocytes in vitro. Finally, we have determined that mature adipocytes in the ear region arise from the NC during mouse development. Given that clonogenic NC cells include multipotent progenitors yielding chondrocytes, myofibroblasts and neural/melanocytic cells (Baroffio et al., 1991; Trentin et al., 2004), it is tempting to speculate that the NC might be a source of mesenchymal stem cells giving rise to adipocytes, chondrocytes and possibly other mesenchymal cells, as described in adult bone marrow (Caplan, 1991; Owen, 1988; Pittenger et al., 1999). A major challenge now is to discover how and when the adipocytic lineage segregates in NC-derived cells. Analysis of the progeny of single NC cells should allow us to determine whether adipocytes arise from multipotent progenitors or from early committed cells.
Acknowledgments
We thank members of the Dani and Ailhaud laboratories for critical advice and comments on the manuscript. This research was supported by CNRS and by funding under the Sixth Research Framework Programme of the European Union, Project FunGenES (LSHG-CT-2003-503494). Work at UCL was supported by the UK Medical Research Council and the Wellcome Trust.
References
- Abderrahim-Ferkoune A, Bezy O, Chiellini C, Maffei M, Grimaldi P, Bonino F, Moustaid-Moussa N, Pasqualini F, Mantovani A, Ailhaud G, et al. Characterization of the long pentraxin PTX3 as a TNFalpha-induced secreted protein of adipose cells. J Lipid Res. 2003;44:994–1000. doi: 10.1194/jlr.M200382-JLR200. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development. 2003;130:4567–4579. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- Ailhaud G, Hauner H. Handbook of Obesity. New York: Marcel Dekker; 2003. [Google Scholar]
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Baroffio A, Dupin E, Le Douarin NM. Clone-forming ability and differentiation potential of migratory neural crest cells. Proc Natl Acad Sci USA. 1988;85:5325–5329. doi: 10.1073/pnas.85.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroffio A, Dupin E, Le Douarin NM. Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development. 1991;112:301–305. doi: 10.1242/dev.112.1.301. [DOI] [PubMed] [Google Scholar]
- Billon N, Jolicoeur C, Tokumoto Y, Vennstrom B, Raff M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor alpha 1 (TRalpha1) EMBO J. 2002;21:6452–6460. doi: 10.1093/emboj/cdf662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Dani C, Smith AG, Dessolin S, Leroy P, Staccini L, Villageois P, Darimont C, Ailhaud G. Differentiation of embryonic stem cells into adipocytes in vitro. J Cell Sci. 1997;110:1279–1285. doi: 10.1242/jcs.110.11.1279. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Dulac C, Cameron-Curry P, Ziller C, Le Douarin NM. A surface protein expressed by avian myelinating and nonmyelinating Schwann cells but not by satellite or enteric glial cells. Neuron. 1988;1:211–220. doi: 10.1016/0896-6273(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Dupin E, Baroffio A, Dulac C, Cameron-Curry P, Le Douarin NM. Schwann-cell differentiation in clonal cultures of the neural crest, as evidenced by the anti-Schwann cell myelin protein monoclonal antibody. Proc Natl Acad Sci USA. 1990;87:1119–1123. doi: 10.1073/pnas.87.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Creuzet S, Le Douarin NM. The contribution of the neural crest to the vertebrate body. Adv Exp Med Biol. 2006;589:96–119. doi: 10.1007/978-0-387-46954-6_6. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fauquet M, Ziller C. A monoclonal antibody directed against quail tyrosine hydroxylase: description and use in immunocytochemical studies on differentiating neural crest cells. J Histochem Cytochem. 1989;37:1197–1205. doi: 10.1177/37.8.2569003. [DOI] [PubMed] [Google Scholar]
- Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269:70–80. doi: 10.1016/j.ydbio.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976;7:105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obes Rev. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Kauffman RG. The histology of developing porcine adipose tissue. J Anim Sci. 1986;63:642–658. doi: 10.2527/jas1986.632642x. [DOI] [PubMed] [Google Scholar]
