Abstract
Background
Tranexamic acid (TXA) is an antifibrinolytic that has been shown to decrease blood loss and transfusion rates after hip and knee arthroplasty, with only limited evidence to support its use in shoulder arthroplasty. This study was conducted to determine whether intravenous (IV) TXA is more effective than placebo in reducing blood loss after primary total shoulder arthroplasty (TSA).
Methods
In this prospective, double-blind, placebo-controlled, randomized clinical trial, patients undergoing primary anatomic and reverse TSA were randomized to receive 1 g of intravenous TXA or a placebo of an equivalent volume of intravenous normal saline administered 10 minutes before the incision. The primary outcome measurement was calculated postoperative blood loss. Secondary outcomes included transfusion rates, weight of hemoglobin loss, hospital length of stay, and thromboembolic events.
Results
The study enrolled 110 patients, 2 of whom were excluded because they did not have a postoperative hemoglobin measurement, and the remaining 108 patients (52 for TXA, 56 for placebo) were analyzed. There were no significant differences between TXA and placebo groups in preoperative characteristics. For the primary outcome, the TXA group had significantly lower postoperative blood loss of 1100.9 ± 367.4 mL compared with 1274.5 ± 460.0 mL for the placebo group (P = .03). For secondary outcomes, TXA had lower weight of hemoglobin loss compared with placebo (152.2 ± 57.3 g vs. 178.0 ± 65.8 g; P = .03). No patients in the TXA or placebo groups required a transfusion.
Conclusions
Intravenous TXA reduced blood loss after primary TSA compared with placebo.
Keywords: Tranexamic acid, Total shoulder arthroplasty, Reverse total shoulder arthroplasty, Blood loss, Blood transfusion, Hospital length of stay, Randomized control trial
Anatomic and reverse total shoulder arthroplasty (TSA) is associated with the risk of moderate blood loss that can lead to transfusions. Average estimated intraoperative blood loss has been reported in the range of 354 mL to 361 mL, not accounting for additional blood loss postoperatively in soft tissues or surgical drains.4, 15 Transfusion rates of 2.4% to 9.5% have been reported for primary TSA.4, 11, 15, 16
Tranexamic acid (TXA) is a synthetic antifibrinolytic agent that is an established method of reducing blood loss and transfusion requirement for patients undergoing total hip and knee arthroplasty.2, 3, 7, 13, 22 TXA can be administered intravenously (IV), topically (intra-articularly), or orally, with most available literature addressing IV and topical administration. Systematic reviews and meta-analyses of the total hip and knee arthroplasty literature demonstrate an approximately 30% decrease in blood loss and a 50% decrease in the transfusion rate with topical or IV administration of TXA compared with placebo.2, 3, 7, 13, 22 Moreover, the literature demonstrates no increased rate of thromboembolic or other complications associated with TXA administration for hip and knee arthroplasty.7, 22 Several recent studies have begun to explore the use of TXA in TSA patients to potentially reduce blood loss and transfusion.1, 6, 8, 14, 19
This study was conducted to determine whether IV TXA is more effective than placebo in reducing blood loss after primary TSA. We hypothesized that IV TXA would significantly reduce blood loss after TSA.
Materials and methods
Study design and patients
This prospective, double-blind, placebo-controlled, randomized clinical trial of IV TXA compared with placebo was registered at clinicaltrials.gov with registration number NCT02569658. The study was designed to determine the superiority of TXA over placebo by comparing 2 parallel groups: IV TXA and IV saline. A 1:1 allocation ratio of patients to TXA and placebo was used. There were no changes to the trial design during the study.
Patients were eligible for study inclusion if they were undergoing a unilateral primary anatomic or reverse TSA by the study authors at a single institution. Exclusion criteria were allergy to TXA, acquired disturbances of color vision, preoperative use of anticoagulant therapy within 5 days of surgery, history of arterial or venous thromboembolic disease (including deep venous thrombosis, pulmonary embolism, stroke, transient ischemic attack), ongoing pregnancy or breast-feeding, recent myocardial infarction (within 6 months before surgery), cardiac stent placement, renal impairment, hemophilia, refusal of blood products, revision TSA, TSA performed for the indications of acute proximal humeral fracture, or prior open shoulder surgery, including failed open reduction and internal fixation of proximal humeral fractures. Patients who underwent prior arthroscopic shoulder procedures were eligible to participate.
