Abstract
Caveolin-1 has a critical role in orchestrating the membrane organization of B cells. In its absence, signaling via the B cell antigen receptor and B cell tolerance are impaired, which results in autoimmunity.
In this issue of Nature Immunology, Minguet et al. identify a novel role for the scaffolding protein caveolin-1 (Cav1) in organizing the membrane localization of immunoglobulin M (IgM) and IgD B cell antigen receptors (BCRs)1. Cav1-deficient B cells exhibit defective signaling via the BCR, which results in impaired immune responses to T cell–independent immunogens. Surprisingly, despite this defect, over time Cav1-deficient B cells undergo population expansion, which leads to the production of autoantibodies and frank auto-immunity. The authors identify a defect in receptor editing in Cav1–/– mice, which suggests that impaired B cell central tolerance might contribute to autoimmunity. Although there is a growing literature on the importance of fine membrane organization of receptors and co-receptors for BCR signal transduction, the functional consequences of perturbing this organization in vivo have yet to be explored. Moreover, although impaired BCR signaling in patients with primary B cell immunodeficiencies, such as combined variable immunodeficiency (CVID), is closely linked to autoantibody-mediated disease2, there is a dearth of animal models that recapitulate this association. Cav1-deficient mice thus represent a new model of autoimmunity in the setting of B cell immunodeficiency.
High-resolution microscopy has revealed that IgM-BCRs and IgD-BCRs localize into distinct clusters that associate differentially with co-receptors such as CD19 on the surface of resting B cells3,4. Disruption of the actin cytoskeleton with drugs is sufficient to trigger signaling in a BCR-dependent manner, which indicates that physical segregation of BCRs away from downstream signal-transduction machinery in resting B cells is important for the restriction of signaling in the basal state3. However, the molecular mechanisms by which such fine membrane organization is established, as well as the in vivo relevance of BCR segregation, are not well understood. Here, Minguet et al. take advantage of the well-described proximity-ligation assay to probe the localization of IgMBCRs and IgD-BCRs relative to each other as well as to lipid-raft components in both resting B cells and antigen-stimulated B cells1. In Cav1-deficient B cells, IgM-BCRs and IgD-BCRs are mislocalized in the resting state and IgM-BCRs fail to translocate into lipid rafts after antigenic stimulation. This altered membrane organization of BCRs leads to diminished BCR signal transduction in Cav1–/– B cells, which results in poor responses to T cell–independent immunogens in Cav1–/– mice5. Despite their impaired BCR signaling, Cav1-deficient mice develop an autoimmune disease characterized by the formation of germinal centers with large numbers of activated B cells, autoantibody production and immunocomplex glomerulonephritis. The spontaneous formation of germinal centers and autoantibody production are at least partly B cell intrinsic in this model and might result from defective receptor editing that leads to the escape of self-reactive B cells into the periphery. The Cav1–/– mice represent a unique genetic tool with which to explore how disruption of membrane organization affects not only BCR signaling but also in vivo B cell function.
It has long been postulated that defective central-tolerance mechanisms should produce autoimmune disease. Indeed, defective signaling via the T cell antigen receptor (TCR) results in impaired negative selection (and/or impaired generation regulatory T cells) in the thymus and drives systemic autoimmune disease in mice6. Well-described examples include the Skg mouse model of inflammatory arthritis, in which a severely hypomorphic allele encoding the tyrosine kinase Zap70 is sufficient to produce rheumatoid-arthritis-like pathology and serum rheumatoid factor in response to an innate immune stimulus7. Susceptibility to autoimmunity appears to lie at a ‘sweet spot’ of partial T cell immunodeficiency. This is perhaps best exemplified by an allelic series of Zap70-hypomorphic variants in which mice with severely or modestly impaired TCR signaling remain healthy, while those with an intermediate phenotype develop a type 2 helper T cell–mediated inflammatory disease that resembles that of patients with partial T cell immunodeficiency6,8. It is possible that impaired central tolerance in this context permits the escape of self-reactive T cells that remain just functional enough to cause damage in the periphery. Alternatively, it has also been suggested that TCR signaling in response to self-peptide–major histocompatibility complex is important for maintaining peripheral T cells in a quiescent state, and disruption of TCR signaling in peripheral T cells paradoxically drives autoimmunity under such circumstances6.