- Hausman GJ, Campion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res. 1980;21:657–670. [PubMed] [Google Scholar]
- Hausman GJ, Wright JT, Jewell DE, Ramsay TG. Fetal adipose tissue development. Int J Obes. 1990;14(Suppl. 3):177–185. [PubMed] [Google Scholar]
- Hole N. Embryonic stem cell-derived haematopoiesis. Cells Tissues Organs. 1999;165:181–189. doi: 10.1159/000016698. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- Lahav R, Dupin E, Lecoin L, Glavieux C, Champeval D, Ziller C, Le Douarin NM. Endothelin 3 selectively promotes survival and proliferation of neural crest-derived glial and melanocytic precursors in vitro. Proc Natl Acad Sci USA. 1998;95:14214–14219. doi: 10.1073/pnas.95.24.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet M-A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:163–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8:971–974. doi: 10.1016/s0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Dev Suppl. 1988;103:155–169. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- MacDougald OA, Lane MD. Adipocyte differentiation. When precursors are also regulators. Curr Biol. 1995;5:618–621. doi: 10.1016/s0960-9822(95)00125-4. [DOI] [PubMed] [Google Scholar]
- Mandrup S, Lane MD. Regulating adipogenesis. J Biol Chem. 1997;272:5367–5370. doi: 10.1074/jbc.272.9.5367. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell IM, Graham A. Trunk neural crest has skeletogenic potential. Curr Biol. 2002;12:767–771. doi: 10.1016/s0960-9822(02)00818-7. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Sakamoto T, Watanabe K, Muguruma K, Ikeya M, Nishiyama A, Arakawa A, Suemori H, Nakatsuji N, Kawasaki H, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–888. doi: 10.2337/diabetes.49.6.883. [DOI] [PubMed] [Google Scholar]
- Mountford P, Zevnik B, Duwel A, Nichols J, Li M, Dani C, Robertson M, Chambers I, Smith A. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS. Mesectodermal capabilities of the trunk neural crest of birds. J Embryol Exp Morphol. 1982;70:1–18. [PubMed] [Google Scholar]
- Nataf V, Mercier P, Ziller C, Le Douarin NM. Novel markers of melanocyte differentiation in the avian embryo. Exp Cell Res. 1993;207:171–182. doi: 10.1006/excr.1993.1177. [DOI] [PubMed] [Google Scholar]
- Negrel R, Grimaldi P, Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci USA. 1978;75:6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59:89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- Phillips BW, Vernochet C, Dani C. Differentiation of embryonic stem cells for pharmacological studies on adipose cells. Pharmacol Res. 2003;47:263–268. doi: 10.1016/s1043-6618(03)00035-5. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Elabd C, Delteil F, Astier J, Vernochet C, Saint-Marc P, Guesnet J, Guezennec A, Amri EZ, Dani C, et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem Biophys Res Commun. 2004;315:255–263. doi: 10.1016/j.bbrc.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Rohwedel J, Guan K, Wobus AM. Induction of cellular differentiation by retinoic acid in vitro. Cells Tissues Organs. 1999;165:190–202. doi: 10.1159/000016699. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald A. Adipocyte differentiation from the inside out. Nat Rev. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci. 2003;4:806–818. doi: 10.1038/nrn1221. [DOI] [PubMed] [Google Scholar]
- Shah NM, Groves AK, Anderson DJ. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982;29:53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Student AK, Hsu RY, Lane MD. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin A, Glavieux-Pardanaud C, Le Douarin NM, Dupin E. Self-renewal capacity is a widespread property of various types of neural crest precursor cells. Proc Natl Acad Sci USA. 2004;101:4495–4500. doi: 10.1073/pnas.0400629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wdziekonski B, Villageois P, Vernochet C, Phillips B, Dani C. Use of differentiating embryonic stem cells in pharmacological studies. Methods Mol Biol. 2006;329:341–351. doi: 10.1385/1-59745-037-5:341. [DOI] [PubMed] [Google Scholar]
- Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]