Patients who met eligibility criteria and consented to participate in the study by an informed consent process were randomized to receive 10 mL of IV normal saline placebo or 1 g of IV TXA diluted in 10 mL normal saline (X-Gen Pharmaceuticals, Inc., Horseheads, NY, USA). This dose of TXA was chosen because it is standard practice at our institution to administer 1 g IV TXA 10 minutes before the incision for total hip and knee arthroplasty.
A random number algorithm was used for the randomization process. The TXA or placebo was placed into an unlabeled 10-mL syringe by research pharmacy staff who were not involved in the patient's care to ensure identical appearance and blinding between TXA and placebo. A blinded research assistant delivered the syringe to the blinded anesthesiology staff, who administered the syringe was administered 10 minutes before the incision. All patients, surgeons, anesthesiologists, and clinical staff were blinded to the patient's study group allocation. Study group allocation remained blinded by the research pharmacists until the time of data analysis.
Data acquisition was performed by a research assistant blinded to patient study group allocation. Patient demographics and preoperative characteristics were recorded and compared between the TXA and placebo groups. Included were age, sex, American Society of Anesthesiologists (ASA) Physical Status Classification, body mass index, and preoperative hemoglobin.
Surgical technique and postoperative care
Anatomic or reverse TSA was performed by 5 fellowship-trained attending surgeons. Anesthesia involved interscalene regional anesthesia combined with general anesthesia. A deltopectoral approach was used, followed by implantation of uncemented anatomic or reverse TSA implants, according to attending surgeon preference. Drains were used postoperatively based on surgeon preference and removed on postoperative day 1 in all patients.
Patients underwent standard postoperative care, including admission to the hospital for at least 1 night. Patients were monitored by a hospitalist while in the hospital and received occupational therapy. Patients had sequential compression devices for deep venous thrombosis prophylaxis during their hospital stay. The patients underwent daily complete blood count, including measurement of hemoglobin, for as long as they remained in the hospital. Patients underwent transfusion if their postoperative hemoglobin dropped below 7.0 g/dL or for higher hemoglobin values only for specific medical indications specified by the consulting hospitalist attending.
Outcomes
The primary outcome for this study was postoperative blood loss based on a formula accounting for initial patient hemoglobin, the lowest postoperative hemoglobin, and patient blood volume approximated based on patient sex, height, and weight.5, 20 This method of calculating blood loss is intended to account for intraoperative and postoperative losses, including bleeding into soft tissues. It is felt to be more accurate than methods such as estimated blood loss and surgical drain losses.5, 20 The blood loss calculation formulas are as follows:
Where Hb(loss) is loss of hemoglobin in grams, Hb(i) is preoperative hemoglobin, Hb(e) is the lowest postoperative hemoglobin, and Hb(t) is total amount of transfused hemoglobin. BV is predicted blood volume, which is calculated as follows:
Where Ht is height in meters and Wt is weight in kilograms.
Secondary outcomes included transfusion rates, weight of hemoglobin loss, intraoperative estimated blood loss, and hospital length of stay. Postoperative complications, including thromboembolic events, were noted for a 90-day postoperative period. Complications were noted on routine postoperative follow-up examinations. A research assistant who was not involved in patient care and was blinded to patient study group allocation recorded data after patients were discharged from the hospital.
Statistical analysis
Power analysis was performed before study initiation to determine sample size. Using estimated intraoperative blood loss averaging 361 ± 220 mL for TSA from a recent report4 and an estimated 30% reduction of blood loss based on total hip and knee arthroplasty literature,2, 3, 7, 13, 22 we determined that a sample size of 110 patients (55 patients per group) was necessary to achieve power of 80% at an α of 5%.
Descriptive statistics are presented with mean ± standard deviation for continuous data and as frequency with percentages for categoric data. Data were tested for normality by Kolmogorov-Smirnov testing and found to be normally distributed, and therefore, parametric tests were used. Two-sample unpaired Student t tests were used to compare continuous variables, including calculated blood loss. The χ2 and Fisher exact tests were used as appropriate based on expected values to compare categoric data. Significance was set at P < .05. Analyses were performed using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA).