In contrast to autoimmune disease that arises from impaired TCR signaling, the development of systemic autoimmunity in the setting of impaired BCR signaling is relatively rare in genetic mouse models. Indeed, the most well-described B cell–intrinsic mutations that lead to autoimmunity in mice produce enhanced BCR signaling9. The Src family kinase Lyn, the immunoreceptor-tyrosine-based-inhibition-motif–containing inhibitory receptors CD22 and FcγRIIb and the effector phosphatase Shp1 are all part of a common inhibitory signaling pathway that serves to repress BCR signal transduction in B cells. Disruption of this inhibitory pathway genetically (as in Cd22–/–, Fcgr2b–/–, Lyn–/–, PtprcE613R/E613R or Ptpn6mv/mv mice) results in substantially enhanced BCR signaling in response to endogenous antigens and the generation of lupus-like autoimmune disease9. Central tolerance is intact in such mice; the hyper-responsive BCR signaling breaks peripheral tolerance in a Toll-like receptor–dependent manner, although the precise checkpoint that is breached remains incompletely defined.
One of the few examples in which impaired BCR signaling leads to systemic autoimmunity is the polygenic lupus-prone NZM2410 strain. One of the alleles that contributes to autoimmunity in this strain was mapped over a decade ago to the gene encoding Ly108 (Slamf6), a member of the SLAM family of receptors that signal through the adaptor SAP. Ly108 has high expression in immature B cells and can modulate BCR signal strength; the NZM2410-derived allele of Slamf6 produces weaker BCR signaling than the C57BL/6 allele does in immature B cells and might impair central tolerance that permits the escape of self-reactive B cells into the periphery10. However, the association between impaired BCR signaling and auto-immunity is very unusual in animal models. Indeed, although mice harboring germline mutations in genes encoding key components of the BCR signaling machinery, including Btk and others, have been extensively characterized, the majority of such mutations suppress autoimmunity rather than ‘breaking’ immunotolerance. Thus, Cav1-deficient mice represent an important and unusual model.
In contrast to reduced BCR signaling in mice, such reduced signaling in humans is closely linked to autoimmunity. CVID is the most common primary human immunodeficiency. It is characterized by recurrent bacterial infections, an impaired antibody response to vaccination and low serum immunoglobulin concentrations2. It can be caused by germ line mutations in a growing list of genes that prominently includes Tnfrsf13b, Tnfrsf13c, Icos and Ctla4, as well as genes encoding interleukin 21 (IL-21) and its receptor IL-21R. A subset of patients with CVID have mutations in genes encoding molecules critical for BCR signaling (for example, CD19, CD21, CD81 and BLK)2. In contrast to the prototypical severe B cell immunodeficiency Bruton’s X-linked agammaglobulinemia, which is caused by germ-line null mutations in BTK, CVID is closely linked autoantibody-mediated diseases such as autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura. The cellular mechanism by which tolerance is broken in patients with CVID is now being explored. Cloning and recombinant expression of BCRs from the immature-transitional B cell compartments of patients with CVID has shown a high frequency of self-reactive BCRs, suggestive of a defect in central tolerance11. Indeed, analysis of use of the distal joining region in the rearranged BCR light chains cloned from patients with CVID has provided direct evidence of a defect in receptor editing12.
Impaired BCR signaling might be a feature of common polygenic human autoimmune diseases that do not arise on the background of obvious immunodeficiency. Many cases of autoimmune hemolytic anemia and idiopathic thrombocytopenic purpura are diagnosed before the underlying immunodeficiency is recognized. Recently, it has been argued that the clinical syndrome of CVID lies on a continuum with monogenic diseases of immune-system dysregulation2. This raises the possibility that subclinical immunodeficiency, with associated polygenic or monogenic mutations that result in impaired BCR signaling, might have a role in at least some cases of isolated systemic autoimmunity. PTPN22 R620W is a common human genetic polymorphism that confers a small but significant risk for systemic autoimmunity. However, it is associated with discrepant altered BCR signaling phenotypes in mice and humans and its effect therefore remains controversial13. The population expansion of self-reactive transitional and naive B cells has been observed in patients with systemic lupus erythematous, rheumatoid arthritis or type 1 diabetes, which has led to speculation that impaired central B-cell tolerance might be a primary defect in these populations11.