Results
Patient enrollment and baseline data
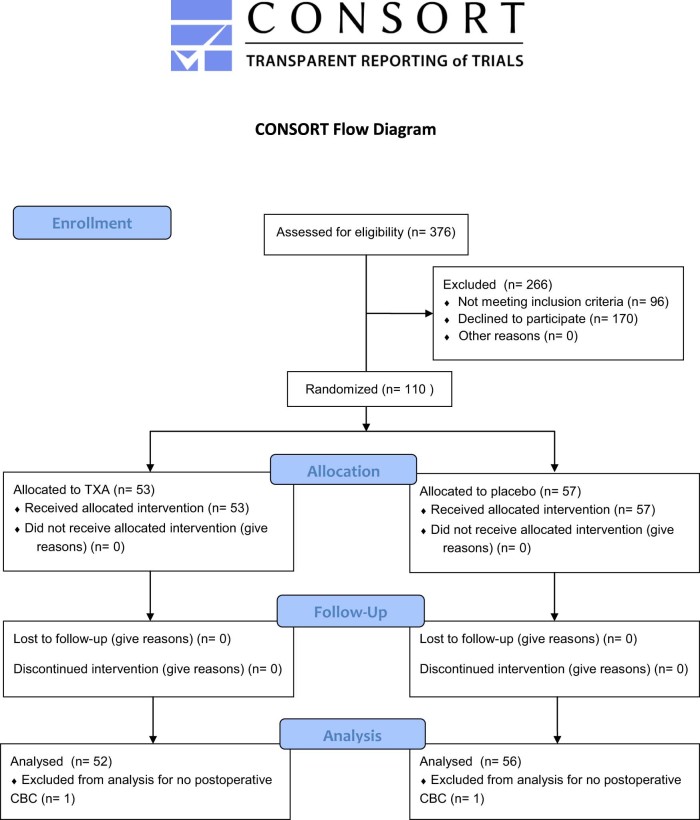
During the enrollment period from September 2015 to November 2016, 376 patients underwent primary anatomic or reverse TSA. A Consolidated Standards of Reporting Trials flow diagram is shown in Fig. 1. Of these, 92 patients were ineligible based on exclusion criteria, and 170 declined to participate. After randomization, there were 53 patients in the TXA group and 57 in the placebo group. All patients received the study drug according to their randomly assigned group. Postoperative hemoglobin values were not taken for 2 patients, and they could not be analyzed further. The remaining 108 patients (52 TXA and 56 placebo) were analyzed. No patients were lost to follow-up.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for patients in the study. (TXA, tranexamic acid; CBC, complete blood count.)
The enrolled patients were a mean age of 66.4 ± 10.1 years, and 47.2% were male (51 of 108). Reverse TSA was performed in 54.6% of patients (59 of 108). Baseline preoperative data did not significantly differ between TXA and placebo (Table I). Operative time from incision to closure was 101.9 ± 21.4 minutes (median, 99; range, 62-149 minutes), which did not differ between the TXA and placebo groups (P = .70).
Table I.
Preoperative and intraoperative characteristics for total shoulder arthroplasty patients in placebo and tranexamic acid groups
| Variable | Placebo |
TXA |
P value |
|---|---|---|---|
| (n = 56) | (n = 52) | ||
| Age, yr | 65.2 ± 9.2 | 67.7 ± 10.9 | .18 |
| Male | 28 (50.0) | 23 (44.2) | .57 |
| Weight, kg | 88.4 ± 19.1 | 84.2 ± 19.6 | .24 |
| Height, m | 1.7 ± 0.1 | 1.7 ± 0.1 | .90 |
| Body mass index, kg/m2 | 29.7 ± 5.2 | 29.0 ± 5.0 | .47 |
| Predicted blood volume, L | 5.2 ± 1.0 | 4.9 ± 1.1 | .23 |
| Preoperative hemoglobin, g/dL | 13.9 ± 1.2 | 13.7 ± 1.3 | .20 |
| Preoperative platelet count, ×109/L | 234.8 ± 58.8 | 247.7 ± 62.1 | .27 |
| Operative time, min | 102.7 ± 21.6 | 101.1 ± 21.4 | .70 |
| Reverse shoulder arthroplasty | 30 (53.6) | 29 (55.8) | .85 |
| ASA score | 2.8 (1.5) | 2.8 (0.9) | .93 |
TXA, tranexamic acid; ASA, American Society of Anesthesiologists.
Data are presented as mean ± standard deviation for continuous data and as frequency (%) for categoric data.
Primary outcome
The postoperative blood loss after TSA was significantly lower in the TXA group than in the placebo group (1100.9 ± 367.4 mL vs. 1274.5 ± 460.0 mL; P = .03; Table II). There was no statistically significant difference in blood loss by use of a drain (P = .34) between the 5 attending surgeons (P = .25) and between reverse and anatomic TSA (P = .30). Increased surgical time was correlated with increased blood loss (P = .04).
Table II.
Primary and secondary outcomes for total shoulder arthroplasty patients in placebo and tranexamic acid groups
| Variable | Placebo |
TXA |
P value |
|---|---|---|---|
| (n = 56) | (n = 52) | ||
| Primary outcome | |||
| Postoperative blood loss, mL | 1274.5 ± 460.0 | 1100.9 ± 367.4 | .03 |
| Secondary outcomes | |||
| Total hemoglobin loss, g | 178.0 ± 65.8 | 152.2 ± 57.3 | .03 |
| Estimated blood loss, mL | 221.6 ± 139.2 | 208.2 ± 122.2 | .59 |
| Hospital length of stay, d | 1.8 ± 1.2 | 1.8 ± 1.0 | .95 |
| Transfusion | 0 (0) | 0 (0) | 1.0 |
| Thromboembolic complications | 1 (1.8)* | 0 (0) | 1.0 |
| Reoperation and readmission | 0 (0) | 0 (0) | 1.0 |
TXA, tranexamic acid.
Data are presented as mean ± standard deviation for continuous variables and as frequency (%) for categoric variables.
One patient in the placebo group experienced a deep venous thrombosis.
Secondary outcomes
The TXA group had lower total hemoglobin loss compared with placebo (152.2 ± 57.3 g vs. 178.0 ± 65.8 g; P = .03; Table II). There was no difference in length of stay (1.8 ± 1.0 days for TXA vs. 1.8 ± 1.2 days for placebo; P = .95; Table II), estimated blood loss as recorded in the operative report (208.2 ± 122.2 mL for TXA vs. 221.6 ± 139.2 mL for placebo; P = .59; Table II), or in operative time between reverse (105.8 ± 21.0 minutes) and anatomic TSA (98.7 ± 21.4 minutes; P = .09). No patients in the TXA of placebo groups required a transfusion or experienced infection, readmission, or return to the operating room by 90 days postoperatively (Table II). A postoperative deep venous thrombosis occurred in 1 patient in the placebo group, but there were no other thromboembolic complications.
Discussion
TXA is widely used in total hip and knee arthroplasty due to multiple studies suggesting that it reduces postoperative blood loss and transfusion rate without increasing rates of complications.2, 3, 7, 13, 22 In recent years, there has been emerging interest in translating TXA to TSA patients because transfusion rates of 2.4% to 9.5% have been reported for primary TSA.4, 11, 15, 16 We report results of a prospective, double-blind, placebo-controlled randomized clinical trial of TXA vs. placebo for primary TSA, finding that TXA reduces blood loss after TSA.
Several studies addressed TXA in TSA with retrospective study designs.1, 6 Abildgaard et al1 retrospectively compared cohorts of primary TSA patients who received 1 g IV TXA before incision (during years 2014-2015) with a prior group of patients that did not receive TXA (during year 2013). They found that the TXA group had lower blood loss, drain output, and change in hemoglobin for primary anatomic and reverse TSA. Similarly, Friedman et al6 retrospectively compared patients receiving 20 mg/kg IV TXA before incision for primary anatomic and reverse TSA (years 2009-2012) with a prior group of patients that did not receive TXA (years 2007-2009). They found that the TXA group had reduced blood loss, 21% shorter recovery room time, and 16% shorter hospitalization. These retrospective studies suffer from limitations inherent in their design that could bias results, including lack of blinding, lack of randomization, and importantly, changes in surgical and postoperative procedures between an earlier cohort and a more recent cohort that are major confounding variables in their conclusions.
Three randomized studies have been performed in TSA patients using TXA to date.8, 14, 19 Gillespie et al8 compared TSA patients receiving 100 mL saline placebo vs. 2 g TXA in 100 mL saline solution administered by topical application into the surgical wound at the end of the case for 5 minutes. The topical TXA group had reduced blood loss (108 mL vs. 170 mL) as determined by estimated intraoperative blood loss plus loss occurring in surgical drains. This study also noted that TXA decreased changes in hemoglobin, with no transfusions or complications noted. Although the authors demonstrated the effectiveness of topical TXA, their method of blood loss calculation did not account for additional blood loss in the soft tissues that was not evacuated by the postoperative drain. The authors therefore likely under estimated the true total blood loss, and we believe our method of calculating blood loss more accurately represents postoperative blood loss.