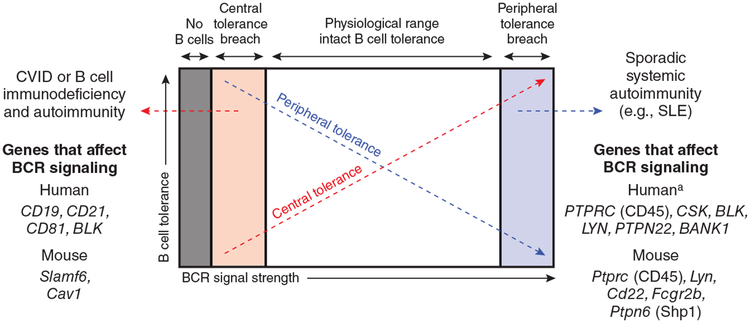
Cav1-deficient mice represent a valuable model of autoimmunity that arises in the context of B cell immunodeficiency. But why are such mouse models rare, and why do mice fail to recapitulate human phenotypes? One possibility is that, similar to defects in TCR signaling associated with autoimmunity, the degree of impaired BCR signaling must be ‘just so’: not too severe and not too mild (model, Fig. 1). The autoimmunity in patients with CVID is not completely penetrant, occurring in about 25% of cases. Moreover, it is now thought that the majority of CVID cases are polygenic2. Such observations raise the possibility that autoimmunity in this setting requires genetic modifiers that are not present in monogenic mouse models. It might be that abnormal central tolerance is necessary but not sufficient to cause frank autoimmune disease; additional defects in peripheral B cell tolerance and/or an abnormal T cell compartment might be required. It will be important to genetically delineate the B cell–intrinsic and B cell–extrinsic contributions to autoimmunity in the Cav1-deficient mouse to determine whether multiple perturbations of the immune system have a role in disease pathogenesis, especially as Cav1-deficient T cells, similar to B cells, exhibit defective TCR localization and signaling14,15. Whole-exome sequencing is opening the doors to the identification of important genetic modifiers that modulate the phenotype of monogenic human immunodeficiencies. The generation of mice with similar ‘modifier alleles’ might be necessary for more accurate modeling of autoimmunity that arises in the setting of B cell immunodeficiency.
Figure 1.
Model for the development of B cell autoimmunity at the extremes of BCR signal strength. Severe impairment in BCR signaling results in a complete block in B cell development in the bone marrow and agammaglobulinemia without autoimmunity (gray zone) (for example, Bruton’s X-linked agammaglobulinemia). In contrast, genetic mutations that modestly impair BCR signaling subvert central tolerance mechanisms such as receptor editing and negative selection, which allows the escape of autoreactive clones into the periphery and drives autoantibody production in CVID (pink zone)2. Genetic mutations that result in the disruption of critical inhibitory pathways that restrain BCR signaling break peripheral tolerance and, notably, produce lupus-like autoimmunity in mouse models (blue zone)9. aAlthough BCR signaling is thought to be enhanced in B cells from patients with lupus, and multiple single-nucleotide polymorphisms in and around genes encoding molecules that regulate BCR signaling are associated with human systemic lupus erythematous (SLE)16, the functional consequences of such mutations remain controversial (for example, PTPN22) or undefined13.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Julie Zikherman, Division of Rheumatology, the Rosalind Russell and Ephraim P. Engleman Arthritis Research Center and the Department of Medicine, University of California San Francisco, San Francisco, California, USA..
Clifford A Lowell, Department of Laboratory Medicine, University of California San Francisco, San Francisco, California, USA..
References
- 1.Minguet S et al. Nat. Immunol 18, 1150–1159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogaert DJA et al. J. Med. Genet 53, 575–590 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Mattila PK et al. Immunity 38, 461–474 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Depoil D et al. Nat. Immunol 9, 63–72 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Medina FA, Williams TM, Sotgia F, Tanowitz HB & Lisanti MP Cell Cycle 5, 1865–1871 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Liston A, Enders A & Siggs OM Nat. Rev. Immunol 8, 545–558 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi N et al. Nature 426, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Siggs OM et al. Immunity 27, 912–926 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi CM, Hicks R & Diamond B J. Immunol 174, 1775–1781 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Kumar KR et al. Science 312, 1665–1669 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Meffre E Ann. NY Acad. Sci 1246, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romberg N, Ng Y-S, Cunningham-Rundles C & Meffre E Blood 118, 5977–5978 (2011). [Google Scholar]
- 13.Rawlings DJ, Metzler G, Wray-Dutra M & Jackson SW Nat. Rev. Immunol 17, 421–436 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomassian T et al. J. Immunol 187, 2993–3002 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schönle A et al. Blood 127, 1930–1939 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Morris DL & Vyse TJ Curr. Opin. Rheumatol 29, 423–433 (2017). [DOI] [PubMed] [Google Scholar]