Pauzenberger et al14 recently randomized patients to 1 g IV TXA or saline, finding reduced drainage, estimated blood loss, early postoperative pain by visual analog scale, and hematoma formation. Similarly, Vara et al19 reported that IV TXA reduced blood loss in primary reverse TSA. Our study confirms these results, also finding Level I evidence that TXA reduces blood loss after primary shoulder arthroplasty, although none of our patients required a transfusion compared with the 9.8% transfusion rate reported by Vara et al.19 Surgeons can therefore consider using TXA to reduce blood loss during TSA by whichever route of administration is best for their practice based on the available literature showing significant decreases in blood loss from IV as well as topical administration.
The present study has limitations. Although we found statistically significant decreases in blood loss for patients receiving TXA for TSA compared with placebo, our results do not establish that TXA provides a benefit to patients or health systems in improved outcomes, reduced transfusion rates, shorter lengths of stay, or cost savings. The use of TXA in hip and knee arthroplasty has demonstrated cost savings of as much as $83.73 per patient based on transfusion costs alone.9, 12, 18 After accounting for reduction in operating room, laboratory, blood, and room and board costs, Gillette et al9 was able to show $879 of cost savings per patient in the setting of a primary hip or knee arthroplasty. The transfusion rates in the groups not being treated with TXA were much higher, at 17.5% to 43%.12, 18 Transfusion rates are lower for TSA compared with hip and knee arthroplasty, so demonstrating cost-effectiveness may be more challenging.
Next, calculations for blood loss and change in hemoglobin before postoperative day 5 are at risk of inaccuracy due to hemodilution.10 Nevertheless, this is routinely performed in hip and knee arthroplasty and is unlikely to alter the significance of the study conclusions because the same methodology was applied to both groups and the hospital length of stay was equivalent between the two.10 Calculated blood losses for hip and knee arthroplasty have included values closer to 1300 mL compared with 1100 mL for TSA in our study group.5, 20 In addition, the lack of transfusions and complications in the study population makes our study underpowered to assess differences for these uncommon secondary study outcomes.17, 21, 22
Finally, the study's inclusion and exclusion criteria may limit generalizability of study findings to other scenarios. The exclusion criteria generally included medical contraindications for TXA and patients not undergoing primary TSA, including prior open shoulder surgery and revision TSA. The latter was used to achieve the necessary sample size of relatively uniform patient populations for the TXA and placebo groups. Future studies investigating TXA in revision TSA will be necessary, particularly because these patients may experience greater blood loss than primary TSA and might derive greater benefit from TXA.4, 11, 15, 16
Conclusion
IV TXA reduced blood loss after primary TSA compared with placebo.
Disclaimer
The TXA used in this study was donated by X-Gen Pharmaceuticals, Inc. (Horseheads, NY, USA), which had no other role in the study including no role in study design, patient enrollment, data collection, data analysis, and manuscript preparation.
Brian J. Cole receives royalties from Arthrex, DJ Orthopaedics, Saunders/Mosy-Elsevier, SLACK Incorporated, and Elsevier; is a paid consultant for Arthrex, Regentis, and Zimmer; has stock or stock options in Carticept and Regentis; receives research support from Arthrex, Medipost, and Zimmer; receives other financial support from Athletico, Ossur, Smith & Nephew, and Tornier; and serves of the boards for the American Journal of Orthopedics, Arthroscopy, the Journal of Bone and Joint Surgery, the Journal of the American Academy of Orthopaedic Surgeons, the American Academy of Orthopaedic Surgeons, the American Orthopaedic Society for Sports Medicine, the American Shoulder and Elbow Surgeons, Arthroscopy Association of North America, and the International Cartilage Repair Society. The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
The Rush University Medical Center Institutional Review Board approved this study on April 10, 2015 (ORA Number: 14102308-IRB01).
References
- 1.Abildgaard J.T., McLemore R., Hattrup S.J. Tranexamic acid decreases blood loss in total shoulder arthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:1643–1648. doi: 10.1016/j.jse.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Alshryda S., Mason J., Sarda P., Nargol A., Cooke N., Ahmad H. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H) J Bone Joint Surg Am. 2013;95:1969–1974. doi: 10.2106/JBJS.L.00908. [DOI] [PubMed] [Google Scholar]
- 3.Alshryda S., Mason J., Vaghela M., Sarda P., Nargol A., Maheswaran S. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K) J Bone Joint Surg Am. 2013;95:1961–1968. doi: 10.2106/JBJS.L.00907. [DOI] [PubMed] [Google Scholar]
- 4.Chalmers P.N., Gupta A.K., Rahman Z., Bruce B., Romeo A.A., Nicholson G.P. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29:856–860. doi: 10.1016/j.arth.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Fillingham Y.A., Kayupov E., Plummer D.R., Moric M., Gerlinger T.L., Valle Della C.J. The James A. Rand Young Investigator's Award: a randomized controlled trial of oral and intravenous tranexamic acid in total knee arthroplasty: the same efficacy at lower cost? J Arthroplasty. 2016;31(9 Suppl.):26–30. doi: 10.1016/j.arth.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 6.Friedman R.J., Gordon E., Butler R.B., Mock L., Dumas B. Tranexamic acid decreases blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg. 2016;25:614–618. doi: 10.1016/j.jse.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi R., Evans H.M., Mahomed S.R., Mahomed N.N. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie R., Shishani Y., Joseph S., Streit J.J., Gobezie R. Neer Award 2015: a randomized, prospective evaluation on the effectiveness of tranexamic acid in reducing blood loss after total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:1679–1684. doi: 10.1016/j.jse.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Gillette B.P., Maradit Kremers H., Duncan C.M., Smith H.M., Trousdale R.T., Pagnano M.W. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl.):137–139. doi: 10.1016/j.arth.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Barrena E., Ortega-Andreu M., Padilla-Eguiluz N.G., Pérez-Chrzanowska H., Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96:1937–1944. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 11.Gupta A.K., Chalmers P.N., Rahman Z., Bruce B., Harris J.D., McCormick F. Reverse total shoulder arthroplasty in patients of varying body mass index. J Shoulder Elbow Surg. 2014;23:35–42. doi: 10.1016/j.jse.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Johansson T., Pettersson L.G., Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76:314–319. [PubMed] [Google Scholar]
- 13.Ker K., Beecher D., Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database Syst Rev. 2013;(7) doi: 10.1002/14651858.CD010562.pub2. CD010562. [DOI] [PubMed] [Google Scholar]
- 14.Pauzenberger L., Domej M.A., Heuberer P.R., Hexel M., Grieb A., Laky B. The effect of intravenous tranexamic acid on blood loss and early post-operative pain in total shoulder arthroplasty. Bone Joint J. 2017;99-B:1073–1079. doi: 10.1302/0301-620X.99B8.BJJ-2016-1205.R1. [DOI] [PubMed] [Google Scholar]
- 15.Saltzman B.M., Chalmers P.N., Gupta A.K., Romeo A.A., Nicholson G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23:1647–1654. doi: 10.1016/j.jse.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Shields E., Iannuzzi J.C., Thorsness R., Noyes K., Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23:1449–1453. doi: 10.1016/j.jse.2014.01.052. [DOI] [PubMed] [Google Scholar]
- 17.Tan J., Chen H., Liu Q., Chen C., Huang W. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184:880–887. doi: 10.1016/j.jss.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 18.Tuttle J.R., Ritterman S.A., Cassidy D.B., Anazonwu W.A., Froehlich J.A., Rubin L.E. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29:1512–1515. doi: 10.1016/j.arth.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Vara A.D., Koueiter D.M., Pinkas D.E., Gowda A., Wiater B.P., Wiater J.M. Intravenous tranexamic acid reduces total blood loss in reverse total shoulder arthroplasty: a prospective, double-blinded, randomized, controlled trial. J Shoulder Elbow Surg. 2017;26:1383–1389. doi: 10.1016/j.jse.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Wong J., Abrishami A., Beheiry El H., Mahomed N.N., Roderick Davey J., Gandhi R. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 21.Wu Q., Zhang H.A., Liu S.L., Meng T., Zhou X., Wang P. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol. 2015;25:525–541. doi: 10.1007/s00590-014-1568-z. [DOI] [PubMed] [Google Scholar]
- 22.Yang Z.G., Chen W.P., Wu L.D. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–1159. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]